Abstract
Paired-pulse depression was studied at the glutamatergic synapse between retinal afferents and thalamocortical cells in the rat dorsal lateral geniculate nucleus. The main objective of this study was to examine the contributions of the pre- and postsynaptic sites to this depression by comparing AMPA- and NMDA-receptor-mediated responses. Equal depression of the two receptor components would indicate involvement of presynaptic mechanisms, while differences in depression would indicate involvement of postsynaptic mechanisms. Pharmacologically isolated AMPA- and NMDA-receptor-mediated currents were recorded using the whole-cell patch-clamp technique in acute thalamic slices. Both the AMPA and the NMDA components showed pronounced depression when retinal afferents were activated by paired pulses. The depression decayed within 5 s. The AMPA component was more strongly depressed than the NMDA component at paired-pulse intervals ranging from 20 to 200 ms, suggesting the involvement of postsynaptic mechanisms. For intervals of 500 ms and longer, the depression of the two components was identical, suggesting the involvement of purely presynaptic mechanisms. The degree of depression measured without the use of pharmacological tools produced similar results, thus excluding the involvement of presynaptic ionotropic glutamate receptors. Cyclothiazide, a blocker of AMPA-receptor desensitisation, reduced the difference in depression between the two components, suggesting that desensitisation of the AMPA receptors is a postsynaptic mechanism that contributes to the difference in depression between the AMPA and the NMDA components.
The efficiency of synaptic connections in the brain changes as a function of recent activity (Zucker, 1989, 1999). This can be expressed by the phenomenon of short-term depression, which may be implicated in the processes of gain control and temporal filtering by synaptic transmission (Markram & Tsodyks, 1996; Abbott et al. 1997; Chance et al. 1998; Fortune & Rose, 2000). Insights into the properties of synaptic depression and the underlying mechanism may therefore be important for an understanding of the dynamics of information processing in neural circuits.
Short-term depression is usually assumed to be due to presynaptic processes, like depletion of the readily releasable pool of vesicles (Dittman & Regehr, 1998; Stevens & Wesseling, 1998; Wang & Kaczmarek, 1998) and reduction in the release probability of the individual vesicles (Betz, 1970; Korn et al. 1984). The latter mechanism probably involves a variety of factors including inactivation of presynaptic Ca2+ channels (Jia & Nelson, 1986; Wu & Saggau, 1995; Dittman & Regehr, 1996; Forsythe et al. 1998), modulation of the release machinery (Scanziani et al. 1992; Hsu et al. 1996) and negative feedback through presynaptic metabotropic receptors (Scanziani et al. 1997; von Gersdorff et al. 1997). It has been suggested, however, that postsynaptic receptor desensitisation (Otis et al. 1996; Rozov et al. 2001) and receptor modification by intracellular messenger systems (Mennerick & Zorumski, 1996) contribute to synaptic depression.
The retinal input to the thalamocortical cells in the dorsal lateral geniculate nucleus is mediated through ionotropic glutamate receptors of the NMDA and non-NMDA type (Heggelund & Hartveit, 1990; Scharfman et al. 1990; Sillito et al. 1990). The non-NMDA receptors involved are of the AMPA type (Kielland & Heggelund, 2001). The retinogeniculate connection shows a pronounced short-term depression (Turner & Salt, 1998; von Krosigk et al. 1999), which presumably plays an important role in the transfer of visual signals to the thalamic cells and the subsequent relay to visual cortical areas.
In this project we compared the paired-pulse depression of AMPA- and NMDA-receptor-mediated currents at the synapse between retinal afferents and thalamocortical cells to evaluate the role of pre- and postsynaptic mechanisms. It has been shown that the manipulation of factors that reduce presynaptic glutamate release leads to a parallel reduction of the AMPA- and NMDA-receptor-mediated postsynaptic response components (Perkel & Nicoll, 1993; Tong & Jahr, 1994). Thus, if paired-pulse depression is due exclusively to presynaptic mechanisms, the AMPA and the NMDA components should exhibit depression of a similar degree and time course.
We found that both the degree and time course of paired-pulse depression were similar for the two types of receptor components at relatively long paired-pulse intervals (>500 ms), suggesting that mainly presynaptic mechanisms are involved under these conditions. For shorter paired-pulse intervals, however, the AMPA component was more strongly depressed than the NMDA component, suggesting the involvement of postsynaptic mechanisms under these conditions. In the study presented here, we have demonstrated that the postsynaptic effects could be accounted for, at least in part, by desensitisation of the AMPA receptor. Some of these results have been published previously in abstract form (Kielland & Heggelund, 2000).
METHODS
Coronal brain slices were made from the dorsal lateral geniculate nucleus of Wistar rats that were 14-17 days old, according to the guidelines, and with the approval, of the Animal Care Committee in Norway. The rats were deeply anaesthetised with halothane and killed by rapid decapitation. A block of the brain was dissected out and cooled to below 5 °C in a saline solution containing (mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 1.25 NaH2PO4 and 25 d-glucose, bubbled with 5 % CO2-95 % O2. The block was cut into 300 μm slices using a vibratome. The slices were stored submerged in the saline solution at room temperature (≈24 °C) until recording. The slices were used for recording within 4 h.
During experiments, slices were held submerged in a small chamber (volume ∼1.5 ml). The chamber was perfused at a rate of 1.5 ml min−1 with the same type of solution as used during preparation of the slices. Picrotoxin (80-100 μM) was added to the external solution to block GABAA-receptor-mediated synaptic currents. The cells were visualised with differential interference contrast microscopy using infrared light. Cells with a rounded soma and three or more primary dendrites were defined as thalamocortical cells (Zhu & Uhlrich, 1997). Whole-cell voltage-clamp recordings were obtained with glass electrodes filled with (mm): 80 Cs2SO4, 8 NaCl, 0.5 MgCl2, 0.5 CaCl2, 3 Na2ATP, 10 Hepes, 10 EGTA and 0.1 D-600 (a blocker of L-type Ca2+ channels). The pH was adjusted to 7.2 with CsOH. Cs2SO4 was added to block GABAB currents and to make the cells electronically more compact by blocking K+ channels. The access resistance (5-20 MΩ) was monitored continuously during the experiment, and it varied by less than 15 % during an experimental series. When cells were clamped at -40 to -60 mV the leak current was between 0 and -100 pA. Postsynaptic currents were evoked by electrical stimulation of the optic tract with bipolar electrodes, using current pulses of 150 μs duration (20-200 μA). The stimulus artefacts are truncated in the figures. The recorded electrical signals were filtered at 5 kHz and sampled at 10 kHz with an HEKA EPC 9 amplifier and Pulse software (HEKA Elektronik, Lambrecht, Germany). The amplitude of the postsynaptic currents was calculated as the difference between the peak synaptic current and the baseline holding current, or the decaying current of a preceding synaptic event. Offline analysis was performed using Igor Pro (Wave Metrics, Lake Oswego, OR, USA). Unless stated otherwise, Student's paired t test was used to test for statistically significant differences using SPSS software (SPSS, Chicago, IL, USA). Cyclothiazide was obtained from RBI (Natick, MA, USA) and prepared as a stock solution of 100 mm in DMSO. Control experiments for the effects of vehicles were negative. Picrotoxin was obtained from Sigma (St Louis, MO, USA). 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) and d-2-amino-5-phosphonovalerate (d-APV) were obtained from Tocris Cookson (Bristol, UK).
RESULTS
Depressions of AMPA and NMDA components have partially different time course
Electrical stimulation of the optic tract evoked a pure excitatory postsynaptic current (EPSC) in thalamocortical cells when GABAA receptors were blocked by perfusion with picrotoxin and GABAB receptors were blocked by intracellular Cs+. Two successive, suprathreshold electrical pulses were given to the optic tract. The magnitude of the depression was quantified by the paired-pulse depression ratio (PPD), i.e. the peak amplitude of the second EPSC (EPSC2), divided by the peak amplitude of the first EPSC, (EPSC1): EPSC2/EPSC1.
The waveform of the EPSCs had two components, an early fast AMPA-receptor component (Kielland & Heggelund, 2001) and a later longer-lasting NMDA-receptor component. Paired-pulse tests demonstrated that the depression could be considerably more pronounced for the early component than for the later component (Fig. 1A), suggesting a difference in the mechanism underlying this depression between the AMPA and NMDA components. To compare the depression of the two components, the AMPA- and the NMDA-receptor-mediated EPSCs were examined separately in the same cell through sequential application of a non-NMDA-receptor antagonist (CNQX, 5 μM) and an NMDA-receptor antagonist (d-APV, 50 μM). The antagonists were applied in a different order on different cells to balance eventual sequence effects. These experiments demonstrated that the AMPA and the NMDA components could exhibit depression with different characteristics, as illustrated in Fig. 1B and C by the results from one cell at a paired-pulse interval of 100 ms. In this case, the pure AMPA component had a PPD of 0.32 and the pure NMDA component had a PPD of 0.62. This suggests that different mechanisms are involved in the depression of the two components.
Figure 1. Short-term depression was stronger for AMPA-receptor-mediated currents than for NMDA-receptor-mediated currents.
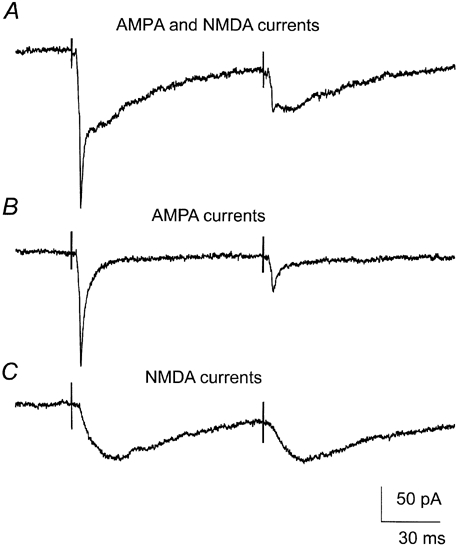
Excitatory postsynaptic currents (EPSCs) recorded in thalamocortical cells after two successive electrical stimulations of the optic tract. A, note the change in shape between the first and the second EPSC. The early fast AMPA component was reduced more than the longer lasting NMDA component. B, the AMPA component was isolated by application of the selective NMDA-receptor antagonist d-2-amino-5-phosphonovalerate (d-APV, 50 μM). C, the NMDA component was isolated by application of the selective AMPA-receptor antagonist cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5 μM). The trace with the isolated NMDA component shows less depression than the corresponding trace with the isolated AMPA component. Each figure is an average of five sweeps. The cell was clamped at -40 mV.
To characterise the difference in depression between the two components, we determined the time course of the paired-pulse depression for the pharmacologically isolated currents (Fig. 2A). The depression for both components was determined at a series of paired-pulse intervals between 20 ms and 9 s. The AMPA component exhibited maximal depression at 20 ms intervals, with an average PPD of 0.36 (s.e.m. = 0.04, n = 7). The depression gradually decayed with prolongation of the interval and completely disappeared after 5 s (Fig. 2B). The NMDA component exhibited maximal depression at a stimulus interval of 50 ms, with an average PPD of 0.61 (s.e.m. = 0.05, n = 9). For this component there was no significant change in the degree of depression in the interval range between 20 and 500 ms, but for further increases in paired-pulse interval the depression gradually decayed. Like the depression of the AMPA component, the depression of the NMDA component was completely gone after 5 s. The depression of the AMPA component was significantly stronger than that of the NMDA component in the paired-pulse interval range between 20 ms and 200 ms (P ≤ 0.002 for paired-pulse intervals of 20, 50, 100 and 200 ms). The difference was largest at 20 ms and gradually decreased with increasing length of the interval. Between 500 ms and 5 s there was no significant difference in depression between the two receptor components. Accordingly, there was a clear difference between the depressions of the two components that was limited to the shorter paired-pulse intervals.
Figure 2. The depression was more pronounced for the AMPA component at short paired-pulse intervals.
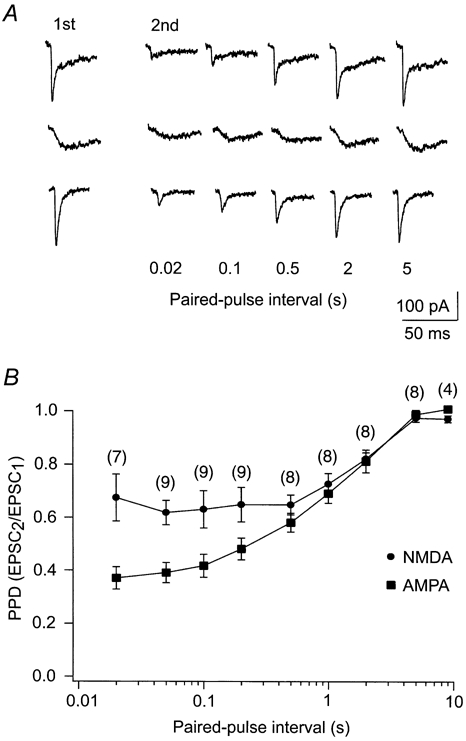
Time course of depression for the AMPA and NMDA components using paired-pulse stimulation. A, data from a representative experiment showing single EPSCs at different paired-pulse intervals. The EPSCs in the upper row were recorded without antagonists, the EPSCs in the middle row were recorded with CNQX, and the EPSCs in the lower row were recorded with d-APV. B, comparison of the average paired-pulse depression ratio (PPD) between the AMPA and the NMDA components. The abscissa is logarithmic. Error bars are s.e.m. The number of cells studied at each paired-pulse interval is given in parentheses.
One methodological factor that might have contributed to the difference in depression between the two components is a possible change in the magnesium-induced blockade of the NMDA-receptor-mediated current due to incomplete space-clamp. Since the duration of the NMDA current lasted for at least 100 ms in these experiments, there was an inward synaptic current at the start of the second EPSC at the shortest paired-pulse intervals. Therefore, depending upon the speed of the clamp, it is possible that the membrane at the site of the receptors was depolarised relative to the holding potential during the second EPSC. Since the magnesium-induced blockade of NMDA receptors is sensitive to membrane potential, we tested whether a change in membrane potential could account for the smaller synaptic depression of the NMDA component compared to the AMPA component. In the same cells we compared the degree of depression exhibited by the NMDA component at membrane potentials of -40 mV and +30 mV at a paired-pulse interval of 50 ms. At +30 mV, the magnesium-induced blockade is removed (Mayer et al. 1984; Nowak et al. 1984). We found no difference in depression between the two holding potentials (Fig. 3A, n = 10).
Figure 3. Manipulating the magnesium-induced blockade of the NMDA receptor did not affect paired-pulse depression.
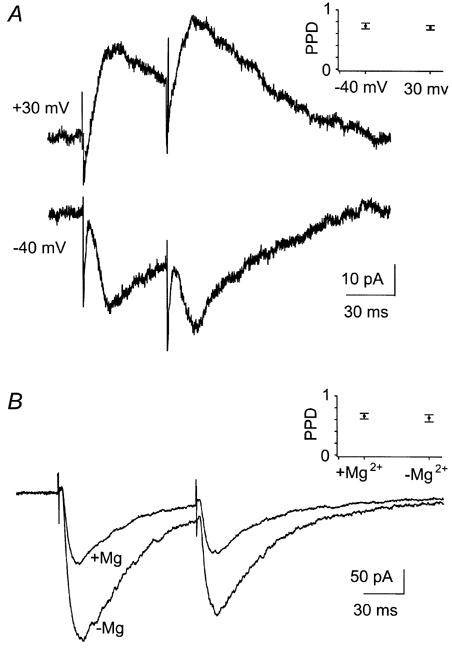
A, EPSCs of the NMDA component in a cell voltage clamped at +30 mV and -40 mV. B, EPSCs in another cell before and after washout of Mg2+. The cell was clamped at -40 mV. Both figures show an average of three sweeps. The AMPA component was blocked by CNQX (10 μM). The two insets show the average PPD of all cells (n = 10 in A, n = 6 in B) before and after manipulation of the magnesium-induced blockade for the respective experiments. Error bars are s.e.m.
In a second test for whether or not a change in the amount of magnesium-induced blockade affected the degree of depression, we compared, for the same cells at a paired-pulse interval of 100 ms, the depression of the NMDA component before and after removal of Mg2+ from the extracellular medium (Fig. 3B). The amplitude of the NMDA-receptor-mediated EPSCs increased on average by 81 % (s.d. = 28 %), but the degree of depression was not significantly changed by removal of extracellular Mg2+ (n = 6). It is therefore unlikely that the difference in depression between the AMPA and NMDA components was due to a different amount of magnesium-induced blockade between first and second EPSCs.
The difference in depression between AMPA and NMDA components was not due to presynaptic ionotropic glutamate receptors
Presynaptic receptors of the AMPA, kainate and NMDA type at glutamatergic terminals have been described, and it has been suggested that they have effects on synaptic efficiency (MacDermott et al. 1999). Although such presynaptic receptors have not been demonstrated at the retinogeniculate synapse, their presence cannot be ruled out. It is therefore possible that the difference we found between the depression exhibited by the AMPA and NMDA components could be due to selective blockade of presynaptic receptors by the receptor antagonists we used to separate the two components. To check this hypothesis, measurements of the time course of the depressions for the two components were repeated with an alternative method that did not involve pharmacological separation of the AMPA and NMDA components. We used the fact that AMPA-receptor-mediated EPSCs have a faster rise and decay time than NMDA-receptor-mediated EPSCs at the temperature we used (≈24 °C, see Fig. 1). Consequently, by measuring amplitudes of the dual-component EPSCs at different points in time we could estimate the AMPA and NMDA components in the same EPSC without the use of receptor blockers. The AMPA component was measured at the early sharp peak of the EPSCs, while the NMDA components were measured 20 ms after the stimulus artefacts (Fig. 4, inset).
Figure 4. Effects of the antagonists on potential presynaptic receptors do not contribute to the difference in depression between the AMPA and the NMDA component.
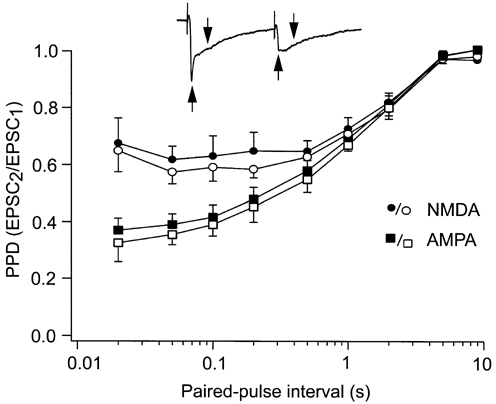
Paired-pulse depression measured during pharmacological isolation of AMPA and NMDA components (filled symbols) was compared with depression measured without pharmacological tools (open symbols). In the latter case the separation of the two components was based on differences in the time course of the two receptor-mediated currents. The inset trace shows the time point for measurement of the AMPA component (upward arrow) and the NMDA component (downward arrow) in the dual-component EPSCs. The abscissa is logarithmic. Error bars are s.e.m.
The results showed the same time course of depression for the two components with this method as that obtained using pharmacological isolation of the components. The depression was stronger for the AMPA component than for the NMDA component at the shorter inter-pulse intervals, whereas the depression was similar for the two components at the longer inter-pulse intervals (Fig. 4). This suggests that the difference in short-term depression between the two components was not due to presynaptic ionotropic glutamate receptors. The degree of depression obtained with this method was slightly stronger than that obtained with the pharmacologically isolated EPSCs (P ≤ 0.001, all intervals pooled, ANOVA). However, this increased depression was about the same for both components across the whole range of paired-pulse intervals tested, suggesting that the increased depression was due to methodological rather than biological factors.
Desensitisation of AMPA receptors contributes to the difference in depression between the two components
Desensitisation reduces the AMPA-receptor-mediated EPSCs at this synapse in rat (Kielland & Heggelund, 2001) and mouse (Chen & Regehr, 2000). We therefore examined the possibility that desensitisation of the AMPA receptors could contribute to short-term depression of the AMPA component, and eventually explain the difference in depression between the two components at the shorter paired-pulse intervals. To test this hypothesis we repeated the measurements of the time course of the depression for the AMPA-receptor component under conditions where a blocker of AMPA-receptor desensitisation, cyclothiazide, was added to the extracellular solution (Partin et al. 1993; Trussell et al. 1993; Yamada & Tang, 1993). The NMDA receptors were blocked by d-APV (50 μM).
When cyclothiazide (40 μM) was added, the peak amplitude and the decay time of the AMPA-receptor-mediated EPSCs increased, as described previously (Kielland & Heggelund, 2001). Moreover, the degree of depression was reduced, as illustrated by the example shown in Fig. 5A. In this cell, the PPD changed from 0.21 before, to 0.38 after cyclothiazide application at a paired-pulse interval of 50 ms. The cyclothiazide effect gradually decreased with increasing paired-pulse interval. The PPD changed from 0.26 to 0.47 at 200 ms and from 0.63 to 0.65 at 500 ms. This resembles the difference in depression we observed between the AMPA and NMDA components. However, the application of cyclothiazide did not seem to completely remove the difference in depression between the two components (Fig. 5B, n = 7). Cyclothiazide reduced the PPD of the AMPA component by 0.14, 0.09 and 0.04 for the paired-pulse intervals 50, 200 and 500 ms, respectively. For these same intervals, the difference in PPD between the AMPA and the NMDA components in the experiments without cyclothiazide was 0.23, 0.16 and 0.07, respectively.
Figure 5. Desensitisation of the AMPA component contributes to the difference in depression between the AMPA and the NMDA component.
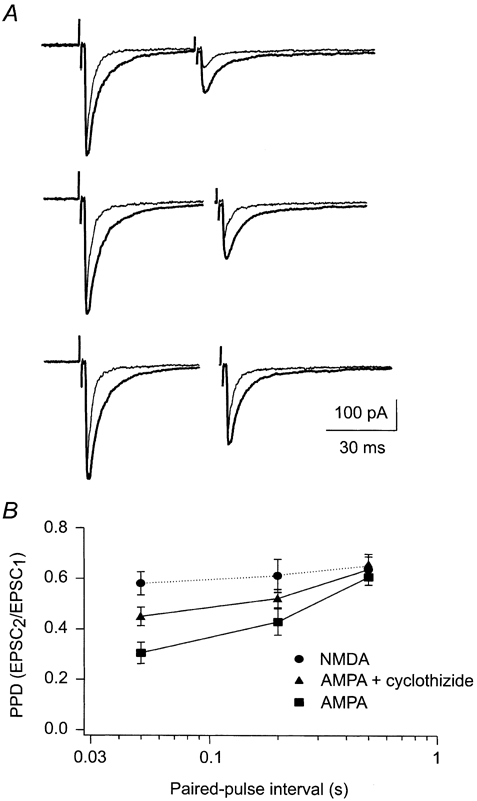
A, recording of AMPA-receptor-mediated EPSCs evoked in a cell at paired-pulse intervals of 50 ms (upper trace), 200 ms (middle trace) and 500 ms (bottom trace). Thin traces are control recordings, and thick traces are recordings made with cyclothiazide in the extracellular solution. Cells were voltage clamped at -60 mV and d-APV (50 μM) was added. B, comparison of the depression of the AMPA component in control conditions and in the presence of cyclothiazide plotted together with the depression of the NMDA component. The abscissa is logarithmic. Error bars are s.e.m.
Even though blocking of desensitisation by cyclothiazide did not completely abolish the difference in depression between the AMPA and the NMDA components, the desensitisation seemed to follow the same time course as the difference in depression between the two receptor components. This supported further the conclusion that the desensitisation of AMPA receptors can at least partly explain the differences in depression between AMPA and NMDA components.
To test whether cyclothiazide acted specifically on the desensitisation of the AMPA receptor, and not more generally on the synaptic transmission (e.g. on the transmitter release), we examined the effect of cyclothiazide on the EPSCs mediated by NMDA receptors. In these experiments, AMPA receptors were blocked by CNQX (10 μM). As illustrated in Fig. 6, we found that cyclothiazide (40 μM) had no effect on the depression of the NMDA-receptor-mediated EPSCs (n = 4), suggesting that under the experimental conditions used here, cyclothiazide specifically modulated the AMPA receptors.
Figure 6. The NMDA component was not affected by cyclothiazide.
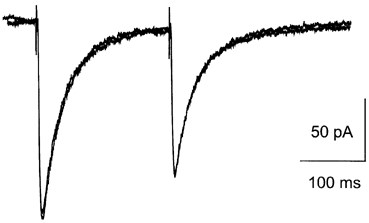
NMDA-receptor-mediated EPSCs in a representative cell before and after application of cyclothiazide. Traces for the two conditions are superimposed. The traces are average of 10 sweeps at paired-pulse interval of 200 ms. The cell was clamped at -40 mV and the extracellular solution contained CNQX (10 μM). Neither peak current nor short-term depression was altered by cyclothiazide.
DISCUSSION
The occurrence of paired-pulse depression was demonstrated in the retinogeniculate synapse using voltage-clamp recording. A comparison of the depression for the AMPA- and NMDA-receptor-mediated EPSCs suggests the involvement of both pre- and postsynaptic mechanisms. At the shortest paired-pulse intervals the AMPA component was considerably more depressed than the NMDA component, but this difference gradually disappeared with increasing length of the interval between the paired pulses. This suggests that the depression is a combination of a longer-lasting presynaptic effect and a shorter-lasting postsynaptic effect specific to the AMPA component. The latter could, at least in part, be explained by desensitisation of the AMPA receptor.
We used cyclothiazide, a potent blocker of AMPA-receptor desensitisation (Partin et al. 1993; Trussell et al. 1993; Yamada & Tang, 1993), to prove that desensitisation contributed to the depression of the AMPA component. In some other types of synaptic connections cyclothiazide has also been shown to have presynaptic effects. These effects generally lead to an increase in synaptic depression (Diamond & Jahr, 1995; Dittman & Regehr, 1998). Accordingly, a potential presynaptic effect of cyclothiazide in our experiments should have resulted in increased paired-pulse depression of the AMPA component, instead of the reduced depression we observed. However, it has been demonstrated that cyclothiazide reduces synaptic depression in a calyceal synapse in the rat brainstem (Bellingham & Walmsley, 1999). In that experiment, cyclothiazide affected the depression over a 100 ms period after a synaptic event, while in our experiments the effect was seen for up to 500 ms. Moreover, they also found paired-pulse depression to be more pronounced for the NMDA component than for the AMPA component. These differences suggest that different mechanisms are involved in the two types of synapses. In addition, in our experiments cyclothiazide did not change the response of the NMDA-receptor-mediated EPSCs. This is further evidence against a presynaptic effect, and makes it likely that cyclothiazide has a specific effect on postsynaptic AMPA receptors.
The difference in depression of the AMPA and NMDA components together with the reduction of the AMPA-receptor-mediated depression evoked by cyclothiazide suggest that the difference between the two components is due to desensitisation. However, the difference between the two components was not completely abolished by cyclothiazide. This could reflect an incomplete block of desensitisation by the drug, since we had no control for the completeness of the block. In fact in a similar study in mice published recently, a higher concentration of cyclothiazide was used (75 μM vs. 40 μM in our study), and in that study (Chen et al. 2002) the NMDA component was more strongly depressed after cyclothiazide treatment than was the AMPA component. Alternatively, the residual difference of depression could be due to other mechanisms like differential regulation of the two receptors by intracellular messenger systems (e.g. it has been shown that phosphorylation by protein kinase C preferentially potentiates the NMDA receptor; Lozovaya & Klee, 1995).
In most studies of short-term depression, the major emphasis has been on presynaptic mechanisms (Zucker, 1989, 1999; von Gersdorff et al. 1997; Hashimoto & Kano, 1998). However, in addition to the result from the retinogeniculate synapse presented here, desensitisation has been shown to determine depression in a calyceal synapse in the chick cochlear nucleus (Trussell et al. 1993; Otis et al. 1996), and in a synapse between pyramidal and bipolar cells in the neocortex of rat (Rozov et al. 2001). Large differences in recovery time from desensitisation have been found in these types of synapses. In the cochlear nucleus the recovery time was 100 ms, in the synapse between pyramidal and bipolar cells it lasted up to 5 s, and in the retinogeniculate synapses we found that the desensitisation lasted up to 500 ms.
It is likely that desensitisation will vary considerably between synapses because it is dependent upon the subunit composition of the receptor complex and morphology of the synapse, which are factors with large variability in the brain. Differential gene expression, alternative splicing and RNA editing lead to large variety of different AMPA receptors that have various intrinsic recovery times from desensitisation (Colquhoun et al. 1992; Lomeli et al. 1994; Angulo et al. 1997). Moreover, the time course of glutamate in the synaptic cleft is important for desensitisation because only micromolar levels of transmitter are sufficient to keep the receptors in a desensitised state (Kiskin et al. 1986; Trussell & Fischbach, 1989). It has been suggested that clearance of glutamate from the synaptic cleft is dependent upon the morphology of the synapse (Trussell et al. 1993; Clements, 1996; Otis et al. 1996). The terminals of the retinal afferents can have several release sites, which can be localised close together and encapsulated in a common glial structure, the glomerulus (Sherman & Guillery, 1996). This morphology could prolong clearance of glutamate from the synaptic cleft. Furthermore, it has been suggested that closely spaced synaptic sites promote desensitisation due to diffusion of glutamate between the sites (Trussell et al. 1993).
In a recent in vivo study of the retinogeniculate connections in cat, Usrey et al. (1998) showed that two retinal spikes evoked with inter-spike intervals of 3-30 ms conferred on the second spike an increased probability of evoking an action potential. They suggested that the effect was due to presynaptic facilitation. This is inconsistent with our findings, which demonstrate depression instead of facilitation in this type of synapse, suggesting that the effect can be related to postsynaptic mechanisms.
Retinal ganglion cells in vivo are spontaneously active. In the unanaesthetised rat this activity seems to be above 2 Hz, and the visually driven activity can reach several hundred hertz (Brown & Rojas, 1965). In our in vitro condition the degree of paired-pulse depression of the NMDA component varied little over inter-pulse intervals up to 500 ms, whereas that of the AMPA component varied substantially during this interval. Although our recordings were carried out in a slice preparation at room temperature, the findings suggest that the NMDA component will be in a tonically depressed state at the firing rates of the retinal input in vivo, whereas the degree of depression of the AMPA component can be modulated depending upon the firing rate in the retinal afferent. Since the depression of the AMPA component is strongest at very short inter-pulse intervals, this means that the short-term depression would have the strongest effects on the EPSCs evoked by the highest firing rates of the retinal input. Under these conditions the thalamocortical cells will typically have a membrane potential closer to the firing threshold, such that weaker EPSCs will be sufficient to generate action potentials in the thalamocortical cell. The short-term depression could fine-tune the amplitude of the EPCS to the appropriate level under these conditions such that even facilitation of transmission (Usrey et al. 1998) could occur.
Acknowledgments
We wish to thank Dr Morten Raastad for reading the manuscript. The project was financially supported by the Norwegian Research Council.
REFERENCES
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Lambolez B, Audinat E, Hestrin S, Rossier J. Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. Journal of Neuroscience. 1997;17:6685–6696. doi: 10.1523/JNEUROSCI.17-17-06685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. Journal of Physiology. 1970;206:629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Rojas JA. Rat retinal ganglion cells: receptive field organization and maintained activity. Journal of Neurophysiology. 1965;28:1073–1090. doi: 10.1152/jn.1965.28.6.1073. [DOI] [PubMed] [Google Scholar]
- Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. Journal of Neuroscience. 1998;18:4785–4799. doi: 10.1523/JNEUROSCI.18-12-04785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Blitz DM, Regehr WG. Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron. 2002;33:779–788. doi: 10.1016/s0896-6273(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends in Neurosciences. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Jonas P, Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. Journal of Physiology. 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. Journal of Neuroscience. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. Journal of Neuroscience. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Rose GJ. Short-term synaptic plasticity contributes to the temporal filtering of electrosensory information. Journal of Neuroscience. 2000;20:7122–7130. doi: 10.1523/JNEUROSCI.20-18-07122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. Journal of Physiology. 1998;506:391–405. doi: 10.1111/j.1469-7793.1998.391bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund P, Hartveit E. Neurotransmitter receptors mediating excitatory input to cells in the cat lateral geniculate nucleus. I. Lagged cells. Journal of Neurophysiology. 1990;63:1347–1360. doi: 10.1152/jn.1990.63.6.1347. [DOI] [PubMed] [Google Scholar]
- Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Jia M, Nelson PG. Calcium currents and transmitter output in cultured spinal cord and dorsal root ganglion neurons. Journal of Neurophysiology. 1986;56:1257–1267. doi: 10.1152/jn.1986.56.5.1257. [DOI] [PubMed] [Google Scholar]
- Kielland A, Heggelund P. AMPA and NMDA sensitive currents show different short-term plasticity in the lateral geniculate nucleus of rat. Society for Neuroscience Abstracts. 2000;26:523. [Google Scholar]
- Kielland A, Heggelund P. AMPA receptor properties at the synapse between retinal afferents and thalamocortical cells in the dorsal lateral geniculate nucleus of the rat. Neuroscience Letters. 2001;316:59–62. doi: 10.1016/s0304-3940(01)02337-0. [DOI] [PubMed] [Google Scholar]
- Kiskin NI, Krishtal OA, Tsyndrenko AY. Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neuroscience Letters. 1986;63:225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS, Burnod Y, Triller A. Regulation of efficacy at central synapses. Journal of Neuroscience. 1984;4:125–130. doi: 10.1523/JNEUROSCI.04-01-00125.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Klee MR. Phorbol diacetate differently regulates the N-methyl-d-aspartate (NMDA) and non-NMDA receptors-mediated components of the rat hippocampal excitatory postsynaptic currents. Neuroscience Letters. 1995;189:101–104. doi: 10.1016/0304-3940(95)11463-7. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annual Review of Neuroscience. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Postsynaptic modulation of NMDA synaptic currents in rat hippocampal microcultures by paired-pulse stimulation. Journal of Physiology. 1996;490:405–407. doi: 10.1113/jphysiol.1996.sp021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Otis T, Zhang S, Trussell LO. Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. Journal of Neuroscience. 1996;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Perkel DJ, Nicoll RA. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. Journal of Physiology. 1993;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. Journal of Neuroscience. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Lu SM, Guido W, Adams PR, Sherman SM. N-methyl-d-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proceedings of the National Academy of Sciences of the USA. 1990;87:4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Murphy PC, Salt TE. The contribution of the non-N-methyl-d-aspartate group of excitatory amino acid receptors to retinogeniculate transmission in the cat. Neuroscience. 1990;34:273–280. doi: 10.1016/0306-4522(90)90137-s. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wesseling JF. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron. 1998;21:415–424. doi: 10.1016/s0896-6273(00)80550-4. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994;12:51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Fischbach GD. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989;3:209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurons in vitro. Journal of Physiology. 1998;510:829–843. doi: 10.1111/j.1469-7793.1998.829bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395:384–387. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. Journal of Neuroscience. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Krosigk M, Monckton JE, Reiner PB, McCormick DA. Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus. Neuroscience. 1999;91:7–20. doi: 10.1016/s0306-4522(98)00557-0. [DOI] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. Journal of Physiology. 1995;485:649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. Journal of Neuroscience. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Uhlrich DJ. Nicotinic receptor-mediated responses in relay cells and interneurons in the rat lateral geniculate nucleus. Neuroscience. 1997;80:191–202. doi: 10.1016/s0306-4522(97)00095-x. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annual Review of Neuroscience. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Current Opinion in Neurobiology. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]


