Abstract
This study investigated intramuscular triacylglycerol (IMTG) and glycogen utilisation, pyruvate dehydrogenase activation (PDHa) and acetyl group accumulation during prolonged moderate intensity exercise. Seven endurance-trained men cycled for 240 min at 57 % maximal oxygen consumption (V̇O2,max) and duplicate muscle samples were obtained at rest and at 10, 120 and 240 min of exercise. We hypothesised that IMTG utilisation would be augmented during 2-4 h of exercise, while PDHa would be decreased secondary to reduced glycogen metabolism. IMTG was measured on both muscle samples at each time point and the coefficient of variation was 12.3 ± 9.4 %. Whole body respiratory exchange ratio (RER) decreased from 0.89 ± 0.01 at 30 min to 0.83 ± 0.01 at 150 min and remained low throughout exercise. Plasma glycerol and free fatty acids (FFAs) had increased compared with rest at 90 min and progressively increased until exercise cessation. Although plasma glucose tended to decrease with exercise, this was not significant. IMTG was reduced at 120 min compared with rest (0 min, 15.6 ± 0.8 mmol kg−1 d.m.; 120 min, 12.8 ± 0.7 mmol kg−1 d.m.) but no further reduction in IMTG was observed at 240 min. Muscle glycogen was 468 ± 49 mmol kg−1 d.m. at rest and decreased at 120 min and again at 240 min (217 ± 48 and 144 + 47 mmol kg−1 d.m.). PDHa increased above rest at 10 and 120 min, but decreased at 240 min, which coincided with reduced whole body carbohydrate oxidation. Muscle pyruvate and ATP were unchanged with exercise. Acetyl CoA increased at 10 min and remained elevated throughout exercise. Acetylcarnitine increased at exercise onset but returned to resting values by 240 min. Contrary to our first hypothesis, significant utilisation of IMTG occurred during the first 2 h of moderate exercise but not during hours 2-4. The reduced utilisation of intramuscular fuels during the last 120 min was offset by greater FFA delivery and oxidation. Consistent with the second hypothesis, PDHa decreased late in moderate exercise and closely matched the estimates of lower carbohydrate flux. Although the factor underlying the PDHa decrease was not apparent, reduced pyruvate provision secondary to diminished glycolytic flux is the most likely mechanism.
Fat and carbohydrates are the primary metabolic fuels utilised by contracting skeletal muscles. Fat and carbohydrate are stored within muscle fibres as intramuscular triacylglycerol (IMTG) and glycogen, respectively, and as adipose triacylglycerol and liver glycogen, which are transported to skeletal muscle as plasma free fatty acids (FFAs) and glucose via the circulation. Although numerous studies have addressed substrate metabolism over a range of exercise intensities and durations, little is known regarding the relative proportion of these substrates late in prolonged moderate intensity exercise lasting 4 h. Indeed, few studies have examined substrate utilisation during exercise lasting longer than 90-120 min.
Prolonged exercise of moderate intensity is associated with a time-dependent decrease in carbohydrate oxidation and greater fat oxidation (Ahlborg & Felig, 1974; Romijn et al. 1993). The reduction in carbohydrate oxidation late in prolonged exercise is a function of decreased muscle glycogen utilisation which results from reduced muscle glycogen availability (Gollnick et al. 1974), and attenuated blood glucose use secondary to decreased delivery (Ahlborg & Felig, 1974; Ahlborg et al. 1982). Whilst plasma FFA oxidation increases with duration and contributes considerably to fat metabolism (Ahlborg & Felig, 1974; Romijn et al. 1993), no study to date has carefully examined IMTG oxidation after 120 min of exercise. In fact, controversy exists as to whether IMTG is even utilised during exercise (Kiens & Richter, 1998; Roepstorff et al. 2002).
IMTG is stored in lipid droplets that exist in close proximity to the mitochondria (Boesch et al. 1997) and teleologically would seem to be a suitable metabolic substrate. Isotopic tracer studies have reported that approximately one-half of the lipids utilised during prolonged moderate intensity exercise are derived from plasma FFAs, with the remaining lipid (≈50 %) thought to be derived from ‘local stores’ (Havel et al. 1964, 1967; Romijn et al. 1993; van Loon et al. 2001). These findings have led researchers to conclude that IMTG is the additional lipid source fueling oxidative pathways during prolonged moderate exercise.
Although some studies using the biopsy technique have reported significantly reduced IMTG following prolonged (90-120 min) moderate intensity (60-65 % maximal oxygen consumption (V̇O2,max) exercise (Hurley et al. 1986; Cleroux et al. 1989; Phillips et al. 1996a), controversy exists as to the extent of IMTG oxidation because others have detected no such change despite employing similar exercise protocols (Keins et al. 1993; Wendling et al. 1996; Starling et al. 1997; Keins & Richter, 1998; Bergman et al. 1999; Guo et al. 2000; Roepstorff et al. 2002; Steffensen et al. 2002).
This discrepancy in the literature is probably related to the large variability in the IMTG measured from muscle samples. For example, we previously reported an IMTG coefficient of variation (c.v.) in untrained individuals of 23.5 % (n = 13) between three biopsies sampled from the same leg at the same time, while the c.v. for glycogen was 3-10 % (Wendling et al. 1996). The reality may be that the detection of subtle changes in IMTG, which would be expected during 90-120 min of moderate exercise, may not be possible using chemical extraction techniques despite an energetically significant contribution of IMTG to ATP production.
The first purpose of this study was to better define substrate utilisation during 4 h of moderate intensity (55 % V̇O2,max) exercise by measuring the contribution of the intramuscular substrates (IMTG, glycogen) and acetyl group accumulation, estimating the contribution of plasma FFAs and glucose and measuring pyruvate dehydrogease activation (PDHa). Consistent with the greater fat oxidation and lower carbohydrate oxidation observed later in exercise, we hypothesised that IMTG utilisation would be augmented during 2-4 h of exercise, and this would occur in coordination with lower PDHa secondary to reduced glycogen metabolism. The second purpose of this study was to examine whether the variability of the IMTG measure is reduced in endurance-trained athletes compared with untrained individuals.
METHODS
Subjects
Seven endurance-trained males with cycling experience volunteered to participate in this study. Their mean (± s.d.) age, weight and V̇O2,max were 25 ± 3 years, 77 ± 4 kg and 61.7 ± 1.7 ml kg−1 min−1, respectively. Written informed consent was obtained from each subject after a detailed explanation of the experimental procedures and associated risks were outlined both verbally and in writing. The ethics committees of both institutions approved the study which was performed according to the Declaration of Helsinki.
Pre-experimental protocol
Subjects completed an incremental test to volitional exhaustion on an electromagnetically braked cycle ergometer (Lode Excalibur, Quinton Instruments, Seattle, WA, USA) to determine their maximal oxygen consumption (V̇O2,max). At least 2 days later, subjects returned to the laboratory to perform a practice ride. Subjects were instructed to consume a light mixed meal 2 h before arriving at the laboratory. Subjects cycled for 2-3 h at 55 % V̇O2,max on the same cycle ergometer as used previously. The practice ride was completed to familiarise the subjects with the laboratory environment and to ensure subjects were able to complete the prolonged exercise without premature fatigue. Respiratory gas samples were collected and analysed on-line (Quinton Q-Plex 1, Quinton Instruments, Seattle, WA, USA) at 30 min intervals throughout the practice ride.
Experimental protocol
Subjects arrived at the laboratory having consumed a light mixed meal 2 h previously. They were asked to consume the sort of meal (≈ 60 % carbohydrate) that they would normally consume prior to a long training ride and to refrain from alcohol and caffeine consumption and exercise for 24 h prior to testing. Upon arrival at the laboratory subjects voided and rested quietly on a couch. A Teflon catheter was inserted into a forearm vein and a resting blood sample (≈ 6 ml) was obtained. The catheter was kept patent by flushing with 0.9 % saline. The subject's legs were then prepared for needle biopsy under local anaesthesia (2 % lidocaine without adrenaline (epinephrine)).
Six muscle samples were obtained from the vastus lateralis throughout the experiment, four from one leg and two from the other. A resting muscle sample was obtained from leg 1 while the subject remained on the couch. Subjects commenced cycling at a power output corresponding to 55 % V̇O2,max and were asked to maintain a pedal frequency between 75 and 100 r.p.m. After 10 min a second muscle sample was obtained from leg 1 while the subject remained on the cycle ergometer. Two separate muscle samples were obtained at 2 (leg 2) and 4 h (leg 1) of exercise. Duplicate muscle samples were obtained from different incisions on the same leg, with the first sample being taken from a site proximal to the second. Muscle samples were immediately frozen in liquid N2, removed from the needle while frozen and stored in liquid N2 until analysis. The time taken to freeze the first biopsy was always < 20 s from exercise cessation and was used for all measurements. The second sample obtained at 2 and 4 h was frozen within 60 s of exercise cessation and was used for IMTG measures only. Venous blood samples (≈6 ml) were obtained at 10 and 30 min of exercise and at 30 min intervals thereafter. Prior to blood sampling, expired gases were collected for 3 min and analysed on-line. The last minute of data was recorded. Subjects were permitted to drink water ad libitum throughout exercise and a fan was directed onto subjects to facilitate heat loss.
Analysis
One portion of heparinised whole blood was immediately deproteinised 1 : 2 with 0.6 % (w/v) PCA. The PCA extract was stored at −20 °C and subsequently analysed for blood glucose, lactate and glycerol (Bergmeyer, 1974). A second portion of whole blood was centrifuged and 400 μl plasma was added to 100 μl NaCl and incubated at 56 °C for 30 min to inactivate lipoprotein lipase. The plasma was subsequently analysed for FFAs via a colorimetric method (Wako NEFA C test kit, Wako Chemicals, VA, USA). A final portion was centrifuged and the supernatant removed for the determination of insulin by radioimmunoassay (Coat-a-Count insulin test kit, Diagnostics Products, CA, USA).
A small piece of frozen wet muscle (10-20 mg) was removed and analysed for PDHa as described by Putman et al. (1993). The remaining muscle was freeze dried, dissected free of connective tissue, blood and visible fat under magnification and powdered.
One aliquot (≈5 mg) of freeze-dried muscle was extracted in a volume of 0.5 m PCA (1 mm EDTA) and neutralised with 2.2 m KHCO3. The extract was used for the determination of adenosine triphosphate (ATP), phosphocreatine (PCr), creatine and lactate by spectrophotometric assays (Bergmeyer, 1974; Harris et al. 1974). Pyruvate was determined in the extract fluorometrically (Passoneau & Lowry, 1993) and acetyl CoA and acetylcarnitine were determined by radiometric assays (Cederblad et al. 1990). Glycogen content was determined in a second aliquot (2-3 mg) of freeze-dried muscle according to the methods of Harris et al. (1974). IMTG content was determined from a 5-7 mg aliquot of powdered tissue from duplicate biopsies. The biopsies at 0 and 10 min were treated as duplicates to alleviate the need for an additional biopsy at 0 min and thus reduce undue stress to the subjects. We anticipated negligible IMTG use early in exercise and felt this would not affect our results. Briefly, the IMTG was extracted and the chloroform phase evaporated (Frayn & Maycock, 1980). After reconstitution, phospholipids were removed upon the addition of silic acid. The IMTG was saponified and the free glycerol was assayed fluorometrically (Bergmeyer, 1974). All metabolite and PDHa measurements were normalised to the highest total creatine content from the six samples obtained for each subject to correct for non-muscle contamination.
Calculations
Whole body carbohydrate and fat oxidation rates were estimated using the following equations:
(Péronnet & Massicotte, 1991). Plasma FFA oxidation was calculated as the difference between whole body fat oxidation rate (indirect calorimetry) and IMTG oxidation rate after converting IMTG oxidation to its molar equivalent assuming that the molecular mass of IMTG is 855 g mol−1 (Péronnet & Massicotte, 1991) and that each triacylglycerol molecule contains three fatty acids. Plasma glucose oxidation rate was calculated as the difference between muscle glycogen oxidation rate (biopsy) and whole body carbohydrate oxidation rate. PDH flux was calculated by converting the carbohydrate oxidation rate to mmol pyruvate kg−1 min−1. An active muscle mass of 10 kg was assumed for all calculations (Putman et al. 1993).
Statistical analysis
Data are given as means ± s.e.m. and were analysed using one-way analysis of variance (ANOVA) with repeated measures; specific differences were located using Student-Newman-Keuls post hoc test. Variability is expressed as coefficient of variation (c.v. = s.d./mean × 100) for determinations of two biopsies at each time point. A difference of P < 0.05 was considered significant.
RESULTS
Performance, respiratory and blood responses
Of the seven subjects, five completed the 240 min of exercise whilst two reached volitional exhaustion at 210 min giving a mean exercise time of 231 ± 6 min.
V̇O2 was 2.46 ± 0.13 l min−1 at 30 min of exercise (52 ± 1 % V̇O2,max) and a time-dependent drift was observed such that V̇O2 was elevated (P < 0.01) at 210 and 240 min (Table 1). Respiratory exchange ratio (RER) decreased from 0.89 ± 0.01 at 30 min to 0.83 ± 0.01 at 150 min (P < 0.01) and remained lower thoughout exercise (Table 1). Calculated whole body fat oxidation was higher and carbohydrate oxidation was lower (P < 0.01) at 150 min compared with 30 min and remained lower until the completion of exercise (Fig. 1).
Table 1.
Respiratory, blood metabolite and plasma hormone measures at rest and during 240 min of exercise at 57 % V̇O2,max
| Time (min) | V̇O2 (l min−1) | RER | Blood glucose (mmol l−1) | Blood lactate (mmol l−1) | Blood glycerol (μmol l−1) | Plasma insulin (pmol l−1) |
|---|---|---|---|---|---|---|
| 0 | NM | NM | 4.9 ± 0.4 | 0.69 ± 0.13 | 119 ± 18 | 51.9 ± 5.7 |
| 10 | NM | NM | 5.1 ± 0.6 | 1.00 ± 0.07 * | 128 ± 14 | 24.0 ± 6.4 * |
| 30 | 2.54 ± 0.13 | 0.89 ± 0.01 | 5.0 ± 0.3 | 0.91 ± 0.16 | 147 ± 12 | 14.7 ± 2.7 * |
| 60 | 2.59 ± 0.12 | 0.88 ± 0.01 | 5.1 ± 0.3 | 0.58 ± 0.08 | 211 ± 32 | 14.7 ± 5.4 * |
| 90 | 2.59 ± 0.11 | 0.86 ± 0.02 | 4.9 ± 0.3 | 0.54 ± 0.11 | 301 ± 46 * | 6.9 ± 1.5 * |
| 120 | 2.68 ± 0.10 | 0.85 ± 0.02 | 4.9 ± 0.3 | 0.49 ± 0.09 | 380 ± 49 * | 5.0 ± 0.4 * |
| 150 | 2.62 ± 0.11 | 0.83 ± 0.01 †‡ | 4.7 ± 0.3 | 0.53 ± 0.12 | 501 ± 55 * | 1.5 ± 0.4 * |
| 180 | 2.69 ± 0.12 | 0.83 ± 0.02 †‡ | 4.5 ± 0.3 | 0.59 ± 0.15 | 608 ± 51 * | 1.4 ± 0.3 * |
| 210 | 2.72 ± 0.16 † | 0.82 ± 0.01 †‡ | 4.3 ± 0.3 | 0.63 ± 0.17 | 655 ± 36 * | 1.4 ± 0.2 * |
| 240 | 2.91 ± 0.16 † | 0.81 ± 0.01 †‡§ | 4.0 ± 0.3 | 0.65 ± 0.23 | 701 ± 58 * | 0.9 ± 0.1 * |
Values are means ± s.e.m., n = 7.
Significantly different from 0 min
significantly different from 30 min
significantly different from 60 min
significantly different from 90 min, P < 0.05.
NM, not measured.
Figure 1. Whole body carbohydrate and fat oxidation rates during 240 min moderate exercise in men.
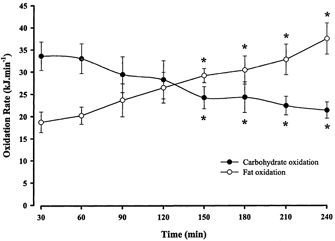
Values are means ± s.e.m., n = 7. * Significantly different (P < 0.05) from rest. To convert oxidation rates to grams per minute divide value for carbohydrate by 16.19, and for fat by 40.80.
Resting blood glucose averaged 4.9 ± 0.4 mmol l−1 at rest and decreased to 4.0 ± 0.3 mmol l−1 at 240 min, but this decrease did not reach significance (P = 0.08) (Table 1). Blood lactate was elevated (P < 0.01) from rest at 10 min of exercise but returned to basal levels by 30 min and was unchanged thereafter (Table 1). Plasma FFAs were higher (P < 0.01) at 90 min of exercise compared with rest. Thereafter, FFA levels increased significantly at each time point, reaching a peak of 1.66 ± 0.32 mmol l−1 at 240 min (Fig. 2). Blood glycerol was higher (P < 0.01) than resting levels after 90 min and continued to rise until cessation of exercise (Table 1). The resting plasma insulin concentration was 51.8 ± 5.7 pmol l−1; it was decreased (P < 0.01) at 10 min and remained depressed throughout exercise (Table 1).
Figure 2. Plasma FFA concentration before and during 240 min moderate exercise in men.

Values are means ± s.e.m., n = 7. * Significantly different (P < 0.05) from rest.
IMTG
We report a coefficient of variation between duplicate samples (17 pairs) of 12.3 ± 9.4 % (mean ± s.d.). Resting IMTG averaged 15.6 ± 0.8 mmol kg−1 d.m. and was significantly reduced (P < 0.01) at 120 min. We observed no further decrement in IMTG following 240 min of exercise (Fig. 3).
Figure 3. IMTG content in vastis lateralis before and during 240 min moderate exercise in men.

Values are means ± s.e.m., n = 7. * Significantly different (P < 0.05) from rest.
Muscle metabolites
Muscle ATP and pyruvate were unchanged from rest during exercise (Table 2). Muscle PCr was lower (P < 0.05) than resting values at 10 min of exercise and remained depressed throughout exercise (Table 2). In contrast, muscle creatine was elevated (P < 0.05) at all exercise time points compared with rest (Table 2). Muscle lactate increased (P < 0.05) in the first 10 min of exercise but returned to resting concentrations by 120 min and plateaued until completion of exercise (Table 2). Muscle glycogen averaged 468 ± 49 mmol kg−1 d.m. at rest, decreased (P < 0.01) to 217 ± 48 mmol kg−1 d.m. by 120 min and was further reduced (P < 0.05) at 240 min (Table 2). Muscle acetylcarnitine increased (P < 0.05) from rest at 10 and 120 min of exercise, but returned to resting values by 240 min (Fig. 4). Acetyl CoA was higher (P < 0.05) at all exercise time points compared with rest (Fig. 4).
Table 2.
Muscle metabolite concentrations at rest and during 240 min exercise at 57 % V̇O2,max
| 0 min | 10 min | 120 min | 240 min | |
|---|---|---|---|---|
| ATP | 23.6 ± 0.5 | 24.7 ± 1.1 | 24.0 ± 1.1 | 24.0 ± 1.1 |
| PCr | 83.4 ± 2.8 | 70.1 ± 5.3 * | 60.6 ± 6.6 * | 60.8 ± 3.4 * |
| Creatine | 49.3 ± 1.6 | 62.7 ± 3.8 * | 72.1 ± 5.5 * | 71.9 ± 6.1 * |
| Lactate | 8.7 ± 1.3 | 14.1 ± 2.8 * | 6.3 ± 1.4 | 7.2 ± 1.3 |
| Pyruvate | 0.08 ± 0.02 | 0.13 ± 0.03 | 0.10 ± 0.03 | 0.12 ± 0.03 |
| Glycogen | 468 ± 49 | NM | 217 ± 48 * | 144 ± 47 *† |
Values are means ± s.e.m., n = 7. All values are expressed as mmol kg−1 d.m.
Significantly different (P < 0.05) from 0 min
significantly different (P < 0.05) from 120 min.
NM, not measured.
Figure 4. Muscle acetylcarnitine (above) and acetyl CoA (below) contents before and during 240 min moderate exercise in men.
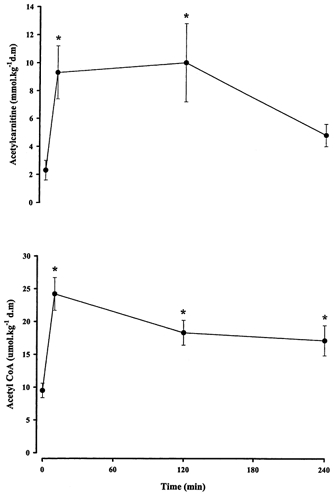
Values are means ± s.e.m., n = 7. * Significantly different (P < 0.05) from rest.
PDHa
Resting PDHa averaged 0.96 ± 0.14 mmol min−1 kg−1 w.m. and increased (P < 0.01) to 2.00 ± 0.23 mmol min−1 kg−1 w.m. at 10 min of exercise. PDHa remained elevated above resting levels throughout exercise, but by 240 min PDHa was lower (P < 0.05) than 10 and 120 min (Fig. 5). The calculated flux through PDH at 120 and 240 min was 1.93 and 1.47 mmol min−1 kg−1 w.m., respectively.
Figure 5. PDHa before and during 240 min moderate exercise in men.
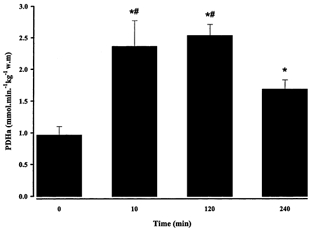
Values are means ± s.e.m., n = 7. * Significantly different (P < 0.05) from rest, # significantly different from 240 min (P < 0.05).
DISCUSSION
This study was undertaken to examine the variability of the IMTG measurement in muscle samples of trained men and to investigate IMTG utilisation and carbohydrate metabolism during 240 min of moderate exercise. The present study demonstrated a substantial reduction in the c.v. of the IMTG measurement in trained men compared with values previously obtained in untrained individuals (Wendling et al. 1996). During exercise from 0 to 120 min IMTG and muscle glycogen were significantly reduced, and considerable utilisation of extramuscular fuels was evident (Fig. 6). No further reduction in IMTG was observed from 120 to 240 min and muscle glycogen degradation was attenuated. It is likely that the origin of almost all of the fat oxidised after 120 min of exercise is from extramyocellular depots and this is consistent with the observed increase in FFA availability and plasma glycerol. PDHa was reduced at 240 min compared with other exercise measures (10, 120 min) and this was in accordance with the reduced whole body carbohydrate oxidation late in exercise. The mechanism underlying the reduction in PDHa is not readily apparent.
Figure 6. Relative contribution of endogenous and blood-borne substrates to energy production during 240 min moderate exercise in men.
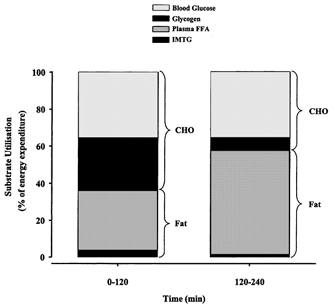
CHO, carbohydrate. Total caloric expenditure between time frames varied (0-120 min, ≈6185 kJ; 120-240 min, ≈6450 kJ). The contribution of acetylcarnitine to total energy expenditure between 120 and 240 min was 1.16 %.
Fat metabolism
Circulating FFAs and IMTG supply the fat fuel required by the muscle for ATP production during exercise. Despite the results from isotope tracer (Romijn et al. 1993; van Loon et al. 2001) and nuclear magnetic resonance (NMR) spectroscopy (Krssak et al. 2000; Décombaz et al. 2001) studies that suggest pronounced IMTG use during exercise, controversy exists regarding whether IMTG is oxidised during exercise. The controversy exists as the bulk of the literature examining IMTG utilisation directly reports no use of IMTG during 90-120 min of exercise at ≈ 65 % V̇O2,max (Keins et al. 1993; Wendling et al. 1996; Starling et al. 1997; Keins & Richter, 1998; Bergman et al. 1999; Guo et al. 2000).
It is commonly perceived that these discrepancies are due to the methodological limitations associated with the muscle biopsy technique. This method does not discriminate between IMTG and fat interlaced between muscle fibres, nor does it account for the variability in IMTG content at different sites resulting from the disparity in fibre type storage (Essén et al. 1975). In a previous study, we reported a between-biopsy (three biopsies) c.v. of 23.5 ± 14.6 % in mainly untrained subjects (Wendling et al. 1996). In that study cycling for 90 min at 65 % V̇O2,max induced a 6 mmol kg−1 d.m. (22.8 %) reduction in IMTG, but the decrease in IMTG was of the same order of magnitude as the between-biopsy variability. Here we report a c.v. of 12.3 ± 9.4 % in 17 paired biopsies. The improved reliability of our measurement is related to the use of trained men as subjects. Trained subjects are less likely to store fat between muscle fibres than untrained individuals (Szczepaniak et al. 1999) and this observation is supported by our finding of no obvious adipose tissue contamination in any of the ≈40 muscle samples. Based on these data in trained men, a 12 % or greater reduction in IMTG (1.9 mmol kg−1 d.m. for the present study) is required for changes to be considered meaningful. Moreover, these conclusions remain consistent when a single (either of the duplicate samples) muscle biopsy is sampled at each time point. These results may explain the controversy of earlier biopsy studies that reported non-significant changes in IMTG use during prolonged moderate exercise.
We recommend that future studies investigating IMTG utilisation recruit trained individuals to serve as subjects to minimise the variability of the IMTG measure and the associated problems observed in untrained subjects. Duplicate samples are not necessary when assessing IMTG utilisation in trained men, whereas the reliability of the IMTG measure in endurance-trained women has not been assessed. Based on previous work that has demonstrated poor repeatability in an untrained population (Wendling et al. 1996), we advocate the use of duplicate biopsies in untrained individuals.
In agreement with previous studies using chemical analysis (Hurley et al. 1986; Cleroux et al. 1989; Phillips et al. 1996a) and more recently 1H-NMR spectroscopy (Krssak et al. 2000; Brechtel et al. 2001; Décombaz et al. 2001) we provide direct evidence of IMTG use during prolonged exercise in trained men during the initial 2 h of cycling at ≈55 % V̇O2,max (Fig. 3). A novel finding of the present study was that further IMTG depletion did not occur in the final 120 min of exercise. Such a finding did not support our original hypothesis that IMTG use would be elevated later in exercise, and was surprising given the progressive increase in whole body fat oxidation (Fig. 1). It has been proposed previously that IMTG is utilised early in exercise to account for the sluggish delivery of adipose-derived FFAs to the contracting muscle (Romijn et al. 1993). Although we have no kinetic measures of glycerol and FFAs, we observed marked and progressive increases in plasma glycerol (Table 2) and plasma FFAs (Fig. 2) after 90 min of moderate exercise. These data support the contention that the progressive increase in peripheral lipolysis and delivery of FFAs to the active muscle results in a shift towards enhanced oxidation of extramuscular fat which would obviate the requirement for IMTG hydrolysis. Indeed, we estimate that exogenous FFAs supplied 39 % (59 g) of the total energy for ATP production in the first 120 min of exercise, and this increased to 59 % (94 g) in the second 120 min when FFA availability was greater (Fig. 6). Thus, trained subjects seem to preferentially utilise plasma FFAs over IMTG when both substrates are available. Interestingly, when plasma FFA mobilisation and oxidation were reduced by the ingestion of a nicotinic acid analogue, IMTG utilisation was enhanced in endurance-trained men (Coyle et al. 1998). Taken together, these data indicate that trained muscle is able to utilise both intra- and extramuscular fat stores and a reciprocal relationship between IMTG and exogenous FFAs use may exist.
Supporting the possibility of enhanced FFA uptake with delivery is the finding of increased FFA uptake when plasma FFAs are increased with Intralipid and heparin infusion (Odland et al. 1998). Also, FFA uptake was shown to increase linearly with FFA delivery in the trained thigh (Turcotte et al. 1992; Kiens et al. 1993), which may be partly due to increased fat transporter content (Kiens et al. 1997; Turcotte et al. 1999). It has been suggested that FFA delivery may be reduced in the trained state because of reduced sympathoadrenal activity (Hartley et al. 1972), but glycerol Ra, an index of whole body lipolysis, was similar before and after endurance training despite ≈50 % reduction in plasma adrenaline after training (Phillips et al. 1996b). Moreover, in rodents, endurance training increases FFA oxidation and esterification and reduces IMTG utilisation during contractions when exogenous FFA availability is adequate (Dyck et al. 2000). Overall, these data are entirely consistent with our findings in trained men that demonstrate reduced IMTG utilisation with concomitant increases in plasma FFA oxidation secondary to greater FFA delivery late in moderate exercise.
A major limitation of estimating IMTG utilisation using any of the basic approaches (e.g. biochemical, isotope tracer kinetics and 1H-NMR spectroscopy) is the distinct possibility of TG/FFA cycling. A recent study using dual-tracer pulse-chase procedures in the contracting rat soleus reported increased palmitate oxidation and an equal concomitant increase in esterification (Dyck & Bonen, 1998). However, in humans, the rate of FFA incorportation into the IMTG pool was only ≈10 % of the IMTG oxidation rate (Guo et al. 2000). This raises the possibility that a small amount of exogenous FFAs entering the cytoplasm is directed towards storage in the cytoplasmic IMTG pool, which would mask the hydrolysis of IMTG, thus resulting in an underestimation of IMTG utilisation.
The mechanisms regulating the reduction in IMTG hydrolysis are unknown. Hormone-sensitive lipase (HSL) was recently identified as the enzyme responsible for IMTG hydrolysis in rat skeletal muscle (Langfort et al. 1999, 2000) and modest increases in HSL activation have been demonstrated during exercise in untrained humans and with adrenaline infusion in adrenalectomised patients (Kjær et al. 2000). Aside from these data, which suggest that HSL is subject to dual control by contractions and adrenaline via increases in intracellular cyclic 5′-AMP, no other information regarding the regulation of HSL in skeletal muscle exists. One attractive hypothesis for the regulation of HSL is feedback inhibition by long-chain fatty acyl CoA (LCFA CoA). In vitro studies have demonstrated direct non-competitive negative feedback of HSL by oleoyl-CoA (Jepson & Yeaman, 1992). The possibility exists that LCFA CoA inhibits HSL activity after binding to a specific site on the enzyme, thus preventing further mobilisation of IMTG stores. Long-chain fatty acyl-CoA synthetase (FACS) catalyses esterification of long-chain fatty acids with coenzyme A, the first step in fatty acid metabolism. Evidence from studies in adipocytes suggest FACS resides in the plasma membrane and long-chain fatty acids are immediately esterified upon entry to the cytoplasm (Gargiulo et al. 1999). Although tenuous, the findings of reduced IMTG hydrolysis and greater plasma-derived FFA oxidation (and presumably LCFA-CoA accumulation) support the possibility of feedback inhibition of HSL. Clearly, further studies investigating LCFA CoA content and the regulation of HSL in human skeletal muscle during exercise are warranted.
Carbohydrate metabolism
The reduced carbohydrate oxidation observed in the second 120 min of exercise was a function of attenuated muscle glycogen use (Table 2). Muscle glycogen utilisation is most rapid in the early stages of exercise and decreases later in exercise secondary to reduced glycogen availability (Gollnick et al. 1974). Moreover, increased availability of plasma FFAs is associated with attenuated glycogen use during whole body exercise (Costill et al. 1977). Our findings of reduced glycogen use and elevated plasma FFAs are entirely consistent with these data. Glucose uptake is reduced late in prolonged exercise as arterial glucose concentrations decline (Ahlborg & Felig, 1974). We estimated no reduction in the contribution of plasma-derived glucose to total energy expenditure (Fig. 6), and this is not surprising given the maintenance of euglycaemia until exercise cessation.
PDH permits entry of carbohydrate into the mitochondria by catalysing the decarboxylation of pyruvate to acetyl CoA. Although numerous studies have previously examined the acute regulation of PDH and its role in intramuscular fuel selection, no study had examined PDH activation during exercise longer than ≈60 min. We observed an increase in PDHa at 10 and 120 min of exercise and although PDHa remained elevated from rest at 240 min, PDHa was lower than at the previous exercise time points. The reduction in PDHa at 240 min was closely linked to the reduction in whole body carbohydrate oxidation, which is consistent with previous studies that report a close match between PDH activation, estimated PDH flux, and carbohydrate oxidation (Constantin-Teodosiu et al. 1991; Gibala et al. 1998; Howlett et al. 1998). PDH activity is controlled by the relative activities of PDH kinase (PDK) and PDH phosphatase (PDP) which inhibit and activate PDH, respectively. During exercise, PDK is inhibited by pyruvate and a high NAD/NADH ratio and stimulated by a high ATP/ADP ratio, whereas PDP is stimulated by Ca2+. In the present study the apparent mechanism(s) mediating the decreased PDHa at 240 min are not readily apparent. The muscle ATP/ADP ratio was unchanged (Table 2) and the NAD/NADH ratio not measured. We have no measure of Ca2+ in the present study, but muscle fatigue in the single mouse fibre is partly caused by failure of the sarcoplasmic reticulum to release Ca2+ (Allen & Westerblad, 2001) whilst mitochondrial Ca2+ uptake is reduced with exhaustive exercise in rodents (Tate et al. 1978). The possibility exists that reduced Ca2+ may contribute to reduced PDH activation late in fatiguing exercise. Based on the reduced glycogen utilisation and negligible lactate accumulation between 120 and 240 min, pyruvate flux was decreased late in exercise despite the absence of change in whole muscle pyruvate concentration (Table 2). Because pyruvate is a substrate for PDH and an inhibitor of PDK (Ki = 0.5-2.0 mm), we attribute the reduction in PDHa to reduced substrate availability. Indeed, the observation that the calculated flux through PDH closely matched the measured PDH activation over the last 120 min (1.47 vs.1.68 mmol kg−1 d.m. min−1, respectively) supports the notion that pyruvate flux is an important regulator of PDH during prolonged exercise. The likelihood is that we were unable to measure the subtle changes in pyruvate that mediate changes in PDH activation.
Alternatively, PDH activity may be reduced late in exercise secondary to rapid increases in PDK protein and activity. Transcriptional activity of PDK4 in response to exercise is rapid, with three- to sevenfold increases occurring immediately after 60-90 min exhaustive knee extensor exercise (Pilegaard et al. 2000). PDK4 mRNA, protein and total activity have been shown to increase within 15-24 h on a low carbohydrate diet or during fasting (Peters et al. 2001; Spriet et al. 2001). Whether the greater PDK4 mRNA translates to increased protein and total activity during a prolonged exercise bout is yet to be determined.
Acetyl group accumulation
Accumulation of acetyl groups is observed during exercise when the formation of acetyl CoA is greater than acetyl CoA utilisation by the tricarboxylic acid (TCA) cycle (Constantin-Teodosiu et al. 1991; Howlett et al. 1998). The excess acetyl CoA units are transferred to carnitine to regenerate free CoA, which is essential for continued PDH activity and many other mitochondrial reactions. In the present study we observed an increase in acetyl group accumulation early in exercise (Fig. 4) that was maintained until 120 min, after which acetylcarnitine decreased at 240 min. Similar levels have previously been reported at fatigue following a low carbohydrate diet (Putman et al. 1993). The decrease in acetylcarnitine coincided with decreased PDHa and whole body carbohydrate oxidation. These data suggest that the reduction in carbohydrate oxidation (flux through PDH) results in an inability to supply sufficient acetyl CoA to fuel oxidative metabolism. Thus, in spite of increased fat metabolism, acetyl units derived from acetylcarnitine were required to fuel oxidative metabolism late in exercise.
Conclusion
In summary, this study describes the time course for fuel utilisation, PDH activation and acetyl group accumulation during 4 h of moderate intensity exercise. The between-biopsy IMTG variability in trained men (12.3 %) was less than the net decrease in IMTG content deeming our results meaningful. During the first 120 min of exercise a significant proportion of the substrate oxidised was derived from IMTG and muscle glycogen, but plasma FFAs and glucose constituted the primary fuel sources. No further reduction in IMTG occurred in the final 120 min of exercise, and this was accompanied by substantial increases in plasma FFA oxidation. It is possible that IMTG was used early in exercise to offset the sluggish delivery of plasma FFAs whereas the increased delivery and oxidation of plasma FFAs obviated the requirement for IMTG hydrolysis late in exercise. Whole body carbohydrate oxidation was decreased in the final 120 min of exercise as a function of reduced muscle glycogen degradation and PDH activation. Although an explanation for the reduced PDH activation was not readily apparent, reduced pyruvate availability secondary to diminished glycolytic flux is the most likely regulator. Despite the compensatory increase in extramuscular fat metabolism late in exercise, ATP derived from acetylcarnitine stores was required to fuel oxidative metabolism.
Acknowledgments
We thank Drs Graham Jones and Kieran Killian for their expert medical assistance. This study was supported by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institute of Health Research.
REFERENCES
- Ahlborg G, Felig P. Lactate and glucose exchange across the forearm, legs and splanchnic bed during and after prolonged leg exercise. Journal of Clinical Investigation. 1982;69:45–54. doi: 10.1172/JCI110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Journal of Clinical Investigation. 1974;53:1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DC, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. Journal of Physiology. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. American Journal of Physiology. 1999;276:E106–117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods in Enzymatic Analysis. New York: Academic Press; 1974. [Google Scholar]
- Boesch C, Slotboom J, Hoppeler H, Kreis R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magnetic Resonance in Medicine. 1997;37:484–493. doi: 10.1002/mrm.1910370403. [DOI] [PubMed] [Google Scholar]
- Brechtel K, Niess AM, Machann J, Rett K, Schick F, Claussen CD, Dickhuth HH, Haering H-U, Jacob S. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS) Hormone and Metabolic Research. 2001;33:63–66. doi: 10.1055/s-2001-12407. [DOI] [PubMed] [Google Scholar]
- Cederblad G, Carlin JI, Constantin-Teodosiu D, Hultman E. Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Analytical Biochemistry. 1990;185:274–278. doi: 10.1016/0003-2697(90)90292-h. [DOI] [PubMed] [Google Scholar]
- Cleroux J, Van Nguyen P, Taylor AW, Leenen FHH. Effects of β1- vs. β1+β2- blockade on exercise endurance and muscle metabolism in humans. Journal of Applied Physiology. 1989;66:548–554. doi: 10.1152/jappl.1989.66.2.548. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E. Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiologica Scandinavica. 1991;143:367–372. doi: 10.1111/j.1748-1716.1991.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Costill DL, Coyle EF, Dalsky G, Evans W, Fink W, Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. Journal of Applied Physiology. 1977;43:695–699. doi: 10.1152/jappl.1977.43.4.695. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Jeukendrup AE, Wagenmakers AJM, Saris WHM. Intramuscular triglyceride oxidation during exercise acetely increases with reduced plasma FFA mobilization and oxidation. FASEB Journal. 1998;10:A143. [Google Scholar]
- Décombaz J, Schmitt B, Ith M, Decarli B, Diem P, Kreis R, Hoppler H, Boesch C. Post-exercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001;281:R760–769. doi: 10.1152/ajpregu.2001.281.3.R760. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Bonen A. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxidation. American Journal of Physiology. 1998;275:E888–896. doi: 10.1152/ajpendo.1998.275.5.E888. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Miscovic D, Code L, Luiken JJFP, Bonen A. Endurance training increases FFA oxidation and reduces triacylglycerol utilization in contracting rat soleus. American Journal of Physiology. 2000;278:E778–785. doi: 10.1152/ajpendo.2000.278.5.E778. [DOI] [PubMed] [Google Scholar]
- Essén B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre type in human skeletal muscle. Acta Physiologica Scandinavica. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. Journal of Lipid Research. 1980;21:139–144. [PubMed] [Google Scholar]
- Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE. Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. Journal of Lipid Research. 1999;40:881–892. [PubMed] [Google Scholar]
- Gibala MJ, Maclean DA, Graham TE, Saltin B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. American Journal of Physiology. 1998;275:E235–242. doi: 10.1152/ajpendo.1998.275.2.E235. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibers after exercise of varying intensity and at varying pedalling rates. Journal of Physiology. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. Journal of Applied Physiology. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjs L-O. Glycogen, glycolytic intermediates and high energy phosphates determines in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical and Laboratory Investigation. 1974;33:109–120. [PubMed] [Google Scholar]
- Hartley LH, Mason JW, Hogan RP, Jones LG, Kotchen TA, Mougey EH, Wherry FE, Pennington LL, Ricketts PT. Multiple hormonal responses to prolonged exercise in relation to physical training. Journal of Applied Physiology. 1972;33:607–610. doi: 10.1152/jappl.1972.33.5.607. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Carlson LA, Ekelund L-G, Holmgren A. Turnover rate and oxidation of different free fatty acids in man during exercise. Journal of Applied Physiology. 1964;19:613–618. doi: 10.1152/jappl.1964.19.4.613. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Pernow B, Jones NL. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. Journal of Applied Physiology. 1967;23:90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Parolin ML, Dyck DJ, Hultman E, Jones NL, Heigenhauser GJF, Spriet LL. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. American Journal of Physiology. 1998;275:R418–425. doi: 10.1152/ajpregu.1998.275.2.R418. [DOI] [PubMed] [Google Scholar]
- Hurley BF, Nemeth PM, Martin WH, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. Journal of Applied Physiology. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Jepson CA, Yeaman SJ. Inhibition of hormone-sensitive lipase by intermediary lipid metabolites. FEBS Letters. 1992;28:197–200. doi: 10.1016/0014-5793(92)81328-j. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essén-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. Journal of Physiology. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Kristiansen S, Jensen P, Richter EA, Turcotte LP. Membrane associated fatty acid binding protein (FABPPM) in human skeletal muscle is increased by endurance training. Biochemical and Biophysical Research Communications. 1997;231:463–465. doi: 10.1006/bbrc.1997.6118. [DOI] [PubMed] [Google Scholar]
- Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. American Journal of Physiology. 1998;275:E332–337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- Kjær M, Howlett K, Langfort J, Zimmerman-Belsing T, Lorentsen J, Bülow J, Ihlemann J, Feldt-Rasmussen U, Galbo H. Adrenaline and glycogenolysis in skeletal muscle during exercise: a study in adrenalectomized humans. Journal of Physiology. 2000;528:371–378. doi: 10.1111/j.1469-7793.2000.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Shulman GI. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. Journal of Clinical Endocrinology and Metabolism. 2000;85:748–754. doi: 10.1210/jcem.85.2.6354. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase by contractions in rat skeletal muscle. Biochemical Journal. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochemical Journal. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJF, Wong D, Hollidge-Horvat MG, Spriet LL. Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. American Journal of Physiology. 1998;274:R894–902. doi: 10.1152/ajpregu.1998.274.4.R894. [DOI] [PubMed] [Google Scholar]
- Passoneau JA, Lowry OH. Enzymatic Analysis: A Practical Guide. Totawa, NJ, USA: Humana; 1993. [Google Scholar]
- Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Canadian Journal of Sports Science. 1991;16:23–29. [PubMed] [Google Scholar]
- Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJF, Spriet LL. Human skeletal muscle PDK activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. American Journal of Physiology - Endocrinology and Metabolism. 2001;281:E1151–1158. doi: 10.1152/ajpendo.2001.281.6.E1151. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnapolsky MA, Heigenhauser GJF, Grant SM. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. American Journal of Physiology. 1996a;270:E265–272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnapolsky MA, Heigenhauser GJF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. Journal of Applied Physiology. 1996b;81:2182–2191. doi: 10.1152/jappl.1996.81.5.2182. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. American Journal of Physiology - Endocrinology and Metabolism. 2000;279:E806–814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Putman CT, Spriet LL, Hultman E, Lindinger MI, Lands LC, McKelvie RS, Cederblad G, Jones NL, Heigenhauser GJF. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. American Journal of Physiology. 1993;265:E752–760. doi: 10.1152/ajpendo.1993.265.5.E752. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup I, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. American Journal of Physiology - Endocrinology and Metabolism. 2002;282:E435–447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. American Journal of Physiology. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Tunstall RJ, Watt M, Hargreaves M, Cameron-Smith D. Time course of a 40 hour fast on pyruvate dehydrogenase activation and kinase expression in human skeletal muscle. Medicine and Science in Sports and Exercise. 2001;33:S98. [Google Scholar]
- Starling RD, Trappe TA, Parcell AC, Kerr CG, Fink WJ, Costill DL. Effects of diet on muscle triglyceride and endurance performance. Journal of Applied Physiology. 1997;82:1185–1189. doi: 10.1152/jappl.1997.82.4.1185. [DOI] [PubMed] [Google Scholar]
- Steffensen CH, Roepstorff C, Madsen M, Kiens B. Myocellular triacylglycerol breakdown in females but not in males during exercise. American Journal of Physiology - Endocrinology and Metabolism. 2002;282:E634–642. doi: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by 1H spectroscopy: validation in vivo. American Journal of Physiology. 1999;276:E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- Tate CA, Bonner HW, Leslie SW. Calcium uptake in skeletal muscle mitochondria. II. The effects of long-term chronic and acute exercise. European Journal of Applied Physiology and Occupational Physiology. 1978;39:117–122. doi: 10.1007/BF00421716. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. American Journal of Physiology. 1992;262:E791–799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Swenberger JR, Tucker MZ, Yee AJ. Training-induced elevation in FABPPM is associated with increased palmitate use in contracting muscle. Journal of Applied Physiology. 1999;87:285–293. doi: 10.1152/jappl.1999.87.1.285. [DOI] [PubMed] [Google Scholar]
- van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. Journal of Physiology. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling PS, Peters SJ, Heigenhauser GJF, Spriet LL. Variability of triacylglycerol content in human skeletal muscle biopsy samples. Journal of Applied Physiology. 1996;81:1150–1155. doi: 10.1152/jappl.1996.81.3.1150. [DOI] [PubMed] [Google Scholar]


