Abstract
The whole-cell configuration of the patch-clamp technique, immunoprecipitation experiments and unilateral naris occlusions were used to investigate whether the voltage-gated potassium channel Kv1.3 was a substrate for neurotrophin-induced tyrosine phosphorylation and subsequent functional modulation of current properties in cultured rat olfactory bulb (OB) neurons. Membrane proteins of the OB included all three Trk receptor kinases, but the truncated form of the receptor, lacking an intact kinase domain, was the predominant form of the protein for TrkA and TrkC, while TrkB was predominantly found as the full-length receptor. Acute (15 min) stimulation of OB neurons with bath application of 50 ng ml−1 brain-derived neurotrophic factor (BDNF), which is a selective ligand for TrkB, caused suppression of the whole-cell outward current and no changes in the kinetics of inactivation or deactivation. Acute stimulation with either nerve growth factor or neurotrophin-3 failed to evoke any changes in Kv1.3 function in the OB neurons. Chronic exposure to BDNF (days) caused an increase in the magnitude of Kv1.3 current and speeding of the inactivation and deactivation of the channel. Acute BDNF-induced activation of TrkB receptors significantly increased tyrosine phosphorylation of Kv1.3 in the OB, as shown using a combined immunoprecipitation and Western blot analysis. With unilateral naris occlusion, the acute BDNF-induced tyrosine phosphorylation of Kv1.3 was increased in neurons lacking odour sensory experience. In summary, the duration of neurotrophin exposure and the sensory-dependent state of a neuron can influence the degree of phosphorylation of a voltage-gated ion channel and its concomitant functional modulation by neurotrophins.
Recent evidence has shown that neurotrophins, growth factors and cytoplasmic protein kinases have a neuromodulatory role in addition to their well-studied roles in growth and differentiation (Schlessinger & Ullrich, 1992; Lev et al. 1995; Levine et al. 1995; Berninger & Poo, 1996, 1999; Sherwood et al. 1997; Kafitz et al. 1999). Neurotrophins, namely nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3) are preferred ligands for TrkA, TrkB and TrkC receptor kinases, respectively. Trk receptors, when bound by their preferred ligands, phosphorylate key tyrosine residues within the intracellular domain of the receptor that serve as a scaffold to which various signalling proteins are recruited and activated (for review see Kaplan & Miller, 2000). Phosphorylation of tyrosine residues, as a result of intercellular communication, has been shown to modulate the activity of voltage- and ligand-gated ion channels, either via direct protein-protein interaction with the tyrosine kinase or through a combination of cell signalling events involving activation of a protein tyrosine kinase (e.g. Huang et al. 1993; Wang & Salter, 1994; Lesser & Lo, 1995; Lev et al. 1995; Berninger & Poo, 1996, 1999; Holmes et al. 1996; Yu et al. 1997; Bolton et al. 2000). Interestingly, tyrosine phosphorylation can produce rapid, short-term changes in ion channel function or long-term changes in ion channel targeting or ion channel protein synthesis.
The voltage-gated potassium channel Kv1.3 is most highly expressed in T-lymphocytes, the dendate gyrus, the olfactory bulb and the olfactory cortex (Kues & Wunder, 1992). Pharmacological evidence has indicated that Kv1.3 carries 60–80 % of the outward current in olfactory bulb neurons (Fadool & Levitan, 1998) and the channel is predominantly expressed in the mitral and granule nerve cell layers in a developmentally regulated manner (Fadool et al. 2000). We also know from previous experiments that Kv1.3 is a molecular target for multiple phosphorylation by four different tyrosine kinases: epidermal growth factor kinase, insulin receptor kinase and src kinase, as well as an unknown kinase that is revealed under pervanadate treatment (Bowlby et al. 1997; Fadool et al. 1997, 2000; Fadool & Levitan, 1998). Each of these tyrosine kinases is expressed in the olfactory bulb and could use Kv1.3 as a substrate for tyrosine phosphorylation to modulate information processing in the olfactory system. The adult olfactory system is unique in its ability to regenerate neurons from a basal cell type, which must continually reestablish circuitry back to the olfactory bulb in order to process odour information leading to sensory perception (Graziadei & Monti-Graziadei, 1978). This regenerative property of the olfactory system has spurred investigations exploring the expression patterns and function of growth factors in this region of the nervous system as a means to understand growth factor-dependent neural development and differentiation and neuronal survival. In the past couple of years the regenerative power of the olfactory system has come into focus as a potential avenue to treat spinal cord injuries (Ramon-Cueto et al. 1998; Bartolomei & Greer, 2000; Franklin & Barnett, 2000; Imaizumi et al. 2000a,b; Ramon-Cueto, 2000; Fry, 2001; Lu et al. 2001; Raisman, 2001). The neurotrophin family of growth factor receptors may play central roles in neuroprotection and enhanced regenerative recovery following spinal cord or nerve injury and animal trials of the delivery of ligands that activate these receptors to the injured site have been performed (Blesch et al. 1998; Zigova et al. 1998; Liu et al. 1999; Namiki et al. 2000; Novikova et al. 2000a,b; Bamber et al. 2001).
Given the high level of TrkB receptors expressed on the dendrites of the mitral cells in the olfactory bulb and the reported release of BDNF ligand from neighbouring granule cells (Masana et al. 1993; Katoh-Semba et al. 1998; Mackay-Sim & Chuah, 2000; Carter & Roskams, 2002), we have developed a cultured olfactory bulb (OB) neuron preparation to explore the degree of modulation of electrical properties in these neurons by BDNF activation of TrkB. In our patch-clamp study we performed acute (min) and long-term (days) treatment of OBNs with neurotrophins and demonstrate that the two protocols have opposite effects on the same voltage-gated ion channel, Kv1.3. Secondarily, BDNF phosphorylation of Kv1.3 is affected by sensory deprivation to the olfactory bulb, suggesting that the sensory-dependent state of a neuron can influence the extent of phosphorylation of an ion channel and concomitant functional modulation by BDNF.
METHODS
Solutions, reagents and antibodies
Olfactory bulb neuron (OBN) patch pipette solution contained (mm): 145 KCl, 10 Hepes, 10 EGTA, 2 MgCl2, 0.20 NaATP and 0.5 GTP (pH 7.3). OBN bath recording solution contained (mm): 150 NaCl, 5 KCl, 2.6 CaCl2, 2 MgCl2, 10 Hepes and 100 nm tetrodotoxin (TTX) (pH 7.3). Patch pipette and bath recording solutions were formulated to maximize Kv1.3 currents. The calculated EK using the Nernst Equation at room temperature and incorporating the concentration of potassium (KOH) used to adjust the pH of the solution was −39 mV. Nonidet-P40 protease and phosphatase inhibitor (NP40 PPI) solution contained: 20 mm Tris base, 150 mm NaCl, 1 % nonidet-P40, 10 % glycerol, 1 mm phenylmethylsulphonyl fluoride (PMSF), 10 μg ml−1 aprotinin, 1 μg ml−1 leupeptin and 0.5 mm sodium orthovanadate (pH 8.0) (Yuen & Mobley, 1999). pH adjustment for both these solutions was made using 12 n HCl. Homogenization buffer contained (mm): 320 sucrose, 10 Tris base, 50 KCl and 1 EDTA (pH 7.8). Phosphate-buffered saline (PBS) contained (mm): 136.9 NaCl, 2.7 KCl, 10.1 Na2HPO4 and 1.8 KH2PO4 (pH 7.4). pH adjustment of PBS was made using 5 n KOH.
Human recombinant neurotrophin-3 (NT3) and neuronal growth factor (NGF 2.5s) were purchased from Upstate Biochemicals (Lake Placid, NY, USA). Human recombinant brain-derived neurotrophic factor (BDNF) was purchased from Promega (Madison, WI, USA). Tissue culture reagents were purchased from Gibco/BRL (Gaithersburg, MD, USA). All salts were purchased from Sigma Chemical Co. (St Louis, MO, USA) or Fisher Scientific (Houston, TX, USA). Margatoxin (MgTx), a selective blocker of Kv1.3, was a generous gift from Dr Reid Leonard, Merck Research Laboratories (Rahway, NJ, USA).
AU13, a rabbit polyclonal antiserum, was generated against the 46 amino acid sequence 478 MVIEEGGMNHSAFPQTPFKTGNSTATCTTNNNPNDCVNIKKIFTDV 523, representing the unique coding region of Kv1.3 between the amino terminus and transmembrane domain 1. The purified peptide was produced by Genmed Synthesis (San Francisco, CA, USA) and the antiserum was produced by Cocalico Biologicals (Reamstown, PA, USA). This antibody was used for immunoprecipitation (1 : 1000) and Western blot detection (1 : 1500) of Kv1.3 in the olfactory bulb. Tyrosine-phosphorylated proteins were immunoprecipitated and detected on Western blots (1 : 1000) with the mouse monoclonal antibody 4G10 (12.5 μg ml−1; Upstate Biotechnology, Inc., Lake Placid, NY, USA). Polyclonal antibodies directed against a peptide corresponding to an amino acid sequence at the carboxyl terminus of the precursor form of human TrkA and porcine TrkC were purchased from Santa Cruz and used for Western blot analysis at 1 : 1000 and 1 : 500, respectively (Santa Cruz, CA, USA). Monoclonal antiserum directed against amino acids 156–322 of human TrkB was purchased from Transduction Laboratories (San Diego, CA, USA) and used at 1:800 for Western blots.
Primary cell culture
Olfactory bulbs (OBs) were harvested from postnatal day 1 (P1) Sprague-Dawley rats and neuronal primary cultures were prepared as described previously (Fadool et al. 2000). OBs were harvested from the P1 rats by decapitation without anaesthesia according to Florida State University Laboratory Animal Resources and American Veterinary Medical Association (AVMA)-approved methods. For chronic stimulation experiments, medium was supplemented with 100 ng ml−1 of NT3, NGF or BDNF at 1 day in vitro (DIV 1). Chronic stimulation cultures were maintained up to DIV 11 for electrophysiological recordings.
Naris occlusions
Neonatal unilateral anosmia was established by naris occlusion using a modification of the techniques described by Meisami (1976) and Philpot et al. (1997), as approved by Florida State University Laboratory Animal Resources and AVMA-approved methods. P1 Sprague-Dawley rats were separated from the mother and anaesthetized with hypothermia for 7 min. The left naris was cauterized by insertion of a heated 2 mm metal probe, twice for 3 s. As a control measure, the heated 2 mm metal probe was applied to the shank of the nose twice for 3 s to serve as the matched sham treatment. Occluded and sham-treated litter mates were warmed to 37 °C following cauterization and returned to the mother. Litters were culled at P1 to 12 animals by decapitation to ensure equal nursing of occluded animals during the several day period of tissue healing. Gloves and minimal contact ensured a zero rejection rate by the mother.
Membrane preparation
Adult (P60) Sprague-Dawley rats were killed by exposure to a rising concentration of CO2 according to Florida State University Laboratory Animal Resources and AVMA-approved methods. Olfactory bulbs, cerebellum and cerebral hemispheres were quickly harvested after decapitation. Brain tissues were immediately homogenized in homogenization buffer for 50 strokes with a Kontes tissue grinder (size 20) on ice. The mixture was centrifuged twice at ≈2400g for 30 min at 4 °C in an Eppendorf model 5416 centrifuge to remove cellular debris. The supernatant was then centrifuged in a Beckman ultracentrifuge (model L8-M, Beckman, Westbury, NY, USA) at 110 000g for 2.5 h at 4 °C. The resulting pellet was resuspended in homogenization buffer and tip sonicated on ice 3 times for 20 s with a Tekmar Sonicator (setting 50; Tekmar, Cincinnati, OH, USA). Protein concentration was determined by Bradford assay and samples were stored at −80 °C until use.
Immunoprecipitation
Twenty-five days following unilateral naris occlusion, occluded and sham-treated rats were killed by exposure to a rising concentration of CO2. For immunoprecipitation of tyrosine-phosphorylated proteins or Kv1.3 channel protein from the OB, the OBs from either control or unilateral naris-occluded animals (P25) were exposed, but not removed from the cranium. In this in situ state, brains with intact OBs were treated with either PBS or 100 ng ml−1 BDNF in PBS for 20 min in a 37 °C incubator. OBs were then harvested by homogenization using 50 strokes by a Kontes tissue grinder (size 20) in ice-cold NP40 PPI solution. Lysis was continued on a Roto-Torque slow speed rotary mixer (model 7637; Cole Palmer, Vernon Hills, IL, USA) for 30 min at 4 °C. The lysates were clarified by centrifugation at 14 000g for 10 min at 4 °C and precleared for 1 h with 3 mg ml−1 protein A-sepharose (Amersham-Pharmacia, Newark, NJ, USA), which was followed by another centrifugation step to remove the protein A-sepharose. Tyrosine-phosphorylated proteins or Kv1.3 channel protein were immunoprecipitated from the clarified lysates by overnight incubation at 4 °C with 12.5 μg ml−1 4G10 antibody or AU13 (1 : 1000), followed by a 2 h incubation with protein A-sepharose and centrifugation as above. The immunoprecipitates were washed 4 times with ice-cold NP40 PPI solution. Lysates and washed immunoprecipitates were diluted in sodium dodecyl sulphate (SDS) gel loading buffer (Sambrook et al. 1989) containing 1 mm Na3VO4 and stored at −20 °C for subsequent analysis.
Membrane proteins or immunoprecipitated proteins were separated on 8–10 % acrylamide gels by SDS-PAGE and electrotransferred to nitrocellulose blots. Blots were blocked with 5 % nonfat milk and incubated overnight at 4 °C in primary antibody against Kv1.3 or 4G10 antibody. They were then incubated with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit secondary antibody (1 : 3000) (Amersham-Pharmacia) or HRP-conjugated goat anti-mouse secondary antibody (1 : 3000) (Sigma) for 90 min at room temperature. Enhanced chemiluminescence (ECL; Amersham-Pharmacia) exposure on Fugi Rx film (Fisher) was used to visualise labelled proteins. The film autoradiographs were analysed by quantitative densitometry using a Hewlett-Packard PhotoSmart Scanner (model 106-816, Hewlett Packard, San Diego, CA, USA) in conjunction with Quantiscan software (Biosoft, Cambridge, UK).
Electrophysiology
OBNs were voltage clamped at room temperature in the whole-cell recording configuration. Electrodes were fabricated from Jencons glass (catalogue no. M15/10, Jencons Ltd, Leighton Buzzard, Bedfordshire UK), fire-polished to approximately 1 μm tip diameter, and coated near the tip with beeswax to reduce the electrode capacitance. Pipette resistances were between 9 and 14 MΩ. All voltage signals were generated and data were acquired using a Microstar DAP 800/2 board (Microstar Lab, Bellevue, WA, USA). The amplifier output was filtered at 2 kHz, digitized at 2–5 kHz and stored for later analysis. Patches were held at −80 mV and stepped to +40 mV for a pulse duration of 400 ms, at a stimulating interval of 30 s for peak outward current amplitude, inactivation and deactivation measurements. Current-voltage relationships were assessed while holding the neuron at −90 mV. Neurons were then stepped from −80 to +40 mV in 20 mV increments by stimulating for 400 ms every 30 s.
Acute stimulation of OBNs was achieved by bath application of 50 ng ml−1 of BDNF, NT3 or NGF after control measurements had been acquired for approximately 10 min. Peak outward current amplitude, inactivation, deactivation and current-voltage relationships were measured prestimulation (time 0), 5, 15 and 30 min after acute stimulation. Differences in these biophysical measurements were analysed between control (time 0) and post-neurotrophin stimulation (time 15 min) groups within single cells by Student's paired t test with statistical significance at the 0.95 confidence level. In the studies involving MgTx block of Kv1.3 current before BDNF treatment, picomolar concentrations of MgTx were pre-applied to the bath for 10 min, as in the study of Fadool & Levitan (1998). In the studies of chronic (days) application of neurotrophins, biophysical measurements were compared across both time and treatment dimensions with a two-way analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) follow-up test with statistical significance at the same confidence level.
All electrophysiological data were analysed using software written in house (DapClamp) in combination with the analysis packages Origin (MicroCal Software, Northampton, MA, USA) and Quattro Pro (Borland International, Scotts Valley, CA, USA). The effective magnitude of the remaining capacitance, after transmural and stray capacitance was minimized, was electrically compensated through the capacitance neutralization circuit of the Axopatch 200B amplifier. Likewise, series resistance compensation was used to reduce the effect of pipette resistance electrically. Data traces were subtracted linearly for leakage conductance. The inactivation of the macroscopic current (τInact) was fitted to the sum of two exponentials (y = y0 + A1 exp(-x/τ1) + A2 exp(-x/τ2)) by minimizing the sums of squares, where y0 was the Y offset, τ1 and τ2 were the inactivation time constants, x is the time, and A1 and A2 were the amplitudes. The two inactivation time constants were combined by multiplying each by its weight (A) and summing as described previously (Fadool et al. 2000). The deactivation of the macroscopic current (τDeact) was fitted similarly, but to a single exponential (y = y0 + A1 exp(-x/τ1)). The voltage-dependent activation of Kv1.3 was determined by fitting current-conductance relationships with a Boltzman expression. The Boltzman equation used for fitting was: y = [(A1 — A2)/(1 + exp(X — Xo)/dx] + A2, where the steepness of the voltage dependence, κ, was determined as dx, A1 was the initial y value and A2 was the final y value. X is the time and Xo is the centre between the two limiting values A1 and A2. V1/2 was defined as the voltage at half-maximum activation.
RESULTS
Expression of neurotrophin receptors
Neurotrophins and other growth factors have been studied in the olfactory system for many decades yet reports describing expression of the neurotrophin family of receptors are not consistent (Guthrie & Gall, 1991; Merlio et al. 1992; Masana et al. 1993; Eide et al. 1996; Katoh-Semba et al. 1996; Roskams et al. 1996; Yan et al. 1997; Horikawa et al. 1999; Mackay-Sim & Chuah, 2000; Carter & Roskams, 2002). Membrane preparations of the adult olfactory bulb were therefore prepared and electrophoretically separated by SDS-PAGE followed by Western blot analysis. Nitrocellulose membranes were probed with antisera for TrkA, TrkB and TrkC receptors to confirm expression of these proteins in the OB. While all three Trk receptors were expressed in the olfactory bulb, only TrkB demonstrated a greater concentration of full-length Trk receptor protein (140-145 kDa) over that of its truncated form (90-95 kDa), which lacks a kinase domain (Fig. 1). This interesting pattern of expression allowed us to hypothesize that modulation of Kv1.3 in the native olfactory bulb, which occurs via a phosphorylation-dependent process by Trk receptor activation, would not be likely to involve either TrkA or TrkC, since they were expressed predominantly in a form lacking kinase activity.
Figure 1.
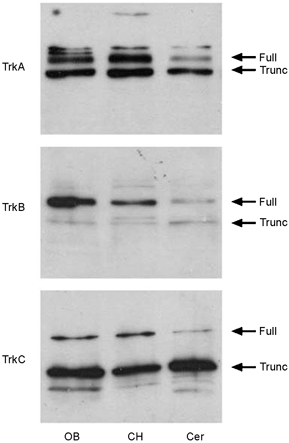
Expression of TrkA, TrkB and TrkC in the adult rat olfactory bulb (OB), cerebral hemisphere (CH) and cerebellum (Cer)
Membrane proteins were separated and visualized by SDS-PAGE followed by Western blot analysis with anti-TrkA (1 : 1000), anti-TrkB (1:800) and anti-TrkC (1 : 500). Arrows indicate appropriate molecular weight for endogenous full-length Trk receptors (Full) and truncated Trk receptors (Trunc) without an intact kinase domain.
Kv1.3 current is acutely suppressed by BDNF stimulation of olfactory bulb neurons
Olfactory bulb neurons were acutely stimulated with NGF, BDNF or NT3 to determine whether neurotrophic factors produced changes in the functional properties of Kv1.3 as expressed in native neurons. Neurons were voltage clamped in the whole-cell configuration at −80 mV and stimulated with a 400 ms depolarizing voltage step to +40 mV using 30 s interpulse intervals for periods up to 30 min. This relatively long delay between stimulations was necessary to prevent cumulative inactivation of Kv1.3, which exhibits a very slow C-type inactivation kinetics (Marom et al. 1993). The neuron was allowed to stabilize for 3–5 min, after which peak current magnitude and rate of inactivation and deactivation were measured (pre-neurotrophin). Neurotrophin (50 ng ml−1) was bath applied for 15 min, current recordings were repeated and kinetic measurements were recalculated. Acute bath application of BDNF significantly suppressed the magnitude of the whole-cell macroscopic current (Fig. 2 and Table 1; Student's paired t test). A study of the time course for BDNF-induced Kv1.3 current suppression was performed by voltage clamping neurons at −80 mV and stimulating with the voltage protocol above. At the third stimulation to +40 mV (time = 0 min), 50 ng ml−1 of BDNF was bath applied and current was monitored for 15 min or for the duration of the patch recording. Current magnitude during BDNF treatment (In) was normalized to that of the first depolarizing pulse (In/I1) and plotted over time as in Fig. 3A. These data indicate that the acute effect of BNDF in OB neurons occurs on the scale of minutes and plateaus within 10 min of application. Neurons that were pretreated for at least 10 min with 100 pm MgTx by applying the Kv1.3 pore blocker to the culture bath failed to respond to BDNF (Fig. 3A). In a separate series of experiments, families of current-voltage relations were generated before and after a 15 min growth factor application. Current-voltage (I-V) relations and conductance-voltage plots indicated that there were no shifts in either the I-V relations or voltage at half-activation (V1/2) following growth factor application. These results indicate that the BDNF-induced current suppression was not attributable to shifts in voltage dependence (Fig. 3B-E). In accordance with the predominant expression of truncated forms of TrkA and TrkC receptor protein, application of NGF and NT3 had no functional effect on the OBN current magnitude or kinetics (Fig. 2A and C). None of the neurotrophins affected the rate of inactivation or deactivation kinetics of Kv1.3 in the olfactory bulb neurons (Table 1). Whether a particular neuron was neurotrophin responsive did not appear to be dependent upon the number of days in vitro (DIV; data not shown). However, since total voltage-gated outward current increased with DIV in untreated neurons, all tests for a given neurotrophin were performed within a 6 h recording window. We do not know whether the quantity of other voltage-gated potassium channels changes over DIV. We do know from previous confocal imaging studies (Fadool et al. 2000) that Kv1.3 channel increases with DIV, presumably during growth of the soma and neuronal processes over time in culture. Increased total Kv1.3 current over DIV, however, was not a factor for our paired analysis (pre- versus post-neurotrophin stimulation) but starting control current and deactivation for a particular tested group could vary dependent upon DIV (Table 1).
Figure 2.
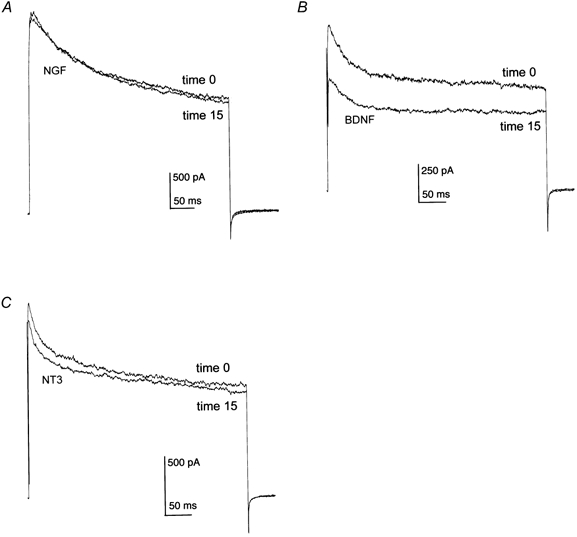
Effects of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) on olfactory bulb neuron (OBN) outward current
A-C, representative whole-cell outward current responses elicited in rat OBNs by voltage steps to +40 mV from a holding potential of −80 mV. Control outward currents were recorded at time 0 while neurotrophin-stimulated outward currents were recorded 15 min after bath application of NGF (50 ng ml−1; A), BDNF (50 ng ml−1; B) or NT3 (50 ng ml−1; C).
Table 1.
Properties and modulation of whole-cell outward current in olfactory bulb neurons by acute stimulation with 50 ng ml−1 NGF, BDNF or NT3
| Treatment | Peak current (pA) | τInact (ms) | τDeact (ms) |
|---|---|---|---|
| NGF (n = 4) | |||
| Pre | 2710 ± 479 | 262 ± 81 | 3 ± 0.8 |
| Post | 2809 ± 455 | 330 ± 73 | 3 ± 0.8 |
| BDNF (n = 7) | |||
| Pre | 1612 ± 379* | 230 ± 29 | 6 ± 2 |
| Post | 1340 ± 335 | 193 ± 44 | 7 ± 2 |
| NT3 (n = 6) | |||
| Pre | 970 ± 150 | 304 ± 40 | 11 ± 5 |
| Post | 1055 ± 95 | 367 ± 44 | 13 ± 4 |
τInact, inactivation time constant; τDeact, deactivation time constant. Whole-cell outward current was evoked by a 400 ms depolarizing step from −80 to +40 mV. Values are means ± s.e.m. and n is the number of neurons sampled. Time constant values were estimated from exponential fits to the inactivating or deactivating portions of the currents. * Value after BDNF treatment (15 min) is significantly different from pretreatment value by Student's paired t test at P < 0.05.
Figure 3.
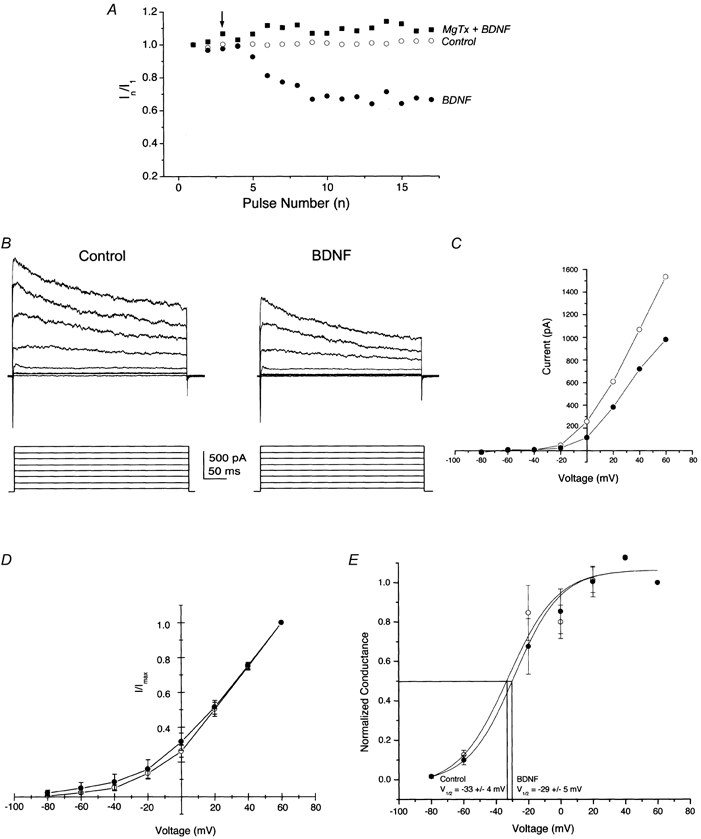
Time course and voltage independence of BDNF acute current suppression of Kv1.3
A, time plot of representative whole-cell outward current responses elicited by an OBN presented with either 50 ng ml−1 BDNF (•) or 1 % bovine serum albumin (control, ○) at the arrow. MgTx + BDNF (▪) represents responses elicited from an OBN that was first pretreated for 10 min with bath-applied 100 nm margatoxin and then stimulated as above with BDNF. OBNs were recorded for 10 min before the experiment to assure stability of the recording. Current magnitude is normalized to the current elicited at pulse 1 (In /I1). Voltage stimulation protocol as in Fig. 2 using a 60 s interpulse duration. B, representative traces of OBN outward currents recorded from a single OBN before (Control) and after (BDNF) 15 min bath application of BDNF. Neuron was held at −80 mV and stepped in 20 mV depolarizing increments from −80 to +60 mV with a stimulation duration of 400 ms and an interpulse interval of 30 s. This is not the same BDNF-treated neuron as in A, due to the 4 min acquisition time for the family of voltage steps. C, current magnitude is plotted against the voltage step (in mV) for the neuron in B, before (○) and after BDNF treatment (•). D, current magnitude is normalized to the current elicited at +60 mV (I/Imax) and plotted against the voltage step (in mV) for seven experiments as in B. E, data for the population of neurons in D were converted to conductance-voltage relations under control (○) and BDNF-treated conditions (•). V1/2 is the voltage at which one-half of the conductance is activated. Line represents the best fit of the data to a Boltzman function. In A-E, error bars represent the s.e.m.
Long-term exposure (3 days) of olfactory bulb neurons to BDNF augments Kv1.3 current magnitude and speeds inactivation kinetics
Chronic stimulation of TrkA, TrkB and TrkC receptors in OB neurons was studied by supplementation of the growth media with 100 ng ml−1 NGF, BDNF, NT3 or vehicle control for up to 72 h. Using the same whole-cell voltage protocol as that described for acute neurotrophin stimulation, peak current magnitude and rate of inactivation and deactivation were compared for control and neurotrophin-supplemented neurons after 24 or 72 h. In contrast with the BDNF-induced current suppression observed under acute stimulation, supplementation with any of the three neurotrophins for the extended period of 24 h induced an increase in peak current amplitude with no changes in kinetics of inactivation or deactivation as compared with that found in time-matched controls. Peak mean current for NGF-, BDNF- and NT3-treated neurons was increased by 23 (n = 10), 37 (n = 11) and 50 % (n = 9), respectively. These increases in total whole-cell current were statistically significant by Student's t test. Extension of chronic neurotrophin stimulation to 3 days revealed a continued increase in total whole-cell current in BDNF-treated neurons and no significant difference in currents elicited by NGF or NT3 (Fig. 4 and Table 2) compared with time-matched controls. Additionally, BDNF induced significant alternation of current inactivation kinetics. During this 72 h interval, chronic treatment with BDNF induced a 39 % increase in peak current compared with time-matched, vehicle-treated controls, a 50 % decrease in the inactivation rate of the current and a 14 % decrease in the deactivation rate (ANOVA, SNK follow-up test, Table 2). The mean change in peak current magnitude from day 1 to day 3 for vehicle control-treated neurons presumably reflects normal cell growth and increased membrane surface for expression of voltage-gated channels in the absence of growth factor supplementation. Conversion of whole-cell currents to current density ratios (current density = current/ membrane capacitance; I density = I/Cm), to normalize any increased size of neurons in the extended BDNF-treated conditions, still demonstrated that BDNF-treated neurons had approximately twice the current density of time-matched controls (Fig. 5, insets).
Figure 4.
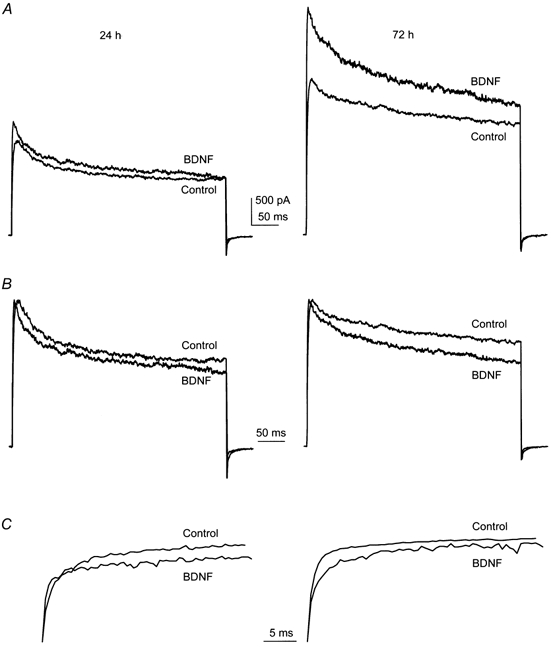
Representative whole-cell current traces 24 and 72 h after chronic neurotrophin stimulation of OBN cultures
A-C, OBN cultures were chronically supplemented from DIV 1 (day 1 in vitro) to DIV 3 with 100 ng ml−1 of BDNF. Whole-cell outward current responses were elicited by voltage steps to +40 mV from a holding potential of −80 mV. Voltage-clamp traces are shown which are representative of changes in peak outward current magnitude (A), inactivation kinetics (B) and deactivation kinetics (C) at 24 and 72 h of chronic stimulation. Current traces in B and C are from the same set of recordings as in A, but were normalized to peak current amplitude or minimal tail current amplitude to allow better visualizeation of changes in the kinetics of τInact and τDeact, respectively.
Table 2.
Whole-cell outward current magnitude, τInact and τDeasct in olfactory bulb neurons chronically stimulated with 100 ng ml−1 NGF, BDNF or NT3
| 24 h | 72 h | >200h | |
|---|---|---|---|
| Peak current amplitude (pA) | |||
| Control | 1758 ± 190(14) | 3188 ± 533(6) | 3851 ± 345(10) |
| NGF | 2157 ± 156(10) | 3025 ± 328(7) | 3596 ± 155(5) |
| BDNF | 2407 ± 287(11) | 4428 ± 290(7)* | 4563 ± 306(6)* |
| NT3 | 2630 ± 346(9) | 2732 ± 350(6) | 3496 ± 305(5) |
| Current kinetic properties (ms) | |||
| Control | |||
| τInact | 183 ± 51(14) | 240 ± 50(6) | 171 ± 29 (10) |
| τDeact | 2 ± 0.4 | 2 ± 0.5 | 2 ± 0.4 |
| NGF | |||
| τInact | 152 ± 38 (10) | 179 ± 45(7) | 134 ± 8(5) |
| τDeact | 2 ± 0.4 | 2 ± 0.6 | 1 ± 0.1 |
| BDNF | |||
| τInact | 160 ± 37(11) | 120 ± 40(7)* | 117 ± 29(6)* |
| τDeact | 2 ± 0.3 | 2 ± 0.4 | 1 ± 0.1 |
| NT3 | |||
| τInact | 150 ± 34 (9) | 128 ± 33(6)* | 149 ± 37 (5) |
| τDeact | 2 ± 0.5 | 0.5 ± 0.1 | 1 ± 0.7 |
Voltage stimulation protocol and calculation of the kinetics of inactivation and deactivation as in Table 1. *Neurotrophin treatment is significantly different from control for a given biophysical property by AN OVA, completely randomized design, with SNK follow-up test at P < 0.05. Values represent means ± s.e.m. with the number of neurons sampled in parentheses.
Figure 5.
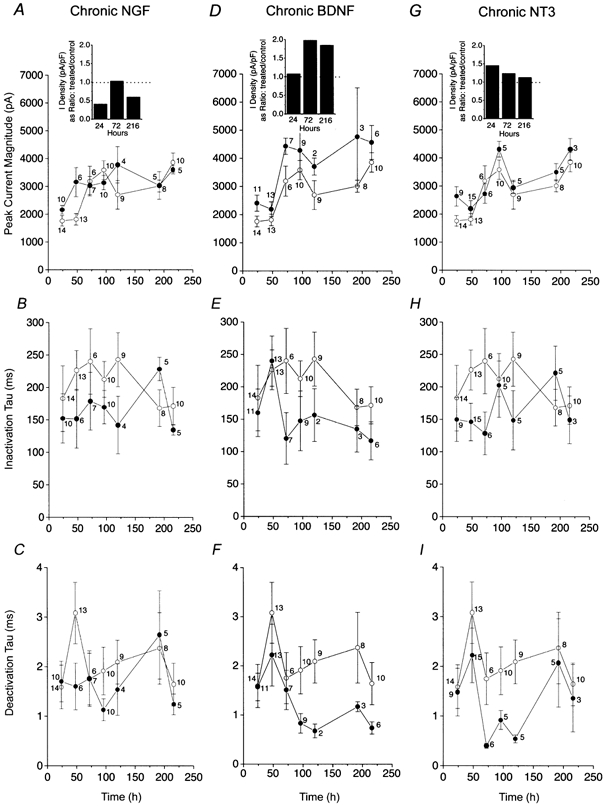
Plots of whole-cell outward current magnitude and kinetics of inactivation and deactivation for OBNs chronically stimulated with neurotrophins
A-I, OBNs were supplemented from DIV 1 to DIV 11 with 100 ng ml−1 of NGF, BDNF or NT3. Voltage stimulation protocol and kinetic fitting are as in Fig. 4. Peak current magnitude (A, D and G), τInact (B, E and H) and τDeact (C, F and I) were calculated for 24–216 h of supplementation with NGF (A, B and C), BDNF (D, E and F) or NT3 (G, H and I). ○, control; and •, neurotrophin supplemented. Insets in A, D and G, histogram plots comparing the current density (in pA pF−1) in neurons that were treated versus control as reported over the time of supplementation in hours. Dotted line represents a ratio of 1.0; no difference.
These initial experiments with chronic neurotrophin stimulation for more than 24 h suggested that BDNF consistently increased peak current magnitude and decreased rate of inactivation whereas NGF and NT3 appeared to demonstrate no consistent modulation of current amplitude after the first day of chronic supplementation. To confirm these findings, experiments were extended through a 10 day window and neurons were supplemented with neurotrophic factors for periods of 24, 48, 72, 96, 120, 192 or 216 h before recording. Data demonstrate that only BDNF-treated neurons exhibited consistent elevation in whole-cell current and speeding of the inactivation and deactivation kinetic rates of the current, which continued to each increase compared with the time-matched controls over this 10 day testing protocol (Fig. 5 and Table 2, ANOVA, SNK follow-up test). As with acute BDNF-treated neurons, families of current-voltage relations were generated after 24, 72 and > 216 h of chronic growth factor application and converted to conductance plots (Fig. 6B, D and F). Current- conductance relationships indicated that there was a slight (10 mV) shift in the voltage at half-activation (V1/2) following chronic exposure of OBNs to BDNF. Whether this magnitude of left-shifted V1/2 represents a physiologically significant shift is not clear, so it is not possible to discern whether long-term exposure to the neurotrophin could alter voltage dependence.
Figure 6.
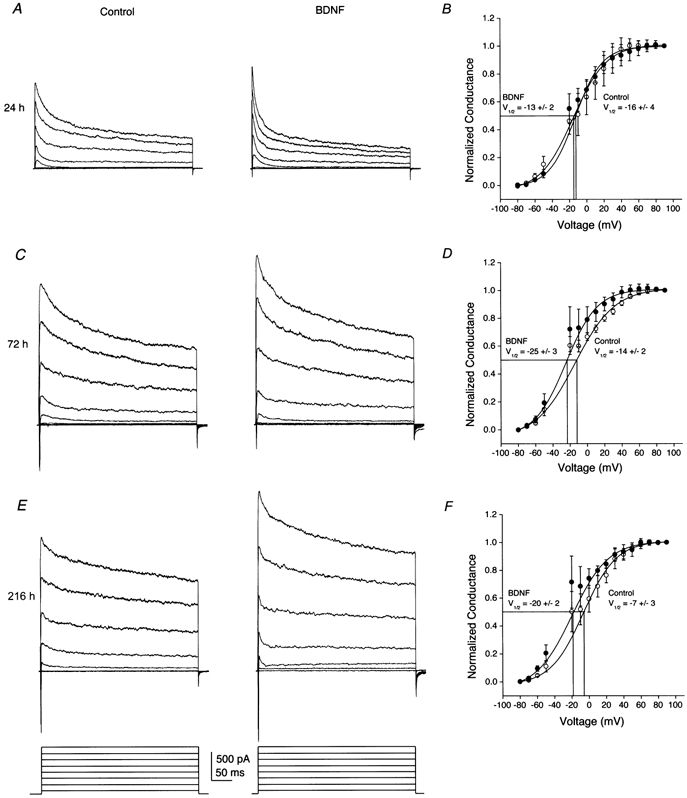
Voltage independence of BDNF enhancement of whole-cell outward current in OBNs
A, C and E, OBNs were supplemented from DIV 1 to DIV 11 with 100 ng ml−1 BDNF or 1 % bovine serum albumin (control). Neurons were held at −80 mV and stepped in 20 mV depolarizing increments from −80 to +60 mV with a stimulation duration of 400 ms and an interpulse interval of 30 s. Shown are representative whole-cell current traces from an OBN supplemented with BDNF (right panels) for 24 (A), 72 (C) or 216 h(E) and the appropriate time-matched controls (left panels). B, D and F, plotted conductance versus voltage relations where neurons were held at −80 mV and stepped in 10 mV depolarizing increments from −80 to +90 mV with a stimulation duration of 400 ms and an interpulse interval of 30 s. Conductances were normalized to that calculated at +90 mV and plotted against the voltage step (in mV) for OBNs recorded after 24 (B), 72 (D) or 216 h (F) of chronic BDNF supplementation, respectively. Points were fitted to a Boltzman function to determine the voltage at half-activation,(V1/2). Error bars represent the s.e.m. for 5 to 10 neurons. ○, control; and •, BDNF.
Tyrosine phosphorylation of Kv1.3 channel in olfactory bulb neurons
Because neurotrophins can participate in a number of signalling pathways and may have effects on neuronal currents that are independent of tyrosine phosphorylation (Kafitz et al. 1999), we used an immunoprecipitation and Western blot strategy to measure the effect of BDNF on the tyrosine phosphorylation of Kv1.3 in the olfactory bulb. As shown in Fig. 7, we first examined the developmental expression of TrkB at various postnatal days in order to select an appropriate time point at which to test tyrosine phosphorylation of Kv1.3. TrkB was expressed in membrane preparations harvested over a 2 month interval and was expressed in the adult (P60) at levels one-third to one-half as great as those at earlier postnatal ages. Since P11-P32 consistently contained moderate levels of TrkB full-length protein, and these time points could also be used for subsequent naris occlusion experiments where animals had to recover from cauterization (see next section below), P20-P32 was selected as the time range over to measure BDNF-induced Kv1.3 tyrosine phosphorylation. Second, the antiserum generated against Kv1.3 (α-AU13; see Methods), as well as several other anti-Kv1.3 antisera that were either internally generated (α-AU11 and α-AU12) or commercially available (Alomone Laboratories), were tested for the ability to recognize cloned Kv1.3 protein as transiently expressed in HEK293 cells and visualized in Western blots. α-AU13 specifically recognized Kv1.3 at serial dilutions of the antiserum from 1 : 500 to 1 : 10 000 (Fig. 8) and was therefore the antibody used in all subsequent experiments. α-AU13 was further characterized in native olfactory bulb membranes, where the appropriate molecular weight band was preabsorbed by incubation with the 46 amino acid peptide used to generate the antiserum (data not shown).
Figure 7.
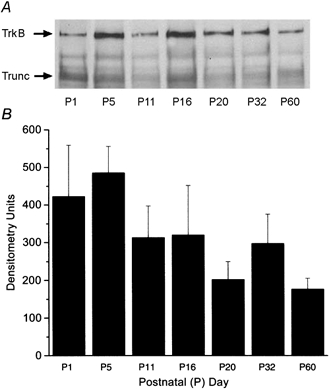
Developmental expression of TrkB in the rat olfactory bulb
A, membrane preparation of rat olfactory bulb harvested at various postnatal ages, separated and visualized by SDS-PAGE followed by Western blot analysis with anti-TrkB (1:800). Full-length receptor (TrkB) or truncated TrkB receptor (Trunc) is indicated at the arrow. B, histogram of densitometry units of postnatal age expression of full-length TrkB as in A. Error bars represent the s.e.m. of three trials.
Figure 8.
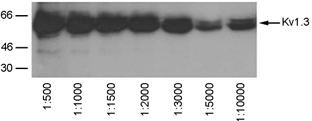
Recognition of Kv1.3 protein by α-AU13 antiserum by Western blot analysis
Cell lysates from Kv1.3 transfected HEK293 cells, separated by SDS-PAGE and visualized by Western blot analysis with various dilutions of the antiserum from 1 : 500 to 1:10 000.
As shown in Fig. 9, acute BDNF treatment of P20-P32 olfactory bulb tissue increased the tyrosine phosphorylation of Kv1.3 without affecting the level of channel or neurotrophin receptor protein. Since BDNF synthesis has been demonstrated to be activity dependent (Isackson et al. 1991; Castren et al. 1992; Rutherford et al. 1998), we hypothesized that tyrosine phosphorylation of Kv1.3 may be modulated under conditions of reduced sensory stimulation to the olfactory bulb. P1 Sprague-Dawley pups were left naris occluded by cauterization of the nasal orifice, killed at P20-P25, and olfactory bulbs were harvested and immunoprecipitated with anti-Kv1.3 antisera (α-AU13) and blotted with anti-phosphotyrosine antisera (α-4G10). Immunoprecipitates prepared from both the left naris occluded and right contralateral control olfactory bulbs demonstrated significant increases in BDNF-induced Kv1.3 tyrosine phosphorylation (Fig. 10A), but the mean increase in tyrosine phosphorylation of Kv1.3 following BDNF stimulation was greater in the naris-occluded, sensory-deprived condition (Fig. 10B). The basal phosphorylation of Kv1.3 in unstimulated, occluded versus non-occluded olfactory bulb was not significantly different.
Figure 9.
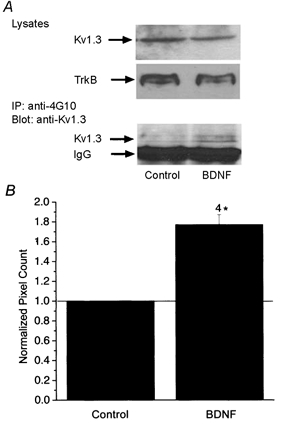
Acute BDNF stimulation increases the tyrosine phosphorylation of Kv1.3 in the rat olfactory bulb
A, olfactory bulbs were stimulated with or without 100 ng ml−1 of BDNF in PBS for 20 min, homogenized, immunoprecipitated with anti-phosphotyrosine antiserum (anti-4G10), separated by SDS-PAGE, and Western blots were probed with anti-Kv1.3 (α-AU13). Total tyrosine phosphorylation of Kv1.3 is shown in the bottom panel. The heavy chain of IgG is also indicated below that of Kv1.3. Ten micrograms of cell lysate were blotted for α-AU13 and α-TrkB, respectively, to confirm equivalent protein expression of the channel and receptor under BDNF-stimulated and unstimulated conditions (top panels). B, histogram of quantitative densitometry of four experiments as in A. Mean pixel density of Kv1.3 tyrosine phosphorylation under control versus BDNF-stimulated conditions. * Significantly different, Arc Sin transformation for percentile data, Student's t test P < 0.05.
Figure 10.
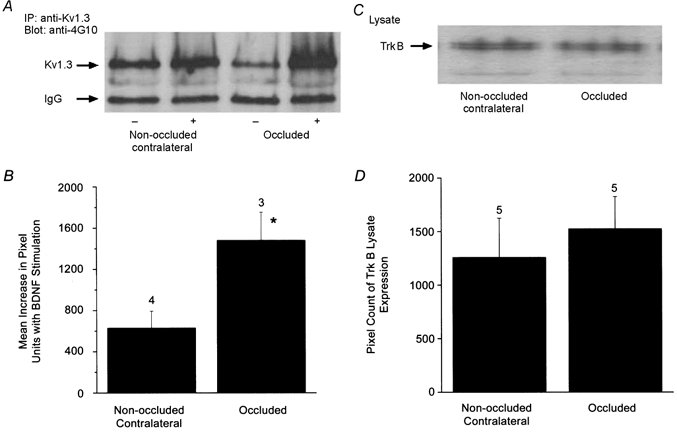
Sensory deprivation by unilateral naris occlusion alters BDNF-stimulated tyrosine phosphorylation of Kv1.3 channel
P1 animals were left naris occluded by cauterization and raised with odour sensory deprivation to this naris from P20 to P25. A, olfactory bulbs contralateral (non-occluded) and ipsilateral (occluded) to the cauterized naris were then harvested, stimulated with BDNF and immunoprecipitated with anti-Kv1.3 and blotted with anti-4G10. Total tyrosine phosphorylation of Kv1.3 is indicated by the arrow. B, histogram of the mean increase in tyrosine phosphorylation of Kv1.3 by acute BDNF stimulation comparing non-occluded versus sensory-deprived conditions (occluded). Pixel values were calculated by quantitative densitometry. The difference in pixel density between unstimulated and BDNF-stimulated olfactory bulbs was plotted for occluded and non-occluded naris conditions, where 0 = no change in Kv1.3 phosphorylation with BDNF treatment. * Significantly different by Student's paired t test, P < 0.05. C, 10 μg of cell lysate were blotted for α-TrkB to confirm equivalent protein expression of the receptor under control versus naris occlusion conditions. Corresponding quantitative densitometry for 5 animals is shown in D. Not significantly different, Student's paired t test, P < 0.05.
DISCUSSION
The OB is the first processing station in the olfactory pathway. Members of the Kv family of potassium channels in the OB partly determine the cell's resting potential, regulate the level of neuronal excitability by influencing the duration of the action potential, determine the frequency of repetitive firing and time the interspike interval (Jan & Jan, 1994). Here we show that BDNF activation of TrkB receptor kinase in these neurons uses Kv1.3 as a substrate for tyrosine phosphorylation and alters the functional properties of this channel based upon the duration of trophic factor stimulation and prior odour sensory experience.
Our patch-clamp study in the olfactory bulb suggests that the neurotrophin BDNF may serve multiple functions. The persistent expression of TrkB receptors in both the developing and adult OB implies that BDNF could influence cell phenotype, synaptic plasticity and neuronal regeneration in the olfactory system (Ming et al. 1999) and simultaneously serve a parallel role to modulate electrical activity at the level of the ion channel Kv1.3 by tyrosine phosphorylation. In these TrkB receptor-expressing OB neurons, the duration of neurotrophin exposure affected the type and presumably mechanism of neuromodulation. Acute stimulation (min) with BDNF elicited a suppression of Kv1.3 current magnitude while chronic exposure to BDNF (days) caused an increase in the current magnitude and speeding of the inactivation and deactivation kinetics of the channel. Neurotrophin signalling is known to be activity dependent; for example, release of BDNF can be upregulated by patterned electrical activity (Berninger & Poo, 1999; Balkowiec & Katz, 2000; Poo, 2001); membrane depolarization can increase the expression of TrkB receptor, which can subsequently increase targeting of voltage-gated ion channels to the plasma membrane (Tongiorgi et al. 1997; Urbano & Buno, 2000); and certain disease states, such as Alzheimer's disease, show a decrease in BDNF synthesis (Yan et al. 1997). We show that, while the expression of TrkB and Kv1.3 is not altered by sensory deprivation to the olfactory bulb induced by unilateral naris occlusion, the BDNF-induced tyrosine phosphorylation of Kv1.3 is increased in neurons lacking odour sensory experience. Hence the experience or sensory-dependent state of a neuron can influence the extent of phosphorylation of an ion channel and concomitant functional modulation by BDNF.
In the OB, acute stimulation of Trk receptors elicited changes in ion channel function that were selective to activation of TrkB and were nonmodulatory upon activation of TrkA or TrkC. Chronic stimulation of Trk receptors also elicited changes in Kv1.3 function that were predominantly selective to TrkB, with the exception of NT3 modulation of Kv1.3 inactivation following 3 days treatment with the growth factor. Although our data cannot definitely demonstrate that there was no cross-activity of NGF or NT3 at TrkB receptor proteins, application of these ligands to the OBNs did not elicit changes in Kv1.3 current magnitude as was observed for BDNF. Our finding that the majority of TrkA and TrkC receptor protein exists as the truncated version that lacks kinase activity could explain why ligands for these receptors failed to modulate or inconsistently modulated Kv1.3 current in the OBNs. The role of truncated, noncatalytic forms of Trk receptor protein in the nervous system (Allen et al. 1994) has not been widely studied, but selective loss of full-length TrkB receptor protein over that of its truncated form has been reported in patients with Alzheimer's disease (Allen et al. 1999) and truncation of neurotrophic receptors in general may be developmentally or traumatically induced (Offenhauser et al. 1995; Brodeur et al. 1997; King et al. 2000).
Our previous work demonstrating acute modulation of Kv1.3 by another receptor tyrosine kinase, insulin receptor kinase, suggested that the BDNF-induced current suppression of Kv1.3 was probably attributable to direct tyrosine phosphorylation of the channel. Kv1.3 has 17 tyrosine residues, six of which lie within good recognition motifs for tyrosine-specific phosphorylation (Songyang et al. 1993; Hunter, 1995). Using a combined immunoprecipitation and Western blot strategy, we demonstrated that Kv1.3 phosphorylation is increased following acute stimulation with BDNF by immunoprecipitating Kv1.3 protein and probing this SDS-PAGE-separated protein with anti-phosphotyrosine-specific antibodies. Although we cannot exclude an indirect mechanism of phosphorylation by cross-talk with another signalling cascade, such as neurotrophin activation of src kinases, we can deduce that modulation of Kv1.3 by BDNF can occur independent of synapse formation, because Kv1.3 plus TrkB cotransfected HEK293 cells can be similarly modulated by including BDNF in the patch pipette during cell-attached recordings (data not shown). A physiological relationship between neurotrophin action and K+ channel function has been previously reported for the G protein-gated inward rectifier Kir3 (Rogalski et al. 2000). Interestingly, BDNF regulation of Kir3 required specific tyrosine residues in the carboxyl terminus of both Kir3.1 and Kir3.4 and did not affect the homotetrameric inwardly rectifying potassium channel. The fact that, under our recording conditions, Kv1.3 comprises as much as 80 % of the outward currents in the OB neurons (Fadool & Levitan, 1998), combined with the fact that homotetrameric Kv1.3 channels transfected with TrkB receptors in HEK293 cells are both suppressed and tyrosine phosphorylated by BDNF (data not shown), implies that BDNF can modulate homotetrameric voltage-gated channels by tyrosine phosphorylation of Kv1.3. It is also important to draw parallels between phosphorylation and expression of Kv1.3 channels (Figs 7–10) and BDNF effects on Kv1.3 currents (Figs 1–6), since potassium currents in OB neurons include other members of the voltage-gated potassium channel family, particularly Kv1.4. Although Kv1.3 phosphorylation cannot be analysed in single-cell culture (only whole OB) because of insufficient protein, lack of BDNF effects in margatoxin-treated neurons supports BDNF modulation of Kv1.3 explicitly over that of other family members. Additionally, one must consider that acute stimulation of central neurons by neurotrophins has been reported that is completely phosphorylation independent. Kafitz et al. (1999) have demonstrated acute, rapid modulation of Na+ currents that occurred in the order of 9 ms, much faster than the most rapid detectable tyrosine phosphorylation, which occurs in approximately 30 s.
Chronic stimulation of OBN cultures with BDNF evoked opposite changes in OB current, namely an increase in outward potassium current as opposed to current suppression. Studies have demonstrated that phosphorylation of TrkB by BDNF is achievable in a 5 min time course and will progressively decline over the course of an hour (Yuen & Mobley, 1999). Unfortunately, the protocol of stimulating whole OBs with BDNF did not permit long chronic exposures to the neurotrophin that could then be assessed biochemically to measure the phosphorylation state of either TrkB or Kv1.3. This protocol of maintaining whole bulbs over days in situ would induce inevitable death of neurons, whereas harvesting cultured OBNs chronically supplemented with neurotrophins did not yield enough protein for immunoprecipitation and SDS-PAGE analysis. So it remains to be determined whether there is a downregulation of either TrkB receptor expression or phosphorylation state that would be consistent with a reduced modulation (suppression) of Kv1.3 current in the OB under chronic BDNF stimulation. The left-shifted conductance plots, however, indicate that chronic BDNF stimulation may alter the voltage dependence of Kv1.3. The lower V1/2 values reported for BDNF-treated cells could account for greater whole-cell currents because the neuron could activate sooner, at lower potentials, than time-matched controls. Another likely interpretation of the mechanism of chronic neurotrophin upregulation can be derived from the fact that the neurotrophin NGF is known to regulate the number and distribution of delayed rectifying K+ channels in PC12 cells and SK-SH neuroblastoma cells (Lesser et al. 1997; Yan et al. 1997). Increased targeting of potassium ion channels to the membrane of OB neurons could account for the increased current magnitude or density observed in BDNF-treated cultures. Equally plausible is the fact that expression or targeting of another class of delayed rectifier could be upregulated under chronic BDNF treatment that would mask a continued Kv1.3 current suppression. Independent of mechanism, our data demonstrate that long-term treatment with neurotrophins, such as in treatment for nerve injury or neurogenerative diseases, upregulates potassium currents in neurons, an effect that is the inverse of acute or rapid exposure. BDNF activation of TrkB receptors can thus have different functional effects depending upon duration of stimulation, a finding also reported by Sherwood & Lo (1999), who compared acute versus chronic effects of BDNF on synaptic transmission in CA1 hippocampal cultures.
Finally, our data in sensory-deprived animals with unilateral naris occlusion demonstrate that odour sensory experience is necessary to maintain kinase activity, as observed in control animals for acute BDNF-induced tyrosine phosphorylation of Kv1.3. Odour sensory deprivation failed to alter either TrkB kinase protein expression (Fig. 10) or Kv1.3 protein expression (Fadool et al. 2000), yet phosphorylation of the channel after acute BDNF stimulation was significantly increased. Most recent data have shown that BDNF levels fall in the granule cell layer following sensory deprivation via naris occlusion (McClean et al. 2001), which implies, for our data, that there must be an increased efficacy of the TrkB kinase to yield increased Kv1.3 tyrosine phosphorylation following occlusion. Alternatively, a different signalling cascade could be activated after occlusion to increase Kv1.3 tyrosine phosphorylation in the presence of BDNF. Independent of mechanism, all increases in tyrosine phosphorylation of Kv1.3 by a variety of receptor and cellular tyrosine kinases have been correlated with a decreased total current and would imply that an increase in basal channel phosphorylation induced by sensory deprivation would decrease Kv1.3 current magnitude. If neurotrophic factors are being researched as potential treatments to increase nerve cell generation and growth following injury or deprivation (Sinson et al. 1996; Zochodne, 1996), it is important also to consider altered receptor kinase or channel functions induced by such deprivation, because potential treatments would be administered from an altered basal state. This patch-clamp study of modulation of voltage-gated potassium current (Kv1.3) in olfactory bulb neurons and the parallel biochemical analysis of the phosphorylated state of Kv1.3 ion channel protein demonstrates that Kv1.3 is tyrosine phosphorylated by acute stimulation with the neurotrophin BDNF. BDNF-evoked Kv1.3 phosphorylation induces current suppression and no changes in inactivation or deactivation kinetics or voltage dependence of the potassium current in these OB neurons. Conversely, chronic stimulation with BDNF over days induces an enhancement of the potassium current, probably by a mechanism that changes the voltage dependence of Kv1.3, and a speeding of both the inactivation and deactivation kinetics. Acute stimulation of OB neurons by BDNF is dependent upon odour sensory experience, where unilateral naris occlusion shortly after birth upregulates the increase in tyrosine phosphorylation of Kv1.3 induced by BDNF. Studies of genetically modified mice lacking TrkB or BDNF indicate that lack of expression of the kinase or ligand does not alter the topographical positioning or number of OB glomeruli (Nef et al. 2001). Unfortunately, functional studies have not been undertaken with such a model, so this is an important future directive in understanding how neurotrophins affect ion channel biophysics and electrical patterning for coding of olfactory information.
Acknowledgments
We would like to thank Ms Davonya Person for technical assistance with the naris occlusions. We are grateful to Drs Marie Wooten and Carol Deutsche for donation of TrkA and Kv1.3 antisera, respectively, for initial trials in our research project. We thank Mr Charles Badland for instructing us in the conversion of our figures to electronic formatting. We would like to thank Dr Steven Jones for mathematical advice in fitting Boltzman relationships. This research was supported by National Institutes of Health R29 DC03387 from the National Institutes of Deaf and Communication Disorders.
REFERENCES
- Allen SJ, Dawbarn D, Eckford SD, Wilcock GK, Ashcroft M, Colebrook SM, Feeney R, MacGowan SH. Cloning of a non-catalytic form of human trkB and distribution of messenger RNA for trkB in human brain. Neuroscience. 1994;60:825–834. doi: 10.1016/0306-4522(94)90507-x. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Wilcock GM, Dawbarn D. Profound and selective loss of catalytic TrkB immunoreactivity in Alzheimer's Disease. Biochemical and Biophysical Research Communications. 1999;264:648–651. doi: 10.1006/bbrc.1999.1561. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. Journal of Neuroscience. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. European Journal of Neuroscience. 2001;13:257–268. [PubMed] [Google Scholar]
- Bartolomei JC, Greer CA. Olfactory ensheathing cells, bridging the gap in spinal cord injury. Neurosurgery. 2000;47:1057–1069. doi: 10.1097/00006123-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Berninger B, Poo M. Fast actions of neurotrophic factors. Current Opinion in Neurobiology. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- Berninger B, Poo M. Exciting neurotrophins. Nature. 1999;401:862–863. doi: 10.1038/44727. [DOI] [PubMed] [Google Scholar]
- Blesch A, Grill RJ, Tuszynski MH. Neurotrophin gene therapy in CNS models of trauma and degeneration. Progress in Brain Research. 1998;117:473–484. doi: 10.1016/s0079-6123(08)64033-9. [DOI] [PubMed] [Google Scholar]
- Bolton MM, Pitman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. Journal of Neuroscience. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby MR, Fadool DA, Holmes TC, Levitan IB. Modulation of the Kv1. 3 potassium channel by receptor tyrosine kinases. Journal of General Physiology. 1997;110:601–610. doi: 10.1085/jgp.110.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM, Nakagawara A, Yamashiro DJ, Ikegaki N, Liu SG, Azar CG, Lee CP, Evans AE. Expression of TrkA, TrkB, and TrkC in human neuroblastomas. Journal of Neurooncology. 1997;31:49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- Carter LA, Roskams AJ. Neurotrophins and their receptors in the primary olfactory neuroaxis. In the Press. [DOI] [PubMed]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proceedings of the National Academy of Sciences of the USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang X-Y, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. Journal of Neuroscience. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Holmes TC, Berman K, Dagan D, Levitan IB. Multiple effects of tyrosine phosphorylation on a voltage-dependent potassium channel. Journal of Neurophysiology. 1997;78:1563–1573. doi: 10.1152/jn.1997.78.3.1563. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Levitan IB. Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. Journal of Neuroscience. 1998;18:6126–6137. doi: 10.1523/JNEUROSCI.18-16-06126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Phillips JJ, Simmen JA. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1. 3. Journal of Neurophysiology. 2000;83:2332–2348. doi: 10.1152/jn.2000.83.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Barnett SC. Olfactory ensheathing cells and CNS regeneration, the sweet smell of success? Neuron. 2000;28:15–18. doi: 10.1016/s0896-6273(00)00080-5. [DOI] [PubMed] [Google Scholar]
- Fry EL. Central nervous system regeneration, mission impossible? Clinical and Experimental Pharmacology and Physiology. 2001;28:253–258. doi: 10.1046/j.1440-1681.2001.03417.x. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM. Differential expression of mRNAs for the NGF family of neurotrophic factors in the adult rat central olfactory system. Journal of Comparative Neurology. 1991;313:95–102. doi: 10.1002/cne.903130107. [DOI] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA, Ren R, Levitan IB. Association of src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- Horikawa I, Takaki M, Ishimaru T, Furukawa M, Kato T, Moriizumi T. TrkA expression in olfactory epithelium and bulb during development. NeuroReport. 1999;10:2205–2208. doi: 10.1097/00001756-199907130-00037. [DOI] [PubMed] [Google Scholar]
- Huang X-Y, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases. The yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Burton WV, Fodor WL, Kocsis JD. Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord. Nature Biotechnology. 2000a;18:925–927. doi: 10.1038/79432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Kocsis JD. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Research. 2000b;854:70–78. doi: 10.1016/s0006-8993(99)02285-4. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan NJ. Potassium channels and their evolving gates. Nature. 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Current Opinion in Neurobiology. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Kaisho Y, Shintani A, Nagahma M, Kato K. Tissue distribution and immunocytochemical localization of neurotrophin-3 in the brain and peripheral tissues of rats. Journal of Neurochemistry. 1996;66:330–337. doi: 10.1046/j.1471-4159.1996.66010330.x. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba RRS, Takeuchi I, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice, a comparison to those of nerve growth factor and neurotrophin-3. Neuroscience Research. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- King VR, Bradbury EJ, McMahon SB, Priestly JV. Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Experimental Neurology. 2000;165:327–341. doi: 10.1006/exnr.2000.7480. [DOI] [PubMed] [Google Scholar]
- Kues WA, Wunder F. Heterologous expression patterns of mammalian potassium channel genes in developing and adult rat brain. European Journal of Neuroscience. 1992;4:1296–1308. doi: 10.1111/j.1460-9568.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Lesser SS, Lo DC. Regulation of voltage-gated ion channels by NGF and ciliary neurotrophic factor in SK- SH neuroblastoma cells. Journal of Neuroscience. 1995;15:253–261. doi: 10.1523/JNEUROSCI.15-01-00253.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser SS, Sherwood NT, Lo DC. Neurotrophins differentially regulate voltage-gated ion channels. Molecular Cell Neuroscience. 1997;10:173–183. doi: 10.1006/mcne.1997.0656. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Differential effects of NGF and BDNF on voltage-gated calcium currents in embryonic basal forebrain neurons. Journal of Neuroscience. 1995;15:3084–3091. doi: 10.1523/JNEUROSCI.15-04-03084.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Himes BT, Solowska J, Moul J, Chow SY, Park KI, Tessler A, Murray M, Snyder EY, Fischer I. Intraspinal delivery of neurotrophin-3 using neural stem cells genetically modified by recombinant retrovirus. Experimental Neurology. 1999;158:9–26. doi: 10.1006/exnr.1999.7079. [DOI] [PubMed] [Google Scholar]
- Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Research. 2001;889:344–357. doi: 10.1016/s0006-8993(00)03235-2. [DOI] [PubMed] [Google Scholar]
- McClean JH, Darby-King A, Bonnell WS. Neonatal olfactory sensory deprivation decreases BDNF in the olfactory bulb of the rat. Brain Research Developmental Brain Research. 2001;128:17–24. doi: 10.1016/s0165-3806(01)00144-4. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Chuah MI. Neurotrophic factors in the primary olfactory pathway. Progress in Neurobiology. 2000;62:527–559. doi: 10.1016/s0301-0082(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Marom S, Goldstein S, Kupper J, Levitan IB. Mechanism and modulation of inactivation of the Kv3 potassium channel. Receptors and Channels. 1993;1:81–88. [PubMed] [Google Scholar]
- Masana Y, Wanaka A, Kato H, Asai T, Tohyama M. Localization of trkB mRNA in postnatal brain development. Journal of Neuroscience Research. 1993;35:468–479. doi: 10.1002/jnr.490350503. [DOI] [PubMed] [Google Scholar]
- Meisami E. Effects of olfactory deprivation on postnatal growth of the rat olfactory bulb utilizing a new method for production of neonatal unilateral anosmia. Brain Research. 1976;107:437–444. doi: 10.1016/0006-8993(76)90243-2. [DOI] [PubMed] [Google Scholar]
- Merlio J-P, Ernfors P, Jaber M, Persson H. Molecular cloning of rat trk C and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- Ming Y, Bergman E, Edstrom E, Ulfhake B. Reciprocal changes in the expression of neurotrophin mRNAs in target tissues and peripheral nerves of aged rats. Neuroscience Letters. 1999;273:187–190. doi: 10.1016/s0304-3940(99)00655-2. [DOI] [PubMed] [Google Scholar]
- Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. Journal of Neurotrauma. 2000;17:1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- Nef S, Lush ME, Shipman TE, Parada LF. Neurotrophins are not required for normal embryonic development of olfactory neurons. Developmental Biology. 2001;234:80–92. doi: 10.1006/dbio.2001.0240. [DOI] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. European Journal of Neuroscience. 2000a;12:776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. BDNF abolishes the survival effect of NT-3 in axotomized Clarke neurons of adult rats. Journal of Comparative Neurology. 2000b;428:671–680. doi: 10.1002/1096-9861(20001225)428:4<671::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Bohm-Matthaei R, Tsoulfas P, Parada L, Meyer M. Developmental regulation of full-length trkC in the rat sciatic nerve. European Journal of Neuroscience. 1995;7:917–925. doi: 10.1111/j.1460-9568.1995.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Philpot B, Lim J, Halpain S, Brunjes P. Experience-dependent modifications in MAP2 phosphorylation in rat olfactory bulb. Journal of Neuroscience. 1997;17:9596–9604. doi: 10.1523/JNEUROSCI.17-24-09596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Raisman G. Olfactory ensheathing cells — another miracle cure for spinal cord injury? Nature Reviews Neuroscience. 2001;2:369–375. doi: 10.1038/35072576. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunje MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. Journal of Neuroscience. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski SL, Appleyard SM, Pattillo A, Terman GW, Chavkin C. TrkB activation by brain-derived neurotrophic factor inhibits the G-protein-gated inward rectifier Kir3 by tyrosine phosphorylation of the channel. Journal of Biological Chemistry. 2000;275:25082–25088. doi: 10.1074/jbc.M000183200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams AJI, Bethel MA, Hurt J, Ronnett GV. Sequential expression of Trks A, B, and C in the regenerating olfactory neuroepithelium. Journal of Neuroscience. 1996;16:1294–1307. doi: 10.1523/JNEUROSCI.16-04-01294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatas T. Molecular Cloning, A Laboratory Manual. New York, NY, USA: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Sherwood NT, Lesser SS, Lo DC. Neurotrophin regulation of ionic currents and cell size depends on cell context. Proceedings of the National Academy of Sciences of the USA. 1997;92:5917–5922. doi: 10.1073/pnas.94.11.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NT, Lo DC. Long-term enhancement of central synaptic transmission by chronic brain-derived neurotrophic factor treatment. Journal of Neuroscience. 1999;19:7025–7036. doi: 10.1523/JNEUROSCI.19-16-07025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinson G, Voddi M, Flamm ES, McIntosh TK. Neurotrophin infusion improves cognitive deficits and decreases cholinergic neuronal cell loss after experimental brain injury. Clinical Neurosurgery. 1996;43:219–227. [PubMed] [Google Scholar]
- Songyang Z, Carraway KL, Eck MJ, Harrison SC, Feldman RA, Mohammadi M, Schlessinger J, Hubbard SR, Smith DP, Eng C, Lorenzo MJ, Ponder BAJ, Mayer BJ, Cantley LC. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1993;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Righi M, Cattaneo A. Activity-dependent dendritic targeting of BDNF and TrkB mRNA in hippocampal neurons. Journal of Neuroscience. 1997;17:9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Buno W. Neurotrophin regulation of sodium and calcium channels in human neuroblastoma cells. Neuroscience. 2000;96:439–443. doi: 10.1016/s0306-4522(99)00552-7. [DOI] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Yu X-M, Askalan R, Keil GJ, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- Yuen EC, Mobley WC. Early BDNF, NT-3, and NT-4 signaling events. Experimental Neurology. 1999;159:297–308. doi: 10.1006/exnr.1999.7148. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Molecular Cell Neuroscience. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Neurotrophins and other growth factors in diabetic neuropathy. Seminars in Neurology. 1996;16:153–161. doi: 10.1055/s-2008-1040971. [DOI] [PubMed] [Google Scholar]


