Abstract
The aim of the study was to verify the hypothesis that trisynaptic actions of group II muscle afferents upon motoneurones are, at least in part, mediated by dorsal horn interneurones exciting the same intermediate zone interneurones that are interposed in disynaptic pathways from group II afferents. Population EPSPs (field potentials) and responses of individual interneurones evoked by group II afferents in the dorsal horn and in the intermediate zone were analysed in order to assess the possibility of a causal relationship between them. When direct actions of group II afferents in the intermediate zone were abolished by presynaptic inhibition, distinct later components of field potentials and delayed interneuronal responses were induced at latencies 0.5-1 ms longer than those seen originally. Both the latency and a marked temporal facilitation define these later group II actions as being evoked disynaptically. Under the same conditions, single stimuli activated more than one half of dorsal horn interneurones, and the second and third stimuli activated all of these interneurones. Responses of dorsal horn interneurones preceded disynaptically evoked responses of intermediate zone interneurones. The study indicates that intermediate zone interneurones may be activated by group II afferents both directly and via dorsal horn interneurones and that synaptic actions of group II afferents upon these interneurones, and their subsequent actions upon motoneurones, may be modulated in parallel at the level of intermediate zone and dorsal horn interneurones.
When synaptic actions of peripheral afferents upon motoneurones are evoked via several parallel interneuronal pathways, the contribution of various neuronal populations to these actions is generally difficult to investigate, except in the simplest pathways. With respect to disynaptic excitatory and inhibitory actions of group II muscle afferents on motoneurones it has been established that they are mediated by intermediate zone interneurones (Cavallari et al. 1987; Edgley & Jankowska, 1987b). Another population of interneurones in pathways from group II muscle afferents, those located in the dorsal horn, have been found to mediate only tri- or polysynaptic actions (Edgley & Jankowska, 1987b; Bras et al. 1989). Whether any interactions between these two interneuronal populations occur has not yet been established. In the present study we have explored the possibility that some dorsal horn interneurones in pathways from group II afferents excite intermediate zone interneurones, thus providing disynaptic input from group II afferents to these neurones and mediating trisynaptic actions of these afferents upon motoneurones. This arrangement is diagrammatically indicated in Fig. 1.
Figure 1. Hypothetical relationships between the dorsal and intermediate zone group II interneurones and GABAergic interneurones mediating presynaptic inhibition of transmission from group II afferents to these interneurones.
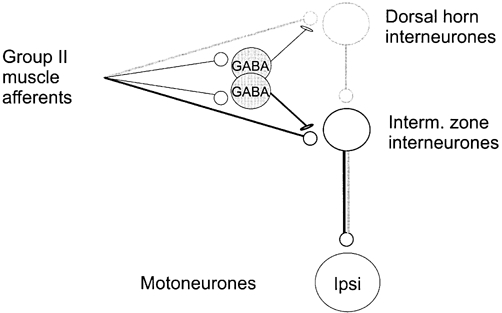
The proposal that all the indicated actions of group II afferents are mediated by axon collaterals of the same fibres is based on results of morphological studies that revealed that the same fibres project to the dorsal horn and to the intermediate zone (Fyffe, 1979; Hongo, 1992). Two distinct subpopulations of GABAergic interneurones modulating transmission to dorsal horn and intermediate zone interneurones, respectively, are proposed to account for the differences in the effectiveness and in the time course of presynaptic inhibition in the dorsal horn and in the intermediate zone (Hammar et al. 2002). Dark lines to and from intermediate zone interneurones indicate the disynaptic pathway and lighter lines to and from the dorsal horn and intermediate zone interneurones, the trisynaptic one. Ipsi, ipsilateral motoneurones. For further explanations see text.
The study is based on observations (Riddell et al. 1995; Jankowska et al. 2000a, 2002; Jankowska, 2001), showing that transmission from group II afferents to neurones located in the intermediate zone and to neurones located in the dorsal horn is differently modulated by presynaptic inhibition. Transmission to intermediate zone neurones may be practically blocked by properly timed conditioning stimuli while transmission to dorsal horn neurones is only partly depressed. Drawing upon these observations, we hypothesized that if dorsal horn group II interneurones excite intermediate zone interneurones, disynaptic excitation of the latter could be seen and analysed under conditions when their monosynaptic excitation is eliminated by presynaptic inhibition.
METHODS
Experiments were performed on nine deeply anaesthetized cats (2.2-3.5 kg) obtained from a supplier accredited by the University of Göteborg. The anaesthesia was induced with sodium pentobarbital (44 mg kg−1i.p.). It was maintained during surgery with several doses of pentobarbital (up to a total dose of 50 mg kg−1d.v.) and then by α-chloralose (up to 60 mg kg−1d.v.). During recording, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, about 0.2 mg kg−1 h−1d.v.) and the animals were artificially ventilated. If any increase in the continuously monitored blood pressure or heart rate occurred, or if the pupils dilated despite the regularly administered doses of chloralose, an additional dose was given at the first sign of these changes. The mean blood pressure was kept at 100-130 mmHg and the end-tidal concentration of CO2 at about 4 % by adjusting parameters of artificial ventilation and of the rate of a continuous infusion of a bicarbonate buffer solution containing 5 % glucose (1-2 ml h−1 kg−1). The core body temperature was kept at about 38 °C by servo-controlled infrared lamps. At the end of the experiment the animals were killed with an overdose of anaesthetic (until cardiac arrest). All the experimental procedures were approved by Göteborg ethics committee and were in accordance with NIH and EU guidelines of animal care. Some of the observations were made in the same experiments as those reported in the companion paper (Jankowska et al. 2002) and the basic experimental procedures were the same. These involved the exposure of the L3-L7 segments of the spinal cord by laminectomy, dissection of a number of peripheral nerves and the use of electrical stimulation of these nerves to activate group II muscle afferents. The following peripheral nerves were dissected; quadriceps (Q), sartorius (Sart), posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius and soleus (GS), extensor digitorum longus and anterior tibial (referred to as deep peroneal, DP) and saphenus (Saph). Extracellularly recorded population EPSPs (field potentials) as well as extracellularly and intracellularly recorded responses of single intermediate zone and dorsal horn interneurones mediated by synaptic actions of group II afferents were analysed. For both purposes, glass micropipettes (1.5-2 μm tip diameter; 1.5-4 MΩ impedance) filled with 2 m solution of NaCl or potassium citrate, were used.
Peripheral nerves were usually stimulated at five times threshold (T), i.e. at intensities near maximal for fast conducting group II afferents, but sometimes also at 3 or 4T. The potentials to be analysed were evoked by one or two stimuli 2.5, 3.3 or 5.0 ms apart. Two to four sequences of 10 or 20 field potentials were averaged. The test stimuli were preceded by 2-5 conditioning stimuli (5T stimuli 2.5 or 3.3 ms apart) in alternating sequences. Intervals between the first conditioning and the first test stimuli were varied between 8 and 300 ms, but were most often 20-40 ms. Effects of conditioning stimuli on intermediate zone field potentials were analysed by comparing changes in the latency and area of the early and later components of the test and conditioned potentials (see Fig. 1 in Jankowska et al. 2002). The latencies were measured from the positive peak of afferent volleys evoked by group I afferents. The areas of the early components of field potentials were measured within a time window of 0.6-0.8 ms from their onset. The areas of the later components were measured within similarly long time windows (from the onset of these components) in records in which the early components were absent. Only field potentials with a sharp rising phase and a distinct peak originally evoked at latencies compatible with latencies of monosynaptically evoked synaptic actions (cf. Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993; Riddell et al. 1995; Riddell & Hadian, 2000b) were analysed.
Extracellularly recorded responses of individual interneurones were analysed by comparing the latency of these responses and the number of spike potentials evoked by successive 20 stimuli, using peri-stimulus-time histograms (PSTH) and cumulative sums (see Jankowska et al. (1997). These were constructed on-line using bins of 20-80 μs depending on the time base of the recordings. The statistical significance of the differences was estimated using Student's t test. Any differences of less than 10 % were considered to be possibly related to measuring errors and functionally not significant.
RESULTS
Disynaptic components of intermediate zone field potentials evoked by group II afferents
Minimal latencies of disynaptic actions of group II afferents in the intermediate zone would be expected to be 0.5-1 ms longer than the latencies of monosynaptic actions. However, differences in the conduction time of the fastest and slower conducting group II afferents from the peripheral nerves to the spinal cord amount to at least 1.5-2.0 ms (Riddell et al. 1995), and the range of latencies of monosynaptically evoked actions of these afferents may be similar (Stauffer et al. 1976; Munson et al. 1982). Monosynaptic actions of slower conducting fibres may thus coincide with disynaptic actions of the fastest conducting fibres. For these reasons one can only rarely see distinct components of field potentials attributable to mono- and disynaptically relayed actions of group II afferents.
One such case is illustrated in Fig. 2C where the first three vertical lines indicate the onset of three components of field potentials evoked in the intermediate zone by stimulation of Q. The first component was evoked by monosynaptic actions of group I afferents, as was indicated by its short segmental latency and by it being induced by stimuli below 2T. The second and third components were evoked by group II afferents, as indicated by their longer latency and by being induced by 4T but not by 2T stimuli. Of the two group II components, the earlier component was evoked at a latency (1.7 ms from group I afferent volleys) that was compatible with monosynaptically evoked actions of group II afferents while the later one was evoked with an additional delay of 0.9 ms.
Figure 2. An example of two distinct components of intermediate zone field potentials evoked by group II afferents in addition to components evoked by group I afferents.
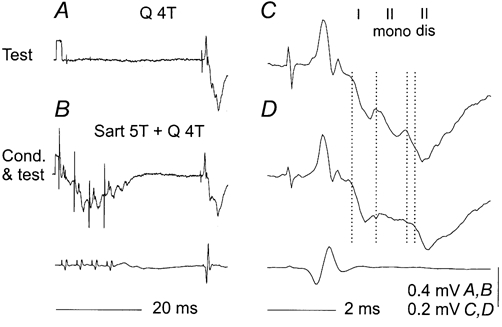
A and B, average records of 20 potentials evoked by four times threshold (4T) stimulation of Q (A), or both Sart 5T and Q 4T (B) at a 32 ms conditioning-testing interval. C and D, expanded (8 × horizontally and 2 × vertically) rightmost parts of records A and B. The dotted lines indicate the onset of potentials evoked by group I afferents and group II afferents, the latter classified as being induced monosynaptically (mono) and disynaptically (dis). It will be noted in D that following the conditioning stimuli, the group I component was practically unaffected and that the monosynaptic actions of group II afferents were reduced to a much greater extent than the disynaptic actions; the latter were primarily only delayed, with their onset at the level of the fourth rather than the third vertical line. Lower traces in B and D, records of incoming volleys from cord dorsum. In this and the following figures the negativity is downwards in microelectrode records and upwards in cord dorsum records.
The later components were usually represented by a not very distinct hump during the declining phase of the early components, as in Fig. 3B and E, or they could not be discerned at all, as in Fig. 3C and F. However, they became as distinct as in Fig. 2D when the monosynaptic components were depressed by conditioning stimuli, as illustrated in Fig. 3. In Fig. 3A the top two records show intermediate zone field potentials evoked by stimuli below threshold (2T) and near maximal (5T) for fast group II afferents of Q. The difference between them thus reflects synaptic actions of group II afferents. In the expanded records in Fig. 3B the group II component of the field potential is contained between the superimposed records of field potentials evoked by 2T and 5T stimuli. When a train of four stimuli at 5T was applied to the same nerve, the group II component was considerably reduced (third record from the top in Fig. 3A). In fact, its early part, that between the two dotted lines in the lower pair of the superimposed expanded records in Fig. 3B, was abolished, disclosing a later part with an additional delay of 0.8 ms, at a latency similar to that in Fig. 2. Another example of the late components disclosed after the early components had disappeared following conditioning stimulation of the same nerve, in this case Sart, is shown in Fig. 3C.
Figure 3. Disynaptic components of field potentials evoked by group II afferents in the intermediate zone after abolition of monosynaptic components.
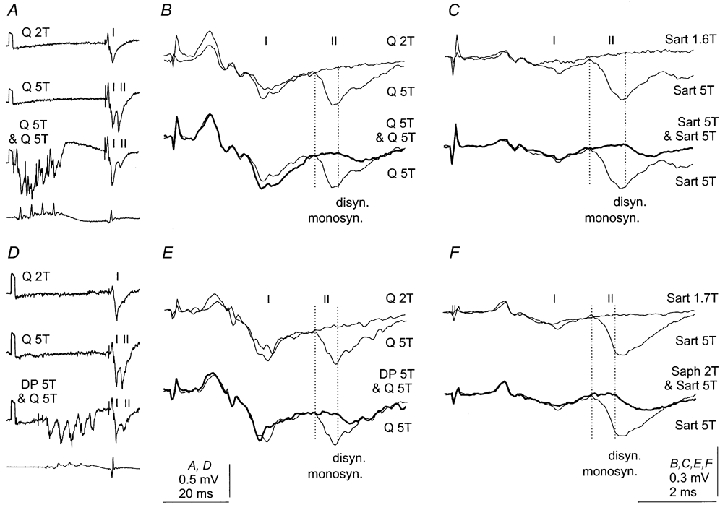
The three top traces in A and D are averaged records of 20 successive field potentials evoked by stimulation of Q without and following conditioning stimulation of Q and DP, respectively; stimulation was either subthreshold (2T) or near-maximal (5T) for group II afferents. Bottom traces in A and D, records from the cord dorsum. B and E, records of field potentials evoked by group II afferents (rightmost parts of middle traces in A and D, expanded 2 × vertically and 12 × horizontally) are superimposed on records of potentials evoked by weaker stimuli (upper pair) and on records in which these potentials were depressed by presynaptic inhibition (lower pair). Dotted lines indicate the original latency of monosynaptic components of field potentials evoked by group II afferents and the latency of potentials concluded to be evoked disynaptically. C and F, records of field potentials evoked from Sart are similarly superimposed. The conditioning stimuli were in this case applied to Sart and Saph, as indicated. Note that the group I component of the field potential in B was enhanced and that those in C, E and F were only marginally affected by the conditioning stimuli.
The later components were most readily disclosed when the early components were eliminated by conditioning stimuli applied to the same nerve from which the tested field potential was evoked (Fig. 3A-C). However, any conditioning stimuli applied to muscle or skin nerves that depressed monosynaptic components made the late components appear, as illustrated in Fig. 3D–F.
The late component might be considered either as reflecting monosynaptic actions of slower conducting group II afferents, assuming that these fibres are less affected by presynaptic inhibition, or as disynaptic actions of fast conducting group II afferents, as postulated in the diagram of Fig. 1. To differentiate between these two possibilities, tests for temporal facilitation were used. They were designed expecting monosynaptic actions of group II afferents to be either unchanged or depressed when double or triple test pulses are used instead of single ones, due either to post-activation depression (Hultborn et al. 1996) or to presynaptic inhibition (Riddell et al. 1995). In contrast, disynaptic actions ought to be facilitated, following double or triple stimuli.
Figure 4 shows that the later components of group II field potentials were clearly facilitated. In both cases illustrated, large monosynaptic group II field potentials (small arrows at the level of the first dotted lines) followed small group I field potentials. Conditioning stimuli abolished the early component (between the dotted lines in the top traces) of the field potentials evoked by the first test stimulus and revealed only traces of the later component (large arrow). After the second and the third test stimuli the later components grew in size. The increase in the amplitude of the late components after successive test stimuli was often associated with a decrease in their latency (by 0.1-0.2 ms). This decrease, which is another feature of temporally facilitated potentials, is indicated in Fig. 4 by decreasing distances between the dotted lines. From now on, the late components with latencies up to 1 ms longer than the latencies of the monosynaptic components will thus be referred to as disynaptic components of group II field potentials.
Figure 4. Temporal facilitation of disynaptic components of group II intermediate zone field potentials.
A, records of the field potential illustrated in Fig. 3A, conditioned by three stimuli at a conditioning-testing interval of 17 ms. B, another intermediate zone field potential, at a conditioning-testing interval of 25 ms; recorded at a more ventral location (depth 3.1 mm, probably lamina VIII) where synaptic actions of group I afferents no longer preceded synaptic actions of group II afferents (Edgley & Jankowska, 1987a). Note that in both cases the monosynaptic components of field potentials, which were originally evoked by group II afferents (small arrows) were abolished by the conditioning stimuli. Note also that the delayed components (large arrows) were larger after the second and third stimuli than after the first stimulus. Other indications are as in Fig. 3.
In addition to the temporal facilitation, the appearance of the late components depended on the relative intensity of the test and conditioning stimuli. Figure 5A and B shows that when the strong conditioning stimuli (5T) obliterated both the early and late components of field potentials evoked by the first stimulus, the late components following the second stimulus became more distinct when the conditioning stimuli were weaker (3-4T in this case). However, when the intensity of the conditioning stimuli was lowered below a certain critical level, the monosynaptic components reappeared, making the disynaptic components difficult to distinguish from them. The most favourable conditions for revealing the disynaptic components thus appeared to be in keeping with the relationships depicted in Fig. 1; when the conditioning stimuli are sufficiently strong to prevent monosynaptic activation of intermediate zone interneurones, but without having too strong a depressive effect on activation of dorsal horn interneurones.
Figure 5. Dependence of disynaptic components of group II field potentials on the intensity of conditioning stimuli and on conditioning-testing intervals.
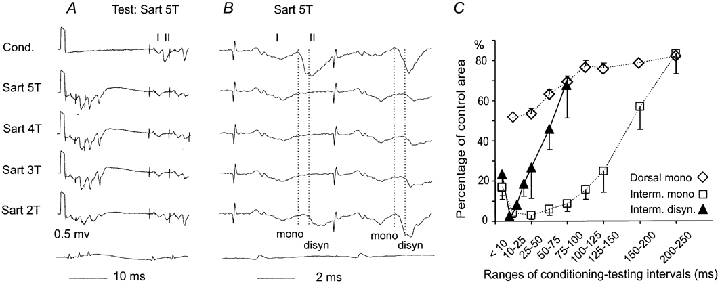
A, from top to bottom, records of a field potential when it was not and when it was preceded by conditioning stimuli of a decreasing intensity, as indicated to the left. B, rightmost parts of records in A expanded 2× vertically and 5 × horizontally. Dotted lines indicate onset of the monosynaptic and the disynaptic components, respectively. C, mean areas of 10 disynaptic components (▴-▴) of intermediate zone field potentials evoked by single stimuli as a function of intervals between 5T conditioning and 5T test stimuli, expressed as a percentage of the area of monosynaptic components of control field potentials. The areas were measured within the same time windows (0.6-0.8 ms from the onset). The range of conditioning-testing intervals for the first five filled triangles were 8-10, 10-20, 20-30, 30-40 and 40-50 ms. Open symbols and dotted lines similarly show the time course of changes in monosynaptic components of intermediate zone (n = 9, □) and dorsal horn (n = 8, ⋄) field potentials (pooled data for effects of stimulation of group II afferents in the same and other nerves, modified from Fig. 5 in Hammar et al. 2002).
The appearance of distinct late components of the field potentials depended also on the intervals between the conditioning and test stimuli. Figure 5C shows that the area of disynaptic components induced by single stimuli was at its smallest at conditioning-testing intervals of about 25-30 ms and increased with longer intervals. As judged by the time course of the depression of the earliest components of intermediate zone field potentials (□), monosynaptic activation of intermediate zone interneurones by group II afferents should remain strongly depressed at conditioning-testing intervals of 50-100 ms. The depression of dorsal horn field potentials (⋄) should on the other hand by then already have declined, increasing the probability of activation of dorsal horn interneurones. Disynaptic components of intermediate zone field potentials mediated by dorsal horn interneurones ought therefore be most conspicuous at such intervals. In practice, the most distinct disynaptic components were seen at 30-50 ms intervals because their latency decreased with increasing intervals and the increasing probability of activation of dorsal horn interneurones. At intervals exceeding 100 ms they were usually continuous with the monosynaptic components and could not be reliably differentiated from them.
In total, distinct components of field potentials of group II origin, fulfilling criteria for disynaptically evoked potentials, were seen in the majority of over 50 field potentials analysed. However, the proportion of potentials in which they were displayed differed depending on the experimental paradigms. The disynaptic components were distinguishable without conditioning stimuli in only four potentials, resembling that illustrated in Fig. 2, being delayed with respect to the onset of the monosynaptic components by 0.8-1.1 ms. When group II field potentials like those illustrated in Fig. 3 were induced by single standard test stimuli of 5T, and conditioning stimuli of 5T abolished the earliest components, late components classified as being evoked disynaptically were disclosed in 20 out of 40 of these potentials. These late components were delayed by 0.5-1.0 ms with respect to the monosynaptic components (evoked at latencies of 2.2-2.7 ms from group I afferent volleys). In the remaining cases the conditioning stimuli either abolished both the early and late components (n = 14) or delayed any potentials that appeared by only 0.2-0.4 ms (n = 6). In the latter cases it was impossible to differentiate between monosynaptic components delayed secondarily to presynaptic inhibition and disynaptic components that may have been unusually effectively evoked.
When field potentials were evoked by two or three weaker test stimuli, like those illustrated in Fig. 4 and Fig. 5, and their monosynaptic components were abolished by conditioning stimuli, late components displaying a clear-cut temporal facilitation were seen in 15 out of 20 of these potentials. In order to assist with the disclosure of temporal facilitation of the late components, the intensity of the test and conditioning stimuli was adjusted for each potential so that neither the early nor the late components remained after the first conditioning stimulus (or so that the late components were very small). Under these conditions, distinct late components were induced by the second and third stimuli at latencies 0.5-1.1 ms longer than the original latencies of the monosynaptic components. The disynaptic components were thus disclosed in a considerable proportion of group II field potentials. However, it should be stressed that the proportions given above applied only to the most successful combinations of conditioning and testing stimuli. Monosynaptic components of field potentials were often abolished by stimulation of some nerves but not others and the effects evoked from a particular nerve depended on the intensity and timing of the test and conditioning stimuli.
Disynaptically evoked responses of intermediate zone interneurones
Changes in monosynaptic and disynaptic components of intermediate zone field potentials evoked by group II afferents were expected to be associated with similar changes in monosynaptically and disynaptically evoked responses of intermediate zone interneurones with group II input. Effects of the same conditioning stimuli as those used for field potentials were therefore analysed in a sample of 21 interneurones of this kind, six of which were antidromically activated from GS or PBST motor nuclei in the L7 segment. All were recorded from extracellularly and one neurone was subsequently penetrated.
Differential depression of monosynaptically evoked responses of group II origin of intermediate zone interneurones
When neuronal responses of group II origin appeared both at a short and at a longer latency, following conditioning stimuli the earlier spikes were either abolished or decreased considerably in number, even when the later responses were only marginally affected. This is illustrated in Fig. 6 with records from an interneurone that ceased to respond just after the onset of group II components of the field potential (between the two dotted lines) but continued to respond at a 1 ms longer latency. The PSTHs and cumulative sums in D show that, if anything, the number of later responses increased and their latency decreased (to the level of the second dotted line). The lower readiness with which the later responses appeared when they were preceded by earlier responses (B as compared to D) could be explained by refractoriness of the interneurone following the earlier spikes.
Figure 6. Effects of conditioning stimulation of group II afferents on responses of an intermediate zone interneurone evoked by group II afferents.
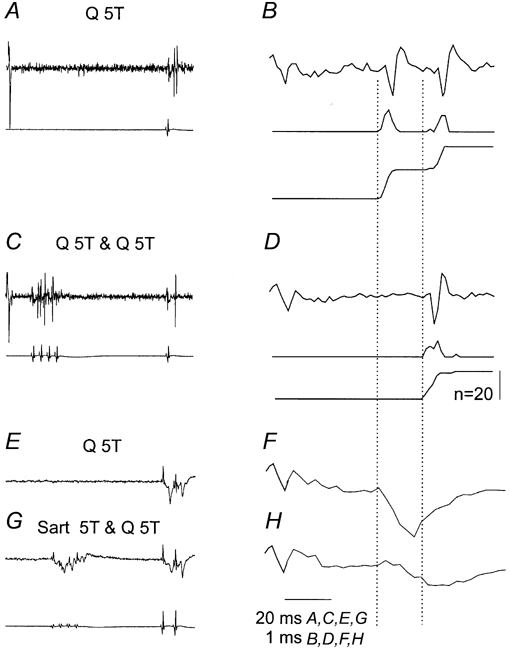
A and B, responses evoked by 5T stimulation of Q alone. C and D, responses evoked by the same stimuli following a short train of conditioning stimuli, at 5T, applied to the same nerve (at a conditioning-testing interval of 57 ms). E-H, corresponding control and conditioned field potentials. A, C, E and G, slow time base records with the corresponding records of afferent volleys from the surface of the spinal cord. B, D, F and H, expanded rightmost parts of records in A, C, E and G and PSTHs and cumulative sums of interneuronal responses evoked by 20 stimuli. Note that after the conditioning stimuli, the early responses disappeared and the neurone only responded at a longer latency. Dotted lines indicate latencies of monosynaptically and disynaptically evoked responses. Note also that the interneuronal spikes coincided with group II components of the field potentials, 0.5-1 ms from their onset. Field potentials were not properly recorded in B and C because low-cut filters were used while recording from interneurones for the purpose of constructing PSTHs.
Intracellular records from the interneurone illustrated in Fig. 6 revealed that the conditioning stimuli considerably reduced the amplitude of monosynaptically evoked EPSPs, which explains their failure to induce early action potentials. As shown in Fig. 7C-G, the test stimuli used to induce the EPSPs were reduced from 5 or 4T to 3T in order to weaken the disynaptic components of these EPSPs. The single sweep records in Fig. 7F and G show nevertheless that some later components followed the earliest components of the EPSPs at a latency compatible with disynaptic coupling and these later components were sometimes present without being preceded by the earlier components. The depression of EPSPs, resulting in the disappearance of the monosynaptically evoked spikes, was evoked after the membrane potential had nearly returned to the resting level following EPSPs evoked by conditioning stimuli. Depression of responses of other neurones was evoked at even longer intervals between the conditioning and test stimuli (see next section).
Figure 7. Effects of conditioning stimuli on intracellularly recorded synaptic actions of group II afferents.
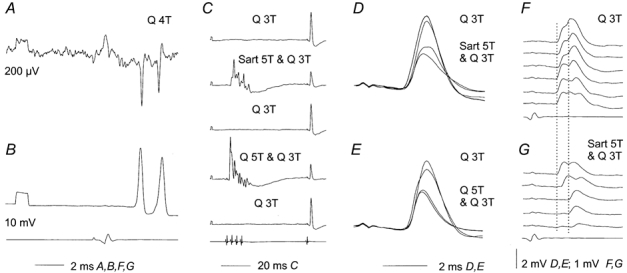
Extracellular (A) and intracellular (B) single sweep records of responses of the intermediate zone interneurone illustrated in Fig. 6, just before and after it was penetrated. C, examples of averages of 20 successive EPSPs evoked by Q group II afferents under control conditions and when weaker test stimuli were preceded by conditioning stimulation of either Sart or Q group II afferents (at 60 ms conditioning-testing intervals). D and E, superimposed expanded records of averaged EPSPs in alternating series of 20 trials in which only test stimuli or both test and conditioning stimuli were used. F and G, examples of single records of EPSPs from the series in D. Dotted lines indicate the onset of the most distinct early and later components of these EPSPs. Note that the distance between these lines corresponds to the distance between the early and late extracellularly recorded spikes in A. Note also that in the three bottom traces in G the early components of the EPSPs are missing.
Figure 8 and Figure 9 show further examples of the differential depression of monosynaptically evoked responses of group II origin in intermediate zone interneurones and, together with Fig. 6, provide further arguments that the depression of these responses is mediated by presynaptic inhibition (see discussion in Jankowska et al. 2002). In the neurone illustrated in Fig. 8, stimulation of Q evoked responses which were apparently evoked monosynaptically (earlier spikes in A) as well as disynaptically (later spikes in A), while stimulation of Sart evoked only later responses (D). Of these, the late responses evoked from Sart and Q were only slightly affected by conditioning stimulation (their number was practically unchanged and the latency increased by 0.4 and 0.3 ms, respectively) while all the early responses induced from Q were abolished by the same stimuli.
Figure 8. Effects of conditioning stimulation of group II afferents on monosynaptically and disynaptically evoked responses of an intermediate zone interneurone.
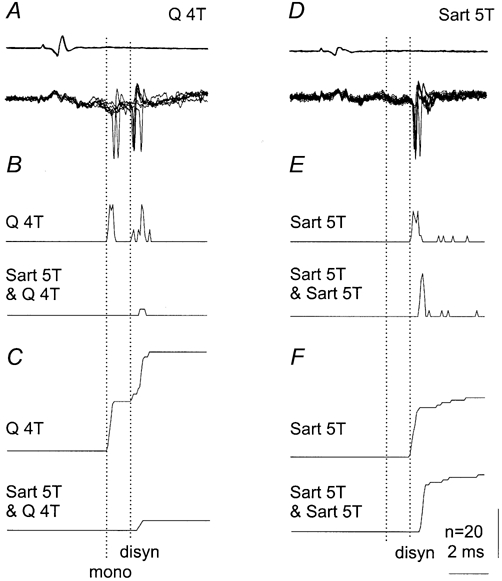
A-C, responses were evoked both mono and disynaptically from Q, at latencies of 2.7 and 3.9 ms (test) and 4.2 ms (conditioned). D-F, responses were only evoked disynaptically from Sart at latencies of 3.4 ms (test) and 3.8 ms (conditioned). A and D, superimposed records of seven subsequent responses (lower traces) and afferent volleys (upper traces) 67 ms following conditioning stimuli. B and E, PSTHs of 20 responses. C and F, cumulative sums of the same responses. Note that the monosynaptically evoked responses from Q were abolished (C) by Sart, while the same conditioning stimuli only delayed the disynaptically evoked responses from Sart (F). Other indications as in Fig. 6.
Figure 9. Effects of conditioning stimulation of group II afferents on extracellularly recorded responses of an intermediate zone interneurone co-excited by group I and II afferents and antidromically activated from the GS motor nucleus.
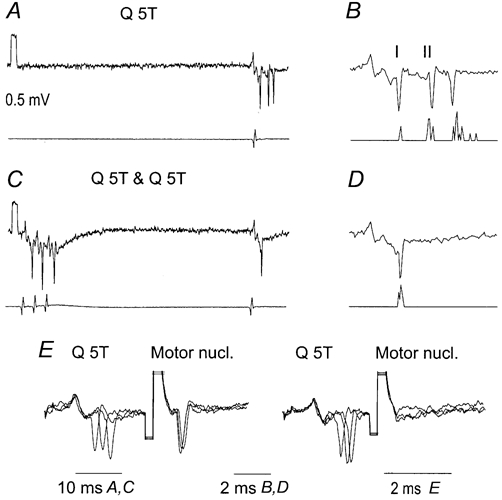
A and C, responses to test stimuli when they were applied alone (A) and when they were preceded by conditioning stimuli at 50 ms interval (C). Lower traces show records of afferent volleys from the cord dorsum. B and D, expanded rightmost parts of records in A and C and PSTHs of responses of the neurone. The responses were evoked at segmental latencies of 1.2, 2.6 and 4.2 ms, respectively. Note that the earliest responses and the earliest (group I) components of the field potentials on which they were superimposed were not depressed by the conditioning stimuli while the late responses were abolished by them. E, collision between synaptically evoked responses and responses following stimuli applied in the GS motor nucleus (a test for antidromic activation of the neurone); three superimposed traces are shown with truncated shock artefacts.
In the interneurone illustrated in Fig. 9, conditioning stimuli sufficiently strong to depress both the early and late responses evoked by group II afferents did not depress responses evoked by group I afferents and, if anything, enhanced them. Similarly differentiated effects of conditioning stimuli on monosynaptically evoked responses from group I afferents (only marginally affected) and group II afferents (abolished) were seen in all other intermediate zone interneurones monosynaptically excited by both group I and group II afferents.
Depending on the combinations of the test and conditioning stimuli, the intensity of these stimuli and the conditioning-testing intervals, the depression of monosynaptically evoked activation of intermediate zone interneurones by group II afferents was either complete (Figs 6, 8, 9 and 10) or partial and was or was not associated with the depression of disynaptically evoked responses of these neurones. The depression of monosynaptically evoked responses was maximal at conditioning-testing intervals of 40-50 ms, and slowly declined at longer intervals, up to 200 ms or more, as illustrated in Fig. 12A and D. The time course of these changes was thus similar to that of the depression of the monosynaptic components of the intermediate zone field potentials (Fig. 5C).
Figure 10. An example of disynaptically evoked responses of an intermediate zone interneurone after the monosynaptically evoked ones had been abolished.
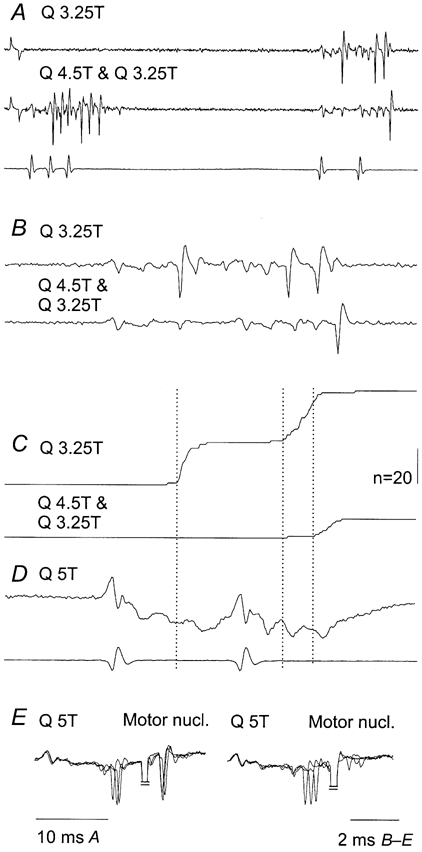
A, extracellular records of responses induced from Q, when this nerve was stimulated with double stimuli (upper trace) and when these stimuli were preceded by a train of conditioning stimuli (lower trace). Note that the responses following the first stimulus were evoked at a longer latency (2.8 ms) than those evoked by the second stimulus (2.2 ms) but that both coincided with the corresponding components of the field potentials shown in D. B, expanded rightmost parts of the records in A. C, cumulative sums of responses evoked by 20 subsequent test stimuli and sequences of conditioning and test stimuli at the same time scale as in B. D, field potentials evoked by somewhat stronger test stimuli at the same time base as in B and C. E, collision of responses evoked from Q and by stimuli applied in the GS motor nucleus, as in Fig. 9E, showing that the neurone projected to these nuclei; three superimposed traces are shown with truncated shock artefacts. Dotted lines indicate the onset of monosynaptically and disynaptically evoked responses. Note that the distance between these lines following the second stimulus (about 1.3 ms) corresponds to a difference in latency of the monosynaptic and disynaptic components of field potentials evoked by second stimuli. Other indications as in Fig. 6.
Figure 12. Timing of the earliest responses of dorsal horn and intermediate zone interneurones with monosynaptic input from group II afferents.
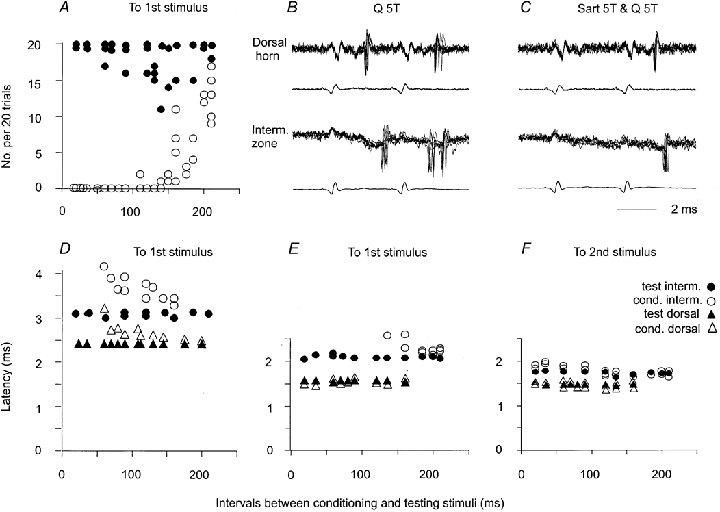
A, number of responses of three intermediate zone interneurones evoked by 20 single stimuli preceded by conditioning stimuli at different conditioning-testing intervals (○) and the number of corresponding responses to test stimuli only (•). B and C, records from two interneurones in the same segment, located at 1.6 mm and 2.35 mm depth, respectively, 200 μm apart rostrocaudally. Seven superimposed traces are shown from sequences in which only test stimuli (Q 5T) or both conditioning and test stimuli (Sart 5T & Q5T) at conditioning-testing intervals of 50 ms were used to activate the neurones. D, latencies of responses of an intermediate zone interneurone (circles) and a dorsal horn interneurone (triangles) recorded in the same experiment as a function of conditioning-testing intervals. The two interneurones belonged to those interneurones that responded to first stimuli at relatively long latencies. E and F, similar data for another pair of interneurones. These interneurones belonged to those responding at minimal latencies to both the first and the second of two stimuli. Filled symbols in A, D, E and F, data for responses evoked by test stimuli alone. Open symbols in A, D, E and F, data for conditioned responses.
Effects of double stimuli
When double test stimuli, 3.3 or 5 ms apart, were used to activate the interneurones instead of single stimuli, distinct responses attributable to disynaptic actions of group II afferents could be evoked by the second stimulus even when conditioning stimuli abolished any responses induced by the first stimulus. In the neurone illustrated in Fig. 10, the first as well as the second test stimulus most likely evoked both di- and monosynaptically induced responses, as indicated by their coincidence with the two components of group II field potentials (Fig. 10D; of the same kind as the field potential shown in Fig. 2). Following conditioning stimuli the first stimulus ceased to evoke any responses whereas the later responses evoked by the second stimulus remained (Fig. 10C, lower trace). They appeared at a latency (3.4 ms) that was 0.6 ms longer than the original latency of the earliest unconditioned responses evoked by the first stimulus and coincided with the disynaptic components of group II field potentials induced by the second stimulus (Fig. 10D). All the arguments for the disynaptic origin of the later components of the field potentials should thus apply to the interneuronal responses coinciding with them. Responses appearing with latencies that were 0.5-1.0 ms longer than the latencies of monosynaptically evoked responses, especially when the latter were abolished, will therefore be referred to as being due to disynaptic actions of group II afferents.
In the whole sample of analysed intermediate zone interneurones, responses coinciding with both mono- and disynaptic components of intermediate zone field potentials were found in 16 of the 21 interneurones, including five of the six interneurones that were antidromically activated from the motor nuclei. When 4-5T stimuli were used, single stimuli evoked both early and late responses in 10 of 14 interneurones (Figs 6-9). When double stimuli were used, late responses were found in a similar proportion of neurones (11 out of 14, some of which were tested with both strong single stimuli and weaker double stimuli). When the first of the two stimuli evoked only occasional or no late responses (Fig. 10), the number of responses that coincided with disynaptic components of the field potentials regularly appeared after the second stimulus in five interneurones. In three other interneurones no late responses appeared following the second stimulus and in six interneurones it was impossible to evaluate the number of possibly disynaptically evoked responses since there was no clear separation between the earlier and later responses induced by the second stimuli. When conditioning stimuli abolished monosynaptic responses, responses coinciding with the disynaptic components of the field potentials were seen in 10 interneurones in which they were otherwise missing or rare.
Indications that dorsal horn interneurones can mediate disynaptic activation of intermediate zone interneurones by group II afferents
In order to be able to mediate disynaptic excitation of intermediate zone interneurones by group II afferents when their monosynaptic excitation is depressed by presynaptic inhibition, the dorsal horn interneurones must fulfil three conditions. First, they must be monosynaptically activated by group II afferents when the test stimuli are preceded by conditioning stimuli. Second, they must be readily activated at conditioning-testing intervals at which intermediate zone interneurones are disynaptically excited and at which disynaptic components of the intermediate zone field potentials are most distinct. Third, they must be activated at segmental latencies of not more than 2 ms by the second or third stimulus to be able to induce disynaptic components of intermediate zone field potentials with latencies of 2.5-3 ms and interneuronal responses with latencies of 3-3.5 ms.
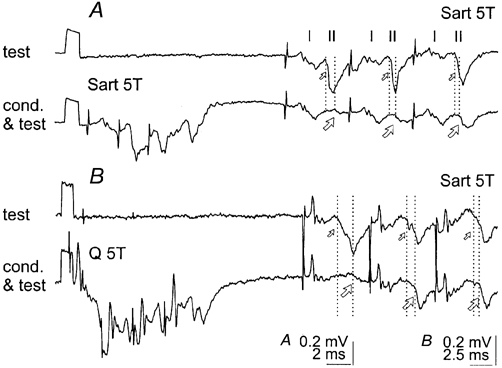
Seven dorsal horn interneurones with group II input were investigated in order to find out whether they complied with these requirements. In four interneurones the conditioning stimuli only increased the minimal latencies of the monosynaptically evoked responses following the first stimulus (by 0.1-0.3 ms) and increased their range (as indicated by a less steep slope of the cumulative sums), but did not prevent the neurones from responding to each stimulus (Fig. 11A and Fig. 12E) at any of the conditioning-testing intervals. In the three remaining interneurones, monosynaptically evoked responses following the first stimulus were abolished by conditioning stimuli at conditioning- testing intervals of 30-60 ms, as exemplified in Fig. 11B and Fig. 12D, but reappeared at conditioning-testing intervals exceeding 80-100 ms. However, responses evoked by the second stimulus remained practically unchanged in all of the investigated neurones. They were induced in all of the trials, and usually with the same minimal latency and a similarly small range of latencies. In some cases the minimal latencies were even shortened (as in the example of Fig. 11B, where the latency was reduced from 2.5 to 2.18 ms at a conditioning-testing interval of 50-60 ms and returned to the original value at longer intervals). This might depend on a smaller number of spikes immediately preceding the second stimulus since the responses due to be induced by the second stimulus no longer coincided with the refractory period and could thus be induced at a shorter latency.
Figure 11. Effects of stimulation of group II afferents on responses of dorsal horn interneurones.
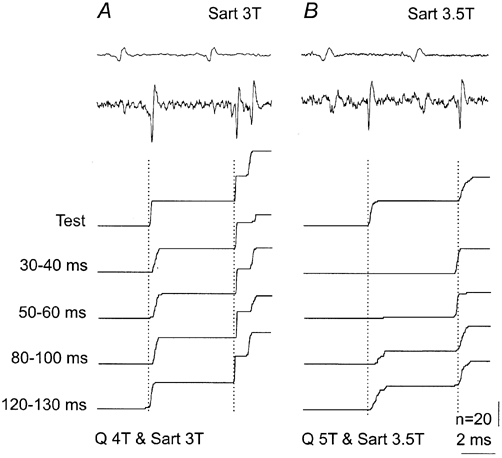
A and B, records from two dorsal horn interneurones with monosynaptic input from Sart group II afferents. These include from the top to the bottom: records of afferent volleys from the surface of the spinal cord, examples of responses of the interneurones and cumulative sums of responses which were not (test) or were preceded by conditioning stimulation of Q with the indicated intervals. Note that conditioning stimuli evoked some depression of monosynaptically evoked responses of these interneurones, but only to the first stimuli. Dotted lines indicate the original latency of responses evoked by the first and second stimuli.
As a population, dorsal horn interneurones would thus be somewhat less effective in mediating disynaptic actions of intermediate zone interneurones, especially at short conditioning-testing intervals, but their effectiveness would increase at intervals of 100 ms or longer.
Figure 12 shows that the latencies of the responses of dorsal horn interneurones, and the effectiveness with which they were activated, were as would be required for evoking disynaptic responses of intermediate zone interneurones. Fig. 12B and C illustrates this with the responses of a dorsal horn and an intermediate zone interneurone located at a distance of less than 1 mm apart. The dorsal horn interneurone originally responded to each stimulus applied to the Q nerve alone. Responses of this neurone were followed by responses of the intermediate zone interneurone. These appeared at latencies of 3.2-3.8 ms and 2.9-3.5 ms with respect to the first and second afferent volleys and about 0.9-1.5 ms and 0.6-1.2 ms after the responses of the dorsal horn interneurone. However, the second stimulus also evoked some responses that preceded spikes of the dorsal interneurone (at a latency of 2.1-2.4 ms). These coincided with the onset of the intermediate zone field potential on which they were superimposed (better seen after the first stimulus) and were classified as being evoked monosynaptically. Following the conditioning stimuli, the first test stimulus failed to activate any of these interneurones whereas the second stimulus activated both of them. All responses of the intermediate interneurone followed the responses of the dorsal horn interneurone (by 0.5-0.7 ms).
Individual interneurones responded within a certain range of latencies. In Fig. 12 D-F, responses of pairs of dorsal horn and intermediate zone interneurones that responded with the longest (D) and shortest (E-F) latencies are therefore compared. In both cases disynaptic responses of the intermediate zone interneurones, which appeared following conditioning stimuli (after the monosynaptic responses disappeared), were induced within 0.5-1.0 ms after responses of the dorsal horn interneurones. They could thus have been induced by dorsal horn interneurones responding with shorter latencies.
DISCUSSION
Relationships between dorsal horn and intermediate zone group II interneurones
All observations reported under Results strengthen the hypothesis that dorsal horn interneurones with monosynaptic input from group II muscle afferents provide disynaptic input from these afferents to intermediate zone interneurones, which in turn may either excite or inhibit α-motoneurones, as outlined in Fig. 1. The main argument in favour of this hypothesis is that intermediate zone interneurones may be disynaptically activated by group II afferents under conditions where monosynaptic excitation of interneurones in the intermediate zone is practically abolished by presynaptic inhibition, while dorsal horn interneurones are readily activated. Other observations in keeping with the hypothesis include the proper time lag between responses of dorsal horn and intermediate zone interneurones (illustrated in Fig. 12), parameters of stimuli needed to induce disynaptic actions of group II afferents (illustrated in Fig. 5) and previously established projections of dorsal horn group II interneurones to the intermediate zone (Bras et al. 1989).
Only small samples of dorsal horn and intermediate zone interneurones were investigated in order to verify that properties of individual interneurones support the conclusions drawn from the analysis of input to and actions of their populations (population EPSPs and effects on motoneurones). Furthermore, even those intermediate zone interneurones that were found to project to motor nuclei were only partly identified, because they might have contacted either α- or γ-motoneurones, or both these kinds of neurones, and they might have been either excitatory or inhibitory. Theoretically, the investigated interneurones could be identified by their actions on motoneurones, using a spike-triggered averaging technique while recording from axons of motoneurones in the ventral roots (Cavallari et al. 1987) or motor units at the muscle level (Fetz et al. 2000). However, the probability of success while combining the experimental paradigm most suitable for the present study with those of spike-triggered averaging would be very low. In addition, no essential differences were seen between the effects of presynaptic inhibition on interneurones that projected to motor nuclei and the effects on other intermediate zone interneurones whose projection area was not identified. Allowing for some differences in the excitability of individual interneurones and in the effectiveness of conditioning stimuli in various preparations, the total population of the investigated intermediate zone group II interneurones appeared to behave in a very similar way. We therefore postulate that the reported properties of individual interneurones reflect properties of both excitatory and inhibitory interneurones in disynaptic pathways between group II afferents and motoneurones and of any other intermediate zone interneurones with monosynaptic and disynaptic input from group II afferents.
Both the hypothesis and the arguments in its favour apply primarily to neurones mediating synaptic actions of fast conducting group II muscle afferents, since such afferents are the main source of the sharply rising field potentials in both the dorsal horn and the intermediate zone which are maximal at about 5T (Edgley & Jankowska, 1987a; Riddell & Hadian, 2000a). These afferents are also the main source of the most synchronous earliest responses of interneurones at these sites (Edgley & Jankowska, 1987b; Lundberg et al. 1987b; Riddell & Hadian, 2000b). Whether the hypothesis extends to neurones mediating synaptic actions of slower conducting group II muscle afferents remains to be established.
Observations lending support to our hypothesis were made in midlumbar segments. However, previous records from interneurones in the L6-S1 segments revealed a clear-cut separation between the earliest and somewhat later components of EPSPs and spike discharges (see Fig. 11 and Fig. 12 in Lundberg et al. (1987b) and Figs 2-5 in Riddell & Hadian, 2000b); the later components appearing at about 3 ms latency and likely to be evoked disynaptically. If the difference in the degree of presynaptic inhibition of actions of group II afferents upon intermediate zone and dorsal horn interneurones in the lower lumbar and sacral segments is as marked as in midlumbar segments, the tests used in the present study might be used to verify that our hypothesis is valid for lower lumbar as well as midlumbar neurones.
As the criteria for disynaptic components of population EPSPs (field potentials) and disynaptically evoked responses of individual interneurones, a latency 0.5-1 ms longer than the latency of monosynaptic responses and temporal facilitation of these responses have been used. Assuming that the monosynaptically and disynaptically evoked synaptic actions of group II afferents are evoked by fibres with the same conduction velocity, a stepwise increase in the latency of this order was expected for the disynaptic actions, considering a 0.3 ms-long additional synaptic delay and at least 0.1-0.2 ms conduction time along axon collaterals of the first order interneurones. When the differences were less than 0.5 ms, it was difficult to differentiate disynaptic actions of the fastest conducting group II afferents from weaker and delayed monosynaptic actions. The proportions of field potentials (about 50 %) and interneurones (60 %) in which we could reasonably confidently recognize disynaptic actions of group II afferents were thus probably underestimated. The criterion of temporal facilitation of longer latency synaptic actions of group II afferents could in addition be used primarily for field potentials (Fig. 3 and Fig. 10) since temporal facilitation of responses of individual interneurones could involve not only dorsal horn but also intermediate zone interneurones, and monosynaptically as well as disynaptically evoked responses. We thus cannot exclude the possibility that most, or even all, intermediate zone interneurones are affected by dorsal horn interneurones with group II input. If not, it would be tempting to consider intermediate zone interneurones with and without disynaptic input from group II afferents as belonging to inhibitory and excitatory fractions of these interneurones, in keeping with a previous proposal of an additional interneurone in inhibitory pathways (Lundberg et al. 1987b). This proposal was based on the observations that spatial facilitation is easier to demonstrate in the inhibitory than in the excitatory pathways from group II afferents.
Alternative sources of disynaptic group II input to neurones in the intermediate zone
Only three areas of terminal branching of group II muscle afferents: in lamina IV, laminae V-VI and laminae VII and IX have been revealed by morphological studies. These correspond to the dorsal horn and intermediate zone areas explored in this study and to motor nuclei and the adjacent areas of the ventral horn. Since similarly strong depression attributable to presynaptic inhibition was found at all sites within the intermediate zone, no intermediate zone interneurones would be likely to mediate the reported disynaptic actions within the intermediate zone. With respect to a contribution of more ventrally located neurones there is no information as yet on any group II-excited interneurones that might be as weakly affected by presynaptic inhibition as dorsal horn interneurones and therefore mediate disynaptic activation of intermediate zone interneurones. In fact, depressive effects of presynaptic inhibition at all the more ventral locations analysed in this study (exemplified in Fig. 4B) were as potent as in the intermediate zone. Furthermore, in view of a decreasing conduction velocity of deeper-projecting collaterals of group II afferents (Fu & Schomburg, 1974; Lundberg et al. 1987a), any ventrally located interneurones activated by them might affect intermediate zone interneurones at latencies longer than the latencies of the reported disynaptic actions of group II afferents. This thus leaves only dorsal horn interneurones as likely to be mediating these actions. However, both intermediate zone and other more ventrally located interneurones might contribute to disynaptic actions of group II afferents under other conditions, when activation of these neurones by group II afferents is not prevented by presynaptic inhibition, or in any other way.
Other actions of group II afferents mediated by dorsal horn interneurones
The diagram in Fig. 1 was not intended to suggest that intermediate zone interneurones are the only target neurones of dorsal horn interneurones. The available evidence in fact suggests the opposite. Projections of dorsal horn group II interneurones extend outside the intermediate zone (to lamina VII and VIII and to the contralateral grey matter; Bras et al. 1989). Even more importantly, many actions of group II afferents are exerted via polysynaptic flexor reflex pathways (Eccles & Lundberg, 1959), even under conditions when direct pathways are blocked by noradrenergic drugs or monoamine-releasing neurones (Lundberg, 1982; Lundberg et al. 1987c). Polysynaptic actions of group II afferents mediated by dorsal horn interneurones must thus to a great extent be exerted via other interneurones. Which particular ipsilateral and crossed group II actions utilize the connections outlined in Fig. 1 remains to be established.
Multiple sites of modulation of synaptic actions of group II afferents
Since group II afferents may use both direct and indirect ways to excite intermediate zone interneurones, this arrangement would greatly increase the probability of activation of these interneurones. Disynaptic actions of group II afferents upon intermediate zone interneurones via dorsal horn interneurones might either be delayed with respect to the direct monosynaptic actions, or coincide with them, and therefore effectively summate and make the generation of action potentials much more secure.
This arrangement would also increase the possibility of controlling reflex actions of group II afferents. In view of somewhat different peripheral and descending sources of input to intermediate zone and dorsal horn group II interneurones (Edgley & Jankowska, 1987b; Davies & Edgley, 1994), information reaching these neurones can be independently integrated, allowing a higher precision of its tuning and of selecting subsets of intermediate zone interneurones best suited to serve the intended movements (Lundberg, 1975; Lundberg et al. 1987c). Strong and long-lasting depression of monosynaptic actions of group II afferents in the intermediate zone by presynaptic inhibition might enable only the earliest nerve impulses in repeatedly activated muscle spindle group II afferents to contribute to direct actions of these afferents on intermediate zone interneurones. The successive nerve impulses might then act upon these interneurones primarily via di- or polysynaptic pathways. Presynaptic inhibition of synaptic actions of group II afferents in the intermediate zone might also be used to depress these actions during centrally initiated movements (Lundberg, 1982; Lundberg et al. 1987c). For instance, presynaptic inhibition induced by group II afferents could be enhanced by collateral actions of reticulospinal tract neurones upon interneurones that mediate this inhibition. The possibility of a triple modulatory control of the operation of the network of the dorsal horn and intermediate zone interneurones by presynaptic inhibition and by monoamine-releasing neurones appears to be of particular interest. Monosynaptic activation of intermediate zone interneurones by group II afferents may be similarly effectively depressed by GABAergic interneurones responsible for presynaptic inhibition (present study) and by noradrenaline-releasing neurones (Bras et al. 1990; Jankowska et al. 2000b). Disynaptic activation might on the other hand be most effectively depressed by serotonin-releasing neurones at the level of dorsal horn neurones (Bras et al. 1990; Jankowska et al. 2000b). Accordingly, when transmission from group II afferents is under negligible presynaptic inhibition and weak noradrenergic but strong serotoninergic control, this would favour monosynaptic actions of group I and II afferents on intermediate zone interneurones, which would in turn directly and perhaps indiscriminately excite and inhibit their target neurones. In contrast, under conditions of strong presynaptic inhibition and strong noradrenergic but weak serotoninergic actions, intermediate zone interneurones could be primarily activated via dorsal horn interneurones and their actions could be more intimately associated with polysynaptic actions mediated by dorsal horn interneurones. The latter neurones could then select only some of the excitatory and/or inhibitory intermediate zone interneurones. Differences in the combined actions of high threshold muscle afferents (including group II afferents) and skin and joint afferents under different conditions might depend on combinations of these modulatory actions.
Acknowledgments
The study was supported by grants from the Parkinson Association for Neurological Movement Analysis Studies and from NIH (NS 40 863). We wish to thank Rauni Larsson for her invaluable assistance during the experiments.
REFERENCES
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. Journal of Comparative Neurology. 1989;290:1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Bras H, Jankowska E, Noga B, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group II muscle afferents in the cat. European Journal of Neuroscience. 1990;2:1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. Journal of Physiology. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. Journal of Physiology. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R, Lundberg A. Synaptic actions in motoneurones by afferents which may evoke the flexion reflex. Archives Italiennes de Biologie. 1959;97:199–221. [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. Journal of Physiology. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. Journal of Physiology. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y. Functions of mammalian spinal interneurons during movement. Current Opinion in Neurobiology. 2000;10:699–707. doi: 10.1016/s0959-4388(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Fu TC, Schomburg ED. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiologica Scandinavica. 1974;91:314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Fyffe R. The morphology of group II muscle afferent fibre collaterals. Journal of Physiology. 1979;296:39–40P. [PubMed] [Google Scholar]
- Hammar I, Slawinska U, Jankowska E. A comparison of post-activation depression of synaptic actions evoked by different afferents and at different locations in the feline spinal cord. Experimental Brain Research. 2002 doi: 10.1007/s00221-002-1098-5. in the Press. [DOI] [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle afferents and Spinal Control of Movement. Oxford, New York, Seoul, Tokyo: Pergamon Press; 1992. pp. 389–394. [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Experimental Brain Research. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. Journal of Physiology. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Bichler E, Hammar I. Areas of operation of interneurons mediating presynaptic inhibition in sacral spinal segments. Experimental Brain Research. 2000a;133:402–406. doi: 10.1007/s002210000429. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. European Journal of Neuroscience. 2000b;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo Lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. European Journal of Neuroscience. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. A relay for input from group II muscle afferents in sacral segments of the cat spinal cord. Journal of Physiology. 1993;465:561–580. doi: 10.1113/jphysiol.1993.sp019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to motoneurones. Journal of Physiology. 2002;542:287–299. doi: 10.1113/jphysiol.2001.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Basic Neurosciences. New York: Raven Press; 1975. pp. 253–265. [Google Scholar]
- Lundberg A. Inhibitory control from the brain stem of transmission from primary afferents to motoneurones, primary afferent terminals and ascending pathways. In: Sjölund B, Björklund A, editors. Brain Stem Control of Spinal Mechnisms. Amsterdam: Elsevier Biomedical Press; 1982. pp. 179–225. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Experimental Brain Research. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Experimental Brain Research. 1987b;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Experimental Brain Research. 1987c;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Munson JB, Sypert GW, Zengel JE, Lofton SA, Fleshman JW. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. Journal of Neurophysiology. 1982;48:1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Riddell JS, Hadian M. Field potentials generated by group II muscle afferents in the lower-lumbar segments of the feline spinal cord. Journal of Physiology. 2000a;522:97–108. doi: 10.1111/j.1469-7793.2000.0097m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Hadian M. Interneurones in pathways from group II muscle afferents in the lower-lumbar segments of the feline spinal cord. Journal of Physiology. 2000b;522:109–123. doi: 10.1111/j.1469-7793.2000.t01-2-00109.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. Journal of Physiology. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EK, Watt DG, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. Journal of Neurophysiology. 1976;39:1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]


