Abstract
We have used a constant [1,2-13C]acetate infusion (0.12 μmol min−1 kg1) for 2 h at rest, followed by 2 h of one-legged knee-extensor exercise at 65 % of leg maximal workload, and 3 h of recovery in six post-absorptive volunteers to quantify whole-body and leg acetate kinetics and determine whether the whole-body acetate correction factor can be used to correct leg substrate oxidation. The acetate whole-body rate of appearance (Ra) was not significantly different at rest, during exercise or during recovery (365-415 μmol min−1). The leg net acetate uptake was similar at rest and during recovery (≈10 μmol min−1), but increased ∼5-fold with exercise. At rest the leg acetate uptake (≈15 μmol min−1) and release (≈5 μmol min−1) accounted for 4 and 1.5 % of whole-body acetate disposal (Rd) and Ra, respectively. When the leg acetate kinetics were extrapolated to the total body skeletal muscle mass, then skeletal muscle accounted for ∼16 and ∼6 % of acetate Rd and Ra. With exercise, leg acetate uptake increased ∼6-fold, whereas leg acetate release increased 9-fold compared with rest. Whole-body acetate carbon recovery increased with time of infusion at rest and during recovery from 21 % after 1.5 h of infusion to 45 % in recovery after 7 h of infusion. Leg and whole-body acetate carbon recovery were similar under resting conditions, both before and after exercise. During exercise whole-body acetate carbon recovery was ∼75 %, however, acetate carbon recovery of the active leg was substantially higher (≈100 %). It is concluded that inactive skeletal muscle plays a minor role in acetate turnover. However, active skeletal muscle enhances several-fold acetate uptake and subsequent oxidation, as well as release and its contribution to whole-body acetate turnover. Furthermore, under resting conditions the whole-body acetate correction factor can be used to correct for leg, skeletal muscle, substrate oxidation, but not during exercise.
Skeletal muscle acetate kinetics has been the subject of several investigations reporting a net uptake of acetate across the arm or leg in humans (Skutches et al. 1979; Pomare et al. 1985; Coppack et al. 1990; Mittendorfer et al. 1998). In addition, acetate uptake and release by the limb have been calculated using tracers (Mittendorfer et al. 1998; Pouteau et al. 1998a). Interpretation of limb acetate kinetics is complicated due to isotopic exchange reactions within the intracellular pools. Acetate is formed from acetyl CoA via acetyl CoA hydroxylase and is converted at the same time to acetyl CoA via acetyl CoA synthase. Both enzymes have been shown to be present in mammalian skeletal muscle (Knowles et al. 1974). However, the intracellular isotopic exchange reactions involving acetate should be the same as for acetyl CoA, which makes acetate a useful indicator for kinetic studies of substrate oxidation. In fact, the determination of the rate of recovery of carbon-labelled CO2 from carbon-labelled acetate allows the calculation of a correction factor for substrate oxidation, the acetate correction factor (Sidossis et al. 1995a,b). When using a 13C- or 14C-labelled substrate in combination with indirect calorimetry, estimation can be made of substrate oxidation. When the carbon-labelled substrate is oxidized, the label should appear in the breath as 13CO2 (or 14CO2) and the oxidation rate of the tracer can be calculated. However, estimates of the oxidation rate of substrates from carbon-labelled tracers have been questioned since the appearance of 13CO2 (or 14CO2) is low at rest. This can be explained by loss of label in the bicarbonate pools and via non-oxidative pathways, thus the carbon label would not appear in CO2 resulting in an underestimation of substrate oxidation rates. The loss of label via non-oxidative pathways mainly occurs via exchange reactions of the tricarboxylic acid cycle (TCA cycle) (Sidossis et al. 1995b; van Hall, 1999). The acetate correction factor accounts for the label fixation that occurs between labelled acetyl CoA entering the TCA cycle and recovery of label in CO2. Indeed, TCA cycle products in which carbon labels from acetate and the substrate palmitate have been found are glucose, lactate/pyruvate and, quantitatively most important, glutamate and glutamine (Sidossis et al. 1995b; Pouteau et al. 1998b; Schrauwen et al. 1998). However, the need to correct oxidation rates for the recovery of infused label does not only concern fatty acid metabolism but also carbohydrate and protein metabolism (Tounian et al. 1996; Toth et al. 2001). Whole-body acetate carbon recovery has been shown to increase with time of infusion (Wolfe & Jahoor, 1990; Sidossis et al. 1995b; Bäurle et al. 1998; Mittendorfer et al. 1998; Schrauwen et al. 1998). It was shown that only after 12 h of [1,2-13C]acetate infusion was a steady state reached in acetate carbon recovery (Mittendorfer et al. 1998). With exercise, acetate carbon recovery is substantially higher, compared with rest (Sidossis et al. 1995a,b; Schrauwen et al. 1998). This implies that the acetate correction factor has to be determined at the exact same time after the start of the infusion as the substrate to be studied, and at the same exercise intensity in order to make a proper correction for substrate oxidation. In analogy with the whole-body acetate correction factor, a regional acetate correction factor is required to determine quantitative regional substrate oxidation. Mittendorfer et al. (1998) have shown that after 3 h primed continuous [1,2-13C]acetate infusion leg and splanchnic acetate carbon recovery was similar to whole-body acetate carbon recovery under resting conditions. They concluded that the whole-body acetate correction factor could be used to correct substrate oxidation by these two tissues. Whole-body and skeletal muscle fatty acid metabolism, and thus fatty acid oxidation, is an important field of research in health, disease and physical activity. Quantitative measures of fatty acid oxidation by skeletal muscle are crucial for a thorough understanding of muscle fatty acid metabolism. Plasma fatty acid metabolism is traditionally studied using a continuous infusion of carbon-labelled fatty acid without prime. Thus, in order to obtain quantitative measures of skeletal muscle fatty acid oxidation, the muscle acetate correction factor has to be determined not with a primed (Mittendorfer et al. 1998), but with an unprimed continuous acetate infusion. So far no studies have been carried out to determine an acetate correction factor for active skeletal muscle. Therefore, in the present study whole-body and skeletal muscle acetate kinetics and carbon recovery were measured during an unprimed, continuous [1,2-13C]acetate infusion, in order to determine whether the whole-body acetate correction factor can be used to correct skeletal muscle substrate oxidation. Skeletal muscle acetate kinetics and carbon recovery were determined using the leg model (van Hall et al. 1999) during 2 h rest, 2 h one-legged knee-extensor exercise at 65 % leg peak power output (Wmax,leg), and 3 h recovery. The one-leg knee-extensor exercise model was used as it is the most frequently used model to study active skeletal muscle energy metabolism. In addition, this model makes it possible to simultaneously study acetate kinetics and carbon recovery in an active and inactive leg, skeletal muscle, without differences in hormonal and metabolite concentrations.
METHODS
Subjects
Six healthy, physically active male subjects, aged 22-28 years, weighing 73-78 kg, participated in the study. The subjects were informed about the possible risks and discomfort involved before giving their voluntary consent to participate. The study was performed according to the Declaration of Helsinki II and was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark.
Protocol
Each subject underwent a preliminary exercise test on the one-leg knee extensor to become familiarized with the knee-extensor exercise and to determine their individual leg peak power output (Wmax,leg). On the day of the experiment the subjects reported to the laboratory at 08.00 h. The subjects changed and remained supine for the next 3 h. After 10 min in the supine position catheters were placed using the Seldinger technique under local anaesthesia (Lidocaine, 20 mg ml−1) in a femoral artery and in the femoral vein of both legs. The tip of each catheter (20G; Ohmeda, Wiltshire, UK) was inserted ∼2-5 cm below the inguinal ligament and advanced ∼8 cm in the proximal direction. Half an hour after placement of the catheters, breath and blood samples were obtained for assessment of background enrichment of breath and blood CO2, and plasma acetate. Immediately after the background samples for 13C enrichment were obtained, a continuous infusion of [1,2-13C]acetate (97 %, 0.12 μmol min−1 (kg body weight)−1) was started, along with a prime of [13C]bicarbonate (98 %, 1.5 μmol (kg body weight)−1). For each individual, the actual rate of infusion of acetate was determined from the acetate concentration and infusion rate of the pump. All isotopes were purchased from Cambridge Isotope Laboratories (Andover, USA), and were dissolved in 0.9 % sterile saline and passed through a 0.2 μm filter into a 250 ml sterile saline bag immediately before infusion. Measurements were carried out 90, 105 and 120 min after the start of the continuous acetate infusion at rest, then after 30, 60, 90, 105 and 120 min of one-legged knee-extensor exercise at 65 % Wmax,leg. The first 30 min of recovery measurements were made every 10 min followed by measurements every 30 min until 3 h of recovery. Blood samples were taken and immediately transferred to ice-cold tubes that contained 10 μl 0.33 m EDTA per millilitre of blood. Blood samples were vortex mixed and centrifuged at 4 °C for 10 min and the plasma immediately frozen in liquid nitrogen and stored at -80 °C until analysed for acetate concentration and enrichment. In addition, another blood sample was taken anaerobically with a 2 ml heparinized syringe (Radiometer, Copenhagen, Denmark) for analysis of haematocrit, haemoglobin and oxygen saturation (OSM3 hemoxymeter, Radiometer, Denmark), and blood pH, O2 and CO2 tension (ABL5, Radiometer, Denmark). From that syringe 0.5 ml of blood was injected into a 20 ml vacutainer to determine blood CO2 enrichment. Together with each blood sample indirect calorimetry measurements were performed (CPX, Medical Graphics Corporation, St Paul, USA) and expired air was collected in a 10 l bag at rest or 20 l bag during exercise (Hans Rudolph, Kansas City, USA) from which a 20 ml vacutainer was filled via a needle for CO2 enrichment.
Blood flow
An ultrasound doppler (model CFM 800, Vingmed Sound, Horten, Norway) equipped with an annular phased array transducer (Vinmed Sound) probe (11.5 mm diameter) operating at an imaging frequency of 7.5 MHz and variable Doppler frequencies of 4.0-6.0 MHz (high-pulsed repetition frequency mode 4-36 kHz) was used. The site for vessel diameter determination and blood velocity measurements in the common femoral artery was distal to the inguinal ligament, but above the bifurcation into the superficial and profunda femoral branch. The femoral artery was isonated at a fixed perpendicular angle. The diameter was determined along the central path of the ultrasound beam where the best spatial resolution is achieved. The blood velocity and flow during exercise were specifically analysed in relation to the muscle contraction force (strain gauge) profile (Rådegran, 1997).
Diet and activity prior to the test
Subjects were asked to maintain their normal activity schedule the week prior to the test. They were, however, not allowed to perform any substantial exercise the day before the experiment. Furthermore, the subjects were asked not to consume any food items with a high natural abundance of 13C such as carbohydrates derived from C4 plants, e.g. maize (corn) and cane sugar, for 1 week before the test.
Analytical procedures
Plasma acetate concentration and enrichment were determined using a gas chromatography-mass spectrometer (GC-MS, Automass II, Finnigan, Paris, France) as described previously (Powers et al. 1995). In short, to 200 μl of plasma 25 μl of 1 mm [1,2-13C-2,2,2-2H3]acetate was added. After derivatization with 0.2 m 2,4-difluoroaniline and 0.2 mN,N-dicyclohexylcarbiimide (Sigma, USA), the acetate derivative was extracted with ethylacetate, dried under a stream of N2 and redissolved in 100 μl ethylacetate. a 1 μl sample was injected into the GC-MS, and isotopic enrichment was determined using electron impact ionisation. Ions were selectively monitored at mass-to-charge ratio (m/z) of 171, 173 and 176, representing the molecular ions of unlabelled, infused and internal standard acetate derivatives, respectively. The concentration of plasma acetate was corrected for the concentration of acetate in the blank to correct for acetate present in the chemicals used. Samples of arterial and venous blood and expired breath for measurement of 13CO2 enrichment were analysed by gas chromatograph-isotope ratio mass spectrometry (GC-IRMS, Deltaplus, Finnigan MAT, Bremen, Germany). The 13C to 12C ratio was determined by split injection (ratio 1:4) of 20 μl of the expired air on the GC-IRMS (GC-column, poraplot Q, Chrompack, The Netherlands). For the determination of blood CO2 enrichment, 0.5 ml of 2.5 m phosphoric acid was added to 0.5 ml blood in the 20 ml vacutainer to release all the CO2. The tubes were brought to pressure with pure helium. The 13C to 12C ratio was determined by split injection (ratio 1:10) of 20 μl of the headspace on the GC-IRMS. The isotopic enrichment of breath or blood CO2 was expressed as the Δo/oo difference between the 13C/12C ratio of the sample and a known laboratory reference standard related to Pee Dee Belemnitella (PDB) limestone.
Calculations
The following equations were used to determine the rate of acetate appearance (Ra) and disappearance (Rd). Whole-body measurements of acetate Ra and Rd were calculated using the non-steady-state equations of Steele (1959) adapted for stable isotopes (Wolfe, 1992).
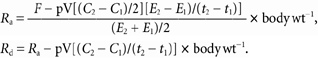 |
E1 and E2 are the arterial isotope enrichments at sample times 1 (t1) and 2 (t2) (expressed in min), respectively. C1 and C2 are the arterial concentrations at times 1 and 2 (expressed in mmol l−1), respectively. pV is the volume of distribution, 0.2 l (kg body weight)−1, assuming that the acute volume of distribution approximates extracellular fluid volume (Bleiberg et al. 1992). Whether pV is appropriate or not for resting, exercise and recovery conditions is obviated by the presence of a near steady-state conditions. The whole-body acetate carbon recovery, the percentage recovery of 13CO2 in expired air from infused [1,2-13C]acetate, was calculated with and without bicarbonate carbon recovery correction.
 |
ECO2 is the 13C/12C ratio in breath CO2, V̇CO2 is the respiratory CO2 production (expressed in μmol min−1 (kg body weight)−1), F is acetate infusion rate (μmol min−1 (kg body weight)−1). The CO2 recovery is a correction for 13CO2 recovery as published previously (van Hall, 1999). At rest 13CO2 recovery was incomplete (≈0.80), the second hour of exercise ∼1.00. However, during the 3 h of recovery from exercise the 13CO2 recovery increased gradually from 0.59, 0.65, 0.76 and 0.97 after 10, 20, 30, 60 and 180 min of termination of exercise, respectively.
The energy derived from acetate (EEacetate) is calculated assuming that all acetate disappearing from the circulation (Rd) is oxidized and acetate energy production is 879 kJ mol−1. The contribution of energy derived from acetate oxidation to total energy production is calculated as:
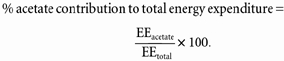 |
Total energy expenditure (expressed in kJ min−1) is calculated without correction for protein oxidation as:
where RER is the respiratory exchange ratio and V̇O2 whole-body oxygen uptake (expressed in l min−1).
The leg net acetate uptake, fractional extraction and uptake were calculated according to the following equations:
 |
The leg acetate carbon recovery, the percentage of recovery of 13CO2 released by the leg from [1,2-13C]acetate taken up by the leg, was calculated according to the following equation:
 |
Cv,CO2, Ca,CO2, Ev,CO2 and Ea,CO2 are the blood CO2 concentration and CO2 enrichment in tracer to tracee ratio (TTR) in the femoral vein and artery, respectively. The leg 13CO2 production is divided by 2 because the oxidation of 1 mol [1,2-13C]acetate produces 2 mol 13CO2. Ca, Cv, Ea and Ev are the plasma acetate concentration and acetate enrichment in TTR in the femoral artery and vein, respectively. Moreover, calculations were made on the assumption that acetate is only carried in plasma, and plasma flow being blood flow × (1 – haematocrit).
Statistical analysis
All values are means ± s.e.m. of five subjects. Statistical analysis of the data was performed using the non-parametric Wilcoxon signed-rank test to determine differences between the resting versus the exercising leg for each measurement. Statistical significance was set at P < 0.05.
RESULTS
Blood flow to resting and active leg
At rest the leg blood flow was ∼30 ml min−1. With exercise the blood flow to the exercising leg increased 13-fold to ∼3.9 l min−1. Blood flow to the resting leg increased ∼2.5-fold to ∼0.8 l min−1 (Fig. 1). This indicates that not a complete resting leg was studied as intended. The blood flow to the resting leg is most likely increased due to muscle activity in that leg needed to stabilize the body, while the other leg is kicking.
Figure 1.

Femoral arterial blood flow at rest, during one-legged knee-extensor exercise and 3 h of recovery. Values are means ± s.e.m., n = 6.
Whole-body and leg acetate kinetics
The arterial acetate concentration and the acetate Ra were similar at rest, during one-legged knee-extensor exercise, and in recovery. However, there was a tendency to somewhat lower acetate Ra in recovery compared with pre-exercise rest (Fig. 2). In contrast to the marginal changes observed in whole-body acetate kinetics, leg acetate kinetics differed markedly between rest, exercise and recovery (Fig. 3). A net acetate uptake by the legs was observed in all five volunteers under all conditions. During exercise, however, both the net leg acetate uptake and the tracer-calculated acetate uptake increased 3- and 5-fold by the resting and active leg, respectively. During one-legged knee-extensor exercise the fractional acetate extraction by the semi-resting leg was similar compared with rest and as a result a 3-fold increase in acetate uptake was observed as acetate delivery was increased. The increase in acetate uptake by the semi-resting leg was proportional to the increase in leg acetate delivery, caused by the increase in blood flow to the semi-resting leg. Fractional acetate extraction by the exercising leg, however, decreased from ∼70 to ∼35 %. Thus, acetate uptake by the exercising leg increased less than acetate delivery to the leg. The leg acetate uptake was 3-fold higher compared with the leg acetate release at rest and in the recovery. During exercise the leg acetate uptake was 2-fold higher than the leg acetate release. The difference in leg acetate uptake and release is also reflected in the contribution of the leg acetate uptake and release to whole-body acetate Rd and Ra, since the systemic rate of acetate turnover did not change. Under resting conditions leg acetate uptake accounted for ∼4 % of the systemic acetate Rd, whereas the leg acetate release accounted for only ∼1.5 % of the systemic acetate Ra. During exercise the contribution of leg acetate uptake to Rd increased ∼6-fold to ∼25 % and contribution of leg acetate release to Ra increased ∼9-fold with ∼13 %. Thus, during exercise the legs became relatively more important as a source of acetate release than uptake from the circulation.
Figure 2.
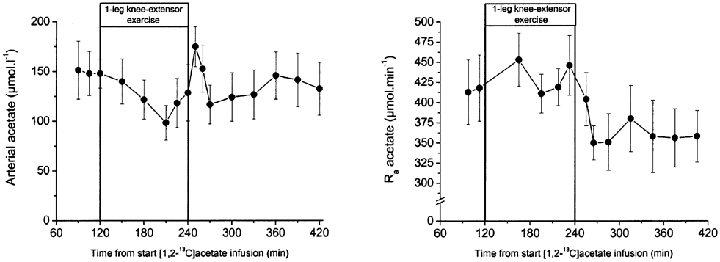
Plasma acetate concentration and whole body acetate rate of appearance (Ra). Values are means ± s.e.m., n = 6.
Figure 3.
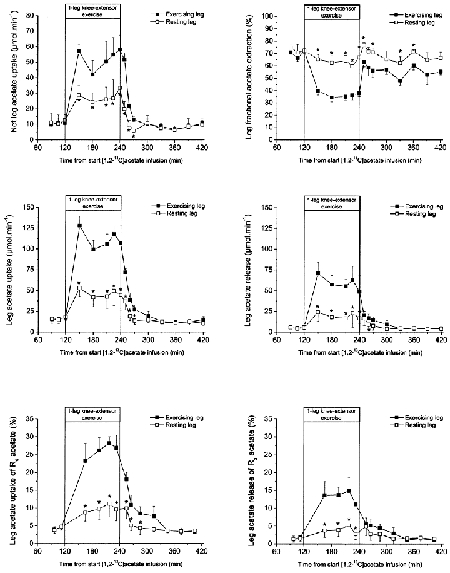
Leg acetate kinetics and the leg contribution to whole body acetate turnover. Values are means ± s.e.m., n = 6.
The mean whole-body energy expenditure was 6.4 ± 0.4, 20.3 ± 0.8 and 6.4 ± 0.7 kJ min−1 at rest, during exercise and recovery, respectively. The contribution of acetate to whole-body energy expenditure was estimated to be 5.3 ± 0.5, 1.9 ± 0.2 and 5.2 ± 0.5 % at rest, during exercise and recovery, respectively, assuming that all acetate extracted from the circulation is oxidized.
Whole-body and leg acetate carbon recovery
The whole-body acetate carbon recovery as CO2 increased with time of infusion at rest both pre- and post-exercise (Fig. 4). During exercise acetate carbon recovery increased with exercise duration to an apparent steady state at 75 % after 1-1.5 h of exercise. After 10 min in the recovery period acetate carbon recovery was similar to the level at rest immediately before exercise. However, acetate carbon recovery after exercise was higher compared with pre-exercise rest when corrected for incomplete CO2 recovery (see Methods and van Hall, 1999). Whole-body and leg acetate carbon recovery were similar under resting conditions both before and after 2 h of exercise. During exercise the acetate carbon recovery by the exercising leg was ∼100 % and higher than the acetate carbon recovery at whole-body level. Acetate carbon recovery in the semi-resting leg increased to near whole-body levels.
Figure 4. The percentage of whole body and leg labelled carbon recovery from [1,2-13C]acetate.
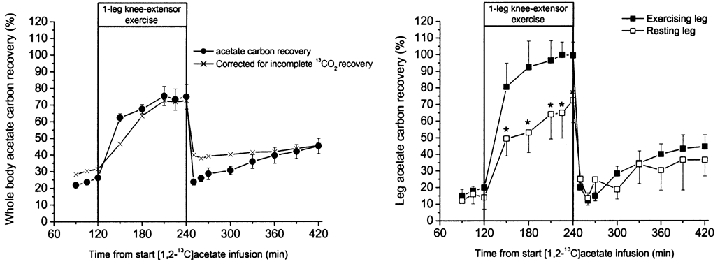
Whole body acetate recovery is the percentage of 13CO2 in expired air of the infused [1,2-13C]acetate, presented uncorrected and corrected for incomplete carbon dioxide recovery. Incomplete carbon dioxide recovery was determined in three of the five subjects as the 13CO2 in expired air of infused sodium H13CO3. Leg acetate carbon recovery is the percentage of 13CO2 released from the leg of the [1,2-13C]acetate taken up by the leg. Leg incomplete carbon dioxide recovery was slightly below 100 % at rest and in recovery and slightly above 100 % during exercise but did not reach significance, therefore leg acetate carbon recovery is not corrected for incomplete 13CO2 recovery (van Hall, 1999). Values are means ± s.e.m., n = 6.
DISCUSSION
The main findings of the present study are as follows. (i) Inactive skeletal muscle plays a minor role in whole-body acetate turnover. Active skeletal muscle enhances several-fold acetate uptake and subsequent oxidation, as well as release and its contribution to systemic acetate turnover. (ii) At rest whole-body acetate carbon recovery is similar to leg acetate carbon recovery. Thus at rest the whole-body acetate correction factor can be used to correct leg, skeletal muscle, substrate oxidation. (iii) During one-legged knee-extensor exercise at 65 % Wmax,leg the acetate carbon recovery is 100 % and higher than the whole-body acetate carbon recovery. Thus, during exercise the whole-body acetate correction factor cannot be used to correct leg substrate oxidation.
The arterial acetate concentration and acetate turnover rate were similar at rest and during exercise and recovery. At rest leg acetate uptake accounted for ∼4 % of systemic acetate Rd, whereas the leg acetate release aaccounted for only ∼1.5 % of systemic acetate Ra. Assuming that the leg muscle mass is 7 kg and that the body total muscle mass is 40 % of body weight, skeletal muscle accounted for ∼16 % of systemic acetate Rd and ∼6 % of Ra. Thus, despite a large tissue mass, skeletal muscle is quantitatively rather unimportant in acetate homeostasis. During one-legged knee-extensor exercise the contribution of the two legs to acetate Rd was 25 and 10 % for the active and semi-resting leg, respectively. Thus, acetate uptake by tissues other then the legs was decreased from 92 % at rest to 65 % during exercise. In addition, non-leg muscles had probably enhanced the acetate uptake via increased activity, for example, the back muscles to support the body while kicking, and trunk muscles for increased respiration. Systemic acetate Rd and Ra was rather similar from rest to exercise, but skeletal muscle acetate uptake and release increased markedly, implying that other tissues have substantially decreased acetate turnover. From the distribution of acetyl CoA synthase and acetyl CoA hydroxylase in mammalian tissues, liver, heart and kidneys are the tissues that are likely to have decreased acetate uptake and release (Knowles et al. 1974). It has been suggested that acetate uptake by the leg is strongly correlated with acetate delivery. This is confirmed in the present study in the semi-resting leg. With the other leg exercising, acetate delivery to the semi-resting leg increased 3-fold and as did acetate uptake, as fractional extraction of acetate by the semi-resting leg was unchanged from rest to exercise. The difference between the semi-resting and exercising leg may be caused by a reduced acetate gradient from plasma to blood by increased muscle acetate content. Alternatively, shunting in the leg could be increased, or because of the high rate of leg perfusion there could be insufficient time for complete exchange.
Substrate oxidation rates are determined by an intravenous continuous infusion of a carbon-labelled substrate for several hours. The appearance of labelled CO2 in breath, or released from a tissue, is a measure of systemic and tissue substrate oxidation. The acetate correction factor can be used to correct for label not appearing as CO2, but fixed in other metabolites mainly via tricarboxylic acid cycle exchange reactions. Acetate carbon recovery changes over time and it takes more than 12 h of infusion to reach a steady state (Mittendorfer et al. 1998). Thus, for proper correction of substrate oxidation rates the acetate correction factor needs to be determined for each time point. Mittendorfer et al. (1998) have shown that, with a primed continuous infusion of [1,2-13C]acetate, after 3 h leg acetate carbon recovery was similar to whole-body acetate carbon recovery. We have confirmed that observation with an unprimed continuous [1,2-13C]acetate infusion to correct for fatty acid oxidation rates, determined with an unprimed continuous [U-13C]palmitate infusion. We further observed that under resting conditions the acetate carbon recovery over the time of infusion at the whole-body level is similar to the leg acetate carbon recovery. Thus, the important message is that under resting conditions, the whole-body acetate correction factor determined over time can be used as a substitute for the leg acetate correction factor, i.e. skeletal muscle. Leg acetate uptake and oxidation accounted for only ∼4 % of the systemic acetate oxidation. Total skeletal muscle accounted for ∼16 % of the systemic acetate Rd, if leg data was extrapolated to body total muscle mass. This implies that substantial changes in leg acetate carbon recovery will have a minor effect on whole-body acetate carbon recovery. This is evidently shown in the present study, as acetate carbon recovery was nearly complete across the exercising leg. However, whole-body acetate carbon recovery was only ∼75 % despite the fact that with exercise the contribution of leg acetate uptake to systemic acetate disposal increased from 4 to 25 %. The opposite is also true, muscle acetate carbon recovery will be obscured if changes occur in acetate carbon recovery in tissues with a high contribution to whole-body acetate carbon recovery, like the liver (Bleiberg et al. 1992; Mittendorfer et al. 1998).
The present study shows that during one-legged knee-extensor exercise the leg acetate carbon recovery was substantially higher compared with that during rest, as shown previously for whole-body acetate carbon recovery (Sidossis et al. 1995a, b; Schrauwen et al. 1998). During one-legged knee-extensor exercise at 65 % Wmax,leg the leg acetate carbon recovery was 100 % compared with only ∼75 % for the whole-body acetate carbon recovery. This implies that during exercise the whole-body acetate correction factor cannot be used to correct leg substrate oxidation. The whole-body acetate correction factor has been shown to be dependent on exercise intensity (Sidossis et al. 1995a). The present study was not designed to investigate the effect of exercise intensity on skeletal muscle acetate recovery. However, it turned out that the supposedly completely resting leg was actually mildly active, which gives us the opportunity to speculate on the effect of skeletal muscle metabolic rate on acetate carbon recovery. The oxygen uptake of the semi-resting leg was ∼12 % of the maximal leg oxygen uptake. Despite this low oxygen uptake and thus activity the acetate carbon recovery was ∼60-70 %. Although the data on the semi-resting leg suggests that leg acetate carbon recovery depends on muscle metabolic rate, it is clear that the leg acetate carbon recovery compared with whole-body acetate carbon recovery is far less affected by metabolic rate. This is no surprise, since during exercise the whole-body acetate carbon recovery is lower due to the contribution of tissues other than the active skeletal muscle with a low acetate carbon recovery as seen under resting conditions. This would explain to a large extent the increase in whole-body acetate carbon recovery with increasing exercise intensity, as the relative contribution of the other tissue decreases to such an extent at high intensities that whole-body acetate carbon recovery nearly completely reflects leg acetate carbon recovery. The relatively high whole-body acetate carbon recovery already observed during low exercise intensities (Sidossis et al. 1995a) together with the high leg acetate carbon recovery in the semi-resting leg of the present study, suggests that even at rather low exercise intensities skeletal muscle acetate carbon recovery reaches 100 %.
The acetate correction factor accounts for label fixation via TCA cycle exchange reactions that can occur in many tissues and organs. Many tissues convert α-ketoglutarate to glutamate and glutamine, and in liver and kidney oxaloacetate is converted to glucose via gluconeogenesis. These metabolites in turn will be converted to other metabolites. Thus, although acetate is in principle completely oxidized, its carbon label does not appear as labelled CO2 in the breath, due to the accumulation in other metabolites. The carbon label of acetate is just temporarily fixed as those metabolites will re-enter the oxidative pathways at a later point in time and the carbon label of acetate will ‘re-’ appear. The pools of these compounds are unlabelled at the start of the labelled acetate infusion, but more labelled acetate compounds will accumulate the longer the acetate infusion. However, a steady state is unlikely to be reached in these pools even after several hours due to the fact that some of these pools are large. Therefore, acetate carbon recovery will increase over time until these pools have reached isotopic equilibrium. During exercise label recovery from acetate is much higher. With exercise the TCA cycle activity increases many fold. Assuming that during exercise the rate of exchange reactions are the same, the rate of the TCA cycle in relation to the exchange reactions is much larger. Thus, relatively less label will be fixed via the exchange reactions leading to higher acetate carbon recovery. An interesting observation in the present study is that in early recovery, immediately after 2 h of exercise, the whole-body acetate carbon recovery was slightly lower compared with that for pre-exercise (Fig. 4A, filled circles). In the case of similar rates of TCA cycle exchange reactions at rest and exercise, one would have anticipated that the whole-body acetate carbon recovery would continue to increase during the 2 h of exercise as has been shown under resting conditions (Mittendorfer et al. 1998), resulting in a substantially higher post- compared with pre-exercise acetate carbon recovery. In a study with an identical protocol, carbon-labelled bicarbonate was infused to establish the bicarbonate carbon recovery factor, correcting for labelled CO2 fixation via carboxylation reactions. From rest to exercise and from exercise to rest substantial changes in bicarbonate carbon recovery were observed (van Hall et al. 1999) as shown previously during bicycle exercise (Wolfe et al. 1984; Tarnapolski et al. 1991; Leese et al. 1994). These changes are caused by changes in the body bicarbonate pool size and turnover rate (Barstow et al. 1990; Leese et al. 1994). Correcting the acetate carbon recovery for bicarbonate carbon recovery (Fig. 4A, crosses) it is apparent that the small, albeit not significant, increase in acetate carbon recovery during exercise is mainly caused by changes in the bicarbonate pools. After 2 h of recovery from exercise, bicarbonate stores are back to pre-exercise conditions. This has been confirmed in a study where whole-body acetate carbon recovery was studied during 2 h at rest, 1 h of bicycle exercise at 60 % V̇O2,max, and 5 h of recovery compared with 8 h at complete rest. Whole-body acetate carbon recovery after 2 h of recovery from bicycle exercise was similar to that under resting conditions (Simonsen et al. 2000).
The use of the acetate correction factor to correct for the underestimation of fatty acid oxidation depends on two assumptions, the acetate infused is directly oxidized via the TCA cycle, and the acetate is metabolized in the same tissues and to the same proportional extent as the substrate. The volunteers from the present study underwent the same procedure and protocol, but with a continuous [U-13C]palmitate infusion (G. van Hall, M. Sacchetti, G. Rådegran & B. Saltin, unpublished observations). The relative contribution of the leg palmitate uptake and the acetate uptake of the active leg to systemic Rd can be seen in Fig. 5. At rest, pre- and post-exercise, the relative utilization of acetate and plasma fatty acids by the leg was similar. During exercise the relative contribution of acetate by the leg is slightly higher than that of plasma fatty acid. However, the difference is unimportant for leg substrate oxidation as the present data indicate that leg acetate correction factor is 100 % during one-legged knee-extensor exercise at 65 % Wmax,leg. Thus, the assumptions involved in the correctness of the acetate correction factor seemed to be fulfilled as therefore were the quantitative measures of substrate oxidation as obtained by the use of carbon-labelled substrates. However, some reservation towards the quantitative measures of substrate oxidation by tracers under resting conditions seemed to be justified. For example after some hours of constant infusion of a 13C-labelled substrate, the actual 13CO2 appearing from substrate oxidation is low and is corrected approximately 5-fold by the acetate correction factor. This implies that one heavily relies on the correctness of the acetate correction factor. Furthermore, across the leg this implies that the actual 13CO2 production is rather low and thus variable for both acetate and substrate oxidation. The longer the period of infusion, the higher the 13CO2 production, and thereby reducing variability. However, the reason for the enhanced 13CO2 production is that over time more of the acetate carbon label is fixed in other metabolites that enter the oxidative pathway. Thus, in time more and more of the 13CO2 produced originates from the oxidation of those metabolites and not from the substrate infused. This means that relatively small changes in substrate oxidation are more difficult to detect, especially in the case of enhanced oxidation of these metabolites compared with the substrate studied.
Figure 5. Leg acetate and palmitate uptake as a percentage of acetate and palmitate Rd.

The leg acetate uptake of the active leg as a percentage of acetate Rd of the present study, see Fig. 3. The palmitate uptake as percentage of palmitate Rd was determined in the same subjects during an identical protocol with a constant infusion of [U-13C]palmitate (G. van Hall, M. Sacchetti, G. Rådegran & B. Saltin, unpublished observations). Values are means ± s.e.m., n = 6.
In summary, acetate is rapidly metabolized in humans. Under resting conditions skeletal muscle does not contribute much to systemic acetate turnover. However, active skeletal muscle becomes more important in both whole-body acetate Ra and Rd. Whole-body and leg acetate carbon recovery is similar at rest and in recovery from exercise. Thus, the whole-body acetate correction factor can be used to correct for leg, skeletal muscle substrate oxidation. However, during one-legged knee-extensor exercise at 65 % Wmax,leg, whole-body and leg acetate carbon recovery are different. This implies that during exercise the whole-body acetate correction factor cannot be used to correct active skeletal muscle substrate oxidation. However, our data indicates that even at rather moderate exercise intensities the leg acetate correction factor is close to 100 %, suggesting that no acetate correction factor needs to be applied to correct substrate oxidation of moderately to highly active skeletal muscles.
Acknowledgments
The Copenhagen Muscle Research Centre is funded by a grant from the Danish National Research Foundation (grant no. 504-14).
REFERENCES
- Balasse EO. Kinetics of ketone body metabolism in fasting humans. Metabolism: Clinical and Experimental. 1979;28:41–50. doi: 10.1016/0026-0495(79)90166-5. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Cooper DM, Sobel EM, Landaw EM, Epstein S. Influence of increased metabolic rate on [13C]bicarbonate washout kinetics. American Journal of Physiology. 1990;259:R163–171. doi: 10.1152/ajpregu.1990.259.1.R163. [DOI] [PubMed] [Google Scholar]
- Bäurle W, Brösicke H, Matthews DE, Pogan K, Fürst P. Metabolism of parenterally administered fat emulasions in the rat: studies of fatty acid oxidation with 1-13C- and 8-13C-labeled triolein. British Journal of Nutrition. 1998;79:381–387. doi: 10.1079/bjn19980063. [DOI] [PubMed] [Google Scholar]
- Bleiberg B, Beers TR, Persson M, Miles JM. Systemic and regional acetate kinetics in dogs. American Journal of Physiology. 1992;262:E197–202. doi: 10.1152/ajpendo.1992.262.2.E197. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Frayn KN, Humphreys SM, Whyte PL, Hockaday DR. Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism. 1990;39:384–390. doi: 10.1016/0026-0495(90)90253-9. [DOI] [PubMed] [Google Scholar]
- Knowles SE, Jarrett IG, Filsell OH, Ballard FJ. Production and utilization of acetate in mammals. Biochemical Journal. 1974;142:401–411. doi: 10.1042/bj1420401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese GP, Nicoll AE, Vaenier M, Thompson J, Scrimgeour CM, Rennie MJ. Kinetics of 13CO2 elimination after ingestion of 13C bicarbonate: the effects of exercise and acid base balance. European Journal of Clinical Investigation. 1994;24:818–823. doi: 10.1111/j.1365-2362.1994.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. American Journal of Physiology, Endocrinology and Metabolism. 1998;274:E978–983. doi: 10.1152/ajpendo.1998.274.6.E978. [DOI] [PubMed] [Google Scholar]
- Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. Journal of Clinical Investigation. 1985;75:1448–1454. doi: 10.1172/JCI111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau E, Dumon H, Nguyen P, Darmaun D, Champ M, Krempf M. Whole-body, peripheral and intestinal endogenous acetate turnover in dogs using stable isotopes. Journal of Nutrition. 1998a;128:111–115. doi: 10.1093/jn/128.1.111. [DOI] [PubMed] [Google Scholar]
- Pouteau E, Maugére P, Darmaun D, Marchini JS, Piloquet H, Dumon H, Nguyen P, Krempf M. Role of glucose and glutamine synthesis in the differential recovery of 13CO2 from infused [2-13C]versus[1-13C]acetate. Metabolism. 1998b;47:549–554. doi: 10.1016/s0026-0495(98)90238-4. [DOI] [PubMed] [Google Scholar]
- Powers L, Osborn MK, Yang D, Kien CL, Murray RD, Beylot M, Brunengraber H. Assay of the concentration and stable isotope enrichment of short-chain fatty acids by gas chromatography/mass spectrometry. Journal of Mass Spectrometry. 1995;30:747–754. [Google Scholar]
- Rådegran G. Ultrasound doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. Journal of Applied Physiology. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Blaak EE, Van Aggel-Leijssen DPC, Borghouts LB, Wagenmakers AJM. Determinants of the acetate recovery factor: implications for estimation of [13C]substrate oxidation. Clinical Science. 2000;93:587–592. [PubMed] [Google Scholar]
- Schrauwen P, Van Aggelen-Leijssen DPC, Van Marken Lichtenbelt WD, Gijsen AP, Wagenmakers AJM. Validation of the [1,2-13C]acetate recovery factor for the correction of [U-13C]palmitate oxidation rates in humans. Journal of Physiology. 1998;513:215–223. doi: 10.1111/j.1469-7793.1998.215by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS, Coggan AR, Gastadelli A, Wolfe RR. A new correction factor for use in a tracer estimations of plasma fatty acid oxidation. American Journal of Physiology. 1995a;269:E649–656. doi: 10.1152/ajpendo.1995.269.4.E649. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. Pathways of free fatty acid oxidation in human subjects – Implications for tracer studies. Journal of Clinical Investigation. 1995b;95:278–284. doi: 10.1172/JCI117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, Bülow J, Al Mulla N, Lyngsøe D, van Hall G. Effect of exercise on acetate correction. International Journal of Obesity. 2000;24:S182. [Google Scholar]
- Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. Journal of Clinical Investigation. 1979;64:708–713. doi: 10.1172/JCI109513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Tarnapolski MA, Atkinson SA, Macdougal JD, Senor BB, Lemon PWR, Schwarcz H. Whole body leucine metabolism during and after resistance exercise in fed humans. Medicine and Science in Sports and Exercise. 1991;23:326–333. [PubMed] [Google Scholar]
- Toth MJ, Maccoss MJ, Poehlman ET, Matthews DE. Recovery of 13CO2 from infused [1-13C]leucine and [1,2-13C2]leucine in healthy humans. American Journal of Physiology, Endocrinology and Metabolism. 2001;281:E233–241. doi: 10.1152/ajpendo.2001.281.2.E233. [DOI] [PubMed] [Google Scholar]
- Tounian P, Schneiter PH, Henry S, Tappy L. Effects of infused glucose on glycogen metabolism in healthy humans. Clinical Physiology. 1996;16:403–416. doi: 10.1111/j.1475-097x.1996.tb00729.x. [DOI] [PubMed] [Google Scholar]
- van Hall G. Correction factors for 13C-substrate oxidation at whole body and muscle level. Proceedings of the Nutritional Society. 1999;58:979–986. doi: 10.1017/s0029665199001299. [DOI] [PubMed] [Google Scholar]
- van Hall G, GonzáleZ-Alonso J, Sacchetti M, Saltin B. Skeletal muscle substrate metabolism during exercise. methodological considerations. Proceedings of the Nutritional Society. 1999;58:899–912. doi: 10.1017/s0029665199001202. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotopes Tracers in Biomedicine Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- Wolfe RR, Jahoor F. Recovery of labeled CO2 during the infusion of C-1 vs. C-2-labeled acetate: implication for tracer studies of substrate oxidation. American Journal of Clinical Nutrition. 1990;51:248–252. doi: 10.1093/ajcn/51.2.248. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Wolfe MH, Nadel ER, Shaw JHF. Isotopic determination of amino acid-urea interaction in exercise in humans. Journal of Applied Physiology. 1984;52:221–224. doi: 10.1152/jappl.1984.56.1.221. [DOI] [PubMed] [Google Scholar]


