Abstract
In this study the effects of perfusing isolated seminiferous tubules of the testes are reported for the first time. Initial perfusion studies (fast rate perfusion) resulted in gross morphological damage to the seminiferous tubules. The recorded transepithelial potential (Vt) was close to 0 mV. Slow perfusion rates eliminated morphological damage to the perfused tubules. These tubules exhibited a Vt of −5.4 ± 1.8 mV which was significantly different (P < 0.0001) from tubules that were perfused at a fast rate. Additional non-perfusion electrophysiological experiments (oil-gap and agar probe techniques) provided the confirmation that tubules not morphologically compromised produced a higher Vt which was not statistically different (P < 0.0001) from slowly perfused tubules. A revised hypothesis on fluid secretion is postulated. In brief, that the seminiferous tubule is solely responsible for the production of its luminal fluid. This hypothesis is contrary to the long standing ‘Tuck’ hypothesis which suggested that the source of luminal fluid in the seminiferous tubule originated from secretions of Sertoli cells as well as from distal testicular structures, e.g. the rete testis.
The seminiferous tubules of the testis provide a specialized chemical milieu in which spermatogonia are transformed from diploid germ cells into haploid spermatids. This luminal milieu is distinctly different from the interstitial fluid and blood plasma and is critical to the process of spermatogenesis. Tuck et al. (1970) were the first to report on the formation of this fluid. Using variations of the micropuncture technique they found that the ionic concentrations of the normal luminal fluid, called free flow fluid (FFF), differed substantially from the fluid that was secreted into oil columns injected under pressure into the lumen of these tubules (the fluid formed in these oil columns was subsequently called primary fluid, PF). In addition, the average transepithelial potential (Vt) was measured and differed in that the FFF was −7.4 mV compared with ±1 mV in the segments of tubules secreting the PF. As a result of these findings they postulated a hypothesis which suggested that the FFF was a mixture of PF and fluids secreted from the distal structures of the rete testis and the tubuli reti. These results remained largely unchallenged until now.
For the first time in this study transepithelial potentials (Vt) recorded from isolated, perfused seminiferous tubules are reported. The rationale for these perfusion experiments emanated from similar perfusion studies on segments of kidney tubules (Burg et al. 1966, 1968; Koeppen, 1989), Malpighian tubules (Isaacson et al. 1989) and various biological tubules (e.g. gastric ducts, Singh et al. 1995) which led to the elucidation of their transepithelial transport mechanisms and contributed enormously to our understanding of how these tissues functioned. With this in mind we used the in vitro perfusion technique on isolated rat seminiferous tubules (Burg et al. 1966). The results from the perfusion study indicated the need to use validation by non-perfusing electrophysiological techniques and also to evaluate Vt. Subsequent to each experiment the tubule's morphology was studied to provide some explanation for the observed variation in Vt.
METHODS
Animal treatment
Adult male Wistar rats, aged between 90 and 120 days, bred at the Medical Research Council, were used for these studies and were maintained in a controlled environment with free access to food and water. Room temperature was thermostatically controlled at 22 °C and a day-night cycle of 12 h was maintained. Rats were allocated randomly to between four and six animals per cage. All experimental protocols were approved by the institution's research ethics committee.
Isolation of seminiferous tubules
Animals were anaesthetized with an intraperitoneal injection of sodium pentobarbitone (60 mg (kg body weight)−1, Sagatal, Rhône-Poulenc Animal Health SA (Pty) Ltd). A mid-line incision was then made through the scrotum, abdominal skin and rectus muscle superior to the pelvis. A single testis was then excised and transferred to a dissection Petri dish filled with cold Ringer solution (for composition see below) which was continuously bubbled with oxygen. The tunica albuginea was subsequently removed and the central portion of the testis was teased apart gently using watchmakers forceps, and seminiferous tubule segments (3-10 mm in length) were isolated. The procedure for the transfer of tubules involved the aspiration of the tubule into a capillary tube, by means of a custom-made mechanical micro-aspirator. The micro-aspirator was made up using a 0.5 ml pipette mechanical aspirator (Glasfirn pi-pump, Germany) which was custom-fitted to a glass capillary tube (i.d. 0.9 mm). The capillary tube was flame bent to an angle of approximately 60 deg so that the open end of the capillary tube was parallel to the floor of the dissection dish during the drafting of dissected segments of seminiferous tubules into the pipette. The open end of the capillary tube was fire polished to prevent the rough edges damaging the tubule. In initial experiments fibres were used to hook the tubules from the dissection medium but this resulted in tubule damage and so this method was abandoned. In the latter case the tubule could be further damaged when lifted out of the medium as the surface tension contributed to the tearing between the germinal epithelium and the myoid layer. Great care was taken to exclude segments that were damaged or stretched. Subsequent to the removal of the testis the animal was killed by i.v. injection of a lethal dose of pentobarbitone.
Electrophysiology
Perfusion technique.
Custom-made micropipettes used for perfusing the seminiferous tubules were fabricated from R6 glass (Drummond Scientific Company, Broomall, PA, USA), using a custom-made microforge, as previously described for the perfusion of renal tubules (Burg et al. 1966, 1968; Isaacson et al. 1989). Figure 1A shows a set of three concentric micropipettes which could be axially changed with respect to one another. The tubule, after being expelled from the mechanical aspirator into the perfusion bath, had one end attached to the outer holding pipette by the gentle aspiration of bath fluid. The snugness of fit of the tubule to the outer holding pipette was augmented by the presence of unpolymerized Sylgard 184 (Dow Corning) lining the inside of the outer holding pipette tip. The latter arrangement allowed the inner perfusion pipette to be moved into the lumen of the tubule after it was attached to the outer holding perfusion pipette.
Figure 1.
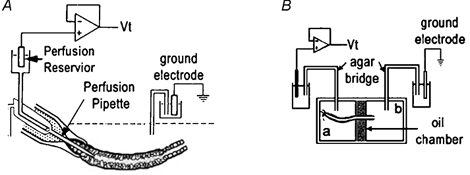
Schematic diagrams of techniques
A, tubules were attached by suction to the holding pipette, followed by the insertion of the inner perfusion pipette. The perfusion flow rate was adjusted by either lowering (slower) or raising (faster) the reservoir attached to the perfusion pipette. The agar probe technique simply involved the replacement of the perfusion pipette with a pipette of similar dimensions filled with agar. B, the oil-gap apparatus consisted of a central oil chamber electrically isolating two lateral chambers (a and b). The Ringer solution in chamber b was in electrical continuum with the luminal fluid and thus measuring the potential between chambers a and b was indicative of the Vt.
Electrical measurements during perfusion.
As the perfusate (Ringer solution) provides a electrical continuum between the perfusate reservoir and the luminal environment the inner perfusion pipette was used to simultaneously perfuse the lumen of isolated seminiferous tubules and to measure the transepithelial potential (Vt). The unpolymerized Sylgard, which lines the inside of the outer perfusion pipette, ensured that the lumen was electrically insulated from the bath Ringer solution. Vt was amplified and displayed on the digital panel meters of an Axoclamp-2A amplifier (Axon Instruments, Inc.). A Pentium computer running pCLAMP 6 software (Axon Instruments, Inc.) and using a Tl-1 Lab Master interface (Axon Instruments, Inc.) was used to digitally capture the data and graphically display the recorded Vt.
All headstages used to measure Vt had an input impedance of greater than 1010 Ω. The bath was grounded to earth via an agar bridge and calomel electrode to the HS2 headstage (Axon Instruments, Inc.; gain: × 1). The entire setup, excluding the amplifier, was placed within an earthed Faraday cage in order to exclude as much electrical noise as possible. Earth loops were avoided by attaching all earths to one point on the oscilloscope (Leader Electronics Corp., Japan).
Aeration and temperature regulation during perfusion.
To avert damaging the fragile tubules by bubbling air into the perfusion bath the Ringer solution was constantly aerated with a 95 %O2-5 %CO2 air mixture in the reservoir above the perfusion bath. Ringer solution flowed into the perfusion bath by gravity via a set of coiled tubes (Narishiga Biowarmer, model MT-2). The tubes were positioned on top of the heater glass of the biowarmer stage, enabling the inflowing Ringer solution to be heated to the same temperature as the Ringer solution in the perfusion bath. The bath was prevented from overflowing by the automated removal of the excess Ringer solution at the other end. Thus, the bath Ringer solution was constantly replaced preventing any temperature and ionic concentration transients in the bath.
Perfusate reservoir.
A 10 ml reservoir, which contained the perfusate, was connected via a tube to the perfusion pipette. The reservoir could be vertically adjusted to regulate the hydrostatic pressure which, in turn, affected the rate of perfusion. The hydrostatic pressure was found to be directly proportional to the rate of perfusion.
Non-perfusing techniques
Agar probe technique.
This technique was designed to position a non-perfusing pipette into the lumen of the seminiferous tubule, which could simultaneously act as a lumen electrode. Essentially, the inner perfusion pipette (Fig. 1A) was replaced with a pipette of similar shape filled with agar (3 g agar per 100 ml perfusate Ringer solution). An agar-filled tube attached the pipette to the perfusion chamber which was filled with 3 m KCl and contained a calomel electrode, which in turn, was attached to the Vt headstage. This arrangement, used in conjunction with the perfusion apparatus, allowed the positioning of the agar probe within the lumen of the seminiferous tubules and permitted the recording of the transepithelial potential (Vt) while not interfering with the luminal environment.
Oil-gap technique.
The oil-gap technique was developed by an entomologist (Ramsay, 1954) and has subsequently been modified to measure Vt in Malpighian tubules (O'Donnell & Maddrell, 1984) and salivary glands (Berridge et al. 1975). The design of the oil-gap technique (Fig. 1B) allows less interference with the physiological status quo of the tubule than any of the other in vitro electrophysiological techniques used in this study. In brief, a segment of tubule (6-10 mm), with one end tied, was carefully dissected from the testis and transferred to the Perspex oil-gap bath which consisted of three compartments, the central one filled with non-conducting liquid, viz. liquid paraffin, while the two lateral compartments were filled with Ringer solution (Isaacson & Nicolson, 1989) (Fig. 1B). In order to measure Vt the tubule was positioned across the three compartments with the two ends bathed in Ringer solution, and each electrically insulated from the other by the liquid paraffin-filled central compartment. The tubule was positioned across these compartments in such a manner that the tied-off end of the tubule was the longer segment, while the open end formed the shorter segment. The temperature of the bath Ringer solution was regulated to 35 °C. The Ringer solution on the tied segment of the tubule was aerated with 5 %CO2-95 %O2. Two agar bridges connected the lateral baths to calomel half-cells which in turn were connected to the headstage of the Axon amplifier (Axoclamp-2A, Axon Instruments) which recorded the interelectrode potential (equivalent to Vt). As far as possible care was taken to avoid the shortcomings of this technique (Isaacson & Nicolson, 1989). The bath was positioned under a stereo dissection microscope (Vickers Instruments, Japan). The entire setup, excluding the amplifier, was placed within an earthed Faraday cage in order to exclude as much electrical noise as possible. The inherent problems with the oil-gap technique were minimized by keeping the open end of the tubules as short as possible (approximately 3–4 mm from the oil gap) as a routine precaution. This limits the potential generated in the tubule distal to the central oil bath, thereby minimizing the subtractive effects of that segment of tubule on the Vt measured across the central oil bath. Isaacson & Nicolson (1989) proved that the segments of the Malpighian tubule on either side of the oil bath acted as opposing batteries connected in series. Thus, the interelectrode potential (i.e. the potential measured across the central oil bath with only the tubule connecting the two outer baths) reflects only the difference in potential generated by tubule segments on either side of the oil bath. Therefore, the standardization of the length of the open-ended portion of the tubule resulted in Vt data which were more uniform in their distribution.
Experimental design
Tubules were generally exposed to two types of experimental technique prior to being processed for their gross morphology.
Perfused tubules.
A set of experiments was designed to test the effects of different perfusion pressures on the morphology of the germinal epithelium of rat seminiferous tubules. Tubules were dissected out and perfused. During these experiments, tubules were perfused under control conditions and no experimental permutations were carried out on the tubules. All tubules were perfused for up to 40 min and then subjected to the tissue-fixing protocol described below.
Non-perfused tubules
Two types of ‘non-perfused’ experiments were carried out: (i) oil-gap experiments and (ii) the luminal agar probe experiments. Subsequent to ≈30 min of Vt recording tubules were taken directly from their experimental set-up and subjected to the tissue-fixing protocol described below.
Controls.
A primary and a secondary control were used during this study. In the primary control a rat (n = 6) was anaesthetized and one testis removed and placed immediately into 2.5 % glutaraldehyde in 0.1 m PO4 buffer. Subsequently, segments of seminiferous tubules (3-5 mm long) were dissected out and placed in this medium on ice for 2 h for initial fixation (primary control study — 12 tubules). The secondary control involved the removal of the seminiferous tubules from the testis, which were then placed for about 30 min in the perfusion bath (12 tubules from 6 rats). The morphology of the primary and secondary control tubules did not differ and was subsequently used to evaluate the morphology of perfused and non-perfused tubules (Fig. 3A and B).
Figure 3.
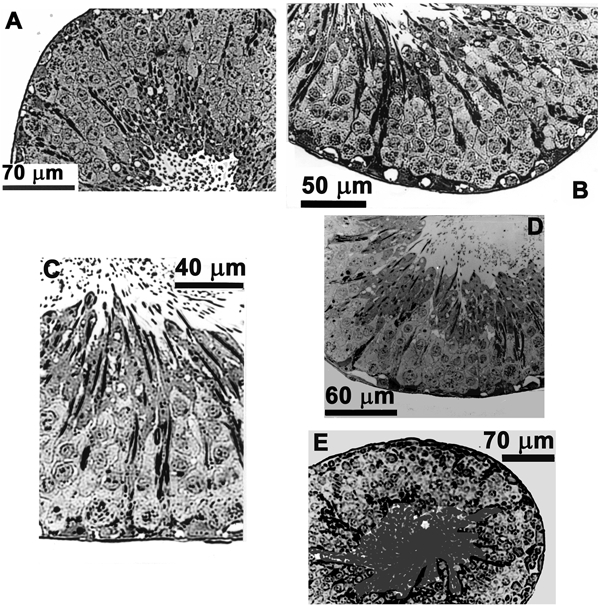
Effects of slow perfusion rates and alternative techniques
A, cross-section of a tubule which, immediately after testis dissection, underwent tissue processing for microscopy. These tubules also served as a primary control. B, this tubule was placed in the perfusion bath along with a perfused tubule. These tubules did not differ morphologically from primary controls and served as a secondary control. C, a slowly perfused tubule (0.14 μl min−1). D, cross-section of a tubule exposed to the agar probe technique. E, a tubule exposed to the oil-gap technique. None of these tubules differed morphologically from the controls.
Tissue fixing.
The perfused and non-perfused tubules were subjected to an experimental electrophysiological procedure before being prefixed for 2 h in 2.5 % glutaraldehyde in a 0.1 m phosphate buffer (20 ml of 0.2 m NaH2PO4, 80 ml of 0.2 m Na2HPO4) (Dym & Fawcett, 1970). All seminiferous tubules were then rinsed twice in a 0.1 m phosphate buffer for a period of 15 min at room temperature. Specimens were then post-fixed in 1 % osmium tetroxide in a 0.1 m buffer for 60 min at room temperature, rinsed twice in distilled phosphate water for a period of 20 min, stained en bloc with 2 % uranyl acetate in 70 % ethanol (this process required two rinses for a period of 15 min) and dehydrated in ascending concentrations of ethanol: two washes in 80 % ethanol for 10 min, two washes in 95 % ethanol for 10 min, three washes in 100 % ethanol for 30 min and finally two washes in acetone for 30 min. The tubules were then impregnated in a 1 : 1 ratio of acetone to Spurr resin (Taab Laboratories Equipment Ltd, UK) for 12 h at room temperature, followed by impregnation with fresh Spurr resin for 1 h at room temperature, and then impregnation with fresh resin for 5 h under a vacuum. Finally, specimens were embedded in oven-dried moulds with fresh resin which was left to polymerize for 16 h at 60 °C. Semithin sections (1 μm thick) were cut using an ultra-microtome (Reichert ultratome-OMU3) and prepared for microscopy.
Data presentation.
All data are presented as the mean ± s.d.
Solutions.
Standard bathing Ringer solution contained (mm): 103 NaCl, 25 NaHCO3, 19 sodium gluconate, 1 sodium acetate, 1.2 NaH2PO4, 5 KCl, 1 CaCl2, 1 MgCl2 and 5.5 glucose at pH 7.4. Perfusate Ringer solution contained (mm): 57 NaCl, 42 sodium gluconate, 1.2 NaH2PO4, 40 KCl, 10 choline chloride, 1 CaCl2, 1 MgCl2 and 5.5 glucose at pH 7.4.
RESULTS
Perfusion studies
During our initial perfusion experiments large quantities of cellular debris emerged from the distal end of the perfused tubule, which resulted in the perfusion of the surrounding myoid layer (Fig. 2A and B). To investigate this phenomenon a series of experiments was designed to test the effects of different perfusion rates on the transepithelial potential (Vt) and the morphology of the tubule.
Figure 2.
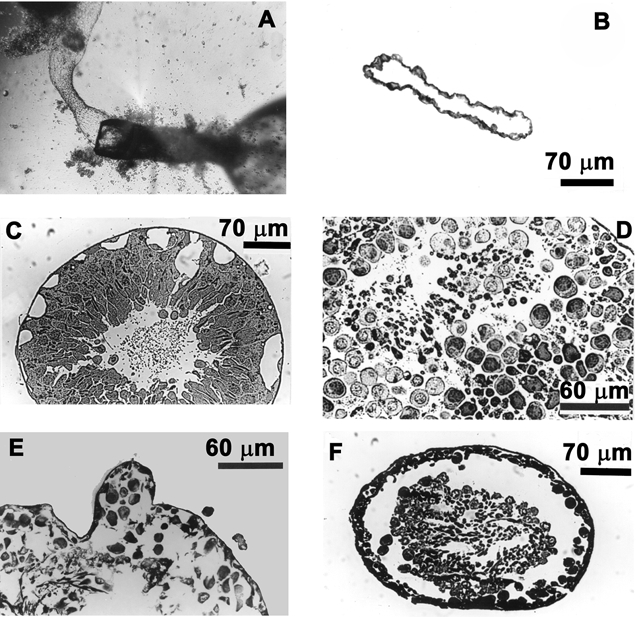
Effects of high perfusate flow rates
A, the perfusion pipette with the attached myoid tubule subsequent to the ejection of the germinal epithelium. B, cross-section of a seminiferous tubule exposed to high perfusate flow rates (0.68 μl min−1). Note that only the outer myoid layer remains resistant to high perfusate flow rates. C, decreasing the flow rate to 0.42 μl min−1 resulted in the formation of large vacuoles leading to the breakdown (D and E) and eventual ejection of the germinal epithelium (F).
High perfusate flow rates destroy germinal epithelium.
In the high perfusate flow rate category, tubules were perfused at 0.68 and 0.42 μl min−1. The morphology of both sets of tubules will be described collectively, as there was no significant difference between them in terms of effects of perfusion on tubular morphology and their Vt (P > 0.05). These tubules exhibited a wide range of morphological damage, which ranged from large vacuoles forming within the germinal epithelium to the stripping off of the entire germinal epithelium (Fig. 2B-F). The most severe morphological damage was represented by spermatids and other cellular debris constantly washed out at the distal open end of the perfused tubule (Fig. 2A), the proximal end of the tubule being attached to the perfusing pipettes. The shearing force of the perfusate appeared to tear the germinal epithelium away from the basement membrane. After some 20–30 min of the extrusion of cellular contents, the outer myoid layer was the only tubular structure left (Fig. 2B). This outer myoid layer was able to withstand high perfusion pressures and also exhibited a small voltage (< −1 mV). The polygonally shaped cells of the perfused myoid layer could be clearly seen through the inverted microscope at low magnification (× 44) and furthermore, based on the fact that they excluded Trypan Blue in the perfusate, they certainly appeared viable throughout the period of perfusion.
The morphological study showed that fast perfusion rates caused shear stresses that resulted in the formation of large vacuoles (Fig. 2C), tearing of the epithelium (Fig. 2F) and massive separation of the cells of the epithelium (Fig. 2D and E), eventuating in the extrusion of the germinal epithelium.
Vt was recorded directly before tubules were subjected to tissue processing for morphology observations. The mean Vt for the tubules perfused at 0.68 and 0.42 μl min−1 was 0.89 ± 3.2 mV (n = 7) and −0.64 ± 1.5 mV (n = 9), respectively. Statistically (one-way ANOVA) these groups were not significantly different from each other (P < 0.0001).
Low perfusate flow rates preserve the germinal epithelium.
In the group of tubules perfused at 0.14 μl min−1 the morphology of the germinal epithelium (Fig. 3C) compared favourably with the morphology of the control tubules (Fig. 3A). These tubules did not develop large vacuoles within the germinal epithelium, there were no signs of tearing of the germinal epithelium from the basement membrane and, perhaps more significant, evaluation of the viability of the apical (lumen) membranes showed spermatids still attached to Sertoli cells. The mean Vt for these tubules was −5.4 ± 1.8 mV (n = 17). The Vt of this group differed significantly (one-way ANOVA, P < 0.0001) from the Vt measured in the high flow rate perfused groups.
Validation using non-perfusing techniques
Two additional electrophysiological methods, which did not involve the perfusion of the tubule, were employed to measure Vt and to corroborate the findings of the perfusion experiments.
Oil-gap technique.
The design of the oil-gap technique (Fig. 1B) allowed less interference with the physiological status quo of the tubule than any of the other in vitro electrophysiological techniques used in this study.
At the end of a Vt recording session these tubules (Fig. 3E) did not differ morphologically from the control tubules (Fig. 3A and B). The mean Vt for these tubules (-5.06 ± 2.4 mV; n = 9) did not differ significantly (P < 0.0001) from the slowly perfused tubules (0.14 μl min−1).
Agar probe technique.
As this technique was identical to the perfusion experiments in all other respects it provided a control for possible perfusion-associated damage to tubules. Use of the agar probe to investigate the Vt did not cause any of the morphological damage seen previously in tubules (Fig. 3D). An additional benefit of this technique is that it did not in any way influence the luminal milieu. Vt measured with this technique yielded a mean of −5.7 ± 1.4 mV (n = 8) which did not differ significantly (P < 0.0001) from Vt measured in the slowly perfused tubules and the oil-gap group of experiments.
In vivo and in vitro perfusion rates
In the current study, we attempt to show that a relationship exists between the damage to the germinal epithelium (whether incurred via physiologically abnormal perfusion rates or injection of any other material) and the Vt. In vivo perfusion rates for single seminiferous tubules have been estimated at 0.017 μl min−1 (1 μl h−1) (Setchell et al. 1994). Although, the lowest in vitro perfusion rate of 0.14 μl min−1 in this study is still many times that of the suggested in vivo rate, we show that there is no significant difference (ANOVA, P < 0.0001) between both the morphology and the recorded Vt of these tubules (0.14 μl min−1) and tubules which have been allowed to perfuse by virtue of their own secretions into the lumen (oil-gap, agar probe techniques and control tubules). However, to avoid perfusion-related morphological damage it would be judicious to perfuse seminiferous tubules at rates closer to the in vivo rate and not higher than 0.14 μl min−1.
Summary of results
The mean Vt values recorded using these non-perfusing electrophysiological techniques did not differ significantly (P < 0.0001) from each other or from the mean Vt of the slowly perfused group (0.14 μl min−1). However, the mean Vt of the combined slowly perfused group and alternative technique groups differed significantly from the mean Vt of the fast-perfused groups (P < 0.0001). The tubules from the fast-perfused groups also showed extensive morphological damage whereas the morphology of the other groups did not differ significantly from their controls.
The Vt of the fast-perfused groups were also much closer to 0 mV than in any of the other groups. The data showed that tubules with very low Vt were not morphologically viable and the integrity of the germinal epithelium had been compromised. As seminiferous tubules are very fragile it is quite possible to damage them during the experimental process. Therefore, Vt could be used as a valuable indicator of the integrity of the isolated perfused tubule.
DISCUSSION
The unique composition of the seminiferous tubule luminal fluid is an essential prerequisite for the process of spermatogenesis. The data in this paper challenges the current thought that fluid within the seminiferous tubules is formed as a result of the dilution between the primary fluid secreted by the germinal epithelium of seminiferous tubules and the fluid secreted by distal structures of the testis, viz. the rete testis and/or the straight tubules of the testis (Tuck et al. 1970).
The current morphological data present the germinal epithelium as fragile and easily compromised by the shear forces of fast-flowing perfusate. Destruction of the germinal epithelium yielded a Vt of ±1 mV. In contrast, slowly perfused tubules and non-perfused tubules with an intact germinal epithelium produced a mean Vt of greater than −5 mV. This suggests that the higher Vt indicates an intact germinal epithelium and that a low Vt is indicative of a compromised epithelium.
Furthermore, there is considerable agreement between the mean Vt values obtained in this study and the literature. The average Vt for oil-gap, agar probe and slow rate perfusion techniques were −5.06, −5.66 and −5.42 mV, respectively. Levine & Marsh (1971) and Gladwell (1977), using microelectrodes (without injecting oil into the lumen), reported mean Vt values of approximately −4.8 and −5.86 mV, respectively, while Tuck et al. (1970), also using microelectrodes, reported Vt of −7.4 mV for free flow fluid and −1.2 mV for primary fluid (oil injected into the lumen). In view of the data presented above, this low Vt value (-1.2 mV) for primary fluid may have arisen as a result of epithelium damage caused by the injection of oil into the tubules. Supporting this line of reasoning Henning & Young (1971) reported that injection pressures of more than 300 mmHg had to be applied to move the oil along the tubular lumen. This, in all probability, disrupted the apical membranes of the Sertoli cells, resulting in the mixing of FFF and intracellular fluids. This view is endorsed by examining the ionic content of the two fluids: in brief, the PF had an ionic content (Na+/K+/Cl−: 38/112/62 mequiv l−1; Tuck et al. 1970) which differed significantly from the FFF (Na+/K+/Cl−: 108/40- 50/120 mequiv l−1; Tuck et al. 1970; Levine & Marsh, 1971). The suspiciously high potassium concentration (112 mequiv l−1) of the PF probably arose from compromised apical Sertoli cell membranes which led to the mixing of fluid between the intracellular and luminal compartments. In another study, perfusion fluid of various electrolytic compositions, injected so as to split the injected oil column in the lumen into two, did not resemble the ionic composition of PF after 90–120 min, although equilibrium conditions prevailed within 30–60 min (Henning & Young, 1971). Furthermore, water and electrolyte secretion decreased to zero within 30–60 min (Tuck et al. 1970; Levine & Marsh, 1975). Both of these latter incidences suggest an epithelium that has been compromised. However, as no morphology study was carried out on tubules injected with columns of oil, the morphological state of the ‘Tuck’ tubules remains unknown.
Additional morphological evidence supporting the theory that fluid secreted by the cells of the seminiferous tubule is not diluted by fluids secreted by distal testicular structures is the existence of an anatomical valve-like structure found at the transitional epithelium between the seminiferous tubules and the tubuli recti which preempts the flow of fluid into the seminiferous tubule (Lindner, 1982). Moreover, in many species the length of seminiferous tubules (rat, ±1 m; man, 30–70 cm) (Wing & Christensen, 1982) obviates a gross dilution mechanism in order to regulate the highly specialized luminal micro-environment which, in turn, is crucial to the process of spermatogenesis (Waites & Gladwell, 1982; Jégou, 1992).
Our data indicate strongly that the injection of oil into seminiferous tubules must have compromised the apical membranes of the Sertoli cells resulting in the formation of a PF which differs in ionic composition from the FFF.
A revised hypothesis
The presented evidence necessitates a revised hypothesis on the formation of luminal seminiferous fluid, viz. that the germinal epithelium of seminiferous tubules secretes a primary fluid which is equivalent to the normally present ‘free flow’ fluid and which is not diluted by secretions of distal testicular structures. The current data suggest that the difference in composition between the hypothetical ‘primary’ and ‘free flow’ fluids probably arose as a result of an experimental artefact.
Acknowledgments
We thank Professor Gerhard van der Horst for his support and advice during this project. We also want to thank the National Research Foundation (South Africa) and the Medical Research Council (South Africa) for their financial support during this study.
REFERENCES
- Berridge MJ, Lindley BD, Prince WT. Membrane permeability changes during stimulation of isolated salivary glands of Calliphora by 5-hydroxytryptamine. Journal of Physiology. 1975;244:549–567. doi: 10.1113/jphysiol.1975.sp010812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB, Grantham J, Ambramow MA, Orloff J. Preparation and study of fragments of single rabbit nephrons. American Journal of Physiology. 1966;210:1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Burg MB, Isaacson L, Grantham J, Orloff J. Electrical properties of isolated perfused rabbit renal tubules. American Journal of Physiology. 1968;215:788–794. doi: 10.1152/ajplegacy.1968.215.4.788. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biology of Reproduction. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Gladwell RT. The effect of temperature on the potential difference and input resistance of rat seminiferous tubules. Journal of Physiology. 1977;268:111–121. doi: 10.1113/jphysiol.1977.sp011849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning RD, Young JA. Electrolyte transport in the seminiferous tubules of the rat studied by the stopped-flow micro-perfusion technique. Specialia. 1971;27:1037–1039. doi: 10.1007/BF02138866. [DOI] [PubMed] [Google Scholar]
- Isaacson L, Nicolson S. A reappraisal of the oil-gap technique for the measurement of transtubular potentials in insect epithelia. Journal of Experimental Biology. 1989;141:429–440. [Google Scholar]
- Isaacson LC, Nicolson SW, Fisher DW. Electrophysiological and cable parameters of perfused beetle Malpighian tubules. American Journal of Physiology. 1989;257:R1190–1198. doi: 10.1152/ajpregu.1989.257.5.R1190. [DOI] [PubMed] [Google Scholar]
- JÉGOU B. The Sertoli cell. Bailliere's Clinical Endocrinology and Metabolism. 1992;6:273–311. doi: 10.1016/s0950-351x(05)80151-x. [DOI] [PubMed] [Google Scholar]
- Koeppen BM. Electrophysiology of collecting duct H+ secretion: effect of inhibitors. American Journal of Physiology. 1989;246:F79–84. doi: 10.1152/ajprenal.1989.256.1.F79. [DOI] [PubMed] [Google Scholar]
- Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. Journal of Physiology. 1971;213:557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine N, Marsh DJ. Micropucture study of the fluid composition of ‘Sertoli cell-only’ seminiferous tubules in rats. Journal of Reproduction and Fertility. 1975;43:547–549. doi: 10.1530/jrf.0.0430547. [DOI] [PubMed] [Google Scholar]
- Lindner SG. On the morphology of the transitional zone of the seminiferous tubule and the rete testis in man. Andrologia. 1982;14:352–362. doi: 10.1111/j.1439-0272.1982.tb02277.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell MJ, Maddrell HP. Secretion by the Malpighian tubules of rhodius prolixus stal: Electrical events. Journal of Experimental Biology. 1984;110:275–290. doi: 10.1242/jeb.110.1.275. [DOI] [PubMed] [Google Scholar]
- Ramsay JA. Active transport of water by the Malpighian tubules of the stick insect, Dixippus morosus (Orthoptera, Phasmidae) Journal of Experimental Biology. 1954;31:104–113. [Google Scholar]
- Setchell BP, Maddocks S, Brooks DE. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Vol. 1. New York: Raven Press Ltd; 1994. pp. 1063–1175. chap. 18. [Google Scholar]
- Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. Journal of Clinical Investigations. 1995;96:2373–2379. doi: 10.1172/JCI118294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck RR, Setchell BP, Waites GMH. The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflügers Archiv. 1970;318:225–243. doi: 10.1007/BF00593663. [DOI] [PubMed] [Google Scholar]
- Waites GMH, Gladwell RT. Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiological Reviews. 1982;62:624–671. doi: 10.1152/physrev.1982.62.2.624. [DOI] [PubMed] [Google Scholar]
- Wing T-Y, Christensen AK. Morphometric studies on the rat seminiferous tubules. American Journal of Anatomy. 1982;165:13–25. doi: 10.1002/aja.1001650103. [DOI] [PubMed] [Google Scholar]


