Abstract
Cerebellar granule neurons express a standing outward (background) K+ current (IK,SO) that regulates the resting membrane potential and cell excitability. As several tandem-pore (2P) K+ channel mRNAs are highly expressed in cerebellar granule cells, we studied whether, and which, 2P K+ channels contribute to IK,SO. IK,SO was highly sensitive to changes in extracellular pH and was partially inhibited by acetylcholine, as reported previously. In cell-attached patches from cultured cerebellar granule neurons, four types of K+ channels were found to be active when membrane potential was held at −50 mV or +50 mV in symmetrical 140 mm) KCl. Based on single-channel conductances, gating kinetics and modulation by pharmacological agents and pH, three K+ channels could be considered as functional correlates of TASK-1, TASK-3 and TREK-2, which are members of the 2P K+ channel family. The fourth K+ channel (Type 4) has not been described previously and its molecular correlate is not yet known. Based on the measurement of channel current densities, the Type 2 (TASK-3) and the Type 4 K+ channels were determined to be the major sources of IK,SO in cultured cerebellar granule neurons. The Type 1 (TASK-1) and Type 3 (TREK-2) activities were relatively low throughout cell growth in culture (1-10 days). Similar to TASK-1 and TASK-3, the Type 4 K+ channel was highly sensitive to changes in extracellular pH, showing a 78 % inhibition by changing the extracellular pH from 7.3 to 6.3. The results of this study show that three 2P K+ channels and an additional pH-sensing K+ channel (Type 4) comprise the IK,SO in cultured cerebellar granule neurons. Our results also show that the high sensitivity of IK,SO to extracellular pH comes from the high sensitivity of Type 2 (TASK-3) and Type 4 K+ channels.
Cerebellar granule (CG) neurons form synapses with Purkinje cells and provide a major excitatory input via parallel fibres in the molecular layer of the cerebellum. Cerebellar granule neurons express an outwardly rectifying K+ current that is responsible for the large negative resting membrane potential recorded in these cells (Watkins & Mathie, 1996). This K+ current is referred to as ‘the standing outward’ current (IK,SO), and is inhibited by activation of G protein-coupled receptors such as the muscarinic receptor (Watkins & Mathie, 1996; Boyd et al. 2000). IK,SO is also inhibited markedly by lowering the pH of the extracellular solution (Millar et al. 2000). Therefore, inhibition of IK,SO by receptor agonists and extracellular acidosis will enhance the excitability of granule neurons and their firing rate, producing an increased excitatory synaptic input to Purkinje cells (Ross et al. 1990; North, 2000).
The electrophysiological and pharmacological properties of IK,SO are similar to those of TASK-1, a member of the tandem-pore K+ channel family (Millar et al. 2000). Thus, they both are active across the physiological range of membrane potentials and show little or no inactivation, characteristics that are typical of background K+ currents (Lesage & Lazdunski, 2000; Goldstein et al. 2001). Both IK,SO and TASK-1 are inhibited by muscarinic receptor activation, by acidosis and by millimolar concentrations of Ba2+, but are not inhibited by K+ channel blockers such as tetraethylammonium (Watkins & Mathie, 1996; Millar et al. 2000). These studies, together with the finding that TASK-1 mRNA is expressed in CG neurons, have led to the reasonable assumption that IK,SO is the functional correlate of TASK-1. More recently, however, another tandem-pore K+ channel (TASK-3) with properties similar to TASK-1 was cloned (Kim et al. 2000; Rajan et al. 2000) and its mRNA also found to be expressed in the cerebellar granule layer (Brickley et al. 2001; Talley et al. 2001). Another tandem-pore K+ channel, TREK-2 (Bang et al. 2000; Lesage et al. 2000), was also expressed in cerebellar granule layer, although its pH sensitivity was different from that of TASK-1 (Bang et al. 2000; Talley et al. 2001). These studies indicate that IK,SO might be composed of several K+ channels, one of which might be TASK-1.
In this study, we sought to determine the molecular identity of IK,SO by studying the electrophysiological and pharmacological properties of resting K+ channels that are active in CG neurons. Specifically, we wished to test the hypothesis that several tandem-pore (2P) K+ channels, including TASK-1, TASK-3 and TREK-2, contribute to the resting K+ conductance in CG neurons and thus the IK,SO. By recording single-channel currents from cultured CG neurons, we have identified four different K+ channels that may give rise to IK,SO. Three of these K+ channels possessed biophysical and pharmacological properties similar to those of TASK-1, TASK-3 and TREK-2. The fourth K+ channel was an acid-sensing K+ channel similar to TASK-1 and TASK-3, but its molecular identity is not known. The contribution of TASK channels to IK,SO became greater as the period of growth of neurons in culture became longer, at least up to 10 days. Our results also show that in cultured CG neurons, the high sensitivity of IK,SO to extracellular pH is mainly due to two types of K+ channels: the K+ channel with properties similar to TASK-3 and the acid-sensing fourth K+ channel.
METHODS
Cerebellar granule neuron culture and isolation
All animals were used in accordance with the Guide for the Care and Use of Laboratory Animals (DHEW Publication no. NIH85-23). The cerebellum was isolated from rapidly decapitated P6-P8 rat pups, and washed with oxygenated physiological buffer solution (PBS) at 4 °C. The cerebellar cortex was cut into 500 μm or thinner sections and incubated for 15 min in a solution containing papain (12 U ml−1; Worthington, Lakewood, NJ, USA), albumin (0.2 mg ml−1) and dl-cysteine (0.2 mg ml−1). After digestion, the tissue was washed twice with PBS solution and resuspended in a solution containing DNase I (1000 Kunitz unit ml−1; Worthington). After gentle trituration of the solution using a fire-polished glass pipette, the suspended cells were gently passed through a 3 cm3/25 g syringe. The suspension was layered on top of sterilized fetal bovine serum and centrifuged at 100 g for 10 min. The pellet was resuspended in plating medium that contains NeuroBasal Media supplemented with B-27 (10 μl ml−1; Life Technologies, Rockville, MD, USA), glutamic acid (2.5 mm), glutamine (20 mm), gentamicin (50 μg ml−1) and fungizone (2.5 μg ml−1). The cells were plated on glass coverslips coated with poly-l-lysine at a density of 1 × 105 cells cm−2. After a 24 h period for cell attachment, the medium was changed every 3 days with new plating medium containing B-27 (20 μl ml−1), glutamine, gentamicin and fungizone in Neurobasal Medium. Cells were kept for 10 days at 37 °C in a humidified incubator gassed with a 95 % air-5 % CO2 mixture. Neurons from 4-week-old rats were freshly isolated using the same protocol except that the suspended cells were plated onto polylysine-coated glass coverslips and used within 4 h.
Transfection in COS-7 cells
Rat TASK-1, TASK-3 and TREK-2 were cloned previously in this laboratory (Kim et al. 1999, 2000; Bang et al. 2000). The coding regions of rat TASK-1, TASK-3 and TREK-2 were subcloned into pcDNA3.1 vector (Invitrogen, Carlsbad, CA). COS-7 cells were seeded at a density of 2 × 105 cells per 35 mm dish 24 h prior to transfection in 10 % bovine serum in Dulbecco's modified Eagle's medium (DMEM). COS-7 cells were co-transfected with a 2P K+ channel DNA and pcDNA3.1/GFP using LipofectAMINE and OPTI-MEM I Reduced Serum Medium (Life Technologies). Green fluorescence from cells expressing green fluorescent protein (GFP) was detected with the aid of a Nikon microscope equipped with a mercury lamp light source. Cells were used 1–10 days after transfection.
Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200, Axon Instruments, Union City, CA, USA). All recordings were performed at room temperature (24 °C). Single-channel currents were digitized with a digital data recorder (VR10, Instrutech, Great Neck, NY, USA), and stored on video tape. The recorded signal was filtered at 5 kHz using an 8-pole Bessel filter (-3 dB; Frequency Devices, Haverhill, MA, USA) and transferred to a computer (Dell) using the Digidata 1200 interface (Axon Instruments) at a sampling rate of 20 kHz. Threshold detection of channel openings was set at 50 %. Whole-cell currents were recorded after cancelling the capacitive transients. Whole-cell and single-channel currents were analysed with the pCLAMP program (version 7). For single-channel analysis, the filter dead time was 100 μs (0.3/cutoff frequency) such that events shorter than 50 μs in duration would be missed. Data were analysed to obtain a duration histogram, amplitude histogram and relative channel activity (relative NPo, where N is the number of channels in the patch, and Po is the probability of a channel being open). NPo was determined from ≈1 min of current recording. The single-channel current tracings shown in the figures were filtered at 2 kHz. In experiments using excised patches, pipette and bath solutions contained 140 mm KCl, 1 mm MgCl2, 5 mm EGTA and 10 mm Hepes. The pH was adjusted using KOH to 7.3. In whole-cell recordings, bath solution contained 135 mm NaCl, 3 mm KCl, 0.5 mm CaCl2, 1 mm MgCl2 and 10 mm Hepes. The pH was adjusted to 7.3 using NaOH. Arachidonic acid was dissolved by sonicating for 5 min (Heat Systems-Ultrasonics, Inc. W-380, Farmingdale, NY, USA) in bath recording solution at a desired concentration. All other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA). For statistics, Student's t test was used with P < 0.05 as a criterion for significance. Data are represented as means ± s.d.
RESULTS
Properties of the standing outward K+ current
Granular cells were identified by their small size (2-3 pF) and cells with short dendrites were used. The standing outward K+ current (IK,SO) in cultured cerebellar granule (CG) neurons has been well characterized previously (Watkins & Mathie, 1996; Millar et al. 2000). IK,SO is a non-inactivating K+ current that is open at rest, and therefore can be identified by a pulse protocol, as illustrated in Fig. 1A. When the membrane potential of the whole cell was held at −20 mV for a few seconds and then repolarized to −80 mV, an instantaneous decrease in current was present. Upon step depolarization, a transient outward current was often present and this was followed by inactivation to a steady-state level that is referred to as IK,SO. In our cultured CG neurons (7-9 days in culture), application of 10 μm ACh reduced IK,SO by 47 ± 6 % (n = 5). ACh reduced the non-inactivating current and not the transient outward current, in keeping with earlier findings (Watkins & Mathie, 1996). When the membrane potential was held at −80 mV and a ramp pulse from −120 mV to −20 mV applied, an outwardly rectifying current was typically observed, as seen in Fig. 1B. Under this condition, where IK,SO should also be fully active, application of ACh also produced a decrease in outward current, as expected. The effect of ACh on IK,SO was rapid and fully reversible. As reported previously, 1 mm TEA failed to affect IK,SO (4.9 ± 6.0 % change, n = 5). Another important property of IK,SO is its sensitivity to extracellular pH (pHo). As shown in Fig. 1C, lowering pHo caused a significant decrease in whole-cell current. On average, decreasing the pHo from 7.3 to 6.3 reduced the current by 78 ± 15 % (n = 5) in CG neurons grown in culture for 7–10 days (Fig. 1D). Raising the extracellular pH produced an increase in current but the effects were generally small compared with the decrease produced by acid pHo. Thus, IK,SO exhibits properties that are similar to those of TASK-1, which is a member of the 2P K+ channel family, shows non-inactivating resting K+ current, and is inhibited by muscarinic receptor agonists and acid conditions (Millar et al. 2000; Talley et al. 2000).
Figure 1.
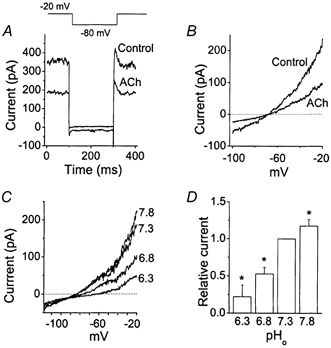
The standing outward K+ current in cerebellar granule neurons
A, whole-cell current was activated by a step pulse protocol as shown. After holding the cell for 4 s at −20 mV, membrane potential was changed to −80 mV and then back to −20 mV. ACh (10 μm) was applied to the extracellular perfusion solution. GTP (100 μm) and ATP (1 mm) were present in the pipette solution for all whole-cell experiments. B, whole-cell current was activated by a 1 s duration ramp pulse protocol (-100 mV to −20 mV). ACh (10 μm) was applied to the extracellular perfusion solution. C, whole-cell currents were recorded at different extracellular pH values. The ramp protocol was the same as that in B. D, the bar graph shows the effect of extracellular pH on the whole-cell current measured at −20 mV. Each bar is the mean ± s.d. of five determinations. *Significant difference from the corresponding control value observed at pH 7.3 (P < 0.05).
Background K+ channels observed in cell-attached patches
To identify all K+ channels that might contribute to IK,SO, we recorded single K+ channel currents from cell-attached patches of cultured CG neurons (n > 1000) using small tip pipettes (≈5 MΩ tip resistance). From these experiments, we found four different types of channels that were active when membrane potential was held constant at values ranging from −100 mV to +80 mV, and designated them Types 1-4. This was done primarily based on the differences in their single-channel conductances and opening characteristics (Fig. 2A and Table 1). All four channels were K+ selective, as judged by the 32–36 mV and 52–56 mV positive shifts when 140 mm K+ in the bath was changed to 35 and 15 mm, respectively, in inside-out conditions (see below). Also, four channel types with kinetics and conductances indistinguishable from those shown in Fig. 2 were observed when KCl in the pipette and bath solutions was replaced with potassium glutamate. When patch pipettes with larger tip diameter were used (2-3 MΩ tip resistance), we could observe simultaneous openings of two of these K+ channels in the same patch membrane and could easily identify them according to their channel kinetic properties (Fig. 2B).
Figure 2.
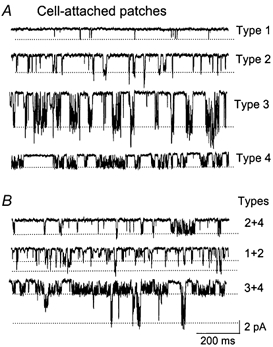
Single-channel currents recorded from cell-attached patches
A, four types of channels with distinct opening kinetics are shown (Type 1-Type 4). Dotted lines indicate the open states. B, cell-attached patches show a mixture of channels, as indicated. The cell membrane potential was held at −50 mV. Pipette and bath solutions contained 140 mm KCl.
Table 1.
Kinetic parameters of native and cloned K+ channels
| Type 1 | TASK-1 | Type 2 | TASK-3 | Type 3 | TREK-2 | Type 4 | |
|---|---|---|---|---|---|---|---|
| Conductance (pS) | |||||||
| 60 mV | 8.6 ± 1.1 | 9.2 ± 1.1 | 16.3 ± 1.2 | 17.1 ± 1.4 | 67 ± 8 | 65 ± 6 | 30 ± 2 |
| −60 mV | 15.6 ± 1.3 | 16.2 ± 1.3 | 33.0 ± 2.4 | 36.0 ± 2.4 | 107 ± 7 | 104 ± 6 | 32 ± 2 |
| Mean open time (ms) | |||||||
| (−60 mV) | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 2.4 ± 0.2 |
Each value represents the mean ± s.d. of 3–6 values. When values determined for the Type 1 K+ channel and TASK-1, the Type 2 K+ channel and TASK-3, and the Type 3 K+ channel and TREK-2 were compared, none was significantly different from each other (P > 0.05). For more details, see text.
During the course of these experiments, we noted that the functional expression of the K+ channels was dependent on the growth period in culture. To assess such growth-dependent changes in K+ channel expression, we first recorded the magnitude of the whole-cell current present at 0 mV under physiological conditions (i.e. 3 mm KCl in the bath solution). As shown in Fig. 3A, the average current increased steadily from ≈150 pA at day 1 to 500 pA at day 8, and did not significantly increase further for the next 48 h. To determine the growth-dependent changes in the functional expression of the four K+ channels, we recorded channel openings in 40–50 cell-attached patches for each day of culture at both −50 and +50 mV in symmetrical 140 mm KCl solution. During the first 1–3 days in culture, a small fraction of cell-attached patches (≈20 %) showed no channel openings. However, during the next 4–10 days in culture, every patch had some channel activity. Figure 3B and C shows the growth-dependent profiles for each of the four types of K+ channels during the 10 days in culture from eight separate cultures. Average channel activity per patch was calculated for each K+ channel type. Type 1 and Type 3 K+ channels were generally low in activity throughout the culture, although Type 1 showed a small increase towards the end of the 10-day period. Channel activity of Type 2 started out low and increased markedly during days 5-8, and remained elevated for the next 48 h. The Type 4 K+ channel was most consistently observed throughout the culture period and showed an increase in activity during days 1-5. These changes in channel activity were generally similar at both −50 mV and +50 mV. The higher averaged channel activity of the Type 4 channel at +50 mV than that at −50 mV was due to the voltage dependence of channel open probability (see below). These results show that the Type 4 K+ channel is mainly responsible for the IK,SO during the first few days in culture, whereas both Types 2 and 4 K+ channels are responsible for the IK,SO after several days in culture. The gradual increase in IK,SO during culture is therefore due to an early increase in Type 4 K+ channel activity and a late increase in Type 2 K+ channel activity. Although the Type 1 channel activity showed a late rise, its contribution to IK,SO, even at this stage, was less than 10 %. All of the single-channel data are based on the measurement of channel density on the cell body of CG neurons. Therefore, a possibility remains that dendrites express different amounts of the four types of K+ channel in CG neurons.
Figure 3.
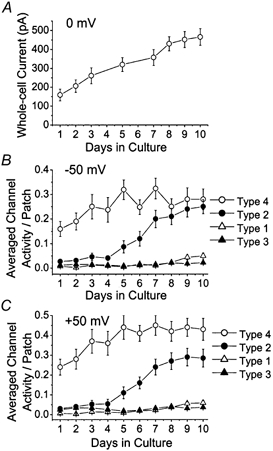
Changes K+ channel activity during culture
A, whole-cell currents were activated by a ramp pulse protocol (-100 to 0 mV) similar to that in Fig. 1B, and the peak currents at 0 mV determined from cells grown in culture for day 1 to day 10. B, approximately 40 patches per day were analysed for four types of K+ channels during cell growth in culture. The channel activity was determined from each cell-attached patch and averaged to give channel activity per patch. Each point is the mean ± s.d. of 35–42 determinations. Cell membrane potential was held at −50 mV. C, same experiment as B except that the membrane potential was at +50 mV to measure outward currents.
The Type 1 K+ channel is similar to TASK-1
As described above, the open probability of the Type 1 channel in CG neurons was typically low (Po < 0.03). Close examination of Type 1 channel kinetics suggested that it might be the functional correlate of TASK-1. Typical single-channel openings of the Type 1 K+ channel in a CG neuron at −60 mV and +60 mV are shown in Fig. 4A. The amplitude histogram of channel openings at −80 mV showed a major single peak at 1.2 ± 0.1 pA (n = 3; Fig. 4B). The openings were short in duration with a mean open time of 0.7 ± 0.1 ms at −60 mV (Fig. 4C). The current- voltage relationship plotted using mean peak values from the amplitude histograms at different membrane potentials showed a weak inward rectification (Fig. 4D). The single-channel conductances were 15.6 ± 1.3 pS at −60 mV and 8.6 ± 1.1 pS at +60 mV (n = 4). In inside-out patches, changing the bath [KCl] from 140 mm to 35 mm and 15 mm shifted the reversal potentials from zero to 34 ± 3 mV and 52 ± 4 mV (n = 3), respectively, close to the calculated Nernst values (36 mV and 58 mV) for a K+-selective ion channel.
Figure 4.
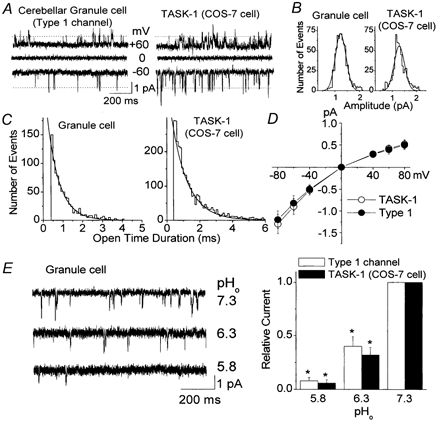
Electrophysiological properties of the Type 1 K+ channel in cerebellar granule neurons
A, openings of the Type 1 K+ channel in neurons and TASK-1 expressed on COS-7 cells are shown at three different membrane potentials in cell-attached patches. The pipette solution contained 140 mm KCl. B, amplitude histograms of Type 1 channel openings in a CG neuron and TASK-1 expressed in a COS-7 cell show single peaks of 1.2 pA (-60 mV). C, duration histograms of Type 1 K+ channel openings in a CG neuron and TASK-1 expressed in a COS-7 cell are shown. D, current amplitudes were determined at each membrane potential and used to plot the current-voltage relationships. Each point is the mean ± s.d. of three determinations. E, outside-out patches from CG neurons were formed and the effect of pHo determined at three pH values. Tracings are inward currents observed at −50 mV in symmetrical 140 mm KCl. Same experiment was done using COS-7 cells expressing TASK-1 (tracings not shown). Channel activity was determined at each pH value and plotted (n = 3). *Significant difference from the corresponding control value observed at pH 7.3 (P < 0.05).
TASK-1 transiently expressed in COS-7 cells showed kinetic properties indistinguishable from those of the Type 1 channel (see Table 1). The pharmacological properties were also similar for Type 1 channel and TASK-1 (Table 2). For example, both TASK-1 and Type 1 channels were unaffected by 1 mm tetraethylammonium (TEA), 1 mm 4-aminopyridine and 100 nm apamin (P > 0.05; n = 5). Type 1 channel currents were reduced by 65 ± 8 % by 3 mm Ba2+ and by 45 ± 8 % with 100 μm quinidine (n = 4). TASK-1 current in COS-7 cells was also reduced to similar degrees by Ba2+ (69 ± 8 %, n = 5) and quinidine (52 ± 6 %; n = 4). Arachidonic acid (10 μm) reduced the Type 1 and TASK-1 K+ channel activities by 32 ± 6 and 36 ± 8 %, respectively (n = 4). TASK-1 is highly sensitive to extracellular acidic conditions due to the presence of a histidine residue in the pore region (Lopes et al. 2001). In three outside-out patches that showed only Type 1 K+ channels, reducing the pH of the bath solution caused a marked decrease in channel activity (Fig. 4E). These effects of pH in CG neurons were not significantly different from those observed with TASK-1 expressed in COS-7 cells (P > 0.05). Recent studies have shown that TASK-1 mRNA is expressed in CG neuron culture and the granular layer of the cerebellum (Brickley et al. 2001; Talley et al. 2001). Therefore, our results show that the Type 1 K+ channel is most likely to be encoded by the TASK-1 gene in CG neurons.
Table 2.
Responses to drugs or native and cloned K+ channels shown as percentage inhibition
| Type 1 | TASK-1 | Type 2 | TASK-3 | Type 3 | TREK-2 | Type 4 | |
|---|---|---|---|---|---|---|---|
| Ba2+ (3 mm) | 65 ± 8 | 69 ± 8 | 62 ± 5 | 58 ± 7 | 45 ± 12 | 52 ± 11 | 95 ± 3 |
| TEA (1 mm) | 6 ± 6 | 8 ± 7 | 3 ± 5 | 2 ± 3 | 2 ± 3 | 0 ± 3 | 90 ± 10 |
| 4-AP(out;1 mm) | 4 ± 6 | 5 ± 5 | 1 ± 4 | 0 ± 3 | 1 ± 3 | 0 ± 4 | 8 ± 9 |
| Apamin (out;100 nm) | 2 ± 5 | 3 ± 4 | 6 ± 7 | 5 ± 6 | 3 ± 4 | 1 ± 3 | 1 ± 4 |
| Quinidine (0.1 mm) | 45 ± 8 | 52 ± 6 | 40 ± 7 | 38 ± 6 | 8 ± 6 | 6 ± 4 | 86 ± 12 |
| Bupivacaine (0.1 mm) | — | — | 58 ± 11 | 54 ± 8 | 52 ± 6 | — | — |
| AA (10 or 20 μm) | 32 ± 6 | 36 ± 8 | 69 ± 6 | 63 ± 7 | (12 ± 4) | (16 ± 6) | (4.9 ± 1.7) |
| GTPγS(10 μm) | — | — | 32 ± 4 | 38 ± 6 | 10 ± 4 | 8 ± 3 | 5 ± 6 |
Each value represents the mean ± s.d. of 3–6 values. Each agent was applied to the cytoplasmic side of the membrane unless indicated otherwise. All mean values above 20 % were significantly different from their respective controls (P < 0.05). Values in parentheses indicate ‘fold’ increase, not percentage inhibition. For more details, see text. AA, arachidonic acid.
The Type 2 K+ channel is similar to TASK-3
The Type 2 K+ channel was observed most frequently in neurons grown in culture for at least 5 days. Figure 5A shows single-channel openings of the Type 2 K+ channel in CG neurons and TASK-3 expressed in COS-7 cells. Amplitude histograms of channel openings showed a single peak at each membrane potential (-80 to +80 mV). Thus, at −60 mV, the amplitude was 2.0 ± 0.1 pA (Fig. 5B). Similar to TASK-1, the Type 2 K+ channel and TASK-3 opened in short bursts with mean open times of 1.7 ± 0.1 ms and 1.8 ± 0.1 ms (n = 4) at −60 mV, respectively (Fig. 5C). The single-channel conductances of the Type 2 K+ channel were 33.0 ± 2.4 pS at −60 mV and 16.3 ± 1.2 pS at +60 mV (n = 3; see Table 1). The single-channel conductances of TASK-3 were 36.0 ± 2.4 pS at −60 mV and 17.1 ± 1.4 pS at +60 mV (n = 3). These values for TASK-3 are slightly higher than those reported in earlier studies, and are due to the low extracellular divalent concentration (1 mm) used in this study (Kim et al. 2000; Rajan et al. 2000). Thus, both channels have nearly identical current-voltage relationships, showing a mild inward rectification in a symmetrical 140 mm KCl solution (Fig. 5D). The current-voltage relationship for TASK-1 is also shown for comparison. In inside-out patches, changing the bath [KCl] from 140 mm to 35 mm and 15 mm, shifted the reversal potentials from zero to 32 ± 3 mV and 53 ± 5 mV (n = 3), respectively. These values are close to the expected potentials calculated from the Nernst relationship (36 mV and 58 mV) for a K+-selective channel.
Figure 5.
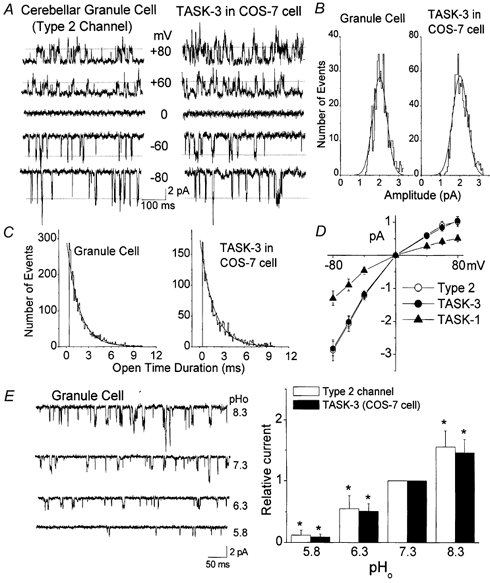
Electrophysiological properties of the Type 2 K+ channel in cerebellar granule neurons
A, openings of the Type 2 K+ channel in neurons and TASK-3 expressed on COS-7 cells are shown at five different membrane potentials in cell-attached patches in symmetrical 140 mm KCl. B, amplitude histograms of Type 2 channel openings in a CG neuron and TASK-3 expressed in a COS-7 cell show single major peaks of 2.0 pA (-60 mV). C, duration histograms of channel openings in a granule cell and a COS-7 cell are shown. D, current amplitudes were determined at each membrane potential and used to plot the current-voltage relationships for Type 2 channel, TASK-3. Current-voltage relationship for TASK-1 is also shown for comparison (n = 3). E, outside-out patches from CG neurons were formed and the effect of pHo determined at four pHo values. Inward currents observed at −50 mV in symmetrical 140 mm KCl are shown. Same experiment was done using COS-7 cells expressing TASK-3 (traces not shown). Channel activity was determined at each pH value and plotted. Each bar represents the mean ± s.d. of five determinations. *Significant difference from the corresponding control value observed at pH 7.3 (P < 0.05).
Similar to the Type 1 K+ channel and TASK-1, the Type 2 channel did not respond significantly to 1 mm TEA, 1 mm 4-aminopyridine and 100 nm apamin when tested in inside-out patches (P > 0.05; n = 5; see Table 1). High concentrations of Ba2+ (3 mm) applied to the cytoplasmic side in inside-out patches blocked the Type 2 K+ channel activity by 62 ± 5 % when the membrane potential was held +50 mV (n = 4). Quinidine (100 μm), lidocaine (1 mm) and bupivacaine (100 μm) produced inhibition of the Type 2 channel activity by 40 ± 7 %, 66 ± 8 % and 58 ± 11 %, respectively (n = 5). As shown in Table 2, the inhibitory effects of these drugs on the Type 2 K+ channel were similar to those found on TASK-3 expressed in COS-7 cells (P > 0.05). Application of 10 μm GTPγS to the cytoplasmic side of inside-out patches produced a 32 ± 4 % decrease in channel activity, suggesting that the G-protein pathway is involved in this inhibitory response. Arachidonic acid (10 μm) produced a 69 % inhibition of the Type 2 K+ channel activity mainly by reducing the open-time duration from 1.7 ± 0.1 ms to 0.5 ± 0.1 ms (n = 4). Methanandamide (1 μm) applied to the extracellular side of the membrane in outside-out patches failed to significantly affect the Type 2 channel activity (n = 6), but caused a marked inhibition from the cytoplasmic side of the membrane (55 ± 8 % inhibition; n = 4).
A well known property of TASK-3 is its sensitivity to pHo (Kim et al. 2000; Rajan et al. 2000). Using outside-out patches containing only Type 2 K+ channels, we studied the effect of pHo on their activity. As shown in Fig. 5E, the Type 2 K+ channels were markedly inhibited by acidic conditions (pH 5.8-7.3) and augmented by alkaline conditions (pH 7.3-8.3). Both the opening frequency and single-channel current amplitude were reduced by a decrease in pHo. The amplitudes measured at −60 mV were 2.2 ± 0.2, 1.7 ± 0.1 and 1.3 ± 0.1 pA at pHo values of 8.3, 7.3 and 6.3, respectively (n = 5). An identical experiment was performed using COS-7 cells expressing TASK-3. In outside-out patches of COS-7 cells, the single-channel conductance of TASK-3 was also reduced by acidic conditions, similar to that observed with the Type 2 K+ channel. At pHo values of 8.0, 7.3 and 6.3, the single-channel current amplitudes measured at −60 mV were 2.3 ± 0.2, 1.8 ± 0.1 and 1.3 ± 0.1 pA, respectively (n = 5). The results also showed that the pHo sensitivities of the Type 2 K+ channel and TASK-3 were not significantly different (Fig. 5E). As reported previously, TASK-3 was reduced by cytoplasmic application of 10 μm GTPγS and by arachidonic acid, similar to those observed with the Type 2 K+ channel (Table 2). These results, together with recent studies showing a high level of expression of TASK-3 mRNA in the cerebellar granule layer, suggest strongly that TASK-3 DNA encodes the Type 2 K+ channel.
The Type 3 K+ channel behaves like TREK-2
TREK-2 was initially cloned from the rat cerebellum cDNA library and is a member of the 2P K+ channel family that is activated by membrane stretch, free fatty acids and acidic pH conditions (Bang et al. 2000; Lesage & Lazdunski, 2000). TREK-2 expressed in COS-7 cells shows distinct channel characteristics, including marked open channel noise, opening in bursts, high single-channel conductance, and an inward rectification that is stronger than that of TASK-1 and TASK-3 (Fig. 6A). Figure 6 shows that the kinetics of the Type 3 channel openings in CG neurons are nearly identical to those of TREK-2. The mean open times were 0.9 ± 0.1 and 1.0 ± 0.1 ms (-60 mV; n = 3) for the Type 3 and TREK-2 channels, respectively (Fig. 6B). The single-channel conductances of the Type 3 K+ channel and TREK-2 were 107 ± 7 pS and 104 ± 6 pS at −60 mV, and 67 ± 8 pS and 65 ± 5 pS at +60 mV, respectively (n = 3; Fig. 6C). In inside-out patches, changing the bath [KCl] from 140 mm to 35 mm shifted the reversal potentials from zero to 33 ± 3 mV (n = 3), as expected for a K+-selective channel.
Figure 6.
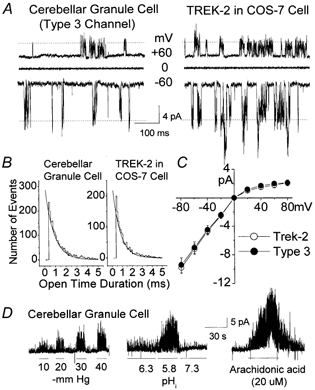
Electrophysiological properties of the Type 3 K+ channel in cerebellar granule neurons
A, openings of the Type 3 K+ channel in neurons and TREK-2 expressed on COS-7 cells are shown at three different membrane potentials in cell-attached patches in symmetrical 140 mm KCl. B, duration histograms of channel openings in a granule cell and a COS-7 cell are shown. C, current amplitudes were determined at each membrane potential and used to plot the current-voltage relationships for the Type 3 channel and TREK-2. Each point is the mean ± s.d. of three determinations. D, inside-out patches were formed from CG neurons and the effects of negative pressure, low pHi and arachidonic acid were examined. Cell membrane potential was held at −30 mV in symmetrical 140 mm KCl.
More notably, the Type 3 K+ channel could be activated by application of negative pressure to the membrane patch and by lowering the pH of the intracellular solution (Fig. 6D). Application of arachidonic acid resulted in a marked, reversible activation of TREK-2. Linoleic and linolenic acids also produced similar effects in three patches from CG cells (not shown). These findings are consistent with our observation in Fig. 3 that TREK-2 activity is generally low under normal conditions and becomes active when they are under stressed conditions. Both Type 3 and TREK-2 were insensitive to 1 mm TEA, 100 μm quinidine, 1 mm lidocaine, 100 μm bupivacaine and 100 μm glybenclamide (P > 0.05; n = 4; Table 2). Ba2+ (3 mm) inhibited the Type 3 K+ channel and TREK-2 by 45 ± 12 and 52 ± 11 %, respectively (n = 4). Methanandamide (1 μm) applied to the extracellular side of the membrane in outside-out patches failed to activate or significantly affect the Type 3 channel activity (n = 4). Recent studies show that TREK-2 mRNA is also highly expressed in the cerebellar granule layer (Talley et al. 2001). Taken together, these results show that the Type 3 K+ channel is most likely to be the functional correlate of TREK-2.
Characteristics of the Type 4 K+ channel
First we show the electrophysiological properties of the Type 4 K+ channel in Fig. 7. In symmetrical 140 mm KCl, channel openings in a cell-attached patch are shown at different membrane potentials (Fig. 7A). Inward currents showed marked flickery openings within each burst, whereas outward currents showed only a few closings within each burst. Removal of Mg2+ from the extracellular solution had no effect on channel opening characteristics, suggesting that a gating mechanism intrinsic to the channel protein is responsible for the flickering openings. Channel openings could be fitted with a single exponential function. The mean open time was 2.4 ± 0.2 ms at −50 mV and 18.4 ± 1.8 ms at +50 mV (n = 3; Fig. 7B). The current- voltage relationship was nearly linear, with a single-channel conductance of 32 ± 2 pS at −60 mV (n = 3; Fig. 7D). Open probability was dependent on membrane potential such that it decreased progressively as the membrane potential became more negative to the reversal potential (Fig. 7E). In inside-out patches, changing the bath [KCl] from 140 mm to 70 mm, 35 mm and 15 mm shifted the reversal potentials from zero to 16 ± 2 mV, 33 ± 3 mV and 55 ± 4 mV (n = 3), respectively. These values are close to the expected potentials calculated from the Nernst relationship (18 mV, 36 mV and 58 mV) for a K+-selective channel.
Figure 7.
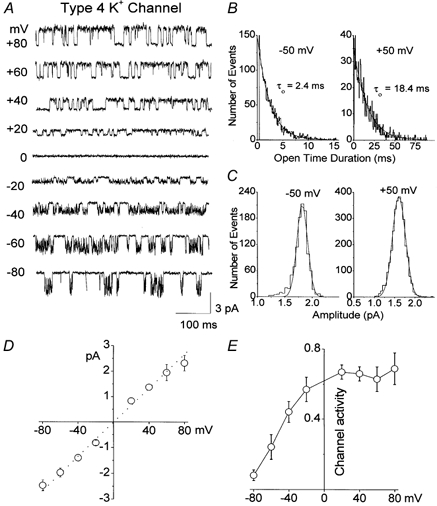
Electrophysiological properties of the Type 4 K+ channel in cerebellar granule neurons
A, channel openings in a cell-attached patch at different membrane potentials are shown. Pipette and bath solution contained 140 mm KCl. B, open-time histograms are obtained from channel openings at −50 mV and +50 mV. C, amplitude histograms are obtained from channel openings at −50 mV (1.7 pA) and +50 mV (1.8 pA). D, current amplitudes were determined at each membrane potential and used to plot the current-voltage relationship. Each point is the mean ± s.d. of three values. E, channel activity is plotted as a function of membrane potential. Each point is the mean ± s.d. of four values.
The Type 4 K+ channel showed a pharmacological profile that was different from those of TASK and TREK-2 channels (Table 2). In inside-out patches, Ba2+ (1 mm) produced a nearly complete inhibition of the Type 4 K+ channel current when applied to the bath solution. Intracellular TEA (1 mm), quinidine (100 μm) and bupivacaine (100 μm) produced 90 ± 10 %, 86 ± 12 % and 52 ± 6 % inhibition of the Type 4 channel activity, respectively (n = 4 each). Interestingly, TEA (1 mm) applied to the extracellular side in outside-out patches had no significant effect on the Type 4 K+ channel activity (P > 0.05; n = 5), consistent with the finding that the whole-cell current is not affected by 1 mm TEA. Thus, unlike the 2P K+ channels described above, the Type 4 channel was highly sensitive to both Ba2+ and TEA, similar to classical inward rectifier (IRK) K+ channels. Other K+ channel blockers such as 4-aminopyridine (1 mm), apamin (100 nm), charybdotoxin (100 nm) and terpiapin (10 nm) failed to affect the Type 4 channel activity (P > 0.05; n = 5 each). Increasing intracellular [Ca2+]i from ≈10 nm to 10 μm and [Na+]i from 0 to 20 mm showed no significant effect (P > 0.05; n = 4). In four patches, application of 100 μm GTPγS to the cytoplasmic side of inside-out patches had no significant effect on the Type 4 channel activity, in contrast to the Type 2 K+ channel, whose activity was reduced by 32 ± 4 %. Interestingly, the Type 4 K+ channel activity was increased 4.9 ± 1.7-fold (n = 5) by intracellular application of 20 μm arachidonic acid, in contrast to the marked inhibition observed with TASK-1 and TASK-3 (Kim et al. 2000). Methanandamide (1 μm) applied to the extracellular side of the membrane in outside-out patches failed to significantly affect the Type 4 channel activity (n = 5). Application of negative (-60 mmHg) or positive pressure (+40 mmHg) to the pipette produced no significant effect on the Type 4 channel activity. On searching the literature, we have not yet found a cloned K+ channel with electrophysiological and pharmacological behaviour similar to that of the Type 4 K+ channel.
The Type 4 K+ channel is also a pHo-sensitive channel
Since IK,SO shows high sensitivity to pHo and the Type 4 K+ channels provide a significant part of IK,SO, we hypothesized that the Type 4 K+ channel is also pHo sensitive. To test this directly, we obtained outside-out patches containing only Type 4 K+ channels and changed the pH of the bath solution in the range 5.8 to 8.3. Figure 8A shows channel openings from an outside-out patch containing several Type 4 K+ channels. The results show that changes in extracellular pH produce large effects on the Type 4 K+ channel activity. The amplitude histograms of the first open level show that the current amplitude is increased at high pHo and reduced at low pHo compared with that at pH 7.3. At pHo values of 5.8, 6.3, 7.3 and 8.3, the channel current amplitudes measured at −50 mV were 1.1 ± 0.1, 1.2 ± 0.2, 1.6 ± 0.2 and 2.1 ± 0.2 pA, respectively (n = 4). Thus, both the frequency of openings and the channel current amplitude (thus the conductance) were affected by pHo. A plot of the relative channel currents at different pHo values shows that the Type 4 K+ channel behaves like TASK-1 and TASK-3 with respect to its response to pHo. We also examined the effect of changes in intracellular pH on the Type 4 K+ channel activity using inside-out patches while maintaining pHo constant at 7.3. Changing pHi from 7.3 to 6.3 and 8.3 produced only a ≈7 % decrease (92.6 ± 0.12 % of control) and a ≈14 % increase (113.6 ± 0.12 % of control) in channel current, respectively. Thus, the Type 4 K+ channel shows a much greater sensitivity to pHo than to pHi. These results indicate that the Type 4 K+ channel belongs to the family of pHo-sensing K+ channels, and further help to explain the basis for the high pHo sensitivity of IK,SO.
Figure 8.
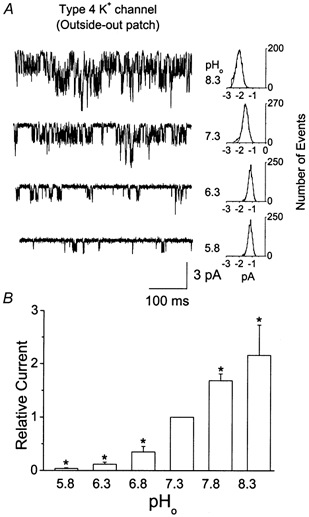
Effect of pH on the Type 4 K+ channel current in cerebellar granule neurons
A, openings of the Type 4 K+ channels in an outside-out patch are shown at different pHo values. Amplitude histograms of the first open level are shown on the right. B, a bar graph shows the relative channel currents at different pHo values. Each bar is the mean ± s.d. of four determinations. *Significant difference from the control value observed at pH 7.3 (P < 0.05)
Are four types of K+ channels present in cerebellar granule neurons from older rat brain?
So far, we have focused on identifying background K+ channels in CG neurons cultured from P6-P8 rats. It is of interest to know whether such channels are also functionally expressed in CG neurons from older rat brain. To address this issue, we freshly isolated CG neurons by protease digestion from 4-week-old rats. Of 112 patches tested, we found 26 patches with Type 1 channel (TASK-1), 20 patches with Type 2 channel (TASK-3), 22 patches with Type 3 channel (TREK-2), eight patches with Type 4 channel and 36 patches with no channel activity. The assignment of channel types was based on current-voltage relationships, and channel opening kinetics (open-time duration and burst activity). All four channel types were found to be K+ selective, as judged by the 32–36 mV shift in reversal potential when [KCl] in the bath solution was changed from 140 mm to 35 mm in the inside-out condition. We were unable to test the pHo sensitivity due to difficulties in obtaining outside-out patches with freshly isolated cells. In 12 cell-attached patches of CG neurons dissociated from a 6-month-old rat, we could clearly identify three patches with Type 1 channel, two patches with Type 2 channel, one patch with Type 3 channel and two patches with Type 4 channel. Recent studies using in situ hybridization have shown that TASK-1, TASK-3 and TREK-2 are expressed in the CG neurons in both young and adult rats (Brickley et al. 2001; Talley et al. 2001). Taken together, these findings strongly suggest that all four types of K+ channels are functionally expressed in adult rat CG neurons. The relative contribution of each channel type to IK,SO in adult CG neurons was not determined in this study.
DISCUSSION
The goal of this study was to characterize the biophysical and pharmacological properties of background K+ channels that give rise to IK,SO in CG neurons and thereby determine whether they might be functional correlates of the recently cloned tandem-pore K+ channels. The similar behaviours of IK,SO and TASK-1 K+ current (Millar et al. 2000) have led to the initial assumption that TASK-1 is the functional correlate of IK,SO in CG neurons. Since the cloning of TASK-1, however, two other members of the 2P K+ channel family (TASK-3 and TREK-2) have been identified, and their mRNA found to be highly expressed in the cerebellar granule layer (Karschin et al. 2002; Talley et al. 2001). Like TASK-1, TASK-3 is active at rest and shows a high sensitivity to extracellular pH. Therefore, one cannot easily distinguish between TASK-1 and TASK-3 simply on the basis of pH sensitivities. TREK-2 is not very sensitive to changes in extracellular pH in the 6.8-8.3 range, but is activated by lowering intracellular pH (Bang et al. 2000; Kim et al. 2001b). Messenger RNA transcripts of TWIK-1 and THIK-2, two putative 2P K+ channels, are also expressed in the cerebellar granular layer (Lesage et al. 1996; Brickley et al. 2001; Rajan et al. 2001). Therefore, the presence of several 2P K+ channels in the granular layer of the cerebellum suggested that the IK,SO could be composed of several 2P K+ channels.
Four types of background K+ channels in CG neurons
We found four types of K+ channels that could serve as background K+ channels in CG neurons. We have analysed the four types of K+ channel with respect to their biophysical and pharmacological properties, to help assign them to their cloned counterparts. Based on single-channel kinetics, conductances, pH sensitivities and responses to various pharmacological agents, we suggest that Type 1, 2 and 3 K+ channels are most likely to represent TASK-1, TASK-3 and TREK-2, respectively. Our results show that native Type 1 and 2 K+ channels are likely to be functional homomers of TASK-1 and TASK-3, respectively. However, we cannot completely rule out the possibility that a TASK-1-TASK-3 heteromer may form in these cells, as such a heteromer has recently been reported to form a functional channel in oocytes and HEK 293 cells (Czirjak & Enyedi, 2002; Talley & Bayliss, 2002). Of the several spliced variants of TREK-2 discovered recently, the isoform present in CG neurons is probably TREK-2c, which is abundantly expressed in the cerebellar granular layer (Gu et al. 2002). We are not yet able to assign the Type 4 K+ channel to a cloned counterpart. Type 1–3 K+ channels found in CG neurons are unlikely to belong to the inwardly rectifying Kir1-3 subfamilies, as nearly all members of the Kir1-3 families are highly sensitive to block by Ba2+ (Isomoto et al. 1997). The four types of K+ channels in CG neurons are not markedly activated or inhibited by intracellular ATP, suggesting that they are not members of the ATP-sensitive K+ channel family (Kir6.x).
We also observed the presence of two additional K+ channels: the Ca2+-activated K+ channel (IK,Ca) and the G-protein-activated K+ channel, usually referred to as IK,ACh. These two types of K+ channels were generally not observed in cell-attached patches unless they were activated by ligands. IK,Ca could be activated by raising [Ca2+]i in inside-out patches, and IK,ACh could be activated by γ-aminobutyric acid and baclofen, as shown previously (Navarro et al. 1996; Nicoll et al. 1990; Slesinger et al. 1997). Both IK,Ca and IK,ACh could be easily distinguished from the four types of resting K+ channels that we describe above, based on well known single-channel kinetics, conductance and rectification. As both IK,Ca and IK,ACh are not active at rest, they are unlikely to contribute to IK,SO.
We were unable to study CG neurons for longer culture periods as the neurons died after day 10. It has been described previously that CG neuron survival and differentiation is greatly prolonged when [K+] in the growth medium is maintained at > 20 mm (Gallo et al. 1987). The longer neuronal survival is believed to be partly due to the adequate supply of Ca2+ to the cells produced by K+-induced depolarization. In this study, CG neurons were grown in medium containing 3 mm KCl, and therefore may have differentiated more slowly compared with cells grown in 25 mm KCl. Nevertheless, we observed a marked increase in whole-cell current in cells grown in 3 mm KCl, a phenomenon also observed in cells grown in high KCl. In this study, we have not examined the density of the four types of K+ channels in CG neurons grown in high K+.
Does the Type 4 K+ channel belong to the 2P K+ channel family?
The finding that the Type 4 K+ channel is also pHo sensitive suggested that this K+ channel may also belong to the subfamily of TASK K+ channels. However, the pharmacological profile suggests that the Type 4 K+ channel probably belongs to a different K+ channel subfamily. This is based on the results that the Type 4 K+ channel is highly sensitive to Ba2+ and TEA, whereas all 2P K+ channels show low sensitivity to these agents. We considered the possibility that one of the several recently cloned 2P K+ channels could encode the Type 4 K+ channel. However, close examination of the electrophysiological and pharmacological properties indicated that none of the functional 2P channels cloned so far show characteristics similar to those of Type 4 K+ channel. For example, TASK-2 and TASK-4 are not sensitive to arachidonic acid and are not inhibited by 1 mm TEA (Reyes et al. 1998; Decher et al. 2001; Girard et al. 2001). TASK-4 is weakly inhibited by acidic pH conditions (Decher et al. 2001). TASK-5 shares 50 % amino acid identity with TASK-3, but fails to form a functional current (Ashmole et al. 2001; Kim & Gnatenco, 2001; Vega-Saenz de Miera et al. 2001). THIK-2 mRNA is highly expressed in the cerebellar granule layer of rat brain, but also does not form a functional K+ channel (Rajan et al. 2001). Whether TWIK-1 forms a functional K+ channel is controversial and this may be due to the extremely low single-channel conductance of TWIK-1. It is clear that TWIK-1 does not form the Type 4 K+ channel whose single-channel openings are clearly visible with a conductance of 32 pS. It has been reported that free fatty acids increase background K+ current in CG neurons (Lauritzen et al. 2000), as well as in other types of neurons (Kim et al. 1995; Colbert & Pan, 1999). The increase in K+ current in the CG neurons by arachidonic acid is probably due to an increase in the open probabilities of TREK-2 and the Type 4 K+ channel, as they are both activated by free fatty acids. The single-channel characteristics of TREK-1 and TRAAK are well defined (Kim et al. 2001a; Fink et al. 1996, 1998; Maingret et al. 1999), and we have not seen either channel in CG neurons, even after application of negative pressure or arachidonic acid. Therefore, we speculate that the Type 4 K+ channel is not one of the cloned 2P K+ channels. On searching the ion channel literature, we have not yet found a study that describes a Type 4-like K+ channel with properties that have we described here. We speculate that the Type 4 K+ channels could belong to an as yet unidentified subfamily of K+ channels. Further studies are clearly needed to address this question.
Is the Type 4 K+ channel sensitive to muscarinic agonists?
In our cultured CG neurons, application of ACh reduced IK,SO by ≈50 % in cells grown for 7–10 days, but by less than 10 % in cells grown for 1–3 days. These results suggest that ACh does not inhibit the Type 4 K+ current, since IK,SO in cells grown for 1–3 days arises mostly from the Type 4 K+ channel activity. However, it is possible that M3 receptors are not expressed or the coupling between the receptor and G-protein is weak in cells grown for 1–3 days. The partial inhibition of IK,SO observed in older cells clearly involves reduction of TASK-3 and TASK-1 currents that are sensitive to G-protein-dependent inhibition (Kim et al. 2000; Millar et al. 2000; Talley et al. 2000). Because the molecular identity of the Type 4 K+ channel is not known, we cannot provide direct evidence showing a lack of inhibition of the Type 4 K+ current by ACh. Nevertheless, our result that Type 4 K+ channels in inside-out patches are not affected by GTPγS is also supportive of our view that it is insensitive to muscarinic receptor agonists.
The mechanism of inhibition of TASK K+ channels by ACh is not clear at present. It has been reported that the activity of phospholipase C (PLC) is important, as the inhibitor of this enzyme (U-73122) partially reduces the inhibition of TASK-1, but intracellular Ca2+ mobilization does not appear to be critical (Boyd et al. 2000; Czirjak et al. 2001). A recent view is that PLC-induced breakdown of polyphosphoinositides may be responsible for the inhibition of TASK-1 (Czirjak et al. 2001). In our cultured granular neurons, application of ATP, which would elevate the level of phosphoinositides in the cell membrane (Berberian et al. 1998), did not increase the Type 4 K+ channel activity. Unlike TASK channels, the activity of the Type 4 K+ channels did not decrease with time (rundown) in inside-out patches, as expected of a channel insensitive to the concentrations of phosphoinositides in the membrane. Direct application of PLC also failed to inhibit the Type 4 K+ channel activity. These observations also suggest that the Type 4 K+ channel is not inhibited by ACh, and is consistent with our result that ACh inhibits only about 50 % of the whole-cell current (IK,SO) in cultured CG neurons. If the Type 4 K+ channel were inhibited by ACh via G-protein, one would observe a larger inhibition of IK,SO. The precise degree of inhibition of IK,SO by muscarinic agonists may therefore depend on the magnitude of each type of K+ channel current that exists in a particular individual neuron.
Physiological significance
Our study clearly shows that IK,SO is the sum of four different K+ channel currents, each with distinct properties in cultured CG neurons and probably also in adult CG neurons. These K+ channels would be involved in setting the resting membrane potential, an important function in excitable cells. TASK-1 and TASK-3 are targets of muscarinic receptor agonists that increase excitability by reducing the resting K+ conductance via G-protein signalling pathways. TREK-2 has been reported to be modulated by Gs-, Gi- and Gq-coupled receptors with positive and negative effects (Lesage et al. 2000). TREK-2 may become particularly important under pathophysiological situation as it is easily activated by conditions that cause cell swelling, decrease in intracellular pH and production of free fatty acids. Most interestingly, three of the four K+ channels are highly sensitive to extracellular pH, suggesting that protons may be an important modulator of CG neuron function. A small reduction in pHo may cause sufficient depolarization of cells to fire and increase excitatory input to Purkinje cells. Changes in pHo during ischaemia and hypoxia in the brain are well established, but whether such changes occur under physiological conditions is not well known. Further studies will be necessary to understand the role of each K+ channel in the synaptic transmission of CG neurons and within the cerebellum.
Acknowledgments
This work was supported by a grant-in-aid from the American Heart Association and National Institute of Health (D.K.) and by the postdoctoral fellowship program of Korea Science and Engineering Foundation (J.H.). We thank Stephen D. Kim for assistance with electrophysiological data analysis.
REFERENCES
- Ashmole I, Goodwin PA, Stanfield PR. TASK-5, a novel member of the tandem pore K+ channel family. Pflügers Archiv. 2001;442:828–833. doi: 10.1007/s004240100620. [DOI] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. Journal of Biological Chemistry. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Berberian G, Hidalgo C, Dipolo R, Beauge L. ATP stimulation of Na+/Ca2+ exchange in cardiac sarcolemmal vesicles. American Journal of Physiology. 1998;274:C724–733. doi: 10.1152/ajpcell.1998.274.3.C724. [DOI] [PubMed] [Google Scholar]
- Boyd DF, Millar JA, Watkins CS, Mathie A. The role of Ca2+ stores in the muscarinic inhibition of the K+ current IK(SO) in neonatal rat cerebellar granule cells) Journal of Physiology. 2000;529:321–331. doi: 10.1111/j.1469-7793.2000.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K+ channels in hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 1999;19:8163–8171. doi: 10.1523/JNEUROSCI.19-19-08163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two pore domain potassium channel subunits. Journal of Biological Chemistry. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Petheo GL, Spat A, Enyedi P. Inhibition of TASK-1 potassium channel by phospholipase C. American Journal of Physiology. 2001;281:C700–708. doi: 10.1152/ajpcell.2001.281.2.C700. [DOI] [PubMed] [Google Scholar]
- Decher N, Maier M, Dittrich W, Gassenhuber J, Bruggemann A, Busch AE, Steinmeyer K. Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Letters. 2001;492:84–89. doi: 10.1016/s0014-5793(01)02222-0. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO Journal. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO Journal. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Kingsbury A, Balazs R, Jorgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. Journal of Neuroscience. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C, Duprat F, Terrenoire C, Tinel N, Fosset M, Romey G, Lazdunski M, Lesage F. Genomic and functional characteristics of novel human pancreatic 2P domain K+ channels) Biochemical and Biophysical Research Communications. 2001;282:249–256. doi: 10.1006/bbrc.2001.4562. [DOI] [PubMed] [Google Scholar]
- Goldstein SAN, Bockenhauer D, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nature. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gu W, Schlichthörl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. Journal of Physiology. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and functions. Japanese Journal of Physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik K-H, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit TASK-5, associated with the central auditory nervous system. Molecular and Cellular Neuroscience. 2002;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Gnatenco C, Kim D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflügers Archiv. 2001a;442:64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang HW, Kim D. TBAK-1 and TASK-1, two pore K+ channel subunits: kinetic properties and expression in rat heart. American Journal of Physiology. 1999;277:H1669–1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. Task-3, a new member of the tandem pore K+ channel family. Journal of Biological Chemistry. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kim D, Gnatenco C. TASK-5, a new member of the tandem-pore K+ channel family. Biochemical and Biophysical Research Communications. 2001;284:923–930. doi: 10.1006/bbrc.2001.5064. [DOI] [PubMed] [Google Scholar]
- Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflügers Archiv. 2001b;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- Kim D, Sladek CD, Aquado-Velasco C, Mathiasen JR. Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells. Journal of Physiology. 1995;484:643–660. doi: 10.1113/jphysiol.1995.sp020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO Journal. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO Journal. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. American Journal of Physiology. 2000;279:793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK-2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi and Gq protein-coupled receptors. Journal of Biological Chemistry. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Zilberberg N, Goldstein SA. Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. Journal of Biological Chemistry. 2001;276:24449–24452. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. Journal of Biological Chemistry. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Barrat L, Southan AP, Page KM, Fyffe REW, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proceedings of the National Academy of Sciences of the USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kennedy ME, Velimirovic B, Bhat D, Peterson AS, Clapham DE. Nonselective and G βγ-insensitive weaver K+ channels. Science. 1996;272:1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiological Reviews. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- North RA. Potassium-channel closure taken to TASK. Trends in Neurosciences. 2000;23:234–235. doi: 10.1016/s0166-2236(00)01592-7. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. Journal of Biological Chemistry. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Muller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histidine as pH sensor. Journal of Biological Chemistry. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. Journal of Biological Chemistry. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- Ross CA, Bredt D, Snyder SH. Messenger molecules in the cerebellum. Trends in Neurosciences. 1990;13:216–222. doi: 10.1016/0166-2236(90)90163-5. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective γ-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proceedings of the National Academy of Sciences of the USA. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. Journal of Biological Chemistry. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. Journal of Neuroscience. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-SaenZ de Miera E, Lau DH, Zhadina M, Pountney D, Coetzee WA, Rudy B. KT3. 2 and KT3.3, two novel human two-pore K+ channels closely related to TASK-1. Journal of Neurophysiology. 2001;86:130–142. doi: 10.1152/jn.2001.86.1.130. [DOI] [PubMed] [Google Scholar]
- Watkins CS, Mathie A. A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. Journal of Physiology. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]


