Abstract
In iontophoresis experiments, a ‘non-specific’ current-induced vasodilatation interferes with the effects of the diffused drugs. This current-induced vasodilatation is assumed to rely on an axon reflex due to excitation of cutaneous nociceptors and is weaker and delayed at the anode as compared to the cathode. We analysed whether these anodal specificities could result from a break excitation of nociceptors. Break excitation is the generation of action potentials at the end of a square anodal DC current application, which are generally weaker than those observed at the onset of a same application at the cathode. In eight healthy volunteers, we studied forearm cutaneous laser Doppler flow (LDF) responses to: (1) anodal and cathodal 100 μA current applications of 1, 2, 3, 4 or 5 min; (2) 100 μA anodal applications of 3 min with a progressive ending over 100 s (total charge 23 mC); these were compared to square-ended 100 μA anodal applications of the same total charge (23 mC) or duration (3 min); (3) a 4 min 100 μA anodal application with a 333 msec break at half time. Results (mean ± S.D.) are expressed as percentage of heat-induced maximal vasodilatation (%MVD). Onset (Tvd) and amplitude (LDFpeak) of vasodilatation were determined. We observed that: Tvd was linearly related to the duration of current application at the anode (slope = 1.01, r2 = 0.99, P < 0.0001) but not at the cathode (slope = 0.03, r2 = 0.02, n.s.). Progressive ending of anodal current did not decrease LDFpeak (63.3 ± 24.6 %MVD) as compared to square-ending of current application of the same duration (36.9 ± 22.2 %MVD) or the same total charge (57.1 ± 23.5 %MVD). A transient break of anodal current did not allow for the vasodilatation to develop until current was permanently stopped. We conclude that, during iontophoresis, anodal break excitation alone cannot account for the delay and amplitude of the vascular response.
The mechanisms of the ‘non-specific’ current-induced vasodilatation that occurs during iontophoresis experiments are still largely unknown and debated. This ‘non-specific’ vasodilatation remains a limiting factor of this otherwise attractive, non-invasive technique of local drug infusion in humans (Grossman et al. 1995; Morris & Shore, 1996; Asberg et al. 1999), since in the study of the vascular effect of a drug, it may interfere with the ‘specific’ effect attributable to the drug itself (Morris & Shore, 1996; Hamdy et al. 2001). A better understanding of the underlying mechanisms of this ‘non-specific’ effect would clarify its limitations; this has led to multiple recent reports. Although current applications are not painful, it is generally admitted that the current-induced vasodilatation during iontophoresis relies on an axon reflex due to excitation of cutaneous nociceptors by the current (Berliner, 1997; Hamdy et al. 2001). Multiple mediators, including calcitonin gene-related peptide (CGRP), substance P, prostaglandins and NO (nitric oxide) can be released from afferent endings following nociceptor excitation, leading to direct or indirect (through sensitisation mechanisms) vasodilatation. Indeed, it has been shown that the current-induced response during iontophoresis is of neural origin since it is abolished under local anaesthesia (Morris & Shore, 1996). Consistently, we recently reported that small capsaicin-sensitive afferents are involved in this response (Durand et al. 2002a). The fact that nociceptors, associated with capsaicin-sensitive fibres, participate in vascular responses to non-painful stimuli has been demonstrated in various experimental human models, such as locally applied pressure (Fromy et al. 1998) or local heat stress (Magerl & Treede, 1996).
Among the different observations about the current-induced vasodilatation, it has been reported that for the same charge, the response at the anode is weaker than that observed at the cathode (Berliner, 1997), and that at the anode the response, appearing only after the current is removed, is delayed as compared to that observed at the cathode (Durand et al. 2002a). Both these two latter observations, reduced amplitude and delay of anodal vs. cathodal vasodilatation for comparable current application, have found no satisfactory explanation. Since the amplitude of the microvascular response increases with the intensity of electrophysiological stimulation of capsaicin-sensitive fibres (Westerman et al. 1987), we hypothesised that these two observations could rely on a common explanation: a make excitation at the cathode and a break excitation at the anode of the cutaneous nociceptors. The expression ‘make/break excitation’ relies on the fact that during prolonged square-wave current applications, action potentials take place mainly at the onset of the current (‘make excitation’) at the cathode and end of the current application (‘break excitation’) at the anode (Accornero et al. 1977). Make/break excitation during monopolar current application is a well-described phenomenon. It is more likely to be present in human sensory than in motor fibres (Stephanova & Mileva, 2000) and is more common in C fibres than in A or B fibres in animal preparations (Accornero et al. 1977; Jones et al. 1995). The difference in neural excitability between the anode and cathode during monopolar square current application is well known (Baker & Bostock, 1989; Wee, 2001) and Accornero et al. (1977) among others, have reported that action potentials are reduced if the break of the current is progressive rather than abrupt.
The purpose of the project was to identify the mechanism of anodal vasodilatation and to test the hypothesis that break excitation is involved. Should a break anodal excitation of cutaneous afferents be the sole underlying excitatory mechanism leading to the late moderate anodal vasodilatation, it would be of major interest in microvascular experiments using iontophoresis since the progressive rather than the abrupt ending of current application could decrease or even abolish the ‘non-specific’ current-induced vasodilatation and thus facilitate the interpretation and use of the technique. As a result, the following hypotheses were tested: (1) if a make/break excitation was involved in the vasodilatory response to current application, the delay for the appearance of the vasomotor response should be strictly proportional to the duration of current application at the anode and independent of it at the cathode; (2) if a break excitation was the explanation for the anodal delay, the progressive instead of abrupt return to zero of the current at the end of current application, should not change the delay for the vascular response; (3) in the hypothesis of break excitation as the sole explanation of the delayed anodal response, a transient break of current application should result in a response within a short delay despite maintained anodal current application; (4) a progressive rather than abrupt ending of anodal current application should lead to an attenuated or abolished vasomotor response as compared to the abrupt ending of a current application of comparable total charge.
METHODS
Eight healthy subjects (27.3 ± 3.9 years old, 166 ± 12 cm, 61.7 ± 11.5 kg, 5 males, 3 females) participated in two different experimental protocols. Subjects were non-smoking volunteers, not involved in regular competitive exercise training and had not been treated with any drug in the last 3 weeks before the beginning of the experiments. They were thoroughly informed of the methods and procedures and gave their written consent to participate in this institutionally approved study, performed according to the Declaration of Helsinki.
Preparation of the subjects
Patients were installed supine in a quiet room with the ambient temperature set at 23 ± 1 °C and left at rest for 15 min before each trial for temperature and cardiovascular adaptation.
Cutaneous blood flow was measured at three different points, on the volar aspect of the forearm using three laser Doppler probes placed at a distance of 5 cm from one another to form an equilateral triangle. We used two specially designed ‘active’ probes (PF 481-1, Perimed, Sweden) to allow for current application, local heating and simultaneous cutaneous blood flow recording. When anodal and cathodal currents were simultaneously applied, each iontophoretic patch was connected to one of the two poles, then one served as the cathode and one as the anode. In experiments with anodal current applications alone, two current suppliers were used and the cathode was positioned on disposable Ag/AgCl adhesive electrodes (Care 610, Kendall, Neustadt, Germany), 5 cm away from the laser probes. The thermostatic holder had a circular chamber of 1 cm2 allowing for the positioning of the specially designed disposable sponge of the iontophoretic electrode. Cutaneous laser Doppler flowmetry (LDF) was measured through a multifibre laser probe (780 nm, 1 mW maximal emission, bandwidth for Doppler shift 20–20000 Hz) at the centre of the sponge. Each sponge was moistened with 0.2 ml of deionised water before each experiment. A third probe (PF408, Perimed, Sweden) was used as a reference to confirm the absence of response to current application at an adjacent unstimulated site. All probes were fixed to the skin with double-sided adhesive rings and covered with an elastic net to improve stability.
Probes were connected to laser Doppler flowmeters (Periflux PF4001, Perimed, Sweden). The two ‘active’ probes were also connected to temperature-regulated heating systems (Peritemp PF4005, Perimed, Sweden) and to regulated current suppliers (Periiont, Micropharmacology System, PF 382 Perimed, Sweden, and A395 R linear stimulus isolator, WPI instruments, UK) allowing for the delivery of regulated intensity currents for programmable durations. The total current charge applied in a defined experiment was expressed as the product of time (seconds) and intensity (mA) and expressed as millicoulombs (mC). The sites and order of current application were chosen randomly within each experiment. Temperature for local heating was set to 44 °C (for 24 min) to cause maximal vasodilatation, since multiple studies support the conclusion that cutaneous vasodilatation is at its maximal level during prolonged local heating to 42–44 °C (Taylor et al. 1984; Johnson et al. 1986; Savage & Brengelmann, 1994; Saumet et al. 1998).
Local cutaneous temperature was measured using a surface thermocouple probe positioned 5 cm from two of the three laser probes. The thermocouple was connected to an electronic thermometer (BAT-12, Physitemp instruments Inc., Clifton, USA). Systemic blood pressure was monitored using a Finapres 2350 (Ohmeda, Englewood, USA) positioned on the second or third finger of the hand contralateral to the sites of LDF measurements.
Procedures
The different procedures are summarised in Table 1.
Table 1.
Summary of the durations of the different procedures of the protocols
| Protocol | Charge (mC) | Rest (min) | Duration of the100 μA current application(min) | Polarity | Ending modality | Recovery(min) | Local heating(min) |
|---|---|---|---|---|---|---|---|
| 1a | 6 | 2 | 1 | A + C | Square | 20 | 24 |
| 1b | 12 | 2 | 2 | A + C | Square | 20 | 24 |
| 1c | 18 | 2 | 3 | A + C | Square | 20 | 24 |
| 1d | 24 | 2 | 4 | A + C | Square | 20 | 24 |
| 1e | 30 | 2 | 5 | A + C | Square | 20 | 24 |
| 2a | 23 | 2 | 3 | A | Progressive over 1.66 min | 20 | 24 |
| 2b | 18 | 2 | 3 | A | Square | 20 | 24 |
| 2c | 23 | 2 | 3.83 | A | Square | 20 | 24 |
| 2d | 24 | 2 | 2 + 2 | A | Square at 2 min for 333 ms and square at 4 min | 20 | 24 |
A, anode; C, cathode.
Protocol 1: response to square-ended 100 μA current applications.
A reference period of 2 min was recorded in resting conditions. Then current application was started at 100 μA on the two active probes, one for the anode, one for the cathode. Application of the current was performed in five different trials, separated by at least 24 h one from another in each subject, for 1, 2, 3, 4 or 5 min randomly. A recovery period of 20 min was recorded to study the long-lasting effects of the current. At the end of this recovery period, local heating to 44 °C was performed for 24 min.
Protocol 2: response to a non-square-ended or to a transient break of anodal current square application.
In this protocol four different procedures were performed. Three of these four procedures, described below as 2a, 2b, 2c were compared to one another to estimate the influence of a non-square-ended anodal current application on the induced vasodilatation, whereas the last procedure (2d) was performed to test the influence of a transient break. For all procedures, after a 2 min reference period, anodal current application of 100 μA was started in a square-wave shape. Current application modality was then performed randomly on each subject according to two of the four procedures and repeated for the two others in another trial at least 3 weeks later. The four procedures were: 2a: after 3 min of constant application, a linear decrease of current intensity for 100 s, resulting in a total current application duration of 4 min 40 s and total current charge of 23 mC; 2b: a square-ending at minute 3 (total current application duration: 3 min, current density 18 mC); 2c: an application of 3 min 50 s corresponding to a total charge application equal to the one applied in protocol 2a when the current was non-squarely-ended (23 mC); 2d: a 4 min current application that was transiently stopped for 333 msec at half time of the current application. This 333 ms break is longer than the latency reported for the break excitation in animal C fibres, on average 63.0 ± 3.3 ms in the study by Jones et al. (1995). Therefore, although performed in a very different preparation, the duration of the break was estimated to be sufficient to allow for the appearance of any possible break excitation. In all procedures a recovery period of 20 min following the end of the latest current application was recorded, then local heating to 44 °C was applied for 24 min as in protocol 1.
Measurements
Data were recorded on a computer via an analogue to digital converter (Biopac System, Inc., California) with a sample rate of 3 Hz, on 16-bit. Due to instantaneous variability of the LDF signal resulting from vasomotion, individual results were averaged over 5 s intervals throughout each experiment. For each experiment, the mean value recorded during the last 30 s of the heating period was used as 100 % of vasodilatation, and the individual data normalised accordingly. Thus, LDF values are expressed as a percentage of maximal vasodilatation (%MVD). Resting LDF (LDFrest) was calculated as the average of the last 5 s of the resting period. Peak response (LDFpeak) to current application was the value recorded at the time when the maximal response of the recovery period was observed on averaged data during the period of current application and subsequent 20 min recorded period. Lastly, in order to search for the onset of vasodilatation, we searched for an inflexion point in the LDF signal. For this purpose, the first derivative of the signal was obtained by iterative subtraction of LDF mean values on every two consecutive 5 s intervals within an experiment. The delay for the onset of vasodilatation (Tvd) was defined as the first of four consecutive 5 s intervals for which the derivate value of LDF was superior to the mean ± 2 standard deviations (s.d.) of the derivate values of the resting period, and measured from stimulation start. The relationship between duration of current application and time for the onset of vasodilatation in protocol 1 was studied with linear regression analysis using the least squares method. All values are expressed as mean ± s.d. Differences were analysed with paired t tests. For all statistical and regression analyses (Prism 2.01, Graphpad Software Inc., USA), a P value < 0.05 was considered significant. Non significant results are reported as n.s.
RESULTS
In all experiments, compared with starting values, no significant changes were observed for skin blood flow recorded at the reference probe, mean systemic arterial blood pressure or local skin temperature.
Subjects did not report painful sensations during any of the protocols, although some sensations could frequently be noted. These sensations were extremely variable from one subject to another or in the same subject between the experiments. Description of the sensation showed no apparent correlation with the amplitude of the vascular response, ranging from nothing to a light tickle, rarely a moderate non-painful pricking sensation. Many subjects were able to identify the start of the current but in most subjects the initial sensation was so light that they were generally unable to say whether the current persisted or not. Indeed, the subjective sensation never increased during current application, nor was it delayed for the exact moment of current start. It is of interest to note that subjects were unable to differentiate which probe was the cathode and which was the anode when anodal and cathodal current were delivered simultaneously during the first experiments. Lastly, they did not feel anything when the current was stopped in any of the experiments, although the Periiont apparatus(Perimed) softly rings as it stops when the total charge is delivered. Thus, the subjects were aware of the exact moment for current ending in some of the experiments (contrary to current start with Periiont or to experiments using the WPI apparatus).
Protocol 1: response to square-ended 100 μA current applications
A typical response to prolonged monopolar stimulation is presented in Fig. 1.
Figure 1.
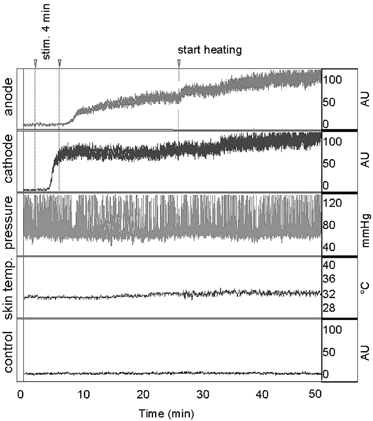
Typical recording at rest (2 min), during and 20 min following a 4 min monopolar current application of 100 μA (indicated by the dotted lines) and during 24 min of local heating
From top to bottom, recordings are: Laser Doppler Flow (LDF) in arbitrary units (AU) at the anode: anode; LDF at the cathode: cathode; systemic arterial pressure: pressure; local skin temperature at a non-heated area 5 cm from heated probes: skin temp.; reference LDF: control. Note that at the anode, the response appears following the end of current application whereas at the cathode the response begins during the current application.
Results for LDFrest and LDFpeak in protocol 1 are reported in Table 2. In brief, no significant difference was found for LDFrest between the various experiments. At the anode, LDFpeak increased from 1 to 5 min of current application duration and reached values not significantly different from those found under the cathode for 5 min of current application duration. At the cathode, no significant difference was found for LDFpeak from 1 to 5 min of current application. The time for the onset of vasodilatation generally occured within 1 to 2 min from the start of current application, whereas Tvd at anodal current delivery routinely occurred within a minute or two after the end of current delivery. Thus, when the data are plotted as in Figure 2, a linear relationship exists between the duration of current application and Tvd at the anode whereas Tvd is independent from the duration of current application at the cathode. It should be noted that the y-intercept calculated for both equations is in the same range, and that the slope at the anode is almost that of a line of identity.
Table 2.
Laser Doppler flow at rest (LDFrest) and peak value (LDFpeak as a percentage of the heating-induced maximal vasodilatation (%MVD), observed after current application in protocol 1 under the anode and the cathode for 100 μA square-ended current application
| Current duration (min) | Anode |
Cathode |
LDFpeak Anode vs. Cathode | ||
|---|---|---|---|---|---|
| LDFrest (%MVD) | LDFpeak (%MVD) | LDFrest (%MVD) | LDFpeak (%MVD) | ||
| 1 | 8.4 ± 3.5 | 18.2 ± 15.9 | 7.1 ± 2.4 | 73.5 ± 18.3 | P < 0.05 |
| 2 | 9.8 ± 6.8 | 33.3 ± 19.7 | 5.8 ± 2.6 | 66.3 ± 19.1 | P < 0.05 |
| 3 | 4.1 ± 1.4 | 30.3 ± 27.5 | 4.2 ± 1.6 | 86.0 ± 38.1 | P < 0.05 |
| 4 | 6.3 ± 2.3 | 41.6 ± 15.5 | 5.3 ± 2.3 | 72.7 ± 11.9 | P < 0.05 |
| 5 | 4.2 ± 1.6 | 51.7 ± 27.1 | 5.0 ± 2.6 | 74.1 ± 23.1 | n.s. |
n.s., not significant
Figure 2.
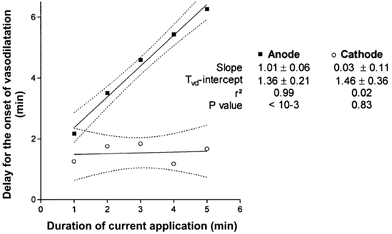
Scatterplot of the mean delay for the onset of vasodilatation (Tvd) from stimulation start and the duration of current application observed for monopolar 100 μA anodal transcutaneous current application at the anode (▪) and the cathode (○)
The table reports the results of linear regression analysis of the presented points. At the anode Tvd is strictly proportional to current application duration, whereas no relationship exists at the cathode.
Protocol 2: Response to a non-square-ended or transiently broken anodal current application
Results for LDFrest, LDFpeak and Tvd in protocols 2a, 2b and 2c are reported in Table 3. For all the experiments, no statistical difference was observed for the LDFrest value. The LDFpeak observed in protocol 2 for the square-ended 3 min anodal current application was in the same range as the one observed for the same condition in protocol 1. The linear decrease of the 3 min current application led to significantly higher LDFpeak values than for square-ended current applications of the same duration. No difference in LDFpeak was observed between progressive decreases of current application and square-ending of a comparable total charge. It should be noted that Tvd values for the square-ended experiments are consistent with those expected from the equation defined in protocol 1 with differences of less than 0.1 min from expected values. However, for a progressive ending of current application, although the current started to decrease at minute 3, Tvd was found 0.8 min later than expected for a stimulation of 3 min in the hypothesis of a break excitation mechanism.
Table 3.
Resting Laser Doppler Flow (LDFrest) and peak LDF (LDFpeak), as a percentage of the heating-induced maximal vasodilatation ( % MVD), resulting from anodal current application and time for the onset of vasodilatation (Tvd) in protocol 2 for progressive decrease of anodal application (2a) and square applications of comparable duration (2b) and comparable charge (2c)
| Protocol | Charge(mC) | Time and modality for the first current decrease(min) | LDFrest (%MVD) | LDFpeak (%MVD) | Tvd (min) | ExpectedTvd (min) |
|---|---|---|---|---|---|---|
| 2a | 23 | 3 min, progressive over 1.66 min | 9.5 ± 4.6 | 63.3 ± 24.6 | 5.16 | 4.4 |
| 2b | 18 | 3 min, square | 7.7 ± 2.8 | 36.9 ± 22.2* | 4.25 | 4.4 |
| 2c | 23 | 3.83 min, square | 9.3 ± 2.2 | 57.1 ± 23.5 | 5.25 | 5.2 |
Expected Tvd is the time calculated from protocol 1 where vasodilatation should have occurred if the delay for anodal vasodilatation had only resulted from a break excitation mechanism. * Signals that the LDFpeak of protocol 2b is significantly different from LDFpeak of protocol 2a.
In protocol 2d, a 333 msec transient break of a 4 min total anodal current application at minute 2, led to a significant increase of LDF from 9.5 ± 3.9 %MVD at rest to 52.2 ± 13.5 %MVD for LDFpeak. Although in the case of break excitation, Tvd was supposed to occur at 3.4 min according to the regression found in the first protocol, despite the transient break, Tvd was found at 5.25 min, 1.85 min later than expected.
DISCUSSION
One goal of this study was to test whether an anodal break excitation was the sole and sufficient explanation for the fact that current-induced vasodilatation at the anode occurred after the current was stopped. Anodal break excitation is a common property of various excitable tissues (Ranjan et al. 1998; Nikolski et al. 2002). Depending on the model used and the tissue studied, this phenomenon has been suggested to depend either on cellular properties of ion channels (Kashiwayanagi et al. 1983) and/or on the occurrence of depolarised areas around the hyperpolarised anode (virtual cathode), specifically around monopolar electrodes. This latter theory is usually described as the ‘bidomain’ model (Roth, 1995).
In the present study, the linear relationship between Tvd and duration of current application at the anode and the constant Tvd at the cathode is consistent with the break excitation hypothesis (Fig. 1). However, the results of the last experiments of protocol 2 in the present study, where a transient break was performed, strongly suggest that break excitation, if present, could not have been the sole cause of the delay for anodal vascular response. According to the results of protocol 1 of the present study, vasodilatation should have been observed within 1.5 to 2 min following the break of current application. This was not the case. Vasodilatation did appear, but only 3.25 min following the transient break, a time corresponding to 1.25 min following the end of the whole 4 min period of anodal current application. Although the methodology used by Jones et al. (1995) is clearly a different one, we assumed that 330 ms was a sufficient delay for action potentials on the neural fibre to be generated as a result of an eventual break excitation. Although the possibility that no break excitation occurred due to insufficient transient break duration cannot be ruled out, the fact that vasodilatation did not appear following the transient break of the current does not exclude a break excitation mechanism but suggests that, if break excitation occurred, the reapplication of the current did interfere with the time for the onset of an eventual response.
The idea that break excitation would not be the sole explanation for the anodal delay is strengthened by the fact that Tvd was changed during progressive decreases of current application as compared to square application of the same duration. We conclude that anodal break excitation is probably not the sole explanation for the delay in the vasodilatation seen with 100 μA anodal current application to the skin.
There are other possible explanations for the delay for the onset of vasodilatation at the anode. Instead of, or in addition to a possible break excitation, it could be suggested, as previously, that the current application interferes with the vasodilator mechanisms resulting from the excitation of nociceptors. As a result of this suggestion, two questions arise. Can nociceptors be activated during, rather than at the end of prolonged anodal current applications? If so and whenever the excitation occurs, how could the vascular response be delayed? On the one hand, Coleridge et al. (1973) have suggested that excitation of unmyelinated C fibres may occur during anodal prolonged currents, although excitation during anodal current application in the range 100–200 μA is debated (Thoren et al. 1977; Hopp et al. 1980). The fact that the generation of action potentials in peripheral fibres requires several times more electrical stimulus intensity at the anode when compared to the cathode is a common finding in humans (Wee, 2001). Animal and human studies (Thoren et al. 1977; Kiernan et al. 2001; Burke et al. 1999) have shown that peripheral nerve responses to depolarising and hyperpolarising currents are both temperature-sensitive. It could be suggested that a progressive increase of local temperature below the probe due to joule-heating progressively facilitated the generation of action potentials during current application explaining the delay for the response at the anode as compared to the cathode. Temperature below the probe was not measured in this study, but estimation of temperature change resulting from the joule-heating is in the range of 10−5 °C according to the equation proposed by Prausnitz (1996), so this hypothesis seems unlikely.
On the other hand, anodal block is the application of low levels of direct positive polarising current in the range 20–300 μA to nerve fibres, which produces a localised reversible block of conduction on the basis of fibre size (Hopp et al. 1980; Seagard et al. 1993; Hopp & Seagard, 1998; Petruska et al. 1998). Then, assuming that the vascular response to current application results from an axon reflex (Berliner, 1997; Hamdy et al. 2001), whatever the nature and time for occurrence of the stimulus of nociceptors is, conduction of the depolarisation in the afferent tree could be blocked by anodal current application. Fibre blockade requires lower intensities in large fibres than in C afferents (Hopp et al. 1980; Seagard et al. 1993). In these latter fibres, in animal models, currents up to 300–350 μA are required to completely block the response (Seagard et al. 1993). However, few experimental data are available in humans and the possible occurrence of an anodal block in humans is debated (Schwartz, 1991; Dreyer, 1993; Wee, 2000). To the best of our knowledge, the current required to block the conduction in C and/or A fibres through large areas of transcutaneous current application is not known in humans.
Lastly, the effect of polarising currents on ion channels of non-neuronal cells participating in vasodilatation (such as endothelial or smooth muscle cells) could also be proposed as a possible mechanism interfering with the vasodilatation, since a large variety of ion channels are voltage-gated. Another potential contributor to the vasodilatation is a slow change in local pH due to current application (Berliner, 1997). Slow progressive local pH changes are observed in the skin or iontophoretic bath during monopolar current applications (Sato et al. 1993; Guffey et al. 1999). The time required to reach sufficient pH changes could be the simplest explanation for the delay for vasodilatation observed at the anode as compared to the cathode. Nevertheless, if pH change was the sole mechanism for the delay for the onset of vasodilatation at the anode, a trigger-type response should be observed. When a sufficient pH change is reached to induce vasodilatation, the delay for the onset of vasodilatation should not increase with longer current application duration. The first part of this study confirms that this was not the case and that the relationship between the onset of vasodilatation and duration of current application was strictly linear.
Observations about the amplitude of the vasodilatation further argue against the sole responsibility of a break excitation mechanism in the current-induced vasodilatation. On the one hand, an anodal break excitation should result in a vasodilatation of constant amplitude whatever the duration of current application. This was not the case. On the other hand, electrophysiological studies (Accornero et al. 1977) and mathematical models (Roth, 1995) show that a progressive rather than an abrupt break of current application decreases neural action potential firing. Since the vasodilator response is proportional to the intensity of afferent fibres stimulation (Blumberg & Gunnar Wallin, 1987; Westerman et al. 1987), progressive ending of current application, assumed to result in a decreased electrophysiological response, is expected to result in a decreased vascular response. Although no electrophysiological recording was performed in our study, contrary to what was expected the progressive ending of current application did not decrease the amplitude of the vasodilatation as compared with square-wave current of comparable charge and duration.
Among the previously discussed mechanisms concerning the delay for the onset of vasodilatation, we have argued against the sole involvement of pH changes in anodal delay for the vasodilatation. We now advocate against the sole participation of anodal break excitation in the changes in amplitude with current duration. This does not necessarily rule out the original hypothesis of break excitation nor of pH changes as effective mechanisms. A combination of the two mechanisms appears as a possible explanation for our results. Indeed, protons are able to sensitise the response of primary afferents (see for review Caterina et al. 1997 and Brain, 2000). A progressive proton accumulation with current duration at the anode (Sato et al. 1993) resulting in a sensitisation of fibres to break excitation could be the underlying mechanism of the increased amplitude of the anodal response to increased duration of current application. We recently provided consistent evidence that a sensitisation mechanism resulted from current application leading to amplification of the current-induced vasomotor response to subsequent current application (Durand et al. 2002b). We suggested that this sensitisation involved prostaglandins but this does not exclude the participation of protons in mechanisms leading to sensitisation. Then, a combination of the two mechanisms, break excitation and proton accumulation, is an interesting but unproven possibility. Complementary experiments to address this problem are provided as supplementary material on the website of the Journal.
Finally, it is of interest to note that the delay for the onset of vasodilatation from current application start under the cathode is similar to its delay from current application ending at the anode. This more than 1 min delay is consistent with what we recently reported with comparable current applications (Durand et al. 2002a) and almost excludes an axon reflex as the direct cause of vasodilatation. Indeed, this reflex would result in a vasodilatation within a few seconds from nociceptor excitation (Blumberg & Gunnar Wallin, 1987; Magerl et al. 1987). Specifically at the anode, in the case of an axon reflex, whatever the cause for the absence of vasodilatation during current application, ending of current application should result in such an ‘immediate’ vasodilatation. We speculate that slow intermediate pathways, such as the ones resulting from cell participation as proposed in neurogenic inflammation (Szolcsanyi, 1996), are essential in our response at the anode and probably also at the cathode. This will require further experiments in the future.
In conclusion, it appears that anodal break excitation is probably not the sole explanation for both the amplitude (reduced as compared to the cathode) and the delay for the onset of vasodilatation (appearing after the end of current application) seen at the anode during current-induced vasodilatation in humans. Many iontophoretic protocols have been used with various intensities and current durations (Hamdy et al. 2001; Eneroth-Grimfors et al. 1991; Delaney et al. 1998; Drummond & Lipnicki, 2001). In studies using 100 μA iontophoresis, the exact cause for the delay for the onset, and amplitude of anodal ‘non-specific’ vasodilatation requires further study. On the one hand, if it involves a proton sensitisation, the use of buffer salts would be an interesting tool to limit the interfering effects of the current. On the other hand, whether an interference of anodal current application with neural conduction or with ion channels participating in vasodilatation exists, is still to be confirmed. In such a case, low current intensities should probably be preferred in iontophoretic experiments.
Acknowledgments
The authors gratefully acknowledge Mr Nick Roscoe (London, UK) for technical assistance, and Dr Nisha Charkoudian (Rochester, USA) for reviewing and correcting the English grammar and style. Sylvain Durand benefits from the financial support of Conseil Régional and of Direction Régionale de la Jeunesse et des Sports, des Pays de la Loire. The protocol was promoted by the ‘Centre Hospitalier Universitaire d'Angers’.
Supplementary material
This paper contains supplementary material entitled:
Proton accumulation plays a role in the amplitude, but not in the time for the onset, of the vascular response to anodal current-induced vasodilatation
Protocols were designed to test the influence of alternate anionic and cationic buffers on the amplitude and time for the onset of anodal current-induced vasodilatation. We used four different vehicles: (a) deionised water, (b) sodium bicarbonate (250 mm in deionised water), (c) sodium acetate (250 mm in deionised water) and (d) sodium chloride (125 mm in deionised water), on eight subjects and performed 5 min anodal current applications. LDFrest was 6.9 ± 3.0, 7.6 ± 3.3, 7.5 ± 2.0, 6.1 ± 3.4 %MVD for (a), (b), (c) and (d), respectively. LDFpeak was 15.8 ± 14.3 %MVD for (b) (P < 0.05 vs. all other solutions). It was 49.8 ± 26.4 %MVD for (c) and 69.8 ± 40.9 %MVD for (d), not significantly different from (a): 65.8 ± 36.5 %MVD. Estimation of the time for the onset of vasodilatation showed no change with the different solutions. In anodal iontophoresis, bicarbonate solution vehicles should probably be preferred to deionised water to decrease the amplitude of the ‘non-specific’ response to current application. The cause for the delay of vasodilatation is still unsolved.
REFERENCES
- Accornero N, Bini G, Lenzy GL, Manfredi M. Selective activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses. Journal of Physiology. 1977;273:539–560. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberg A, Holm T, Vassbotn T, Andreassen AK, Hartmann A. Nonspecific microvascular vasodilation during iontophoresis is attenuated by application of hyperosmolar saline. Microvascular Research. 1999;58:41–48. doi: 10.1006/mvre.1999.2153. [DOI] [PubMed] [Google Scholar]
- Baker M, Bostock H. Depolarisation changes the mechanism of accommodation in rat and human motor axons. Journal of Physiology. 1989;411:545–561. doi: 10.1113/jphysiol.1989.sp017589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner MN. Skin microcirculation during tapwater iontophoresis in humans: Cathode stimulates more than anode. Microvascular Research. 1997;54:74–80. doi: 10.1006/mvre.1997.2025. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Gunnar Wallin B. Direct evidence of neurally mediated vasodilation in hairy skin of the human foot. Journal of Physiology. 1987;382:105–121. doi: 10.1113/jphysiol.1987.sp016358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD. New feelings about the role of sensory nerves in inflammation. Nature Medicine. 2000;6:134–135. doi: 10.1038/72218. [DOI] [PubMed] [Google Scholar]
- Burke D, Mogyoros I, Vagg R, Kiernan MC. Temperature dependence of excitability indices of human cutaneous afferents. Muscle and Nerve. 1999;22:51–60. doi: 10.1002/(sici)1097-4598(199901)22:1<51::aid-mus9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG, Rosenthal F, Dangel A. Stimulation of C-fibers accompanying anodal polarization block of A-fibers in the vagus nerves of cats. Federation Proceedings. 1973;32:355. abstract. [Google Scholar]
- Delaney CA, Murchie KJ, Westerman RA, De Courten MP. Rapid actions of insulin on sensory nerve fonction. NeuroReport. 1998;9:2775–2779. doi: 10.1097/00001756-199808240-00017. [DOI] [PubMed] [Google Scholar]
- Dreyer SJ, Dumitru D, King JC. Anodal block V anodal stimulation. Fact or Fiction. American Journal of Physical Medicine and Rehabilitation. 1993;72:10–18. doi: 10.1097/00002060-199302000-00004. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Lipnicki DM. Repeated local administration of noradrenaline or saline inhibits thermal hyperalgesia in pain-sensitized human skin. British Journal of Clinical Pharmacology. 2001;52:289–295. doi: 10.1046/j.0306-5251.2001.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Fromy B, Bouyé P, Saumet JL, Abraham P. Current-induced vasodilation during 5 min, 0. 10 mA water iontophoresis is delayed from current onset and involves aspirin-sensitive mechanisms. Journal of Vascular Research. 2002a;39:59–71. doi: 10.1159/000048994. [DOI] [PubMed] [Google Scholar]
- Durand S, Fromy B, Bouyé P, Saumet JL, Abraham P. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. Journal of Physiology. 2002b;540:261–269. doi: 10.1113/jphysiol.2001.013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eneroth-Grimfors E, Lindblad LE, Westgren M, Etzell BM, Bevegard S. Iontophoresis of vasoactive drugs. Effect on peripheral blood flow during pregnancy. Acta Obstetrica Gynecologia Scandinavia. 1991;70:25–28. doi: 10.3109/00016349109006173. [DOI] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Saumet JL. Non-nociceptive capsaicin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Research. 1998;811:166–168. doi: 10.1016/s0006-8993(98)00973-1. [DOI] [PubMed] [Google Scholar]
- Grossmann MG, Jamieson MJ, Kellogg DL, Jr, Kosiba WA, Pergola PE, Crandall CG, Shepherd AMM. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvascular Research. 1995;50:444–452. doi: 10.1006/mvre.1995.1070. [DOI] [PubMed] [Google Scholar]
- Guffey JS, Rutherford MJ, Payne W, Phillips C. Skin pH changes associated with iontophoresis. Journal of Orthopedy Sports and Physical Therapy. 1999;29:656–660. doi: 10.2519/jospt.1999.29.11.656. [DOI] [PubMed] [Google Scholar]
- Hamdy O, Abouelenin K, Logerfo FW, Horton ES, Veves A. Contribution of nerve-axon reflex-related to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care. 2001;24:344–349. doi: 10.2337/diacare.24.2.344. [DOI] [PubMed] [Google Scholar]
- Hopp FA, Seagard JL. Respiratory responses to selective blockade of carotid sinus baroreceptors in the dog. American Journal of Physiology. 1998;275:R10–18. doi: 10.1152/ajpregu.1998.275.1.R10. [DOI] [PubMed] [Google Scholar]
- Hopp FA, Zuperku EJ, Coon RL, Kampine JP. Effect of anodal blockade of myelinated fibres on vagal C-fibre afferents. American Journal of Physiology. 1980;239:R454–462. doi: 10.1152/ajpregu.1980.239.5.R454. [DOI] [PubMed] [Google Scholar]
- Jones JF, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C fibres in anesthetized cats and rabbits. Journal of Physiology. 1995;489:203–214. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, O'Leary D, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clinical Physiology. 1986;6:337–346. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Kashiwayanagi M, Miyake M, Kurihara K. Voltage-dependant Ca2+ channel and Na+ channel in frog taste cells. American Journal of Physiology. 1983;244:C82–84. doi: 10.1152/ajpcell.1983.244.1.C82. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Cikurel K, Bostock H. Effects of temperature on the excitability properties of human motor axons. Brain. 2001;124:816–825. doi: 10.1093/brain/124.4.816. [DOI] [PubMed] [Google Scholar]
- Magerl W, Szolcsanyi J, Westerman R, Handwerker H. Laser Doppler measurements of skin vasodilation elicited by percutaneous electrical stimulation of nociceptors in humans. Neuroscience Letters. 1987;82:349–354. doi: 10.1016/0304-3940(87)90281-3. [DOI] [PubMed] [Google Scholar]
- Magerl W, Treede RD. Heat evoked vasodilation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents? Journal of Physiology. 1996;497:837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolski VP, Sambelashvili AT, Efimov IR. Mechanims of make and break excitation revisited: Paradoxical excitation during diatolic stimulation. American Journal of Physiology — Heart and Circulatory Physiology. 2002;2:H565–575. doi: 10.1152/ajpheart.00544.2001. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Hubscher CH, Johnson RD. Anodally focused polarization of peripheral nerve allows discrimination of myelinated and unmyelinated fibre input to brainstem nuclei. Experimental Brain Research. 1998;121:379–390. doi: 10.1007/s002210050472. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR. The effects of electric current applied to skin: A review for transdermal drug delivery. Advanced Drug Delivery Reviews. 1996;18:395–425. [Google Scholar]
- Ranjan R, Chiamvimonvat N, Thakor NV, Tomaselli GF, Marban E. Mechanism of anodal break excitation in the heart. Biophysical Journal. 1998;74:1850–1863. doi: 10.1016/S0006-3495(98)77895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BJ. A mathematical model of make and break electrical stimulation of cardiac tissue by a unipolar anode or cathode. IEEE Transactions on Biomedical Engineering. 1995;42:1174–1184. doi: 10.1109/10.476124. [DOI] [PubMed] [Google Scholar]
- Sato K, Timm DE, Sato F, Templeton EA, Meletiou DS, Toyomoto T, Soos G, Sato SK. Generation and transit pathway of H+ is critical for inhibition of palmar sweating by iontophoresis in water. Journal of Applied Physiology. 1993;75:2258–2264. doi: 10.1152/jappl.1993.75.5.2258. [DOI] [PubMed] [Google Scholar]
- Saumet JL, Abraham P, Jardel A. Cutaneous vasodilation induced by local warming, sodium nitroprusside, and bretylium iontophoresis on the hand. Microvascular Research. 1998;56:212–217. doi: 10.1006/mvre.1998.2099. [DOI] [PubMed] [Google Scholar]
- Savage MV, Brengelmann GL. Reproducibility of the vascular response to heating in human skin. Journal of Applied Physiology. 1994;76:1759–1763. doi: 10.1152/jappl.1994.76.4.1759. [DOI] [PubMed] [Google Scholar]
- Schwartz RG. Electrical sympathetic block: a review of electrotherapy physics. Advances in Therapy. 1991;8:1–5. [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Drummond HA, Van Wynsberghen DM. Selective contribution of two types of carotid sinus ba roreceptors to the control of blood pressure. Circulation Research. 1993;72:1011–1022. doi: 10.1161/01.res.72.5.1011. [DOI] [PubMed] [Google Scholar]
- Stephanova DI, Mileva K. Different effects of blocked potassium channels on action potentials, accommodation, adaptation and anode break excitation in human motor and sensory myelinated nerve fibres: computer simulations. Biological Cybernetics. 2000;83:161–167. doi: 10.1007/s004220000151. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Neurogenic inflammation: reevaluation of axon reflex theory. In: Geppetti P, Holzer P, editors. Neurogenic Inflammation. Boca Raton, FL, USA: CRC Press; 1996. pp. 33–42. [Google Scholar]
- Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. Journal of Applied Physiology. 1984;57:191–196. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- Thoren P, Shepherd JT, Donald DE. Anodal block of medullated cardiopulmonary vagal afferents in cats. Journal of Applied Physiology. 1977;42:461–465. doi: 10.1152/jappl.1977.42.3.461. [DOI] [PubMed] [Google Scholar]
- Wee AS. Anodal excitation of intact peripheral nerves in humans. Electromyography and Clinical Neurophysiology. 2001;41:71–77. [PubMed] [Google Scholar]
- Wee AS, Leis AA, Kuhn AR, Gilbert RW. Anodal block: can this occur during routine nerve conduction studies? Electromyography and Clinical Neurophysiology. 2000;40:387–391. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proton accumulation plays a role in the amplitude, but not in the time for the onset, of the vascular response to anodal current-induced vasodilatation
Protocols were designed to test the influence of alternate anionic and cationic buffers on the amplitude and time for the onset of anodal current-induced vasodilatation. We used four different vehicles: (a) deionised water, (b) sodium bicarbonate (250 mm in deionised water), (c) sodium acetate (250 mm in deionised water) and (d) sodium chloride (125 mm in deionised water), on eight subjects and performed 5 min anodal current applications. LDFrest was 6.9 ± 3.0, 7.6 ± 3.3, 7.5 ± 2.0, 6.1 ± 3.4 %MVD for (a), (b), (c) and (d), respectively. LDFpeak was 15.8 ± 14.3 %MVD for (b) (P < 0.05 vs. all other solutions). It was 49.8 ± 26.4 %MVD for (c) and 69.8 ± 40.9 %MVD for (d), not significantly different from (a): 65.8 ± 36.5 %MVD. Estimation of the time for the onset of vasodilatation showed no change with the different solutions. In anodal iontophoresis, bicarbonate solution vehicles should probably be preferred to deionised water to decrease the amplitude of the ‘non-specific’ response to current application. The cause for the delay of vasodilatation is still unsolved.


