Abstract
Cutaneomuscular reflexes (CMR) have been recorded from the first dorsal interosseous muscle (1DI) of the preferred hand, somatosensory evoked potentials (SEP) were recorded from the contralateral sensory cortex and the sensory nerve action potential (SNAP) was recorded from the median nerve of 15 adult subjects whilst electrically stimulating the digital nerves of the index finger. Subjects performed the following tasks (a) a sustained abduction of the index finger against resistance at 10–20 % maximum voluntary contraction (MVC), and (b) abduction of the index finger as in (a) whilst performing self paced low amplitude tapping of the (i) index finger, (ii) thumb, (iii) middle finger, (iv) little finger and (v) ipsilateral foot. The E2 component of the CMR and the N20/P25 components of the SEP were significantly reduced during finger tapping (P < 0.05). This reduction was independent of which finger was tapping (P > 0.05). There was a significant (qualitative) relationship between the decrease in the size of the E2 component of the CMR and the N20/P25 components of the SEP (χ2 test; P < 0.05). There were no significant changes in E1 and I1 (P > 0.05). The size of the SNAP was independent of task (P > 0.05). The size of the E1, I1, E2 components of the CMR, and the N20/P25 components of the SEP were unaltered during foot tapping (P > 0.05, n = 4). We conclude that the decrease in size of the E2 component associated with finger tapping results from gating of the digital nerve input.
Cutaneomuscular reflexes (CMR) can be evoked by modest, non-painful electrical stimulation of the digital nerves of the finger producing a reflex modulation of the ongoing muscle activity (EMG) recorded during a sustained voluntary contraction of an intrinsic hand muscle (Caccia et al. 1973; Jenner & Stephens, 1982). The CMR recorded from the first dorsal interosseous muscle (1DI) following stimulation of the digital nerves of the index finger is typically triphasic in appearance; there is an initial increase of EMG, (E1) followed by a decrease, (I1), followed by a prominent second increase, (E2). Evidence suggests that the E1 component is mediated via oligosynaptic spinal circuitry (Jenner & Stephens, 1982). The E2 component is mediated via a transcortical pathway, requiring the integrity of the dorsal columns, sensorimotor cortex and the corticospinal tract (Carr et al. 1993; Mayston et al. 1997). Recent findings also suggest that the I1 component is mediated via a transcortical pathway (Mayston et al. 1997).
The reflex effects of cutaneous stimulation on a given muscle are known to depend upon the task being carried out when the reflex is elicited. In the first dorsal interosseous muscle for example, the transcortical E2 component of the CMR evoked by stimulating the digital nerves of the index finger is greater when the subject performs a isolated finger abduction than when the muscle is active during the combined movement of all of the fingers, as in a power grip (Evans et al. 1989).
Somatosensory evoked potentials (SEP) represent the afferent volley evoked by the electrical stimulation of a digital or mixed nerve as it is propagated along the somatosensory pathway to the primary sensory cortex (S1). The SEP is made up of a number of distinct components that reflect activities from different generators. The N20/P25 SEP components recorded from electrodes attached to the scalp over the contralateral sensory cortex are thought to reflect cortically generated activities within the S1. Current evidence suggests that the N20 component represents afferent activity arriving at Broadmann's 3b area. The P25 component is believed to reflect further processing of the afferent activities at Broadmann's area 1 (Desmedt & Tomberg, 1989).
It is well established that cutaneous afferent input to the S1 is attenuated or ‘gated’ during movement (Giblin, 1964; Rushton et al. 1981; Cheron et al. 2000). In man SEP recordings obtained directly from exposed cortex and the scalp have been used to provide evidence that the largest amount of ‘gating’ occurs at a cortical level (Cheron & Borenstein, 1987; Hsieh et al. 1995), whilst the least amount of ‘gating’ occurs at brainstem level (Hsieh et al. 1995).
With this background in mind, in the present study we have examined the effect of performing concurrent small phasic movements of a finger upon the size of the CMR recorded from the 1DI evoked by electrical stimulation of the digital nerves of the index finger during a sustained voluntary contraction of 1DI at 10–20 % of MVC, whilst simultaneously recording the SEP from the contralateral sensory cortex. Evidence is presented which suggests that the decrease in the size of the E2 component of the reflex reported in the present study in association with finger tapping results from ‘gating’ of the digital nerve input at a level above the spinal cord, most likely within the cortex. A preliminary account has been presented to The Physiological Society (Turner et al. 2001).
METHODS
Subjects
Cutaneomuscular reflexes, digital nerve somatosensory evoked potentials, and sensory nerve action potentials were recorded from the preferred hand of 15 healthy subjects, aged 17–49 years (six female). All subjects gave informed consent. The experimental protocols were approved by the local ethics committee, and were in accordance with the guidelines set out in the Declaration of Helsinki, 1964.
Cutaneomuscular reflexes
Reflexes were recorded from the first dorsal interosseous muscle. The surface EMG was recorded using self-adhesive electrodes (TECA NCS Disposable Surface Electrodes, Oxford Instruments Medical, Old Woking, UK) that were placed on the skin over the belly of the muscle, inter-electrode distance 2.5 cm. The subject was asked to abduct the index finger against resistance at 10–20 % maximal voluntary contraction (MVC) using an LED root mean square voltmeter as a visual aid. The EMG was amplified, filtered (20 Hz-5 KHz, Oxford Instruments Medical, Sapphire 4ME) and stored on magnetic tape for future analysis (Racal, Store 4, Southampton, UK).
Digital nerve somatosensory evoked potentials
Recordings were made from the sensory cortex contralateral to the side of stimulation. The scalp was prepared using skinpure abrasive paste (Unimed Electrode Supplies, Farnham, UK). Stick-on silver/silver chloride disc electrodes (10 mm stick-on EEG electrodes, Oxford Instruments Medical) were placed onto the scalp using an adhesive conductive EEG paste (Ten20, Unimed Electrode Supplies). The active electrode was positioned 2.5 cm behind Cz (International 10–20 System) and 7 cm laterally. A reference electrode was placed onto the earlobe ipsilateral to the side of stimulation (Tomberg et al. 1991). The ongoing EEG was amplified, filtered (20 Hz-2 KHz, Sapphire 4ME) and stored on magnetic tape (Racal, Store 4) for further analysis.
Sensory nerve action potentials
Sensory nerve action potentials were recorded using surface electrodes placed onto the skin overlying the median nerve at the wrist (TECA NCS Disposable Surface Electrodes, Oxford Instruments Medical). The SNAP was amplified (20 Hz-2 KHz, Sapphire 4ME) and stored on magnetic tape (Racal, Store 4) for analysis.
Digital nerve stimulation
The digital nerves of the index finger were electrically stimulated using ring electrodes (Oxford Instruments Medical), which were placed either side of the proximal interphalangeal joint. The stimulus was delivered using a constant current stimulator (Sapphire 4ME) at a level 2.5 times above that required for perception (pulse duration 100 μs, frequency 5 Hz). The perception threshold was determined while the subject's hand was relaxed.
Experiment 1
Cutaneomuscular reflexes, digital nerve somatosensory evoked potentials and sensory nerve action potentials were recorded, following digital nerve stimulation of the index finger. Subjects performed the following finger movement tasks: (a) a sustained voluntary abduction of the index finger at 10–20 % MVC using a LED voltmeter as visual feedback, and (b) abduction of the index finger as in (a) whilst simultaneously performing concurrent small self-paced tapping of the (i) index finger, (ii) thumb, (iii) middle finger and (iv) little finger. For each experimental run, subjects were asked to maintain the LED voltmeter monitoring 1DI EMG lit to the 10–20 % MVC level. In the case of the index finger (b)(i) subjects abducted the index finger to the 10–20 % MVC level. Once achieved, subjects were instructed to make small concurrent tapping movements of the index finger throughout the period of digital nerve stimulation. The experimenter carefully monitored the subject and LED voltmeter EMG levels to ensure that the subject continued to abduct whilst performing the movement task.
All subjects performed the finger movement tasks twice, and in a random order. Subjects rested for a few minutes between each task. Data were excluded if the subject was unable to perform the task, or produced a large amount of wrist movement making it impossible to record the afferent volley at the wrist.
Experiment 2
Cutaneomuscular reflexes, digital nerve somatosensory evoked potentials and sensory nerve action potentials were recorded, following digital nerve stimulation of the index finger. In the second experiment four subjects from experiment 1 were asked to abduct the index finger as (a) in experiment 1 whilst simultaneously performing self-paced tapping of the ipsilateral foot.
Analysis
Cutaneomuscular reflexes.
The amplified and filtered EMG signal was rectified and then averaged time-locked to the stimulus for 250 sweeps (SigAvg program; Cambridge Electronic Design, Cambridge, UK). The size of each of the reflex components E1, I1 and E2 was expressed in terms of percentage modulation of background EMG (Nadler et al. 2000). The mean level of ongoing background EMG was found from a 20 ms pre-stimulus period of EMG. A component was considered present if it rose above or fell below the 95 % confidence level of the mean EMG for at least 8 ms (Wohlert, 1996). Because each finger movement task was performed twice in the same recording session, the mean percentage modulation was calculated for each component from the two recordings by taking the percentage modulation measured for each of the 250 sweeps. This was performed for each finger movement task in all subjects.
Digital nerve somatosensory evoked potentials.
The ongoing amplified and filtered EEG signal was averaged time-locked to the stimulus for 250 sweeps. The size of the SEP recorded following electrical stimulation of the digital nerves is particularly small. To improve the signal to noise ratio the two 250 sweep averages were combined using the SigAvg program to give a singe 500 sweep average for each finger movement task. The peak-to-peak amplitude of the N20/P25 components was measured.
Sensory nerve action potentials.
The amplified and filtered signal was averaged time-locked to the stimulus for 250 sweeps (SigAvg program). The size of the SNAP was found by measuring the peak-to-peak amplitude.
Statistical analysis
The effect of finger tapping on the size of the CMR, SEP and SNAP was examined by performing repeated measures analysis of variance (rmANOVA). The χ2 test for association with Yates continuity correction was also employed. Any significant association was further verified by employing Fisher's exact method. The level of statistical significance was set at P < 0.05.
RESULTS
Figure 1 shows the effect of simultaneously making a finger movement on the averaged rectified EMG recorded from 1DI during a sustained voluntary abduction of the index finger for one subject. During simple index finger abduction (Fig. 1A) without simultaneous finger tapping, the stimulus clearly elicits three components; a short latency increase in EMG, E1, followed by a decrease, I1, followed by a second increase, E2, producing per cent EMG modulations of 15.5, 19.2 and 19.5 respectively. In contrast, when this subject performs self-paced tapping of the index finger whilst simultaneously abducting the index finger, there is a clear reduction in the size of the E2 modulation, decreasing from 19.5 to 6.1. The E1 and I1 modulations are unaltered producing per cent EMG modulations of 17.6 and 16.9 respectively. This is shown in Fig. 1B. There are similar effects when the individual performs self-paced tapping of the thumb, middle and little finger (Fig. 1C-E). The size of the E2 component produced by index finger abduction is clearly reduced in all tasks. The per cent EMG E2 modulations produced are 6.0, 0.0 and 12.2 respectively. The E1 and I1 modulations are unchanged producing per cent EMG modulations of 16.9, 21.3 and 15.4 respectively for the E1 component, and 19.7, 16.9 and 14.6 respectively for the I1 component. The size of the sensory volley recorded from the median nerve during the finger movements ranged from 3.0-3.4 μV peak-to-peak. The background EMG level during each of the finger tapping movements ranged from 79–88 μV.
Figure 1.
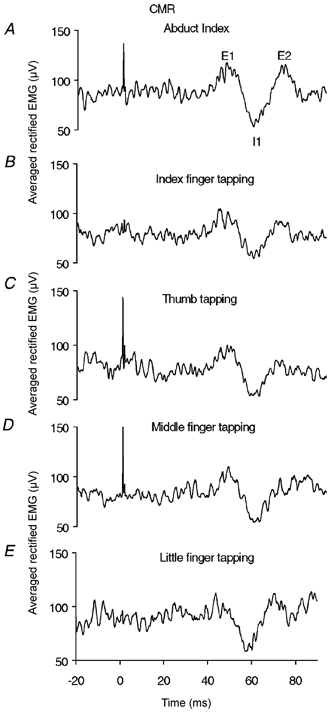
Effect of finger movement on the components of the CMR recorded from 1DI following electrical stimulation of the digital nerves of the index finger
A, cutaneous reflex response recorded from 1DI following stimulation of the digital nerves of the index finger during a sustained voluntary abduction of the index finger, maintained at 10–20 % of the MVC (Abd). A clear cutaneous reflex was observed consisting of an initial rise, E1, followed by a decrease, I1, followed by a second increase, E2, (labelled on the trace). B-E, reflex recorded from 1DI following stimulation of the digital nerves of the index finger during a sustained voluntary abduction of the index finger maintained at 10–20 % of the MVC as in A with concomitant tapping of: B, the index finger (Abd + I); C, the thumb (Abd + T); D, the middle finger (Abd + M); and E, the little finger (Abd + L). In each case the reflex component E2 is clearly reduced or abolished in the case of middle finger tapping whilst E1 and I1 remain unaltered. A-E, show the rectified and averaged EMG time locked to each stimulus, delivered at 5 s−1. 250 sweeps. All recorded in the same session.
Taking all the data together, the mean size of the E2 component decreased when finger tapping was performed whilst simultaneously abducting the index finger in 91 % of all recordings (Fig. 3A). Repeated measures analysis of variance (rmANOVA) revealed a significant decrease in the size of the E2 component recorded from 1DI during a sustained abduction of the index finger whilst simultaneously performing finger tapping compared to the size of the E2 component when simply abducting the index finger (P < 0.05 for each finger tapping movement task). Of the 15 subjects, 73 % reported a decreased appreciation of the stimulus during tapping compared with abduction alone. Although the mean size of the I1 component was reduced particularly during little and middle finger tapping this change was not found to be statistically significantly different to index finger abduction (P > 0.05). The E1 component was unaltered during finger tapping (P > 0.05).
Figure 3.
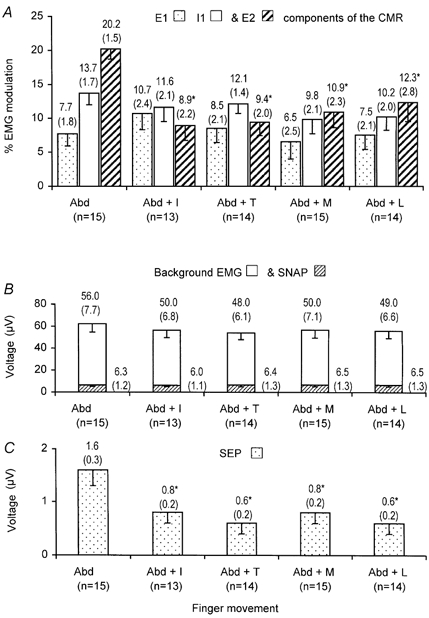
Mean data (−1 s.e.m.) obtained from all subjects showing the effect of finger tapping upon the CMR, SEP, SNAP and EMG
Mean size of each component (top) and s.e.m. (bracketed) are given above each bar for each chart. A, CMR recorded from 1DI, following stimulation the of the index finger digital nerves during a sustained abduction of the index finger alone (Abd) contrasting the CMR obtained during index finger (Abd + I), thumb (Abd + T), middle finger (Abd + M) and little finger (Abd + L) tapping. Stippled bars: mean size of E1 component. Open bars: mean size of the I1 component. Hatched bars: mean size of the E2. component Significant differences were found in the size of the E2 component on comparing the differences between the means obtained during abduction and each finger movement (rmANOVA, P < 0.05, denoted *). Components E1 and I1 were not significantly altered (rmANOVA, P > 0.05 in both cases). B, SNAP recorded from the median nerve at the wrist and background EMG recorded from IDI during index finger abduction following electrical stimulation of the index finger digital nerves for each finger tapping task performed as described in A. Hatched bars: mean size of the SNAP recorded from the median nerve. Open bars: mean size of the background EMG. C, SEP recorded from the contralateral sensory cortex, following electrical stimulation of the index finger digital nerves whilst performing a sustained voluntary abduction of the index finger compared to the effect of abducting the index finger with concomitant tapping of the finger as described in A. Stippled bars: mean size of the N20/P25 component of the SEP. Significant differences in the size of the N20/P25 components were found on comparing the means obtained during abduction and each finger movement (rmANOVA, P < 0.05, denoted *).
On first inspection of the chart illustrated in Fig. 3A, it appears that there is a graded effect in the decrease in the size of the mean per cent E2 EMG modulation. The maximal decrease in the size of the E2 component appears to occur when performing index finger or thumb tapping, whilst little finger tapping appears to have the least effect. However rmANOVA did not reveal a significant difference between which finger was performing the tapping and the resultant decrease in the size of the mean per cent E2 EMG modulation (P > 0.05).
The size of the afferent volley recorded from the median nerve at the wrist and the background EMG levels are shown in Fig. 3B for all subjects. It was found that finger tapping did not significantly alter the afferent volley to the spinal cord (P > 0.05) or background EMG (P > 0.05).
Figure 2 shows the simultaneously recorded SEP obtained from S1 following stimulation of the digital nerves of the index finger in one subject. When the subject performs a simple abduction of the index finger (Fig. 2A), approximately 20 ms following stimulation there is an initial negative rising component, N20, followed by a positive down going component, P25. The peak-to-peak amplitude of the N20/P25 components measured 2.7 μV. However when the subject performs tapping of a finger whilst simultaneously abducting the index finger there is a clear reduction in the size of the N20/P25 components (Fig. 2B-E). The measured peak-to-peak amplitudes being 1.3 μV during index tapping, 1.9 μV during thumb tapping, 1.8 μV during middle and 1.3 μV during little finger tapping.
Figure 2.
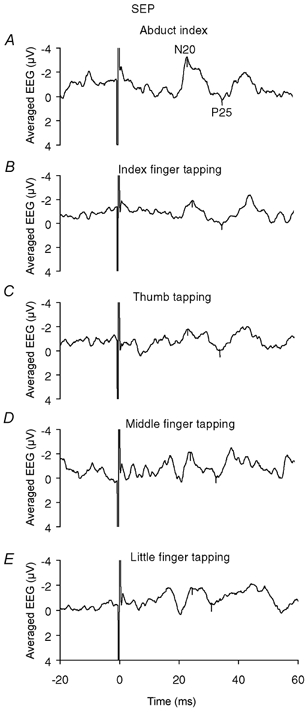
Effect of finger movement on the N20/P25 components of the SEP recorded from the contralateral sensory cortex following electrical stimulation of the digital nerves of the index finger
A, N20/P25 response recorded from the contralateral sensory cortex following stimulation of the digital nerves of the index finger during a sustained voluntary abduction of the index finger, maintained at 10–20 % of the MVC (Abd). An initial negative rise, N20, followed by a positive decrease, P25, was seen, (labelled on the trace). B-E, N20/P25 response recorded from the contralateral sensory cortex following stimulation of the digital nerves of the index finger during a sustained voluntary abduction of the index finger maintained at 10–20 % of the MVC as in A with concomitant tapping of: B, the index finger (Abd + I); C, the thumb (Abd + T); D, the middle finger (Abd + M); and E, the little finger (Abd + L). In each case the response is clearly reduced. A-E, show the averaged EEG time-locked to each stimulus, delivered at 5 s−1. 500 sweeps (a 250 sweep average was obtained on two occasions in the same recording session and combined to give a 500 sweep average).
Combining all data together, the mean size of the amplitude of the N20/P25 components is significantly reduced by finger tapping when compared to simple abduction of the index finger (rmANOVA, P < 0.05 in all finger movements). This is illustrated in Fig. 3C. As with the CMR, pairwise comparisons failed to show that the decrease in the size of the N20/P25 components was dependent upon which finger was tapping (P > 0.05).
The χ2 test for association performed upon the combined data showed a significant qualitative relationship between the decrease in the size of the E2 component of the CMR and the decrease in the size N20/P25 components of the SEP (P < 0.05). Given the small study sample the significance was verified by employing the Fisher's exact method (P < 0.05).
Figure 4 shows the effect of concurrently tapping the ipsilateral foot whilst simultaneously abducting the index finger upon the CMR and SEP for all subjects (n = 4). Taking the data as a whole, the mean size of the E1, I1, E2 components of the CMR and the N20/P25 components of the SEP are unaltered when concurrently tapping the ipsilateral foot in 100 % of all recordings. The size of the components of the CMR recorded from 1DI and the sensory cortex during index finger abduction whilst concurrently tapping the foot showed no significant change when compared to the size of the components of the CMR and SEP recorded when simply abducting the index finger (rmANOVA, P > 0.05 in all cases). This was similarly the case for the SNAP and background EMG levels (P > 0.05 in both cases) also illustrated in Fig. 4.
Figure 4.
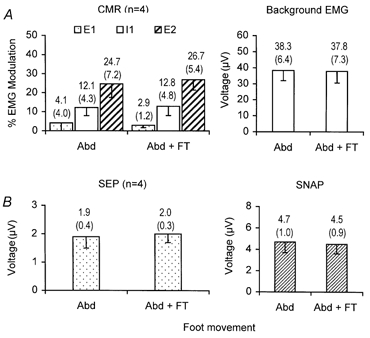
Mean data (-1 s.e.m.) obtained from four subjects showing the effect of ipsilateral foot tapping on the components of the CMR, background EMG, SEP and SNAP
Mean data value (top) and s.e.m. (bracketed) are shown on the chart above each bar. A, CMR and background EMG recorded from 1DI, whilst stimulating the digital nerves of the index finger during sustained abduction of the index finger alone (Abd) contrasting the CMR obtained during foot tapping (Abd + FT). Stippled bars: mean size of the component E1 of the reflex. Open bars: mean size of the I1 component of the reflex. Hatched bars: mean size of the component E2 of the reflex. Open bars (right bar chart): mean size of the background EMG. B, SEP recorded from the contralateral sensory cortex and SNAP recorded from the median nerve at the wrist. Stippled bars: mean size of the N20/P25 component of the SEP. Hatched bars: mean size of the afferent volley.
DISCUSSION
The present study has demonstrated that the E2 component of the CMR recorded from the 1DI muscle is reduced when finger tapping is performed. The reduction in the size of the E2 component was found to be unrelated to which finger is tapping. This effect on the E2 component of the CMR is not seen when ipsilateral foot tapping is performed in place of finger tapping. The E1 and I1 components were found to be unchanged during either finger or foot tapping. It has also been shown that the reduction in the size of the E2 component seen during finger tapping is associated with a reduction in the size of the N20/P25 components of the SEP, reflecting the activities within areas 3b and 1 generated within the S1 in response to the afferent input (Desmedt & Tomberg, 1989).
This decrease in the size of the E2 CMR and N20/P25 SEP components cannot be due to a change in the afferent input to the spinal cord as the size of the SNAP recorded from the wrist was not significantly altered during finger tapping.
The E1 component was unaltered during finger tapping, suggesting that the decrease seen in the present study must have occurred at a level above the spinal cord, as evidence suggests that E1 (Jenner & Stephens, 1982) is spinal whilst I1 and E2 components of the reflex are transcortical in origin (Mayston et al. 1997).
The present findings have shown that during finger tapping there is a significant decrease in the size of the E2 component of the CMR in association with a decrease in the size of the N20/P25 SEP components at the S1. These findings are concordant with the notion that the decrease in the size of the E2 component of the CMR is due to ‘gating’ of the afferent input during finger tapping. Indeed 73 % of the subjects in the present study reported a decreased awareness of the stimulus during tapping compared with abduction alone. There are a number of reports showing ‘gating’ of afferent information at different sites as it is propagated along the somatosensory pathway during movement. In animals ‘gating’ has been shown within the DCN (Ghez & Pisa, 1972), thalamus within VPN (Tsumoto et al. 1975) and S1 (Chaplin & Woolward, 1981). However in man, ‘gating’ of the afferent information is believed to occur within S1 with little contribution from the sub-cortical regions of the brain (Rushton et al. 1981; Hsieh et al. 1995). This suggests that the reduction in the size of the N20/P25 SEP components most likely reflects ‘gating’ of the afferent information within S1.
However Palmeri et al. (1999) have recently demonstrated that the motor cortex (M1) is also able to exert effects on the afferent activity at the level of the DCN and VPN during limb movement in cats. Therefore it is possible that the reduction in the size of the afferent volley arriving at S1 seen in the present study may reflect ‘gating’ of the afferent information within the DCN/VPN imposed by M1 during movement as opposed to S1.
There is also evidence that ‘gating’ of afferent information can be mediated by sensory feedback (Cheyne et al. 1997) as well as corollary discharges to other motor regions when a voluntary movement is initiated (Paus et al. 1996). Thus in the present experiments proprioceptive feedback from the finger movement and cutaneous afferent input generated by the tap may also play a part in the ‘gating’ process. It is likely that both mechanisms contribute to the ‘gating’ effect. How much of a role each mechanism plays could be tested by performing passive finger tapping in place of active finger movement, leaving the sensory feedback but removing the corollary discharge. The role of proprioceptive feedback and cutaneous afferent input could be explored by carrying out experiments in which the fingertips are anaesthetised.
Complementary studies in which four subjects performed ipsilateral foot tapping whilst abducting the index finger showed no reduction in the size of the E2 CMR component or N20/P25 SEP components in the present study. This finding provides evidence that the reduction in the size of the CMR and digital nerve SEP, which occurs during finger tapping, is not unspecific, although further experiments are required to see if less remote areas of the body such as the arm or ipsilateral hand could produce similar effects to the finger tapping performed in this study.
Given the transcortical origin of the I1 and E2 components of the CMR (Mayston et al. 1997) it is surprising that finger tapping did not alter the size of the I1 component of the reflex. Increasingly it has become apparent that the I1 has different characteristics to the E2 component of the CMR. Harrison et al. (2000) have demonstrated that the I1 component shows little habituation in comparison to the E2 component, which habituates much more readily. One possible explanation for these findings may relate to differences in the route of mediation of each component.
In conclusion, the present study has demonstrated that during finger tapping there is a significant decrease in the size of the E2 component of the CMR that is associated with a decrease in the size of the N20/P25 SEP components. The most likely explanation for these findings is that the decrease in the size of the E2 component results from ‘gating’ of the afferent information within the sensory cortex during finger tapping.
Acknowledgments
The authors would like to thank Dr W. L. Merton, and the Department of Clinical Neurophysiology, Portsmouth for their support and encouragement. Additional thanks go to O. Mulki (BSc intercalated medical student) for aiding in the collection of the data obtained in this study.
REFERENCES
- Caccia MR, McComas AR, Upton ARM, Blogg T. Cutaneous reflexes in small muscles of the hand. Journal of Neurology, Neurosurgery and Psychiatry. 1973;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganisation in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Chaplin JK, Woolward DJ. Modulation of sensory responsiveness of single somatosensory cortical cells during movement and arousal behaviours. Experimental Neurology. 1981;72:164–178. doi: 10.1016/0014-4886(81)90135-7. [DOI] [PubMed] [Google Scholar]
- Cheron G, Borenstein S. Specific gating of the early somatosensory evoked potentials during active movement. Electroencephalography and Clinical Neurophysiology. 1987;67:537–548. doi: 10.1016/0013-4694(87)90056-3. [DOI] [PubMed] [Google Scholar]
- Cheron G, Dan B, Borenstein S. Sensory and motor interfering influences on somatosensory evoked potentials. Journal of Clinical Neurophysiology. 2000;17:280–294. doi: 10.1097/00004691-200005000-00006. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Endo H, Tsunehiro T, Weinberg H. Sensory feedback contributes to early movement-evoked fields during voluntary finger movements in humans. Brain Research. 1997;771:196–202. doi: 10.1016/s0006-8993(97)00765-8. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Tomberg C. Mapping early somatosensory evoked potentials in selective attention: critical evaluation of control conditions used for titrating by difference the cognitive P30, P40, P100 and N140. Electroencephalography and Clinical Neurophysiology. 1989;74:321–346. doi: 10.1016/0168-5597(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Evans AL, Harrison LM, Stephens JA. Task-dependent changes in cutaneous reflexes recorded from various muscles controlling finger movement in man. Journal of Physiology. 1989;418:1–12. doi: 10.1113/jphysiol.1989.sp017825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Pisa M. Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Research. 1972;40:145–151. doi: 10.1016/0006-8993(72)90120-5. [DOI] [PubMed] [Google Scholar]
- Giblin DR. Somatosensory evoked potentials in healthy patients and in patients with lesions of the nervous system. Annals of the New York Academy of Sciences. 1964;112:93–142. doi: 10.1111/j.1749-6632.1964.tb26744.x. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Norton JA, Stephens JA. Habituation of cutaneomuscular reflexes recorded from the first dorsal interosseous and triceps muscle in man. Journal of Neurological Sciences. 2000;177:32–40. doi: 10.1016/s0022-510x(00)00326-9. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Shima F, Tobimatsu S, Sun S, Kato M. The interaction of the somatosensory evoked potentials to simultaneous finger stimuli in the human central nervous system. A study using direct recording. Electroencephalography and Clinical Neurophysiology. 1995;96:135–142. doi: 10.1016/0168-5597(94)00251-9. [DOI] [PubMed] [Google Scholar]
- Jenner R, Stephens JA. Cutaneous reflex responses and their central nervous pathways studied in man. Journal of Physiology. 1982;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain. 1997;120:1199–1216. doi: 10.1093/brain/120.7.1199. [DOI] [PubMed] [Google Scholar]
- Nadler MA, Harrison LM, Stephens JA. Acquisition of a new motor skill is accompanied by changes in cutaneomuscular reflex responses recorded from finger muscles in man. Experimental Brain Research. 2000;134:246–254. doi: 10.1007/s002210000453. [DOI] [PubMed] [Google Scholar]
- Palmeri A, Bellomo M, Giuffrida R, Sapienza S. Motor cortex modulation of exteroceptive information at bulbar and thalamic lemniscal relays in the cat. Neuroscience. 1999;88:135–150. doi: 10.1016/s0306-4522(98)00205-x. [DOI] [PubMed] [Google Scholar]
- Paus T, Marrett S, Worsley K, Evans A. Imaging motor-to-sensory discharges in the human brain: An experimental tool for the assessment of functional connectivity. Neuroimage. 1996;4:78–86. doi: 10.1006/nimg.1996.0031. [DOI] [PubMed] [Google Scholar]
- Rushton DN, Rothwell JC, Craggs MD. Gating of somatosensory evoked potentials during different kinds of movement in man. Brain. 1981;104:465–491. doi: 10.1093/brain/104.3.465. [DOI] [PubMed] [Google Scholar]
- Tomberg C, Desmedt JE, Ozaki I. Right or left ear reference changes the voltage of the frontal and parietal somatosensory evoked potentials. Electroencephalography and Clinical Neurophysiology. 1991;80:504–512. doi: 10.1016/0168-5597(91)90132-h. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Nakamura S, Iwama K. Pyramidal tract control over cutaneous and kinesthetic sensory transmission in the cat thalamus. Experimental Brain Research. 1975;22:281–294. doi: 10.1007/BF00234770. [DOI] [PubMed] [Google Scholar]
- Turner LC, Harrison LM, Stephens JA, Mulki O. Finger movement attenuates cutaneomuscular reflexes recorded from the first dorsal interosseous muscle in man. Journal of Physiology. 2001;533.P:52. P. [Google Scholar]
- Wohlert AB. Reflex responses of lip muscles in younger and older women. Journal of Speech and Hearing Research. 1996;39:578–589. doi: 10.1044/jshr.3903.578. [DOI] [PubMed] [Google Scholar]


