Abstract
How patterns of odour-evoked glomerular activity are transformed into patterns of mitral cell action potentials (APs) in the olfactory bulb is determined by the functional connectivity of the cell populations in the bulb. We have used paired whole-cell voltage recordings from olfactory bulb slices to compare the functional connectivity of mitral cells to the known anatomy of the mitral cell network. Both inhibitory and excitatory coupling were observed between pairs of mitral cells. Inhibitory coupling was seen as an increased frequency of small, asynchronous GABAergic IPSPs following APs in the presynaptic cell. Excitatory coupling was short in latency, beginning about 1.3 ms after the presynaptic AP and was mediated by both NMDA and AMPA receptors. Mitral cell pairs were coupled by excitation if and only if their apical dendrites terminated in the same glomerulus. The excitatory coupling between mitral cells resembles conventional fast synaptic transmission in its time course, amplitude and latency, despite the absence of evidence for anatomically defined synapses between mitral cells.
Within a single glomerulus, axons of olfactory receptor neurons (ORNs) expressing the same odorant receptor synapse on the apical dendritic tufts of about 60 mitral and tufted (M/T) cells (Ressler et al. 1994; Mombaerts et al. 1996; for reviews see Shipley et al. 1995; Shepherd & Greer, 1998). M/T cells relay information about the odour-evoked ORN activity they receive in at least three ways (Fig. 1). Within a glomerulus, glutamate release from mitral cell apical dendrites activates other M/T cells (Isaacson, 1999; Schoppa & Westbrook, 2001) as well as periglomerular cells. Periglomerular cells release GABA and other transmitters (Mugnaini et al. 1984) onto the apical dendrites of M/T cells. Thus, the intraglomerular circuitry forms mono- and disynaptic connections among the M/T cells associated with a single glomerulus (Fig. 1). Across glomeruli, but still within the bulb, the long lateral dendrites of active mitral cells propagate APs (Margrie et al. 2001) and release glutamate onto granule cell dendrites which in turn may inhibit other mitral cells located up to 2 mm away (Orona et al. 1984) from the active mitral cell (Fig. 1). Finally, the axons of M/T cells exit the bulb and transmit odour-evoked activity to the olfactory cortex and other downstream areas (Fig. 1). The intra- and interglomerular circuits in which M/T cells participate function to integrate odour-specific patterns of glomerular activity (Cinelli et al. 1995; Friedrich & Korsching, 1997; Yang et al. 1998; Rubin & Katz, 1999; Uchida et al. 2000; Belluscio & Katz, 2001; Meister & Bonhoeffer, 2001) and thus to determine the temporal evolution of odour-specific patterns of M/T cell activity (Friedrich & Laurent, 2001).
Figure 1.
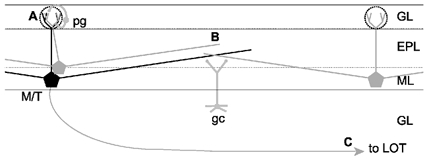
Schematic representation of intra- and interglomerular interactions
A single active olfactory bulb mitral or tufted cell (M/T) will relay its activity to other mitral cells associated with the same glomerulus (A), to mitral cells located in other glomeruli up to 2 mm away (B) and also to downstream brain areas via the lateral olfactory tract (LOT; C). Periglomerular cells (pg) are the main local circuit interneurons mediating intraglomerular inhibition whereas granule cells (gc) are the main local circuit interneurons mediating interglomerular inhibition. Dotted circles indicate borders of individual glomeruli. Dendritic lengths in the drawing are approximately to scale, with the circles around the apical tufts having a diameter of approximately 100 μm. GL, glomerular layer; EPL, external plexiform layer; ML, mitral cell layer; GL, granule cell layer.
Anatomical studies have concluded that there are no direct synaptic connections between mitral cells in mammals (Price & Powell, 1970; Pinching & Powell, 1971b; White, 1972). However, pairs of mitral cells are functionally coupled by both inhibition and excitation. Disynaptic inhibitory (Nicoll, 1969; Isaacson & Strowbridge, 1998), and perhaps excitatory (Didier et al. 2001) connections between mitral cells are mediated by interposed granule and periglomerular cells. Mitral cells also are thought to be coupled via non-synaptic lateral excitation mediated by glutamate spillover from dendritic release sites (Isaacson, 1999; Schoppa & Westbrook, 2001). These connections between mitral cells may serve as a mechanism for generating the slow temporal patterns of mitral cell activity (Friedrich & Stopfer, 2001), and synchronizing the activity of mitral cells associated with the same glomerulus (Carlson et al. 2000; Puopolo & Belluzzi, 2001; Schoppa & Westbrook, 2001). Studies on the synchronization of mitral cell firing in vitro under conditions of increased excitability have provided evidence that mitral cells are functionally connected, and that these connections are specific to mitral cells with apical tufts in the same glomerulus (Carlson et al. 2000; Puopolo & Belluzzi, 2001; Schoppa & Westbrook, 2001).
The mechanisms that underlie the functional connectivity between mitral cells are not well understood. Experiments on self-inhibition and self-excitation of mitral cells (e.g. Friedman & Strowbridge, 2000; Halabisky et al. 2000; Salin et al. 2001) have provided valuable information about the properties of glutamate release from and the activation of glutamate and GABAA receptors on mitral cell dendrites. However, the mechanisms of self and lateral excitation and also self and lateral inhibition may differ substantially. Lateral excitation and inhibition can be investigated in detail only by using paired mitral cell recordings. Moreover, most recent experiments in which lateral coupling between mitral cells was examined directly (Isaacson & Strowbridge, 1998; Schoppa et al. 1998; Isaacson, 1999) have been performed under conditions that favour glutamate release and NMDA receptor activation. Thus, little is known about the properties of these lateral connections under more physiological conditions. Inter- and intraglomerular coupling will have distinct functions in the generation of odour-evoked patterns of mitral cell activity and understanding these functions will be critical to the understanding of the functional circuitry of the olfactory bulb.
Mitral cells associated with the same glomerulus receive similar afferent input from ORN axons (Ressler et al. 1994; Mombaerts et al. 1996) and are thought to show more similar activity patterns than mitral cells associated with different glomeruli (Buonviso & Chaput, 1990). However, this picture may be complicated if intraglomerular inhibition is strong. Knowing the spatial and temporal characteristics of lateral inhibition and lateral excitation is critical for determining how olfactory bulb circuits shape the fast and slow patterns of odour-evoked activity seen in the mitral cell network (Buonviso et al. 1992; Friedrich & Laurent, 2001). Here we use paired whole-cell voltage recordings to examine the properties of lateral inhibition and lateral excitation between coupled pairs of mitral cells. We determine the functional connectivity between single pairs of mitral cells under physiological conditions. Further, we correlate the synaptic interactions observed with the position of the mitral cells in the olfactory bulb, focusing on the question of whether mitral cells that are associated with the same glomerulus interact with each other differently from those that are not.
METHODS
Slice preparation
Horizontal and sagittal olfactory bulb slices (300 μm thick) were prepared from young mice (postnatal days 22-60). Mice were given i.p. injections of anaesthetic (0.1 % ketamine-0.1 % xylazine; ≈3 mg kg−1) until they were non-responsive to foot pinch, and then decapitated. Olfactory bulbs were removed and cut on a vibratome while submerged in ice-cold oxygenated Ringer solution containing (mm): NaCl 125, KCl 2.5, NaHCO3 25, NaH2PO4 1.25, MgCl2 1, glucose 25, CaCl2 2, ascorbate 0.5, pyruvate 1, myo-inositol 2. Slices were transferred to a warm (37 °C) oxygenated incubating bath for 30–60 min and then allowed to equilibrate to room temperature before being transferred to the recording chamber.
Electrophysiology
Whole-cell voltage recordings were obtained from identified mitral cells using infrared differential interference contrast microscopy (Stuart et al. 1993). Slices were superfused continuously with oxygenated Ringer solution containing (mm): NaCl 125, KCl 2.5, NaHCO3 25, NaH2PO4 1.25, MgCl2 1, glucose 25, CaCl2 2, warmed to 34–36 °C. Whole-cell recordings were established using pipettes with resistances of 2–6 MΩ filled with a solution containing (mm): potassium gluconate 120, sodium gluconate 10, Hepes 10, sodium phosphocreatine 10, MgATP 4, NaCl 4, Na2ATP 2 and Na3GTP 0.3 (adjusted to pH 7.3 with KOH). Measurements were made with Axoclamp-2B amplifiers (Axon instruments, Foster City, CA, USA), and data were digitized at 10 kHz using an ITC-16 data acquisition board (Instrutech, Mineola, NY, USA) controlled by custom software written in Igor (WaveMetrics, Lake Oswego, OR, USA). Biocytin (2 mg ml−1, Sigma) and/or the fluorescent dye Alexa 548 hydrazide (20 μm, Molecular Probes) were included routinely in the intracellular solution to allow the morphology of the neurons to be analysed. d-Aminophosphonovalerate (APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and bicuculline were obtained from Tocris Neuramin (Bristol, UK). Reconstructions of mitral cell morphology were performed using Neurolucidia (Microbrightfield, Magdeburg, Germany).
All procedures on animals were performed according to the guidelines of the Max -Planck Society.
RESULTS
Simultaneous whole-cell voltage recordings were obtained from pairs of visually identified mitral cells in olfactory bulb slices. In most cases mitral cell somata were separated by less than 40 μm. Action potentials (APs, 1-20) were elicited in one cell by somatic current injection while the membrane voltage of the second cell was monitored. Groups of 10–50 postsynaptic sweeps were averaged and examined for evidence of changes in membrane potential that corresponded to the time of action potential firing in the presynaptic cell. Current steps were then injected into the second cell and the process was repeated to look for a connection in the opposite direction.
Inhibitory interactions between mitral cell pairs
In about 10 % of pairs tested, activation of trains of APs in one mitral cell resulted in asynchronous, presumed unitary, IPSPs in the second mitral cell (Fig. 2A). The properties of these IPSPs were consistent with those of unitary lateral IPSCs mediated by reciprocal dendrodendritic mitral cell-granule cell synapses (Isaacson & Strowbridge, 1998) and these IPSPs were blocked by bicuculline (20 μm, 8 ± 12 % of control, n = 5). Average lateral IPSPs between mitral cells were small in amplitude, with 6–10 presynaptic APs evoking an averaged response with a peak hyperpolarization of 0.33 ± 0.07 mV (n = 23, Fig. 2C). Single presynaptic APs rarely elicited a measurable response. In part, the small amplitude of the averaged IPSPs was due to variable delay between the presynaptic APs and the evoked IPSPs (Fig. 2A). Trains of presynaptic APs resulted in an increased frequency of unitary IPSPs but the timing of these IPSPs was in most cases not locked to individual presynaptic APs (Figs 2A, 3A and 3C1). IPSPs could usually be detected in both directions, with > 75 % of the connections being reciprocal.
Figure 2.
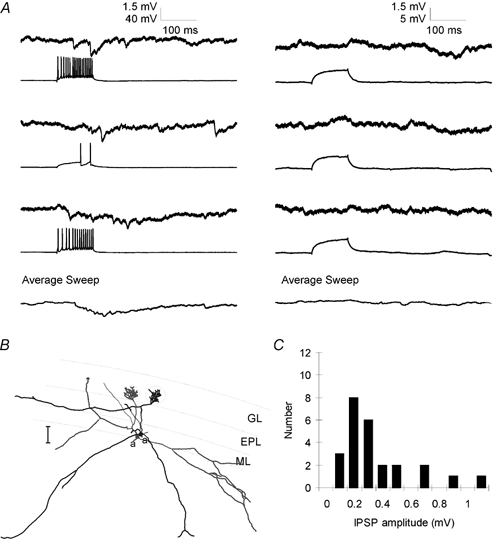
Lateral IPSPs between mitral cells
A, left, single pre- and postsynaptic sweeps from a paired recording from two mitral cells show that stimulation of trains of APs in one mitral cell evoked a series of unitary IPSPs in the other. Individual IPSPs were not well correlated with presynaptic APs and the increase in IPSP frequency outlasted the train of presynaptic APs. Right, sweeps from the same pair of cells in which presynaptic depolarization was subthreshold for AP generation show that the spontaneous rate of unitary IPSPs was low and could not account for the IPSPs observed in part A. Bottom traces show the average of 25 consecutive sweeps. B, reconstructed morphology of the pair of mitral cells from which the data in A were obtained. Axons are indicated by ‘a’. Scale bar is 100 μm. C, histogram showing amplitudes of lateral IPSPs evoked by trains of 6–10 presynaptic APs. Average IPSP amplitude was 0.32 mV.
When mitral cells were coupled by inhibition, the averaged response to repeated presynaptic stimulation was most often a slow rising IPSP with the peak hyperpolarization occurring 100–150 ms after the first AP in a train of APs and decaying over several hundred milliseconds (Fig. 3A, B and C1). In a few mitral cell pairs connected by inhibition (3/25 pairs connected by IPSPs), averaged postsynaptic traces revealed an additional small initial hyperpolarization with a constant latency (3-10 ms) from the first presynaptic AP. These relatively rare, fast inhibitory connections may represent a connection mediated by one of the less numerous types of inhibitory cell found in the bulb, such as van Gehuchten or Blanes cells (Shipley et al. 1995) and were excluded from further analysis. The shape and peak amplitude of the lateral IPSP was very similar for trains of presynaptic APs ranging in duration from 50 to 800 ms (Fig. 3A and B). Thus, APs occurring more than about 50 ms after the beginning of a long train did not produce any additional change in postsynaptic membrane potential. This observation suggests that transmission in the mitral cell to granule cell to mitral cell circuit attenuates markedly over the first 50–100 ms of the train of presynaptic action potentials. Reconstructions of biocytin-filled mitral cells connected by lateral IPSPs revealed that these pairs had broadly overlapping dendritic trees (Fig. 2B and Fig. 3C2), making the location of the synaptic contacts between the mitral cells and the interposed granule cell as yet impossible to determine.
Figure 3.
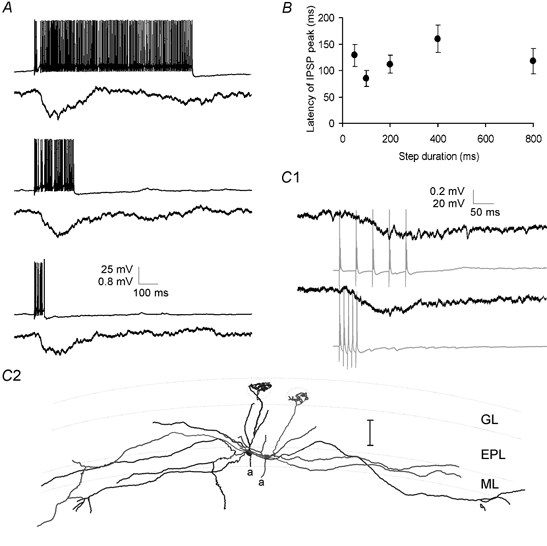
Time course of lateral IPSPs
A, lateral IPSPs were evoked in one mitral cell by trains of APs lasting 50–800 ms in a second cell. The time course and amplitude of the lateral IPSP (average of 7 sweeps) were unaffected by the duration of the train of presynaptic APs (single example sweeps shown). B, group data for four cells showing that in experiments similar to those shown in A the latency from the beginning of the presynaptic current injection to the peak of the lateral IPSP was unaffected by changing the duration of the presynaptic current step. C1, paired recordings from two mitral cells showing inhibitory coupling. Trains of 5 APs evoked at 20 Hz (top) or 100 Hz (bottom) resulted in IPSPs of similar amplitude and time course in the postsynaptic mitral cell. Postsynaptic traces are averages of 6 sweeps. C2, reconstructed morphology of the mitral cell pair from which the data in (C1) were recorded. Scale bar is 100 μm.
Excitatory interactions between mitral cell pairs
Excitatory coupling also was observed between mitral cell pairs and this coupling was always reciprocal (n = 25 pairs, Table 1). The lateral excitation between pairs of mitral cells was large and fast (single AP-evoked response: average amplitude 0.83 ± 0.15 mV, n = 29 connections from 15 pairs, 10–90 % rise time 5.1 ± 0.8 ms, n = 7, average latency from peak of AP to initial rise of the response = 1.3 ± 0.1 ms, n = 15, Fig. 4 and Fig. 5).
Table 1.
Correlated anatomy and connectivity of mitral cells
| Overlapping tufts (n = 19) | Non-overlapping tufts (n = 93) | |
|---|---|---|
| EPSP | 19 | 0 |
| IPSP only | 0 | 18 |
| EPSP and IPSP | 15 (4 undetermined) | 0 |
| No EPSP or IPSP | 0 | 75 |
Figure 4.
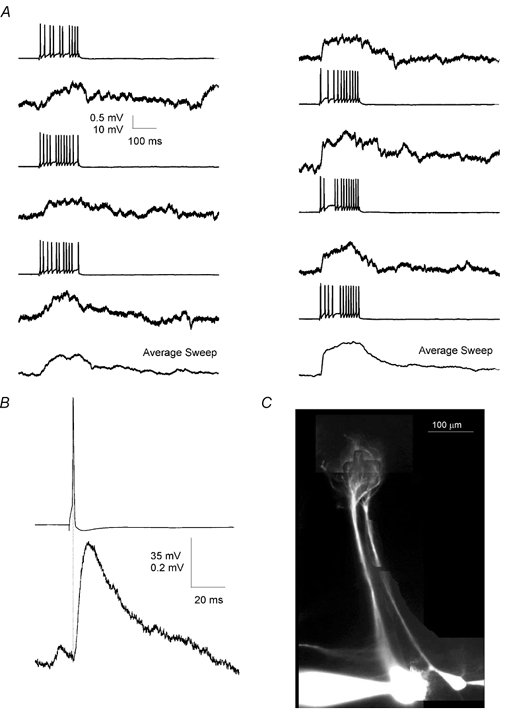
Lateral EPSP between mitral cell pairs
A, reciprocal lateral EPSPs were evoked in two mitral cells by trains of APs in the other mitral cell. Left column shows EPSPs evoked in the first cell by the second cell whereas the right column shows EPSPs evoked in second cell by the first. Bottom traces show the average EPSP evoked in the two directions. B, pre- and postsynaptic sweeps from this pair (n = 8) were aligned in time with respect to the peak of the presynaptic AP and averaged. The postsynaptic EPSP shows short latency and fast rise time. Data are from the connection shown in the right-hand column of A. C, fluorescence image of the two cells from which the data above were obtained. Cells were filled with Alexa 548, 20 μm. The image shows that the cells had nearly parallel apical dendrites and apical tufts that terminated in the same glomerulus.
Figure 5.
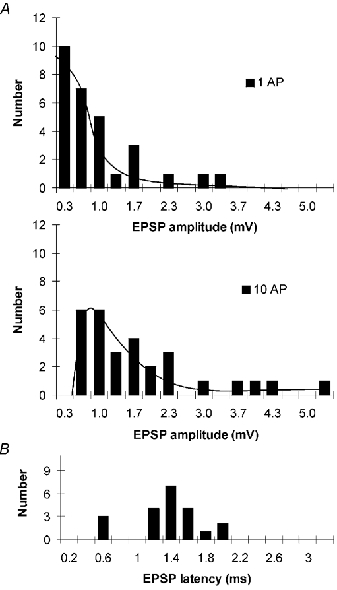
Amplitude and latency histograms of lateral EPSPs
A, histograms showing the amplitudes of lateral EPSPs evoked by single presynaptic APs (upper panel) and trains of 10 APs (lower panel) in mitral cell pairs that were found to have apical dendrites terminating in the same glomerulus. Average single AP-evoked EPSP was 0.83 mV and average EPSP evoked by 10 APs was 1.72 mV. B, histogram showing the latency of the lateral EPSPs. Average latency was 1.34 ms.
Filling connected pairs of cells with either fluorescent tracers or biocytin allowed us to determine whether there was a relationship between mitral cell connectivity and the position or morphology of the mitral cells. Strikingly, in all cases in which morphology was examined (n = 19/19 total pairs, 10/10 pairs filled with fluorescent tracers only, 2/2 pairs filled with biocytin only, 7/7 pairs filled with both fluorescent dye and biocytin), when mitral cell pairs were connected by excitatory connections, the apical dendrites of the two cells terminated with tufts contained in the same glomerulus (Table 1). In none of these 19 pairs was there morphological evidence for axo-dendritic synapses between the mitral cells that were recorded. Mitral cell axons initially projected perpendicularly to the mitral cell layer and were generally cut a short distance from the soma. Axons were never observed to form a close apposition with the soma or dendrite of the other mitral cell.
The above data suggest that excitatory connections between mitral cell pairs occur only between cells with apical dendrites that terminate in the same glomerulus. They do not address the question of whether all mitral cells associated with a given glomerulus are coupled. We examined the morphology of 42 randomly selected pairs of biocytin-filled mitral cells to determine whether all mitral cells with apical tufts sharing the same glomerulus were coupled by excitation. Five pairs of nearby mitral cells were found to have apical tufts in the same glomerulus and excitatory connections were observed between all pairs. That is, there were no mitral cell pairs that were associated with the same glomerulus that were not coupled by excitatory responses. Thus, we conclude that mitral cells are connected by excitatory synapses if, and only if, their apical dendritic tufts are contained in the same glomerulus.
The tight correlation between physiological excitation and convergence of apical tufts suggests that the connection between mitral cells occurs within the glomerulus. To test this hypothesis we used dual somatic and dendritic recordings to identify the site of coupling between mitral cells. After a pair of cells was found to be coupled, one of the somatic electrodes was withdrawn and replaced and a recording was obtained from the apical dendrite of the postsynaptic cell (150-250 μm from the soma). Dendritic single AP-evoked responses recorded in bicuculline were 37 ± 12 % (n = 4, P < 0.05, paired t test, Fig. 6B), larger than those recorded at the soma. Dendritic responses also had shorter latencies (82 ± 8 % of the somatic response, n = 4, P < 0.05, paired t test, Fig. 6B), indicating that the connection between these cells was located in the apical dendrite of the mitral cell, most probably in the apical tuft. Similar experiments have shown previously that synchronous long-lasting depolarizations of mitral cells also originate in the apical tuft (Carlson et al. 2000).
Figure 6.
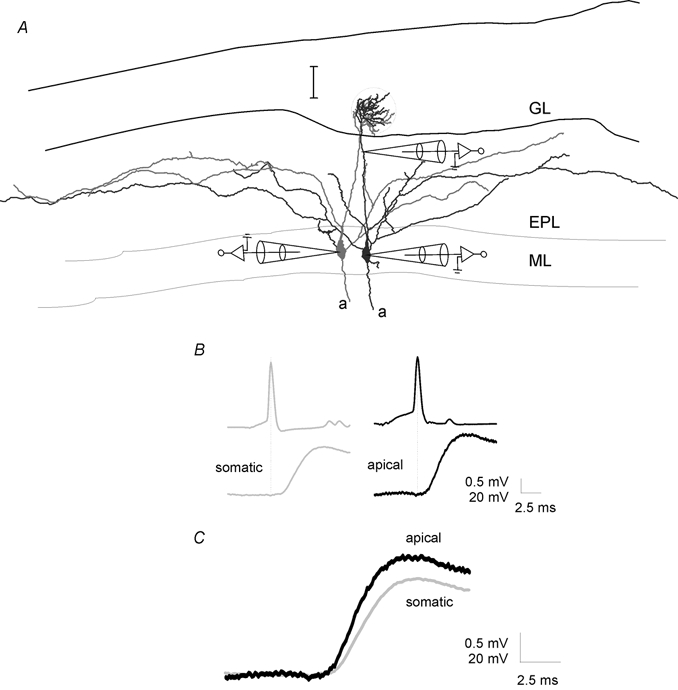
Lateral EPSPs are mediated by synapses on the apical dendrite
A, reconstruction of two mitral cells from which the data in B and C were obtained showing the position of the dendritic electrode. Scale bar is 100 μm. B, lateral EPSPs evoked in bicuculline (20 μm) by single APs were recorded in the soma (left traces) and then, after repatching the cell, 180 μm from the soma, in the apical dendrite (right two traces) of the mitral cell. All traces are averages of 15+ sweeps aligned to the peak of the presynaptic AP. C, same postsynaptic traces as in B shown superimposed and at a larger scale to illustrate latency difference.
In 15 of 19 pairs of mitral cells with tufts in the same glomerulus, clear evidence of IPSPs was also observed following stimulation of action potentials in one of the cells (Fig. 7B, Table 1). In these cases, IPSPs were observed either as a net hyperpolarization following the AP-evoked depolarization (e.g. Fig. 7B) or as an increased rate of unitary IPSPs occurring during the later phases of the excitatory response (e.g. Fig. 8A). In all cases tested (n = 7/7) bicuculline (20 μm) caused an increase in the peak depolarization evoked by trains of APs in mitral cells with co-terminal apical tufts. In contrast bicuculline never revealed excitatory coupling between mitral cells whose apical tufts did not occupy the same glomerulus (n = 10). This result suggests that intraglomerular inhibition is quite prevalent and that its time course is relatively slow. Thus, the net result of intraglomerular connectivity is an initial depolarization lasting tens of milliseconds that is later reduced or reversed by intraglomerular inhibition (see Discussion).
Figure 7.
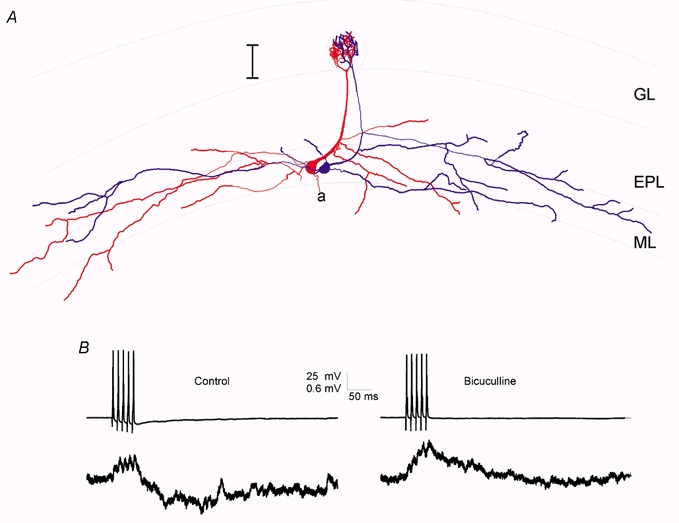
Mitral cells with co-terminal apical dendrites are often connected by both EPSPs and IPSPs
A, reconstruction of two biocytin-filled mitral cells showing that their apical dendritic tufts were located in the same glomerulus. Scale bar is 100 μm. B, pre- and postsynaptic voltage traces from these two cells show that stimulation of APs under control conditions in one cell (left) resulted in an initial depolarization, followed by a longer-lasting hyperpolarization. Right, this hyperpolarization was blocked by addition of bicuculline (20 μm) to the bathing solution.
Figure 8.
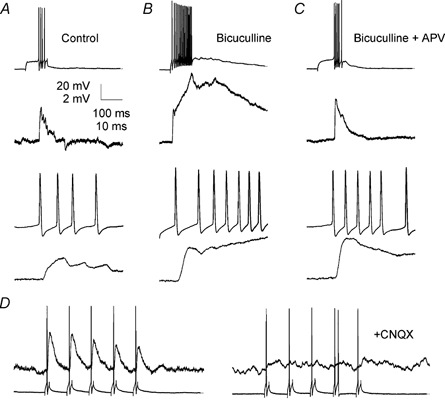
Lateral EPSPs have a large AMPA receptor-dependent component
A, under control conditions presynaptic APs (top) evoked EPSPs in a postsynaptic mitral cell (middle) having its apical tuft in the same glomerulus. Bottom, the same traces are also shown superimposed at an expanded time base. B, addition of bicuculline revealed a large recurrent EPSP in the presynaptic cell (top) and showed that the lateral EPSP observed under control conditions was partially masked by lateral inhibition (middle and bottom). Lateral inhibition reduced the later phases of the lateral EPSP most strongly. C, addition of APV (50 μm) reduced the recurrent EPSP in the presynaptic cell (top) and also reduced the late phases of the lateral EPSP (middle and bottom). The initial peak of the lateral EPSP was, however, unaffected by the APV. D, subsequent to application of bicuculline and APV (left), addition of CNQX (10 μm) blocked the remaining lateral EPSP completely (right).
The properties of the excitatory coupling between mitral cells associated with the same glomerulus are very similar to the properties of classical fast synaptic transmission. However, electron microscopic studies have provided no evidence for the existence of synapses (postsynaptic densities opposed to active zones) between mitral cells in mammals (Price & Powell, 1970). Thus, we investigated whether the excitatory coupling between mitral cells had properties similar to those described for responses due to spillover of synaptically released glutamate onto extrasynaptic glutamate receptors. In previous studies (Kullmann et al. 1996), including studies on mitral cells (Isaacson, 1999), spillover-mediated glutamatergic responses have been characterized by small amplitudes, long rise times (≈50 ms) and complete blockade by NMDA receptor antagonists. In contrast, the amplitude, latency and kinetics of M/T to M/T excitatory coupling were similar to the values reported for other types of synaptic connections (Markram et al. 1997; Feldmeyer et al. 1999). Additionally, the initial peak of single AP-evoked depolarization between mitral cells with co-terminal apical dendrites was not reduced by blockade of NMDA receptors by 50 μm APV (Fig. 8B). Single AP-evoked excitatory postsynaptic potentials (EPSPs) recorded in APV and bicuculline were 115 ± 14 % (P > 0.05, n = 5) of those recorded in bicuculline alone (Fig. 8B and C). Pretreatment with bicuculline was necessary because blockade of NMDA receptors also blocks lateral inhibition between mitral cells (Isaacson & Strowbridge, 1998; Schoppa et al. 1998; Chen et al. 2000; Isaacson, 2001). APV did, however, reduce later components of the excitatory coupling substantially (Fig. 8C). Single AP-evoked EPSPs recorded in CNQX (10 μm) were completely blocked compared to those recorded in bicuculline and APV (4 ± 4 %, P < 0.05, n = 5; Fig. 8D), indicating that these EPSPs were glutamatergic and were mediated by AMPA receptor activation. The CNQX sensitivity of the EPSPs indicates that the coupling between M/T cells is mediated by AMPA or kainate receptors and suggests that the glutamate concentration at the membrane of the postsynaptic mitral cell is sufficient to activate these relatively low affinity receptors.
DISCUSSION
The data presented here describe the properties of unitary connections mediating lateral inhibition and lateral excitation between pairs of mitral cells under physiological conditions. Importantly, we also document a strict correlation between functional connectivity and the position of mitral cells pairs. All mitral cells with apical dendrites terminating in the same glomerulus are reciprocally coupled by a non-synaptic excitatory connection, mediated by AMPA and NMDA receptors. The mutual excitation of mitral cells that receive input from the same glomerulus may be critical for the way in which odour-evoked signals are integrated by olfactory bulb circuits. In contrast, inhibition between mitral cells is less dependent on mitral cell anatomy or position. Lateral inhibition occurs between mitral cells with apical dendrites that occupy the same or different glomeruli. In fact, we observed a higher incidence of inhibitory coupling between mitral cells associated with the same glomerulus than between cells associated with different glomeruli, suggesting that a fraction of the intraglomerular inhibitory connections may be mediated by periglomerular cells.
Fast, non-synaptic excitatory coupling between mitral cells
The observation that mitral cells are coupled by glutamatergic EPSPs is surprising given that electron microscopic studies of olfactory bulb glomeruli have concluded that there are no synaptic contacts between mitral cells in mammals (Price & Powell, 1970; Pinching & Powell, 1971b; White, 1972). However, excitatory coupling provides an explanation for the synchronous oscillations that have been observed between mitral cells having apical tufts in the same glomerulus (Carlson et al. 2000; Puopolo & Belluzzi, 2001; Schoppa & Westbrook, 2001). The lack of anatomical evidence for synaptic connections between mitral cells suggests that the excitatory connection we describe is mediated by spillover of glutamate from the dendritic release sites of one mitral cell onto extrasynaptic glutamate receptors on the second (Aroniadou-Anderjaska et al. 1999; Isaacson, 1999; Carlson et al. 2000; Puopolo & Belluzzi, 2001; Schoppa & Westbrook, 2001). However, the large amplitude, short latency, fast kinetics and large non-NMDA component of the M/T to M/T coupling are different from spillover-mediated responses reported previously (Asztely et al. 1997; Isaacson, 1999). We suggest that excitatory coupling between mitral cells represents a novel variant of spillover-mediated glutamatergic transmission in which the concentration of glutamate reaching the postsynaptic cell is sufficient to activate AMPA and/or kainate receptors in the postsynaptic membrane. AMPA receptor-mediated self excitation has previously been observed (Margrie et al. 2001; Salin et al. 2001; Schoppa & Westbrook, 2001) specifically in mitral cells with intact apical dendrites (Salin et al. 2001). In the case of self-excitation, it is clear that glutamate receptors may be localized near the sites of glutamate release (Sassoe-Pognetto & Ottersen, 2000). The spillover of high concentrations of glutamate from a release site on one mitral cell to receptors on another is, however, unexpected (Asztely et al. 1997; Isaacson, 1999).
Within a glomerulus, the dendritic segments that make up the apical tufts of mitral cells appear to form multiple appositions. Any of these sites of apposition could be the site at which glutamate released from one dendritic release site binds to the AMPA receptors on a postsynaptic mitral cell. The fact that excitatory coupling was always reciprocal suggests either that the densities of presynaptic release sites and postsynaptic receptors are quite high, or else that there is some previously unappreciated structural specialization that mediates synaptic transmission between mitral cells. The large AMPA component, short latency (1.3 ms) and short rise time (5.1 ms) of M/T to M/T responses suggests that the distance from the site of glutamate release to the site where the glutamate binds to postsynaptic receptors is small. Models of diffusion predict that to reach a peak glutamate concentration of approximately 100 μm, and an approximately 30 % open probability of AMPA receptors, a diffusional distance of less than about 300 nm is required (Barbour & Hausser, 1997; Rusakov et al. 1999). The spillover of high concentrations of glutamate onto extrasynaptic non-NMDA receptors may be facilitated by the ensheathing of glomeruli and of dendritic zones within glomeruli by glial processes (Pinching & Powell, 1971a; Chao et al. 1997) as has been suggested to occur at other specialized synapses (Kinney et al. 1997; Mitchell & Silver, 2000). Conversely, the separation of individual glomeruli by glial processes may prevent the spillover of glutamate from a presynaptic site in one glomerulus to a postsynaptic site in another.
Our data suggest that at least one such connection occurs between every pair of mitral cells whose apical dendrites occupy the same glomerulus and thus that there are several hundred of these ‘synapses’ per glomerulus. Such numerous connections are unlikely to have been missed in all of the ultrastructural studies performed on the mammalian olfactory bulb. Because the excitatory coupling occurs if, and only if, mitral cells have apical dendrites terminating in the same glomerulus, excitatory connections between mitral cells are most likely to be mediated by glutamate release from dendritic release sites in the apical dendritic tufts of mitral cells, as has been suggested previously (Carlson et al. 2000; Schoppa & Westbrook, 2001). This conclusion is supported by our observation that the depolarization of a postsynaptic mitral cell is larger and occurs at a shorter latency in the apical dendrite than at the soma. The short average latency from presynaptic AP to postsynaptic EPSP (1.3 ms) suggests that the mitral cells are connected monosynaptically (compare to e.g. Markram et al. 1997; Thomson & Deuchars, 1997; Feldmeyer et al. 1999). These results provide a mechanism for the synchronous long-lasting depolarizations that have been observed in mitral cells with dendrites that occupy the same glomerulus (Carlson et al. 2000; Puopolo & Belluzzi, 2001; Schoppa & Westbrook, 2001) and which show a similar dendritic localization (Carlson et al. 2000). Indeed, when inhibition was blocked, we were able to activate long-lasting depolarizations of postsynaptic mitral cells by stimulation of trains of APs in single presynaptic mitral cells (e.g. Fig. 8).
Non-synaptic coupling as a mechanism for synchronous activation
Several previous studies have provided evidence for excitatory coupling between mitral cells. Synchronous long-lasting depolarizations have been observed in pairs of mitral cells when NMDA currents are enhanced or when inhibition is blocked (Nowycky et al. 1981; Carlson et al. 2000; Puopolo & Belluzzi, 2001; Schoppa & Westbrook, 2001). These synchronous depolarizations seem to originate from the apical tufts, and are blocked by glutamate receptor antagonists (Carlson et al. 2000), but the origin of the glutamate has not been determined. Direct excitatory coupling mediated exclusively by NMDA receptors has also been observed between pairs of simultaneously recorded mitral cells by Isaacson (1999). In contrast to this report, the excitatory coupling between mitral cells with co-terminal apical dendrites described here has a large AMPA receptor component, and a much faster rise time. The experimental conditions used by Isaacson (0 mm Mg2+, TTX, 20–50 ms voltage steps used to elicit release) differ substantially from ours (1 mm Mg2+, no TTX, APs used to elicit release). These differences in conditions may alter glutamate release from mitral cell dendrites and will favour the detection of small amplitude NMDA receptor-mediated EPSCs between pairs of mitral cells. In this previous report, excitatory coupling was seen between more than half of all the pairs tested (Isaacson, 1999). In pairs of mitral cells with somata separated by less than 40 μm, we observed less than 20 % of the pairs to have apical dendrites that terminated in the same glomerulus. Thus, the NMDA receptor-mediated connections described by Isaacson (1999) may represent lateral excitation between mitral cells with apical dendritic tufts that are not associated with the same glomerulus. Interestingly, recent work has suggested that glutamaterigic self-excitation also differs between the apical and lateral dendrites of mitral cells. Glutamate released from lateral dendrites of mitral cells results in self-excitation mediated solely by NMDA receptors, whereas glutamate released from apical dendrites has a substantial AMPA receptor-mediated component (Salin et al. 2001). In conjunction with our results, this suggests that clearance of extrasynaptic glutamate is less efficient in the glomerular neuropil than in the neuropil of the external plexiform layer, or else that much more glutamate is released by mitral cell apical dendritic tufts than by lateral dendrites. Alternatively, mitral cell tufts could contain non-NMDA glutamate receptors that are unusually sensitive to glutamate.
Role of lateral coupling in regulating olfactory bulb activity
Our results suggest that all of the approximately 20 mitral cells (and perhaps the 40 tufted cells) with apical dendrites that terminate in a given glomerulus are coupled by excitatory connections. These same mitral cells are thought to receive similar inputs because particular kinds of ORNs target their axons to individual glomeruli (Ressler et al. 1994; Mombaerts et al. 1996). Thus the mutual connectivity of this population of mitral cells will reinforce their already similar activity patterns. On average, a single AP in a single mitral cell should result in approximately 1 mV (0.83 mV on average, see Fig. 5) of depolarization in all the other mitral cells associated with the glomerulus. If ORN input results in the synchronous firing of a few mitral cells, the intrinsic connectivity of the mitral cell network will serve to amplify and synchronize the mitral cell response. Additionally, we have observed electrical coupling between mitral cell tufts (data not shown) that may also contribute to functional coupling of mitral cells associated with the same glomerulus. The relatively large amplitude coupling between mitral cells and the fact that mitral cells tend to have resting membrane potentials close to threshold (≈ 5 mV) means that amplification of ORN inputs by mutual excitation of mitral cells is substantial. If an ORN input results in the firing of five mitral and tufted cells (about 10 % of those associated with a single glomerulus) these cells will cause an additional 5 mV of depolarization in the remaining 90 % of the mitral cells associated with the same glomerulus. This depolarization, coupled with the ongoing ORN-mediated depolarization will bring nearly all of the mitral cells in this glomerulus to AP threshold. Thus, the glomerulus will fire as a unit with most of its mitral cells having a similar firing pattern. Feedforward inhibition mediated by periglomerular cells may oppose this amplification, provided that periglomerular cells can be activated rapidly enough to prevent the amplification.
Several hundred milliseconds after an odour-evoked train of APs has begun, mitral cells associated with the same glomerulus will mutually inhibit each other via lateral inhibition (Rall et al. 1966). This mutual inhibition will drive mitral cells associated with the same glomerulus to develop dissimilar firing patterns. Such a time-dependent evolution of mitral cell firing patterns has been observed in recordings from mitral cells of zebra fish and may be essential to the discrimination of olfactory stimuli (Friedrich & Laurent, 2001).
Acknowledgments
This work was supported by the Max-Planck Society, the Alexander von Humboldt Foundation and NIDCD. We thank Troy Margrie, Michael Brecht and Andreas Schaefer for helpful comments on the manuscript.
REFERENCES
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Dendrodendritic recurrent excitation in mitral cells of the rat olfactory bulb. Journal of Neurophysiology. 1999;82:489–494. doi: 10.1152/jn.1999.82.1.489. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends in Neurosciences. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. Journal of Neuroscience. 2001;21:2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso N, Chaput MA. Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. Journal of Neurophysiology. 1990;63:447–454. doi: 10.1152/jn.1990.63.3.447. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Chaput MA, Berthommier F. Temporal pattern analyses in pairs of neighboring mitral cells. Journal of Neurophysiology. 1992;68:417–424. doi: 10.1152/jn.1992.68.2.417. [DOI] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. Journal of Neuroscience. 2000;20:2011–2021. doi: 10.1523/JNEUROSCI.20-05-02011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TI, Kasa P, Wolff JR. Distribution of astroglia in glomeruli of the rat main olfactory bulb: exclusion from the sensory subcompartment of neuropil. Journal of Comparative Neurology. 1997;388:191–210. [PubMed] [Google Scholar]
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Cinelli AR, Hamilton KA, Kauer JS. Salamander olfactory bulb neuronal activity observed by video rate, voltage-sensitive dye imaging. III. Spatial and temporal properties of responses evoked by odorant stimulation. Journal of Neurophysiology. 1995;73:2053–2071. doi: 10.1152/jn.1995.73.5.2053. [DOI] [PubMed] [Google Scholar]
- Didier A, Carleton A, Bjaalie JG, Vincent JD, Ottersen OP, Storm-Mathisen J, Lledo PM. A dendro-dendritic reciprocal synapse provides a recurrent excitatory connection in the olfactory bulb. Proceedings of the National Academy of Sciences of the USA. 2001;98:6441–6446. doi: 10.1073/pnas.101126398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. Journal of Physiology. 1999;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Strowbridge BW. Functional role of NMDA autoreceptors in olfactory mitral cells. Journal of Neurophysiology. 2000;84:39–50. doi: 10.1152/jn.2000.84.1.39. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Stopfer M. Recent dynamics in olfactory population coding. Current Opinion in Neurobiology. 2001;11:468–474. doi: 10.1016/s0959-4388(00)00236-1. [DOI] [PubMed] [Google Scholar]
- Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. Journal of Neuroscience. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic gamma-aminobutyric acid (GABA). release in the rat olfactory bulb. Proceedings of the National Academy of Sciences of the USA. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Overstreet LS, Slater NT. Prolonged physiological entrapment of glutamate in the synaptic cleft of cerebellar unipolar brush cells. Journal of Neurophysiolgy. 1997;78:1320–1333. doi: 10.1152/jn.1997.78.3.1320. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Erdemli G, Asztely F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Sakmann B, Urban NN. Action potential propagation in mitral cell lateral dendrites is decremental and controls recurrent and lateral inhibition in the mammalian olfactory bulb. Proceedings of the National Academy of Sciences of the USA. 2001;98:319–324. doi: 10.1073/pnas.011523098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. Journal of Physiology. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. Journal of Neuroscience. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH, Wouterlood FF. Immunocytochemical localization of GABA neurons and dopamine neurons in the rat main and accessory olfactory bulbs. Neuroscience Letters. 1984;47:221–226. doi: 10.1016/0304-3940(84)90517-2. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Research. 1969;14:157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Mori K, Shepherd GM. Blockade of synaptic inhibition reveals long-lasting synaptic excitation in isolated turtle olfactory bulb. Journal of Neurophysiology. 1981;46:649–658. doi: 10.1152/jn.1981.46.3.649. [DOI] [PubMed] [Google Scholar]
- Orona E, Rainer EC, Scott JW. Dendritic and axonal organization of mitral and tufted cells in the rat olfactory bulb. Journal of Comparative Neurology. 1984;226:346–356. doi: 10.1002/cne.902260305. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the periglomerular region of the olfactory bulb. Journal of Cell Science. 1971a;9:379–409. doi: 10.1242/jcs.9.2.379. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the glomeruli of the olfactory bulb. Journal of Cell Science. 1971b;9:347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. Journal of Cell Science. 1970;7:631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- Puopolo M, BelluIZZ O. NMDA-dependent, network-driven oscillatory activity induced by bicuculline or removal of Mg2+ in rat olfactory bulb neurons. European Journal of Neuroscience. 2001;13:92–102. [PubMed] [Google Scholar]
- Rall W, Shepherd GM, Reese TS, Brightman MW. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Experimental Neurology. 1966;14:44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM, Stewart MG. Hippocampal synapses: do they talk to their neighbours? Trends in Neurosciences. 1999;22:382–388. doi: 10.1016/s0166-2236(99)01425-3. [DOI] [PubMed] [Google Scholar]
- Salin PA, Lledo PM, Vincent JD, Charpak S. Dendritic glutamate autoreceptors modulate signal processing in rat mitral cells. Journal of Neurophysiology. 2001;85:1275–1282. doi: 10.1152/jn.2001.85.3.1275. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Ottersen OP. Organization of ionotropic glutamate receptors at dendrodendritic synapses in the rat olfactory bulb. Journal of Neuroscience. 2000;20:2192–2201. doi: 10.1523/JNEUROSCI.20-06-02192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. Journal of Neuroscience. 1998;18:6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31:639–651. doi: 10.1016/s0896-6273(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 1998. [Google Scholar]
- Shipley MT, McLean JH, Ennis M. Olfactory system. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic Press; 1995. pp. 899–926. [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cerebral Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nature Neuroscience. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- White EL. Synaptic organization in the olfactory glomerulus of the mouse. Brain Research. 1972;37:69–80. doi: 10.1016/0006-8993(72)90346-0. [DOI] [PubMed] [Google Scholar]
- Yang X, Renken R, Hyder F, Siddeek M, Greer CA, Shepherd GM, Shulman RG. Dynamic mapping at the laminar level of odor-elicited responses in rat olfactory bulb by functional MRI. Proceedings of the National Academy of Sciences of the USA. 1998;95:7715–7720. doi: 10.1073/pnas.95.13.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]


