Abstract
Fast P2X receptor-mediated excitatory postsynaptic current (EPSC) was identified in pyramidal neurones of layer II/III of somato-sensory cortex in acutely isolated slices obtained from the brain of 17- to 22-day-old rats. The EPSCs were elicited by electrical stimulation of vertical axons originating from layer IV-VI neurones at 0.1 Hz in the presence of bicuculline. When the glutamatergic EPSC was blocked by saturating concentrations of glutamate receptor inhibitors 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]-quinoxaline-7-sulphonamide (NBQX) and D-(-)-2-amino-5-phosphonopentanoic acid (D-AP5), a small EPSC component was recorded from 90 % of neurones tested. This residual EPSC was not affected by selective blockers of nicotinic (hexamethonium) or serotonin (N-(1-azabicyclo-[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide hydrochloride, Y-25130) receptors, but it was reversibly inhibited by the antagonists of P2X receptors NF023 (8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic acid), NF279 (8,8′-[carbonylbis (imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic acid) and PPADS (pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid). Application of ATP (10 μm) or α,β-methylene ATP (10 μm) to pyramidal neurones, acutely isolated from cortical slices, evoked inward currents (30 to 200 pA) in 65 % of cells tested. The relative calcium/caesium permeability (PCa/PCs) of P2X receptors was 12.3 as estimated from the reversal potential of ATP-induced current measured at different extracellular calcium concentrations. We concluded that P2X purinoreceptors are activated during synaptic transmission in neocortex.
Adenosine triphosphate (ATP) is an important neurotransmitter in the nervous system (Ralevic & Burnstock, 1998). The action of extracellular ATP is mediated by two classes of purinoreceptors, represented by distinct families of ionotropic (P2X) and metabotopic (P2Y) receptors (Surprenant et al. 1995; Buell et al. 1996; Collo et al. 1996; Vulchanova et al. 1996). Members of both families are widely expressed throughout the brain and are believed to be involved in mediating transmission between neurones and glial cells (Zimmermann, 1994; Haydon, 2001). Hitherto, P2X receptor-mediated fast excitatory synaptic currents or excitatory potentials have been detected in the medial habenula (Edwards et al. 1992) and in the hippocampus (Pankratov et al. 1998, Mori et al. 2001) as well as in the spinal cord (Bardoni et al. 1997). Yet, purinoreceptors are present in many other areas of the CNS and, in particular, they have been localised in neurones of cerebral cortex (e.g. Kidd et al. 1995; Moore et al. 2000). Moreover, extracellular application of ATP triggered elevation in cytoplasmic Ca2+ concentration in pyramidal neurones of sensorimotor cortex in acute brain slices (Lalo & Kostyuk, 1998). Therefore, functional purinoreceptors are present in neocortical neurones, implying their possible involvement in synaptic transmission.
To elucidate the role of P2X receptors in the synaptic transmission in neocortex we performed a pharmacological dissection of excitatory postsynaptic currents (EPSCs) recorded from pyramidal neurones residing in layer II/III of somato-sensory cortex. Our data demonstrate that ATP-activated P2X receptors mediate a distinct EPSC component in cortical neurones.
METHODS
Slice preparation
All animal procedures were performed according to the principles of the Animals (Scientific Procedures) Act 1986. Whole-cell voltage-clamp recordings were made from pyramidal neurones of somato-sensory cortex in coronal 350 μm thick slices from 17- to 22-day-old Sprague-Dawley rats. Slices were prepared using the technique described previously (Lalo & Kostyuk, 1998; Feldman, 2000). The animals were anaesthetised by halothane inhalation and then decapitated. Brains were dissected out rapidly and placed in physiological saline containing (mm): 135 NaCl, 3 KCl, 1 MgCl2, 2.4 CaCl2, 26 NaHCO3, 1 NaH2PO4, 14 glucose, pH 7.4 gassed with 95 % O2/5 %CO2. Slices were cut at 4 °C and kept at room temperature for 1–4 h before the recordings.
Acute isolation of neurones
To investigate the response to ATP in the cortical pyramidal neurones the cells were acutely isolated using the modified ‘vibrating ball’ technique (Vorobjev, 1991). Layer II/III neurones were dissociated with the aid of a vibrating glass ball (200 μm diameter) moving slowly over the slice surface. The vibration frequency was 100 Hz, vibration amplitude 20–30 μm, the distance of glass ball from the slice surface was adjusted in the range of 10–50 μm to provide the largest outcome of healthy cells. In contrast to the commonly used method of titrating through the glass pipette, the technique employed preserves the cell dendrites.
Fast drug application
A modified ‘square-pulse’ concentration jump method (Lalo et al. 2001) was used for a rapid 200 ms long application of agonist-containing solutions. The tip of the recording pipette, attached to a neurone, was inserted into a glass tube (i.d. 1 mm) through a tiny opening (i.d. 0.6 mm). The lower end of tube was submerged into the external solution in the chamber. The composition of external solution was as follows (mm): 150 NaCl; 5 KCl; 2 CaCl2; 1 MgCl2; 10 Hepes, pH adjusted with NaOH to 7.3. The upper end of the tube was connected via the computer-controlled valves to the sources of negative (-20 mmHg) and positive (+30 mmHg) pressure with the help of the V-shaped plastic tube. Thus, the suction of drug-containing solution filling the chamber or backward washout by clear extracellular solution could be carried out. This technique provides a fast rate of solution exchange and allows the immediate washout of agonist, which is important in view of the rapid desensitisation of P2X receptors (Lalo et al. 2001).
Electrophysiology
Neurones with pyramidal shaped somata were selected using infrared DIC optics, and recordings were made with patch pipettes (3.5-4 MΩ) filled with intracellular solution (mm): 110 CsCl, 10 NaCl, 10 Hepes, 2 MgATP, 0.2 EGTA, pH 7.35. To determine the relative chloride permeability the intracellular CsCl was equimolarly substituted for caesium gluconate in some experiments. The membrane potential was clamped at −80 mV unless stated otherwise. The liquid junction potential was measured with the aid of an EPC-9 patch-clamp amplifier and PULSE software. All voltage dependencies reported were corrected for junction potential values which were correspondingly 3.4 ± 0.1 mV (n = 7) for the CsCl internal solution and 9.8 ± 0.2 mV (n = 7) for Cs gluconate internal solution. All the recordings were made in the presence of 20 μm of bicuculline in the slice superfusing solution at room temperature (22-24 °C).
To measure EPSCs, vertical axons originating from layer IV-VI neurones were stimulated at 0.1 Hz with a bipolar coaxial electrode (Harvard, Edenbridge, UK). The electrode was placed in layer IV approximately opposite the site of recordings, stimulus duration was 100 μs, stimulus amplitude was adjusted to produce a response of about two-thirds of maximal amplitude (typically 8–12 V).
Recordings commenced 15 min after whole-cell access was gained to ensure equilibration between the pipette solution and the cytosol. The series and the input resistances were 4–12 and 300–900 MΩ, respectively, and varied by less than 20 % in the cells accepted for analysis. Currents were monitored using EPC-9 (HEKA, Lambrecht, Germany) filtered at 3 kHz and stored on the disk for off-line analysis. Experiments were controlled by PULSE/PULSEFIT software (HEKA, Lambrecht, Germany) and data were analysed by self-designed software installed on an NT workstation.
Data analysis
All data are presented as mean ± s.d. The EPSC amplitude was determined as the difference between the mean current within a 2 ms time window at the peak of EPSC and the mean current measured before stimulus artefact. The coefficient of variation of EPSC amplitude was calculated as:
where I is the mean EPSC amplitude, σ is the standard deviation (s.d.) of EPSC amplitude and σN is the s.d. of noise amplitude. The value of m = 1/c.v.2 gives the estimate for the mean number of quanta (elementary events) contributing to the EPSC. This parameter is widely used for the assessment of the mechanism of changes in the synaptic transmission efficiency and EPSC amplitude (Kullmann & Nicoll, 1992). The large changes in these values are indicative of the presynaptic changes in the synaptic transmission (i.e. changes in the release of neurotransmitter), whereas the lack of such changes points to the postsynaptic changes (number of postsynaptic receptors activated or changes in their conductance).
The relative calcium permeability of ATP receptors was calculated from the reversal potential of ATP-mediated current in the context of the extended Goldman-Hodgkin-Katz theory (Lewis, 1979). In order to estimate the current-voltage relationship, current amplitudes in each cell were normalised to an amplitude of −80 mV (I/I-80 mV) and averaged over all cells. The current-voltage relation was fitted with a cubic polynomial curve and reversal potential was estimated as the potential at which the fitted curve crossed the zero current line. The permeability ratios for Ca2+ to Cs+ (PCa/PCs) and for Na+ to Cs+ (PNa/PCs) were calculated from values of reversal potential (Erev) at two different extracellular calcium concentrations using the following equation (Edwards et al. 1997):
where
and
It was assumed that the permeabilities of the P2X receptor channel to Na+ and K+ are equal and intracellular calcium concentration is negligible compared to extracellular. The relatively low (2 to 5 mm) concentrations of extracellular calcium were used to avoid the inaccuracy that may result from influence of surface charge (Lewis, 1979) and from inactivation of P2X receptors at high extracellular calcium (Evans et al. 1996) as well.
Drugs
The following compounds were used: 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]-quinoxaline-7-sulphonamide (NBQX); d-(-)-2-amino-5-phosphonopentanoic acid (D-AP5); 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic acid (NF023); 8,8′-[carbonylbis (imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonyl- imino)]bis-1,3,5-naphthalene-trisulphonic acid (NF279); pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS); N-(1-azabicyclo-[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide hydrochloride (Y-25130); and bicuculline, all from Tocris, Bristol, UK. All other chemicals were from the Sigma Chemical Co., Poole, UK.
RESULTS
ESPC components
Stimulation in layer IV of somato-sensory cortex evoked single short-latency EPSCs in layer II/III pyramidal neurones (Fig. 1). In some cells tested the short-latency current was followed by a slow, long-latency current, which could be eliminated by slight lowering of stimulus amplitude. So in each cell tested the stimulus strength was adjusted to produce the single short-latency response. Such responses in the layer II/III pyramidal neurones result from vertical projections sent by neurones of layers IV-VI (Feldman, 2000), which represent the largest excitatory input received by layer II/III from layer IV (Dantzker & Callaway, 2000).
Figure 1.
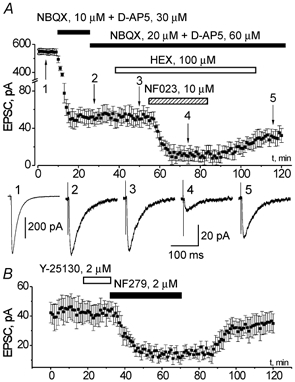
EPSCs elicited in the pyramidal neurone of somato-sensory cortex layer II by field stimulation in the layer IV in the presence of bicuculline, 20 μm
A, top, changes in the amplitude of EPSC following bath application of glutamatergic antagonists NBQX and D-AP5, cholinergic antagonist HEX and P2X receptor antagonist NF023 as indicated on the graph. Each point represents mean ± s.d. for six sequential trials, holding potential −80 mV, stimulation frequency 0.1 Hz. Bottom, the examples of residual EPSC (average of six traces) recorded at moments (1-5) indicated on upper graph. B, changes in the amplitude of non-glutamatergic EPSC following the bath application of serotininergic antagonist Y-25130 and P2X receptor antagonist NF279. Recordings were made at a holding potential of −80 mV in the presence of (μM): NBQX 10, D-AP5 30 and bicuculline 20, stimulation frequency 0.1 Hz. Each point represents mean ± s.d. for six sequential trials.
In 44 out of 50 neurones tested the EPSC recorded in the presence of the GABA receptor antagonist bicuculline (20 μm) was markedly inhibited after bath application of 10 μm NBQX and 30 μm D-AP5, but a small residual EPSC (Fig. 1) remained. The amplitude of residual EPSC averaged 9.7 ± 7.1 % (n = 44) of the total EPSC amplitude measured at a holding potential value of −80 mV. Doubling the concentration of glutamatergic antagonists had virtually no effect on the amplitude of residual EPSC, indicating that it was not due to incomplete inhibition of glutamate receptors, thus representing an activation of non-glutamatergic ionotropic receptors (Fig. 1). The kinetics of this residual EPSC was slower than that of the AMPA receptor-mediated component, but faster than the kinetics of the NMDA-receptor mediated component. The rise and decay time constants of residual EPSC were 3.5 ± 1.5 and 39 ± 11 ms (n = 44), whereas the corresponding parameters for AMPA and NMDA receptor-mediated currents were 1.8 ± 0.7 and 19 ± 5 ms (n = 28) and 6.8 ± 2.6 and 67 ± 16 ms (n = 16) respectively.
To elucidate the nature of this minor component of synaptic input in the cortical pyramidal neurones we tested the sensitivity of residual EPSC to the selective blockers of nicotinic (hexamethonium), 5HT3 (Y25130) and P2X (NF023, NF279, PPADS) receptors. Application of 100 μm of cholinergic antagonist hexamethonium (HEX) in the presence of NBQX, D-AP5 and bicuculline, decreased the amplitude of residual EPSC only marginally, by 4 ± 8 % (n = 6, see Fig. 1). It is noteworthy that at this concentration hexamethonium blocks the nicotinic receptors completely (Fieber & Adams, 1991). The serotoninergic antagonist Y25130, applied at 2 μm concentration, did not affect the residual EPSC in all of the five cells tested (Fig. 1B), although the concentration used exceeds the IC50 for 5HT3 receptors by more than 100-fold (Yakushiji & Akaike, 1992).
In contrast, the residual EPSC was strongly and reversibly suppressed by the purinergic antagonists in all of the 25 cells tested. The specific P2X receptor blocker NF023 (10 μm) reduced the amplitude of residual EPSC by 73 ± 22 % (n = 7, Fig. 1). Other purinergic antagonists, NF279 and PPADS, also exhibited strong blocking action on the residual EPSC. The amplitude of residual EPSC was reduced by 61 ± 18 % (n = 10) in the presence of 2 μm of NF279 (Fig. 1B). Likewise, application of 30 μm PPADS decreased the residual EPSC amplitude by 51 ± 9 % (n = 8, data not shown). Its action was only partially reversible: the EPSC amplitude recovered only to 65 ± 8 % of control after removing of PPADS from the bath solution. At the concentrations applied all the substances are selective for ATP receptors and they do not affect glutamatergic, serotoninergic or cholinergic receptors (Lambrecht et al. 1992; Motin & Bennett, 1995; Damer et al. 1998; Soto et al. 1999). The inhibitory action of all purinergic antagonists tested was not associated with noticeable changes in the coefficient of variation (CV) of the residual EPSCs. The changes in the CV were in the range of 5 ± 10 % (n = 25), implying that the decrease in the residual EPSC amplitude was related to the changes in the activity of postsynaptic P2X receptors rather than to the changes in transmitter release.
Although all recordings were made in presence of the GABAA channel blocker bicuculline, to verify the absence of residual chloride conductance contribution to EPSC we replaced the caesium chloride in the intrapipette solution with caesium gluconate. In the former case the intracellular Cl− concentration was 120 mm and the expected equilibrium potential for chloride ions was about −5 mV, while in the latter case the [Cl−]i and equilibrium potential were 10 mm and −60 mV, respectively. Neither the amplitude nor the current-voltage relationship of EPSC (Fig. 2) were altered significantly when intracellular Cl− ions were substituted for gluconate. The average amplitude of residual EPSC recorded using the caesium gluconate-based pipette solution was 47 ± 17 pA and the reversal potential was 15.5 ± 4 mV (n = 7). The corresponding values for the caesium chloride-based pipette solution was 51 ± 22 pA (n = 44) and 15 ± 5 mV (n = 7). This result demonstrates negligible chloride conductance of the residual EPSC.
Figure 2.
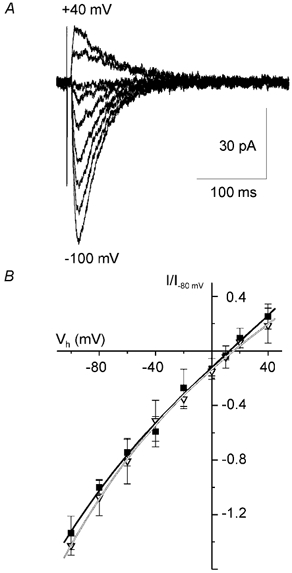
Voltage dependence of P2X-mediated EPSC component in cortical pyramidal neurones
▪, 120 mm [Cl−]i; ▿, 10 mm [Cl−]i. A, representative EPSCs recorded in the presence of (μM): NBQX 20, D-AP5 60 and bicuculline 20 at membrane potentials ranging from −100 to +40 mV. Each trace represents the average of six consecutive sweeps. The intracellular concentration of Cl− was 120 mm. B, voltage-current relationship for the purinergic EPSC measured at different intracellular concentrations of chloride ions. Amplitudes of currents were normalised to the maximal value measured at −80 mV. Each point is the mean ± s.d. for seven cells. Lines represent the cubic polynomial fit. All the measurements were in the presence of (μM): NBQX, 20; D-AP5, 60; and bicuculline, 20. Note the lack of changes in the voltage dependence of EPSC indicating negligible contribution of chloride conductance.
The presumed P2X-mediated residual EPSC showed a reversal potential which is similar to P2X-mediated currents observed in other types of neurones (e.g. Bardoni et al. 1997; Taschenberger et al. 1999). Positive reversal potential might suggest a significant Ca2+-permeability implying that the P2X-mediated postsynaptic current may result in a substantial Ca2+ influx (Edwards et al. 1997 and below).
ATP-induced currents in the dissociated neurones
Additional evidence supporting functional postsynaptic P2X receptors in cortical pyramidal neurones was provided by the experiments on single cells. Using a novel acute isolation technique we were able to test for the ATP-induced currents in neurones retaining their in situ properties, as electrophysiological experiments followed the isolation procedure almost immediately. Fast application of 10 μm ATP evoked inward currents in 67 % of pyramidal neurones (15 out of 22 tested) voltage-clamped at −80 mV (Fig. 3A). The amplitude and kinetic profile of ATP-evoked currents displayed large cell-to-cell variability. The average amplitude of currents evoked by endogenous ATP was 180 ± 90 pA (n = 15) and 70 % of the neurones responding to 10 μm ATP had an amplitude of ATP-mediated current greater than 50 pA. The current rise time varied between 5 and 15 ms, the decay time ranged from 50 to 350 ms. The decay of ATP-mediated inward current was complex and the kinetic differed substantially between cells. The response to ATP was mimicked by application of the specific agonist of P2X receptors α,β-methyleneATP (Ralevic & Burnstock, 1998) in all of the seven cells tested (Fig. 3A). So, participation of homomeric P2X2, P2X5 and P2X6 subtypes seems to be unlikely since they exhibit no sensitivity to α,β-methyleneATP (Collo et al. 1996; Ralevich & Burnstock, 1998). As shown in Fig. 3B, the ATP-mediated currents were reversibly blocked by P2X antagonists. The blockage was significant but not complete: inhibitory effects of PPADS (30 μm) and suramin (50 μm) were 48 ± 8 (n = 6) and 53 ± 14 % (n = 5), respectively. The data on blocking action of PPADS on the ATP-induced currents and residual EPSCs agree closely, the lack of reversibility of the effect on EPSCs may be related to incomplete washout of PPADS from the slice. Pharmacological properties of ATP receptor-mediated currents imply the participation of P2X1 and P2X3 receptors although the P2X4 subtype (insensitive to the all known P2X antagonists but sensitive to α,β-methyleneATP) also may contribute to the ATP-mediated current in the neocortical neurones.
Figure 3.
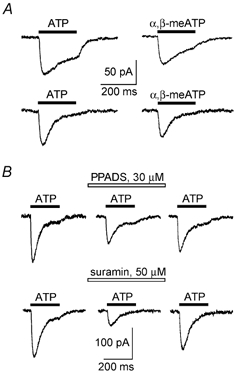
P2X receptor-mediated currents in acutely isolated neocortical neurones
A, the examples of inward currents induced in the pyramidal neurones of somato-sensory cortex layer II/III by the application of ATP (10 μm) and α,β-methyleneATP (10 μm). Pairs of traces were recorded from two different cells. B, action of purinergic antagonists on currents induced by application of ATP (10 μm) to the pyramidal neurones of somato-sensory cortex. Drugs were applied 2 min before application of ATP. Recordings were made with a 5 min time interval at a holding potential of −80 mV.
Calcium permeability of P2X receptors
The voltage dependence of ATP-induced currents (Fig. 4) was similar to the I-V curve for residual (purinergic) EPSC. The reversal potential was 12.5 ± 0.7 mV at an extracellular calcium concentration of 2 mm and it was shifted to 17.1 ± 1.1 mV when [Ca2+]o was elevated to 5 mm. According to the extended constant field theory the evaluated relative permeabilities of ATP receptors in the neocortical neurones were: PCa/PCs = 12.3, PNa/PCs = 0.9. Such very high calcium permeability agrees well with values reported earlier for native P2X receptors in the medial habenula (Edwards et al. 1997) and is close to the data for recombinant P2X1 and P2X4 receptors (Evans et al. 1996; Soto et al. 1996).
Figure 4.
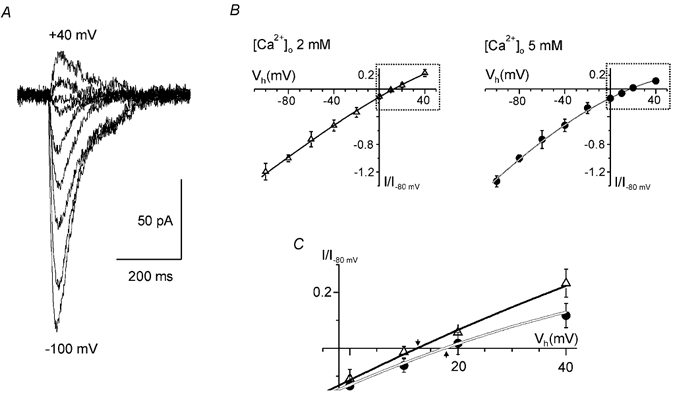
Voltage dependence of ATP-induced currents in isolated cortical pyramidal neurones
▵, 2 mm [Ca2+]o; •, 5 mm [Ca2+]o. A, the examples of inward currents induced in the pyramidal neurones of somato-sensory cortex layer II/III by the application of ATP (10 μm) at membrane potentials ranging from −100 to +40 mV. B, voltage-current relationship for the ATP-induced currents measured at different extracellular calcium concentrations. Amplitudes of currents were normalised to the maximal value measured at −80 mV. Each point is the mean ± s.d. for seven cells. Lines represent the cubic polynomial fit. C demonstrates the superposition of voltage-current relationships in the vicinity of reversal potential (areas outlined in B); arrows represent reversal potential (Erev).
Taken together, the above results provide a strong evidence of participation of P2X receptors in the synaptic transmission in the somato-sensory cortex. The comparison of ATP-mediated fraction of excitatory synaptic input in the cortical pyramidal neurones to its glutamatergic components is shown in Fig. 5. The data demonstrate that the P2X-mediated EPSC component represents a significant fraction of postsynaptic current over a wide potential range.
Figure 5.
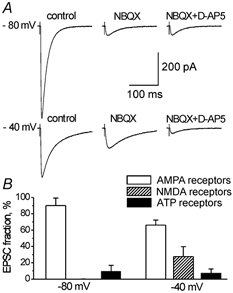
The P2X-receptor mediated fraction of EPSC in the somato-sensory cortex.
A, representative EPSCs recorded in the pyramidal neurone at different membrane potentials after consecutive application of NBQX, 20 μm and D-AP5, 60 μm. Each trace represents the average of six consecutive sweeps. B, diagram demonstrating the relative contribution of the AMPA, NMDA and ATP receptor mediated currents to the total EPSC measured at different membrane potentials. Each column represents the mean ± s.d. for 16 cells. All data were obtained in the presence of bicuculline, 20 μm.
DISCUSSION
In this study we have demonstrated that in 90 % of layer II/III neocortical pyramidal neurones tested in situ in brain slices, ATP-activated P2X receptors mediate a portion of the evoked EPSC. These data substantiate previous findings, which showed that ATP mediates fast excitatory synaptic transmission in the CNS (Edwards et al. 1997; Pankratov et al. 1998; Khakh et al. 2001; Robertson et al. 2001), by demonstrating the P2X-mediated synaptic transmission in the neocortex. This conclusion is based on the results of pharmacological experiments, which found that evoked EPSCs in neocortical pyramidal neurones are sensitive only to glutamate receptor and P2X receptor antagonists, whereas neither the cholinergic antagonist (hexamethonium) nor the serotonin receptor inhibitor (Y25130) were effective. The voltage-dependence of the EPSC component that was sensitive to P2X antagonists was similar to I-V curves obtained for ATP mediated currents on various types of neurones (Bardoni et al. 1997, Taschenberger et al. 1999). The expression of functional P2X receptors was further substantiated by the discovery of ATP-induced inward currents in single pyramidal neurones, acutely isolated from neocortical slices. When facing endogenously applied ATP at 10 μm concentration, 67 % of single neurones responded with an inward current, for which amplitude reached 30–280 pA. A somewhat lower occurrence of ATP-activated currents in single neurones may reflect cellular damage during isolation procedure.
Three P2X receptor subunits (P2X2, P2X4 and P2X6) are predominantly expressed in the CNS, and all three subunits were found in the cortex (Collo et al. 1996; Rubio & Soto, 2001). As P2X receptors assemble as heteromeres, the resulting membrane current response may vary substantially between cells. Indeed, our experiments on isolated cells demonstrated a remarkable variability in the kinetics of ATP-induced inward currents (Fig. 3), indicating differences in P2X subunit expression/assembly in different pyramidal neurones. Furthermore, we have found that a specific blocker of P2X1 receptors NF279 (Rettinger et al. 2000) effectively inhibited the residual EPSC, suggesting functional expression of the P2X1 subunit in the neocortex. Basing on the pharmacological properties of purinergic EPSCs and ATP-evoked currents, one could suggest that they are mediated predominantly by P2X1, P2X3 and P2X4 subtypes, although presence of heteromultimeric P2X1/5 and P2X4/6 receptors may not be excluded.
An important peculiarity of P2X receptors is their substantial Ca2+ permeability, and moreover, in contrast to Ca2+-permeable NMDA receptors, P2X receptors are readily available at resting membrane potentials. In pyramidal cortical neurones the ATP-activated P2X-mediated current has a reversal potential around +15 mV, obliquely indicating high Ca2+ permeability. This is further substantiated by the demonstration of the shift in the reversal potential of ATP-mediated current upon an increase in extracellular Ca2+ concentration. Obviously, the value of relative calcium permeability of P2X receptors (PCa/PCs = 12.3) reported here is just an estimate calculated using GHK theory which has its own limitations. Nevertheless, this value is comparable to PCa/PCs for native and recombinant NMDA receptors (10.4-10.6) and P2X receptors (2-11) obtained by the same technique (Mayer & Westbrook, 1987; Evans et al. 1996; Soto et al. 1996; Edwards et al. 1997). Therefore we may expect that in neocortical pyramidal neurones calcium influx through the P2X receptor may be significant even when compared to the NMDA receptor-mediated calcium signal.
Furthermore, direct recordings of cytosolic Ca2+ concentration ([Ca2+]i) in neocortical neurones have identified substantial [Ca2+]i elevation in response to endogenous application of ATP and purinergic agonists (Lalo & Kostyuk, 1998). These ATP-induced [Ca2+]i signals were at least partially associated with Ca2+ influx through P2X receptors. Therefore these data indicate that P2X receptors in pyramidal neocortical neurones are Ca2+-permeable. Although the contribution of the ATP receptors to the postsynaptic current is not large, their involvement in the Ca2+ influx may be considerable, especially at low membrane potential. Hence this minor component of synaptic transmission may play an important role in cortical pyramidal neurone function. An ATP-mediated transmission may underlie local Ca2+ entry in postsynaptic specialisation, which in turn, may result in local [Ca2+]i signalling. These local Ca2+ signals can be an important part of synaptic plasticity.
Acknowledgments
This research was supported by The Wellcome Trust (short-term travel fellowships to Y.P. and U.L.) and the Howard Hughes Medical Institute (research grant HHMI 55000322 to O.K.). The authors thank Professor D. Tomlinson for helpful comments on the manuscript.
REFERENCES
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. Journal of Neuroscience. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damer S, Niebel B, Czeche S, Nickel P, Ardanuy U, Schmalzing G, Rettinger J, Mutschler E, Lambrecht G. NF279: a novel potent and selective antagonist of P2X receptor-mediated responses. European Journal of Pharmacology. 1998;350:R5–6. doi: 10.1016/s0014-2999(98)00316-1. [DOI] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nature Neuroscience. 2000;3:701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Robertson SJ, Gibb AJ. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology. 1997;36:1253–1268. doi: 10.1016/s0028-3908(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2Xreceptors). expressed in mammalian cells. Journal of Physiology. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Fieber LA, Adams DJ. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. Journal of Physiology. 1991;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nature Reviews in Neuroscience. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Smith WB, Chiu CS, Ju D, Davidson N, Lester HA. Activation-dependent changes in receptor distribution and dendritic morphology in hippocampal neurons expressing P2X2-green fluorescent protein receptors. Proceedings of the National Academy of Sciences of the USA. 2001;98:5288–5293. doi: 10.1073/pnas.081089198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PP. Localization of P2X purinoceptor transcripts in the rat nervous system. Molecular Pharmacology. 1995;48:569–573. [PubMed] [Google Scholar]
- Kullmann DM, Nicoll RA. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992;357:240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Lalo U, Kostyuk P. Developmental changes in purinergic calcium signalling in rat neocortical neurones. Brain Research — Developmental Brain Research. 1998;111:43–50. doi: 10.1016/s0165-3806(98)00120-5. [DOI] [PubMed] [Google Scholar]
- Lalo UV, Pankratov YV, Arndts D, Krishtal OA. Omega-conotoxin GVIA potently inhibits the currents mediated by P2X receptors in rat DRG neurons. Brain Research Bulletin. 2001;54:507–512. doi: 10.1016/s0361-9230(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Lambrecht G, Friebe T, Grimm U, Windscheif U, Bungardt E, Hildebrandt C, Baumert HG, SpatZ-Kumbel G, Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. European Journal Pharmacology. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- Lewis CA. Ion-concentration dependence of the reversal potential and single cell conductance of ion channel at the frog neuromuscular junction. Journal of Physiology. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. Journal of Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. Journal of Comparative Neurology. 2000;421:374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. Journal of Physiology. 2001;535:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motin L, Bennett MR. Effect of P2-purinoceptor antagonists on glutamatergic transmission in the rat hippocampus. British Journal of Pharmacology. 1995;115:1276–1280. doi: 10.1111/j.1476-5381.1995.tb15036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. European Journal of Neuroscience. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G, Damer S, Muller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X1 receptor. Neuropharmacology. 2000;39:2044–2053. doi: 10.1016/s0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Ennion SJ, Evans RJ, Edwards FA. Synaptic P2X receptors. Current Opinion in Neurobiology. 2001;11:378–386. doi: 10.1016/s0959-4388(00)00222-1. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. Journal of Neuroscience. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, GomeZ-hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4:an ATP-activated ionotropic receptor cloned from rat brain. Proceedings of the National Academy of Sciences of the USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch AE. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38:141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends in Neurosciences. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Juttner R, Grantyn R. Ca2+-permeable P2X receptor channels in cultured rat retinal ganglion cells. Journal of Neuroscience. 1999;19:3353–3366. doi: 10.1523/JNEUROSCI.19-09-03353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev VS. Vibrodissociation of sliced mammalian nervous tissue. Journal of Neuroscience Methods. 1991;38:145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji T & Akaike N. Blockade of 5-HT3 receptor-mediated currents in dissociated frog sensory neurones by benzoxazine derivative, Y-25130. British Journal of Pharmacology. 1992;107:853–857. doi: 10.1111/j.1476-5381.1992.tb14536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends in Neurosciences. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


