Abstract
The present studies were designed to increase an existing limitation on the size of the H reflex by accentuating an inhibitory effect of group I afferents in the test volley. They were precipitated by the observation that, during strong voluntary contractions of quadriceps (Q), the late deep peroneal (DP) facilitation of the Q H reflex was suppressed but the facilitation of the ongoing EMG was not. The effects of conditioning stimuli to DP, superficial peroneal (SP) and articular afferents on the excitation of Q motoneurones (MNs) produced by femoral nerve (FN) stimulation were assessed in 11 healthy human subjects using the H reflex of vastus intermedius or the peak of group I excitation in post-stimulus time histograms (PSTHs) of single motor units (MUs) in vastus lateralis. The suppression of the late H reflex facilitation was observed during strong contractions after stimulation of DP and articular afferents, and at rest when DP and SP volleys were combined. In all single MUs tested, the FN-induced peak of excitation was suppressed by DP stimulation during strong Q contractions and by a combination of conditioning volleys (SP with DP or articular) during weak contractions. By themselves these conditioning volleys did not inhibit the background MU discharge even when delivered together. The suppression did not involve the initial bins of the peak; it began 0.7 ms later than the probable onset of monosynaptic Ia facilitation. It is argued that the suppression is not due to presynaptic inhibition of Ia terminals or to recurrent inhibition, but probably reflects convergence between the conditioning volleys and group I afferents in the test FN volley onto interneurones of the disynaptic non-reciprocal group I inhibition. It is concluded that the size of the H reflex is limited by disynaptic inhibition, and that changes in the excitability of this inhibitory pathway can produce prominent changes in the H reflex.
The H reflex has been the main tool in many motor control investigations performed in human subjects. Following the pioneering studies of Magladery et al. (1951) and Paillard (1955), the human H reflex and tendon jerk have been considered monosynaptic reflexes, equivalent to the ventral root discharge produced by dorsal root stimulation, studied extensively in the cat by Lloyd (1943). The first motoneurones (MNs) discharging in the H reflex do so at a latency consistent with a monosynaptic pathway (Magladery et al. 1951). However, based on estimates from post-stimulus time histograms (PSTHs) of the discharge of single motor units (MUs), it has been argued that the duration of the compound group I excitatory post-synaptic potential (EPSP) underlying the H reflex (some 1–2 ms) is so short that the monosynaptic Ia component of the EPSP must be curtailed by oligosynaptic inhibition, and that, consequently, inhibitory pathways activated by the test volley and/or the reflex discharge probably limit the size of the H reflex (Burke et al. 1984).
Transmission in two inhibitory pathways could truncate the monosynaptic Ia excitation. Non-reciprocal group I inhibitory interneurones, activated by group Ib and group Ia afferents (see Jankowska, 1992) produce autogenetic inhibition with an onset ≈0.7 ms after the onset of the facilitation due to the group Ia monosynaptic EPSP in MNs (Pierrot-Deseilligny et al. 1981; Hultborn et al. 1987a). The reflex discharge of low-threshold MNs effectively activates Renshaw cells (Hultborn et al. 1979), thereby producing recurrent inhibition that could prevent the discharge of higher-threshold MNs.
A limitation of the H reflex by a di- or oligosynaptic influence elicited by the test volley would constitute an important limitation on the value of H reflex studies. Indeed MNs recruited last into the reflex will be most dependent on pathways with interposed interneurones, and the changes in the reflex, e.g. during movement, are largely determined by the recruitment of these motoneurones. Therefore, it has been proposed that, in PSTHs of the discharge of single MUs, only those changes affecting the entire excitatory peak and, in particular, the initial 0.5-1.0 ms can be considered to have affected the monosynaptic pathway (e.g. see Katz et al. 1988). This is because the onset of the test excitation, whether in PSTHs or in the H reflex itself, should not be contaminated by non-monosynaptic inputs from afferents in the test volley. However, despite the tacit acceptance of the possibility of di- and oligosynaptic contributions to the reflex discharge, there has been no direct evidence that this does occur and no estimate of the strength of any such limitation on the reflex discharge.
The present studies were designed to increase an existing limitation on the size of the H reflex by accentuating an inhibitory effect of group I afferents in the test volley. It was found that some conditioning stimuli were able to decrease dramatically the size of the quadriceps (Q) H reflex during voluntary contractions, apparently because they facilitate non-reciprocal group I inhibition elicited by the afferent volley of the test reflex. Apart from the implications for the interpretation of changes in H reflex amplitude (see above), these results suggest that autogenetic inhibitory pathways fed by group I (Ia and Ib) afferents can effectively counteract much of the monosynaptic Ia excitation of MNs.
Methods
The experiments were carried out on 11 healthy subjects (aged 22–65 years), all of whom gave written informed consent to the experimental procedures which were performed in accordance with the Declaration of Helsinki, with the approval of the institutional ethics committee. The subjects were seated in an armchair and the examined leg was loosely fixed with the hip semi-flexed (120 deg), the knee slightly flexed (160 deg) and the ankle at 110 deg plantar flexion. Despite the fact that its relatively short afferent pathway would minimise dispersion of the afferent volley (and thus minimise possible effects of Ib afferents activated by the test volley), the Q group was chosen over soleus, because stimulation of the deep peroneal nerve evokes potent excitation in Q MNs (Simonetta-Moreau et al. 1999) without any recurrent inhibition (Meunier et al. 1994).
Voluntary contractions
Experiments were performed during tonic isometric voluntary contractions of different forces. The rectified and integrated EMG activity was displayed on an oscilloscope so that, during a sequence, the subjects could maintain a constant contraction level, expressed as a percentage of the activity measured during a maximal tonic voluntary contraction (MVC) lasting 5 s. Two levels of forces were investigated: (i) a contraction ≥ 5 % MVC. This contraction level could be maintained without effort for a long duration but, because it often involved only one detectable MU, it was probably of little physiological significance; (ii) a force ≥ 10 % MVC, which could not be maintained for a long time without fatigue.
Recording
EMG was recorded by surface electrodes 1 cm apart secured to the skin over the corresponding head of the Q muscle: vastus lateralis (VL) and vastus intermedius (VI), 25–30 and 5–10 cm above the patella, on the lateral and anterior aspects of the thigh, respectively. The H reflex and the ongoing voluntary EMG were recorded from VI using 0.8 cm2 silver plates. The discharge of single MUs in the VL was recorded using differential electrodes DE-2.3 (Delsys Inc., Boston, MA, USA). EMG was also recorded from tibialis anterior (TA) to calibrate the intensity of conditioning stimuli to the deep peroneal nerve.
Test stimulus
The test stimuli for the Q H reflex or the monosynaptic peak in the PSTH were of 1 ms duration, applied to the femoral nerve (FN) with the cathode (2 cm diameter half-ball) in the femoral triangle and the anode on the posterior aspect of the thigh.
Conditioning stimuli
Electrical stimuli of 1 ms duration were delivered through bipolar surface electrodes 2–5 cm apart to stimulate different kinds of afferents: (i) the deep peroneal nerve (DPN) at the fibular neck, at a position that produced dorsiflexion of the foot without eversion and without paraesthesiae in the territory of the superficial peroneal nerve. Only one subject reported paraesthesiae in DP territory, between toes 1 and 2. The intensity of the nerve stimuli was expressed as a multiple of the threshold for an EMG potential in TA (× motor threshold, MT). This stimulation recruited muscle afferents from pretibial flexors, presumably ankle joint afferents and some cutaneous afferents; (ii) the superficial peroneal nerve (SPN), containing mainly cutaneous afferents, ≈5 cm above the lateral malleolus. The intensity of this stimulus was expressed as a multiple of the threshold for perception of radiating paraesthesiae (× perception threshold, PT); (iii) the lateral articular nerve of the knee joint at the level of the joint space, containing mainly joint afferents. Electrodes were placed 2–4 cm apart on the lateral aspect of the joint space, or with the cathode on the lateral aspect of the joint and the anode medial. The intensity of stimulation was adjusted so that it evoked much the same local cutaneous sensation as the local sensation elicited by deep peroneal stimuli at 2 × MT. This relatively strong stimulation was delivered over the joint capsule, remote from muscle, and evoked a sensation that was felt inside the knee joint. Care was taken to ensure that it did not produce a muscle twitch, despite its strength.
Three types of study were undertaken, using the H reflex, measuring the modulation of the ongoing EMG and constructing post-stimulus time histograms (PSTHs) of the discharge of single MUs.
H reflex studies
The H reflex was recorded in VI. Because the sensitivity of H reflexes of small size varies with the amplitude of the unconditioned reflex (Crone et al. 1990), the size of the unconditioned reflex was adjusted to be at the same size (between 10 and 20 % of the maximal M wave, Mmax) at rest and during tonic Q contractions of different forces (see above). When investigating the effects of weak and strong FN stimuli (Fig. 1E), the stimulus intensities (kept constant during weak and strong contractions) were chosen so that there was a stable unconditioned H reflex with the weak intensity during weak contractions, and so that the amplitude of the unconditioned H reflex did not ‘saturate’ with the stronger stimuli during strong contractions. This led us to use a rather narrow range of stimulus intensities: ≈0.9 and 1 × MT for the weak and strong intensities, respectively. Reflex responses were measured as the peak-to-peak amplitude of muscle action potentials. In each experimental run, 20 control and 20 conditioned reflexes were randomly alternated for each conditioning-test interval or conditioning stimulus intensity. Conditioned reflexes were expressed as a percentage of control reflexes. Scheffé‘s test (F test) was used to determine whether the changes evoked by the conditioning stimuli were significant in individual subjects. A non-parametric (Kolmogorov-Smirnov) test was used to compare in the population (eight subjects) changes in the H reflex and in the ongoing EMG (see below) during strong contractions.
Figure 1. Different effects of deep peroneal volleys on EMG and H reflex of quadriceps during strong contractions.
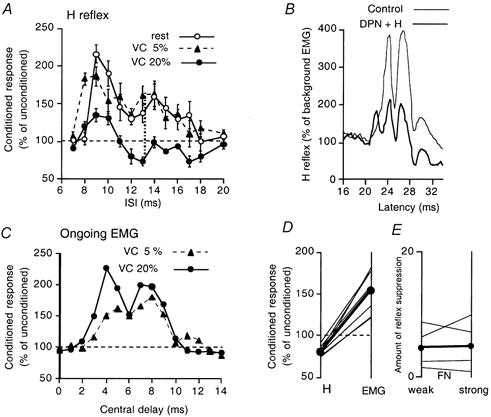
A-C, results in the same subject. A, time course of the deep peroneal (DP, 2 × MT) effects on the quadriceps (Q) H reflex, at rest (open circles), during weak (filled triangles) and strong tonic Q contractions (filled circles). In this and Figs 2A-B and 3A, the size of conditioned reflexes (expressed as a percentage of unconditioned reflexes) is plotted against the interstimulus interval (ISI), and each symbol represents the mean of 20 measurements, vertical bars 1 s.e.m. (unconditioned H reflexes between 10 and 20 % of Mmax). The 6 ms ISI corresponds to a central delay of zero, i.e. when the femoral and peroneal volleys would have arrived simultaneously at the Q MN pool. B, the rectified and averaged H reflex (20 sweeps, 5 kHz sampling rate) during a 10 % contraction, showing control responses (thin line) and conditioned responses (thick line) at the 13 ms ISI. C, changes in the rectified averaged (100 sweeps, 1 kHz sampling rate) ongoing EMG of Q, normalised to the background level, are plotted against the central delay: the latency of the H reflex being 21 ms, the zero central delay (arrival of the DP volley at the segmental level of Q MNs) was 27 (21 + 6) ms. Filled circles and triangles as in A. D, data for eight subjects showing the contrasting effects of the 20 (or 10) % contractions-inhibition of the H reflex (left column) and facilitation of the ongoing EMG (right column)-when measured at their maximum at central delays of between 6 and 12 ms. Each thin line represents one subject and the filled circles and the thick line the mean values. E, data for four subjects comparing the amount of suppression of the Q H reflex facilitation during strong contraction, i.e. the difference between the amount of facilitation during strong (≥ 10 % MVC) and weak (≥ 5 % MVC) contractions (measured at its maximum between 12 and 16 ms ISIs, see the dotted vertical line in A), while using weak (left column) and strong (right column) FN stimulation (see Methods). Thin and thick lines as in D.
Modulation of the ongoing voluntary EMG
Ongoing EMG activity of VI was recorded during voluntary knee extension, filtered (100 Hz-1 kHz), amplified (× 10 000), full-wave rectified and averaged for 50 ms against the conditioning stimulus, using a sampling rate of 1–5 kHz. Conditioned and unconditioned trials (i.e. trials in which the background EMG activity was measured) were randomly alternated (1 s) during short sequences of 50–100 s to avoid muscular fatigue when using ‘strong’ contractions of 20 % MVC. The data recorded during 2–4 sequences were averaged to produce a single run containing 100 conditioned responses. The grand average was expressed as a percentage of the unconditioned EMG activity, which was measured in the alternating 100 control trials and then integrated over 50 ms to provide a measure of baseline EMG.
The latency of the changes in EMG activity produced by DP stimuli was expressed as the central transmission time (‘central delay’), calculated from the expected time of arrival of the DP volley at the same segmental level as the Q MN pool. The calculations involved measuring the latency of the rectified Q H reflex during contraction and adding to this value the difference between peripheral afferent conduction times for group I volleys in the DPN and FN (6-8 ms, depending on the subject's size; Meunier et al. 1990).
Studies of single motor units
Post-stimulus time histograms (PSTHs)
Surface recordings were used. Using the differential DE-2.3 electrodes, it was possible to record from single MUs of VL during contractions of 10–20 % of MVC. Nevertheless, most single MU recordings were made during contractions of less than 5 % MVC, often involving only one visible unit. The EMG potentials of single MUs were converted into standard pulses by a window discriminator with variable trigger levels, and these pulses were fed to a computer which subsequently triggered the stimulators about once every 0.7 s. Stimuli were delivered so that the peak of Ia excitation occurred at the end of the after-hyperpolarization (AHP) after the MU discharge. PSTHs were constructed for the 20–60 ms following conditioning stimulation using 0.2 ms bin widths. Histograms of the firing probability were constructed without stimulation (filled columns on the left of Figs 4-6) and after the conditioning stimulus (open columns on the left of Figs 4-6).
Figure 4. Changes in post-stimulus time histograms of the discharge of a single MU in VL produced by stimuli to the deep peroneal nerve during a quadriceps contraction of 20 % MVC.
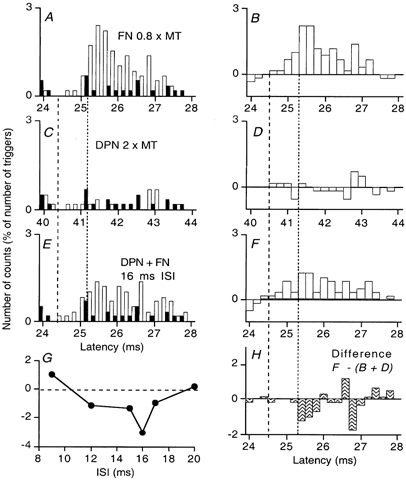
A, C and E, histograms of the discharge without (filled bars) and with (open bars) stimulation of the femoral nerve (FN) by itself (A), the DPN by itself (C) and both nerves, the DP preceding the FN stimulus by 16 ms (E). B, D and F, the difference between the histograms with and without stimulation. H, the suppression of the FN group I excitation, calculated as F - (B + D). In this and Figs 5-6, the number of counts in each bin (expressed as a percentage of the number of triggers) is plotted against the latency after FN stimulation (except in C and D and also E and F in Fig. 5, where the abscissa is the latency after the conditioning stimulation). G, the degree of suppression (calculated as in H) at different ISIs between DPN and FN stimuli. Note the slightly earlier onset of the femoral peak in F than in B, and the lack of suppression on the initial bins of the femoral group I excitation (the bins between the dashed and dotted vertical lines). Same subject as in Figs 1A-C and 3.
Figure 6. Changes in post-stimulus time histograms of the discharge of a single MU in VL produced by stimuli to the lateral articular nerve of the knee joint during weak Q contraction.
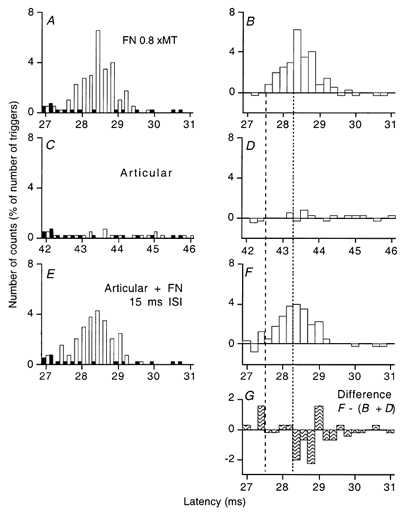
Panels A-F are arranged as in Fig. 4. The articular stimulation by itself had, if anything, a weak facilitatory effect (C, D) but suppressed the femoral group I excitation on combined stimulation (E, F). G, the suppression of the FN group I excitation, calculated as F - (B + D). Note the earlier onset of the femoral peak in F, and that the inhibition of the femoral peak produced by articular stimulation in F and G spared the first four bins of the femoral peak in B (i.e. the bins between the vertical lines).
Organisation of the experiments
The control situation without stimulation, separate stimulation of the femoral nerve and of various other nerves (DP, SP and/or lateral nerve of the knee joint) and combined stimulation of a number of nerves were randomly alternated in the same sequence. To clarify the differences between the results obtained in control and conditioned situations, the control value was subtracted from that observed after conditioning stimulation for each bin in the histogram (open columns on the right of Figs 4-6, in which the number of counts in each bin is expressed as a percentage of the total number of corresponding stimuli delivered during the sequence). The exceptional sequences in which a change in discharge occurred during the control sequence and contributed significantly to the differences seen between the two situations were not retained for further analysis.
Statistical analysis
The statistical analysis of changes in firing probability was confined to the window corresponding to the peak of excitation elicited by femoral stimulation. Within this window of analysis, changes in the femoral group I peak produced by the various conditioning stimuli were compared to the unconditioned femoral peak recorded in the same sequence. Consecutive bins exhibiting an increase (or a decrease) in firing probability with respect to the unconditioned femoral nerve peak were lumped together and tested with a χ2 test to determine the extent to which the distribution of firing probability after conditioning stimulation within this group differed from that in the control situation.
Results
These studies were precipitated by the observation that, during strong contractions, DP stimuli produced different changes in the H reflex and in the ongoing voluntary EMG of Q. This discrepancy suggested that there had been convergence between afferents in the DPN with afferents in the test volley for the H reflex onto inhibitory interneurones projecting to Q MNs. An attempt was made to determine which afferents converged onto these interneurones, and recordings from single MUs were then made to define any such convergence.
Discordance between the deep peroneal-induced modulation of the H reflex and of the ongoing EMG during strong Q contraction
Figure 1A shows the changes in the Q H reflex evoked by DP stimulation at 2 × MT. At rest (open circles), DP volleys produced biphasic facilitation of the Q H reflex, as previously described (Marque et al. 1996), with an early low-threshold non-monosynaptic group I peak at the 9–11 ms interstimulus intervals (ISIs) and a late high-threshold group II peak at the 13–18 ms ISIs.
The filled triangles in Fig. 1A and C show that the H reflex (A) and the ongoing voluntary EMG (C) underwent similar changes during weak tonic Q contractions of ≈5 % MVC. The modulation of the H reflex was much the same as at rest, except that the reflex facilitation started 1 ms earlier, at 8 ms, and the facilitation of the ongoing EMG had a similar time course when its latency was expressed with respect to the central delay (see Methods).
The filled circles in Fig. 1A and C show that the changes in the H reflex and in the ongoing voluntary EMG were quite different during stronger voluntary contractions of ≈20 % MVC: the reflex facilitation was replaced by inhibition at the 12–18 ms ISIs, while the ongoing EMG was facilitated more than with the 5 % contraction (even when, as in the figure, the conditioned EMG was normalised to the enhanced level of the ongoing control EMG). The EMG facilitation had a biphasic time course resembling the reflex facilitation at rest or during weak contractions. During a contraction of 10 % MVC, the evoked changes in the H reflex and ongoing Q EMG were similar to those with contractions of 20 % MVC (illustrated for this subject by the changes in the rectified EMG waveform of the H reflex in Fig. 1B).
Whatever the contraction strength, the H reflex returned to its control value by 20–25 ms (Fig. 1A): there was no inhibition at longer intervals (such as 30–50 ms, not illustrated).
Similar results were observed in the eight subjects so explored (Fig. 1D). The data for individual subjects are shown as thin lines during strong tonic contractions of Q (generally 20 % MVC, except in two subjects in whom 10 % MVC was used because of fatigue). In all subjects, there was a similar discrepancy between the H reflex, which was inhibited (left column), and the ongoing EMG, which was facilitated (right column), both effects being measured at their maxima, at central delays of between 6 and 12 ms (inhibition 80.4 ± 2.2 %, mean ± s.e.m., vs. facilitation 154.3 ± 8.9 %; P < 0.001, Kolmogorov-Smirnov test).
In order to define the characteristics of the suppression further, the effects of varying the intensity of the test stimulus were investigated. Figure 1E compares the suppressive effects of weak and strong FN stimulation on the Q H reflex facilitation produced by increasing the strength of the Q contraction from < 5 to ≥ 10 % MVC. The effect of contraction on the H reflex was measured at its maximum between 12 and 16 ms ISIs (see the vertical dotted line in Fig. 1A). There was no change in the amount of suppression (expressed as a percentage of Mmax) when FN intensity was increased (thick line, mean data for four subjects; thin lines, data for individual subjects, decrease × 1, increase × 1, no change × 2). It is noteworthy that this absence of change in the amount of suppression occurred even though the mean unconditioned reflex amplitude increased from 18 % of Mmax during weak contractions to 32 % during strong contractions (range 8–51 %).
Afferents responsible for the inhibition
DP stimulation at 2 × MT recruits afferents of different modality, and experiments were performed to attempt to identify the afferents responsible for the reflex suppression.
Muscle afferents
In Fig. 2A, the Q H reflex was recorded during a tonic contraction of 20 % MVC when conditioned by DP stimuli, and the intensity of the conditioning stimulus was altered at the 12 ms ISI. There was facilitation at 0.8 × MT (due to the group I heteronymous non-monosynaptic projection; see Marque et al. 1996; Simonetta-Moreau et al. 1999). Inhibition appeared at 1.1 × MT and was maximal at 1.4 × MT. The thresholds for the inhibition and the plateau occurred below those expected for group II muscle afferents (> 1.2 × MT and 3–5 × MT; Simonetta-Moreau et al. 1999). A similar threshold for inhibition (between 1.0 and 1.1 × MT) was found in the other three subjects so investigated. The threshold for group I afferents is much lower (0.6 × MT; Simonetta-Moreau et al. 1999) than that of the reflex suppression. This and the latency of the suppression (≈4-6 ms after the arrival of the group I volley at the segmental level of Q MNs) argue against a significant contribution of group I afferents to the reflex suppression.
Figure 2. Effects of different afferent conditioning volleys on the quadriceps H reflex.
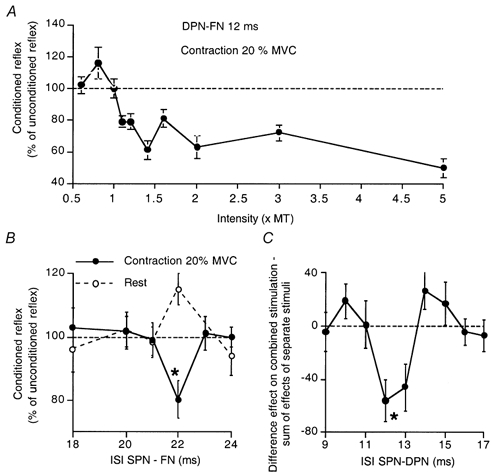
A, the effects of changing the intensity of DP stimuli on the H reflex during a contraction of 20 % MVC, at the 12 ms ISI. Data for one subject. Note the facilitation at 0.8 × MT, with the appearance of inhibition at 1.1 × MT, maximal at 1.4 × MT. B, the effects of SPN volleys on the H reflex of the same subject, at rest (open circles) and during a 20 % tonic Q contraction (filled circles). C, H reflex recorded at rest in another subject. The difference between the effects of combined stimulation of DPN (1.5 × MT) and SPN (5 × PT) and the sum of effects of separate stimuli is plotted against the ISI between SPN and DPN stimuli. There was an inhibition on combined stimulation when the interval between the SP and DP stimuli was 12 and 13 ms, while both SP and DP stimuli facilitated the H reflex at this latency when delivered by themselves (not illustrated). Unconditioned H reflexes between 10 and 20 % of Mmax in A-B, 10 % of Mmax in C. Each symbol is the mean of 20 measurements, vertical bars one s.e.m. Asterisks in B-C indicate a significant (P < 0.05) inhibition.
Cutaneous afferents
The DP stimuli produced a local skin sensation but no radiating paraesthesiae, except in one subject. Purely cutaneous stimuli that mimic the local sensation elicited by the stimulation of the DP nerve at the fibular head do not modify the Q H reflex during strong Q contractions (Forget et al. 1989). The effects of cutaneous afferents from the foot contained in the SP nerve at the ankle were investigated. Figure 2B shows that, at rest (open circles), stimulation at 3 × PT produced a Q H reflex facilitation at the 22 ms ISI, and that this was reversed to inhibition during a strong (20 % MVC) Q contraction (filled circles). Hence, it is likely that cutaneous afferents activated by DP stimulation contribute little to the effects of DP stimulation, but that cutaneous afferents in the SP nerve can produce effects similar to those of DP stimulation on the H reflex facilitation.
Joint afferents
The threshold for the inhibition (1-1.1 × MT; see Fig. 2A) corresponds to a threshold between those for group I and group II afferents, much as has been described for joint afferents in the cat (MacLennan, 1972). In order to explore further the origin of the afferents responsible for the Q H reflex suppression, the lateral articular nerve of the knee joint was stimulated at the level of the joint space (see Methods). Figure 3 shows that such stimulation had effects resembling those of the DP stimulation. During weak contractions at 5 % MVC (filled triangles), facilitation of the Q H reflex started at the 9 ms ISI and lasted only 3 ms. During strong contractions at 20 % MVC (filled circles), the facilitation was replaced by inhibition (Fig. 3A). As with DP stimulation, the same stimulation facilitated the ongoing voluntary EMG at corresponding central delays during the strong contraction (Fig. 3B). Similar results were obtained in the three subjects so explored. Suppression of the homonymous Ia excitation by joint afferents was also observed in the PSTH of a single motor unit (see below, Fig. 6). Given that muscle afferents in the DPN probably play little or no role (see above), it is likely that rapidly conducting joint afferents in the peroneal nerves were largely responsible for the limitation of the femoral group I excitation.
Figure 3. Effects of stimulation of the lateral articular nerve on the quadriceps H reflex.
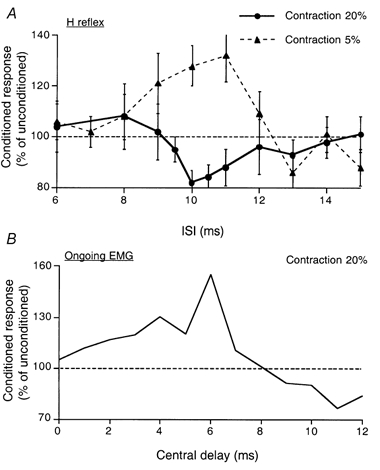
A, time course of the effects of stimulation of the lateral articular nerve of the knee joint on the Q H reflex during weak (5 %, filled triangles) and strong (20 %, filled circles) contractions of Q (each symbol is the mean of 20 measurements, vertical bars one s.e.m.). B, during strong (20 %) contractions the same articular stimulation facilitated the ongoing rectified averaged (100 sweeps, 1 kHz sampling rate) Q EMG (expressed as a percentage of integrated control EMG). Data for the same subject as in Fig. 1A-C.
Inhibitory effects on the H reflex in subjects at rest
The inhibitory effects on the H reflex described above were observed during strong contractions, and this suggests that descending drives and/or the peripheral feedback associated with voluntary contractions might be required to disclose these effects. To rule out these contraction-related effects, changes in the H reflex were investigated at rest after combined stimulation of the DP and SP nerves. For the experiment in Fig. 2C, DP stimulation at 1.5 × MT facilitated the Q H reflex at the 12 ms ISI (129 % of the unconditioned reflex) when delivered by itself (not illustrated). This interval was kept constant, and the effects of SP stimulation at 5 × PT on those produced by the DP stimulation were measured. Stimulation of the SP nerve by itself produced a small facilitation of the reflex (as in Fig. 2B) when delivered 24 ms before the femoral stimulus (i.e. 12 ms before the DP stimulus would have been delivered). Figure 2C shows the difference between the effect on combined stimulation and the sum of the effects of separate stimuli. On combined stimulation of the DP and SP nerves, the facilitation produced by either volley alone was reversed to significant inhibition. Similar results were obtained in the three subjects so investigated. Thus, the suppression of Q H reflex may also be observed at rest when conditioning stimuli are delivered to both the DP and SP nerves.
Studies in single motor units of VL
To define further the mechanism(s) underlying the suppression of the Q H reflex, PSTHs from 17 single MUs in VL were investigated in eight subjects. Modulation of the FN group I peak by the same conditioning volleys as above was explored using two experimental paradigms.
Effects of DP stimulation on the femoral group I excitation during strong contractions
It has previously been shown that DP stimulation at 2 × MT does not suppress the femoral group I excitation in single Q MUs at 16–18 ms ISIs (Marchand-Pauvert et al. 1999, their Fig. 2C-E). However, these experiments were performed during weak contractions involving only a single MU. Given the difference in the effects on the H reflex of weak and strong contractions (see Fig. 1A), the effects of DP stimulation on the femoral-induced excitation were investigated in MUs with a relatively high recruitment threshold (10-20 % MVC). With the differential surface electrodes used (DE-2.3, see Methods), five MUs could be isolated from three subjects during such contractions.
The findings are illustrated in Fig. 4. Histograms with stimulation (open columns) and without it (filled columns) are shown in A, C and E, and the corresponding differences between conditioned and control histograms are shown in B, D and F. The peak of excitation evoked by FN stimulation at 0.8 × MT occurred between 24.6 and 27.2 ms (A, B). If anything, separate stimulation of the DPN produced slight facilitation at around 43 ms (C, D). On combined stimulation, there was a highly significant reduction of the femoral excitation (E, F; 16 ms ISI; P < 0.001). This was not due to inhibition of the MN by the DP volley (see C, D, where there is the slight facilitation at 43 ms, i.e. 27 ms with respect to FN stimulation). The difference (F - (B + D)) between the effect on combined stimulation and the sum of effects of separate stimuli (H) shows that the suppression started at 25.4 ms (dotted vertical line), thus sparing the first 0.8 ms of the femoral group I excitation (starting at 24.6 ms, dashed vertical line). Indeed, in these initial bins there was a trend to facilitation, best appreciated by comparing panels A and E.
In this MU, significant suppression of the femoral excitation was present with combined stimulation at intervals between the DP and femoral volleys of 12–17 ms. This is illustrated in Fig. 4G, where the difference between the effects of combined stimulation and the sum of the effects of separate stimuli is plotted against the ISI. The time course is similar to that of the H reflex suppression during strong contraction shown in Fig. 1A (filled circles, same subject).
A significant reduction of the femoral group I excitation was observed in the other four MUs so explored (P < 0.001 or 0.01). In two MUs (two subjects), the effects of increasing the FN stimulus intensity from 0.7 to 0.8 × MT were investigated, but the results were not consistent (increase in the suppression on combined stimulation with one MU; decrease with the other).
Thus, DP stimulation suppressed the FN-induced peak in single MUs during strong contractions with the same time course as the suppressive effect on the Q H reflex.
Effects of convergence of different peripheral inputs
Suppression of the FN-induced peak could also be disclosed during weak Q contractions (involving only one detectable MU) when there was convergence of different afferent inputs. Relatively weak DP stimuli at 1.5 × MT were then combined with stimuli to the SP nerve at the ankle at 4–5 × PT. Figure 5 illustrates the findings: separate stimulation of the femoral nerve at 0.8 × MT produced prominent excitation beginning at 20.8 ms (A, B), the DP volley had no effect (C, D), and SP volleys produced weak facilitation (E, F). However, there was clear suppression with combined stimulation (G, H) when the SP and DP stimuli preceded the femoral stimulus by 25 and 12 ms, respectively. The suppression of the femoral group I excitation is clarified in panels I-K, which show the algebraic sum of the effects of separate stimuli (I), the effect on combined stimulation (J, same data as in H, but a different scale) and the difference between these two histograms (K). Here again, the profound reduction of the femoral excitation on combined stimulation spared the initial three bins. With the other six MUs (six subjects) so investigated, there was similar suppression, reducing the femoral group I excitation to 50 % of its control value and sparing the initial bins. Neither DP nor SP stimulation produced a suppression in the firing of the MU by itself. When the interval between the two conditioning volleys was varied, the suppression due to convergence of the DP and SP volleys only occurred over a narrow range of ISIs (1-2 ms). This is consistent with the brief duration of the inhibition of the Q H reflex elicited by combined DP and SP volleys at rest (see above, Fig. 2C).
Figure 5. Suppression of the femoral group I excitation due to convergence between superficial peroneal and deep peroneal volleys during quadriceps contraction involving only one motor unit.
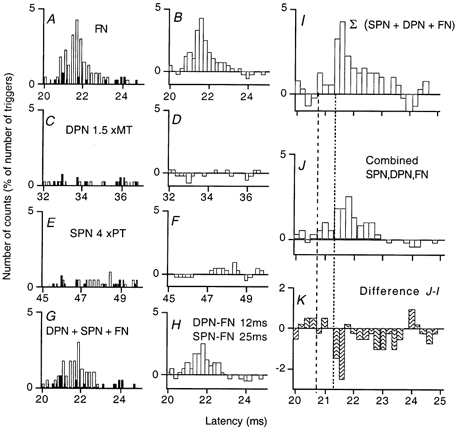
A and B, the FN group I excitation, the histograms in A recorded with and without stimulation, as in Fig. 4A, and that in B representing the difference. C and D, changes in firing probability produced by DPN stimulation at 1.5 × MT by itself. E and F, changes in firing probability produced by superficial peroneal nerve (SPN) stimulation at 4 × PT by itself, weak facilitation at 47.2 ms. G and H, the excitation produced by identical FN volleys when conditioned by both DPN and SPN stimulation (ISIs of 12 and 25 ms, respectively). I, the algebraic sum of the counts in histograms B, D and F. J, same data as in H, but on a different scale. K, difference between the counts in I and J. Note the inhibition of the femoral group I excitation starts at 21.4 ms, sparing the first three bins (i.e. the bins between the vertical lines) containing the onset of the femoral group I excitation in I.
The suppression of the femoral excitation thus results from convergence of two conditioning volleys onto common interneurones. If anything, in the absence of the femoral group I volley, combined stimulation of DPN and SPN produced some facilitation in the PSTH. This indicates that convergence with the femoral group I volley is required for the inhibition to manifest itself.
Effects of stimulation of joint afferents
With one MU, suppression of the FN-induced peak by stimulation of the lateral articular nerve of the knee joint (see Methods) could be disclosed during a weak Q contraction (involving only one detectable MU). This is illustrated in Fig. 6, where the panels are as in Fig. 4. The control femoral peak started at 27.6 ms (A, B). Separate stimulation of joint afferents elicited a small facilitation of MU discharge (C, D). On combined stimulation (E, F, 15 ms ISI), there was facilitation of initial part of the peak (starting at 27.4 ms) followed 1 ms later by highly significant suppression (G; P < 0.001). In four other units, separate stimulation of joint afferents did not suppress the FN-induced peak during weak contractions but, here again, significant suppression appeared when the joint afferent volley was combined with SP (three MUs) or DP (one MU) volleys.
Conclusion
With all 17 MUs explored, significant suppression of the FN-induced peak was produced by one conditioning volley during strong contractions (or a weak contraction for the unit in Fig. 6) or by combining two conditioning volleys during weak contractions. This suppression of the homonymous Ia excitation occurred even though the conditioning volleys by themselves did not inhibit the firing of the MU (and often actually elicited a small facilitation). The suppression of the femoral peak was generally of abrupt onset (Figs 4H, 5K, 6G), but it was difficult to establish precisely when it ended because of the limited duration of the control peak of excitation.
Sparing of the initial component of the group I excitation
With both the H reflex and the PSTHs from single MUs, the suppression spared the onset of the response evoked by FN stimulation. Figure 1B shows the control (thin line) and conditioned (thick line; DP at 2 × MT, 13 ms ISI) rectified averaged H reflex recorded during a tonic contraction of 10 % MVC. During the first 2 ms of the compound H wave (i.e. 21–22 ms) the conditioned response was bigger than the control, but subsequently the H wave was truncated by inhibition. The initial facilitation reflects the facilitation of Q MNs by DP volleys, which is apparent during weak contractions in Fig. 1A (filled triangles) when assessing the whole response. To address this issue further, the effects seen in PSTHs of the discharge of single MUs were examined. In all 17 MUs tested, the initial bins of the peak of the femoral group I excitation were unaffected by the suppression. The mean duration of this initial sparing was 0.7 ± 0.08 ms. Rather than suppression, there was usually a trend to facilitation in these bins, and this advanced the onset of the peak by one 0.2 ms bin (compare B and D in Fig. 4 and Fig. 6, and I and J in Fig. 5). This reflects facilitation of the MN by the conditioning stimulation, and the slightly earlier latency suggests that the PSTH method used in the study (with the femoral stimulation triggered by the previous MU discharge) may have allowed the end of the AHP in the MN to obscure the true onset of the EPSP in the control PSTH.
Discussion
The main finding of the present results is that, during strong voluntary contractions, the investigated conditioning volleys suppressed the Ia excitation of Q MNs (as measured using the H reflex and the peak of excitation in PSTHs from single MUs), but not the ongoing Q EMG or the background firing of the single MUs. It is argued below that this probably occurred because the conditioning volleys increased an existing limitation on the size of the H reflex by potentiating an inhibitory effect of group I afferents in the test volley.
Possible mechanisms underlying the suppression of the homonymous Ia excitation
Such a discrepancy raises the possibility of an inhibitory mechanism acting selectively on the H reflex but not directly on Q MNs. This inhibition could have several origins:
Recruitment of different motoneurones in the H reflex and the ongoing EMG
In isometric voluntary contractions (Milner-Brown et al. 1973) and the H reflex (Buchthal & Schmalbruch, 1970), MNs are recruited in a similar orderly sequence from slow to fast MUs, in accordance with Henneman's ‘size principle’ (cf. Henneman & Mendell, 1981). However, MUs activated in the voluntary contraction may not be recruited into the H reflex recorded during voluntary contractions because of the post-spike AHP, such that the H reflex may involve MUs of higher threshold than those engaged in the voluntary contraction. The discrepancy between the modulations of the EMG and the H reflex could be explained if the net result of the DP (or articular) input was facilitation of early-recruited MNs and inhibition of later recruited MNs. There are several arguments against this hypothesis: (i) in experiments where FN intensity was varied (Fig. 1E) similar results were obtained using H reflexes as small as 8 % of Mmax and voluntary contractions of only 10 % MVC; (ii) a similar suppression was observed at rest when combining DP and SP conditioning volleys and using unconditioned H reflexes of ≈10 % of Mmax (Fig. 2C); and (iii) the hypothesis would not explain why the suppression spared the onset of the H reflex during ‘strong’ contractions of 10 % of MVC (Fig. 1B).
The suppression of the homonymous Ia excitation observed in low-threshold VL MUs (e.g. Figs 5-6) may seem inconsistent with the H reflex facilitation evoked during weak contractions by DP or articular volleys (Figs 1A, 3A, filled triangles). This could reflect the different MN pools studied for the PSTHs (VL) and the H reflex (VI). In the PSTHs, the conditioning volley produced a consistent trend to facilitation in the initial bins of the group I peak (see Results). This facilitation of the purely monosynaptic component of the peak suggests that the same features (facilitation and inhibition) are present in VL and VI MNs, and it is therefore likely that inhibition is stronger in VL MNs where it overwhelms facilitation in the late bins even during weak contractions.
Presynaptic inhibition of the test Ia volley
Presynaptic inhibition of femoral Ia terminals by the group I volley from pretibial flexors (Hultborn et al. 1987a) may be ruled out because the resulting suppression should affect the whole response of Q MNs including its initial purely monosynaptic part (Hultborn et al. 1987a; Katz et al. 1988; Meunier & Pierrot-Deseilligny, 1998). In addition, (i) the duration of the inhibition observed here, only ≈5-10 ms, is too short for presynaptic inhibition of Q Ia terminals elicited by afferents from pretibial flexors (see Hultborn et al. 1987a); and (ii) presynaptic inhibition has been shown to be decreased during voluntary contractions of the target muscle (Hultborn et al. 1987b; Iles & Roberts, 1987; Meunier & Pierrot-Deseilligny, 1989), and the stronger the contraction the greater is this decrease (Iles & Roberts, 1987).
Disynaptic inhibition elicited by the test volley
Evidence for disynaptic inhibition
The latency of the first MNs discharging in the H reflex is consistent with monosynaptic transmission (Magladery et al. 1951). However, it has long been argued that disynaptic non-reciprocal group I inhibition activated by the test volley and/or recurrent inhibition activated by the first MNs to discharge in the reflex have ample time to inhibit higher-threshold MNs and to limit the size of the H reflex (Burke et al. 1984). Indeed, the fact that the inhibitory pathway involves one extra synapse would not prevent disynaptic inhibitory post-synaptic potentials (IPSPs) from suppressing the monosynaptic Ia discharge of MNs. This is possible for two reasons: (i) in individual MNs, the rise time of the EPSP ensures that the last recruited MNs receive monosynaptic input sufficiently late to be inhibited by an IPSP, even though the latter does not begin until 0.5-1.0 ms after the beginning of the EPSP (see Matthews, 1972); and (ii) in the MN pool, the test reflex discharge is desynchronised: even in the cat there is 0.5 ms between the firing of the first and last recruited MNs contributing to the compound monosynaptic reflex (Araki et al. 1960). In man, the afferent pathway is longer and the conduction velocities in Ia afferents slower, and this time has been estimated at 1.5 ms for the Q H reflex (see Fournier et al. 1986). This desynchronisation is not apparent in the compound H reflex because the axons of the last recruited MNs have a more rapid conduction velocity.
Because of the synaptic delay at the interneurone, the inhibitory input would reach the MN after the direct monosynaptic Ia input. Thus, post-synaptic inhibition due to afferents in the test volley should not affect the onset of the femoral excitation, and initial sparing should be demonstrable, as it was. It is conceded that insights from changes in the waveform of the H reflex (as in Fig. 1B) are limited because of the different conduction velocities for individual MUs and the duration of their EMG potentials. It is therefore important that the initial sparing was invariably observed in PSTHs from single MUs. The mean duration of the sparing in single MUs was 0.7 ms, consistent with disynaptic inhibition (Pierrot-Deseilligny et al. 1981; Hultborn et al. 1987a) elicited by afferents in the femoral volley truncating the peak of monosynaptic excitation.
Recurrent or non-reciprocal group I inhibition?
Recurrent inhibition due to activation of Renshaw cells by the first-recruited MNs could be facilitated by the conditioning volleys. However, a similar inhibition was seen in the PSTH for single MUs at corresponding ISIs and, in recordings in which the contraction involved only one detectable motor unit (Figs 5-6), the reflex discharge of this unit could not produce recurrent inhibition of the discharge of that same unit. Even though there may still have been activity in other MUs, it is unlikely that their FN-induced discharge preceded at spinal level that of the lowest-threshold MU being tested, and this is particularly so with the method used in which the stimulation is triggered by the discharge of the test MU. In addition: (i) the peripheral volleys used here activated afferents which have been shown in the cat to inhibit Renshaw cells (group II; Fromm et al. 1977; cutaneous, Wilson et al. 1964); (ii) there are no projections of recurrent collaterals from pretibial flexor MNs onto Renshaw cells directed to Q MNs in humans (Meunier et al. 1994); and (iii) the stronger the tonic contraction the weaker is homonymous recurrent inhibition (Hultborn & Pierrot-Deseilligny, 1979).
In cat MNs, the central delay of the disynaptic reciprocal Ia IPSP is 0.8 ms longer than that of the monosynaptic Ia EPSP (Araki et al. 1960) and, if anything, that of the disynaptic Ib IPSP is somewhat longer (Eccles et al. 1957). The duration of the sparing found in the present investigation (0.7 ms) may therefore seem a little brief. The discrepancy is explained by three factors: (i) the resolution of the method used (0.2 ms bins); (ii) the likelihood that the onset of the monosynaptic peak was slightly disadvantaged by the end of the AHP following the previous MN discharge (see Results); and (iii) the probability that transmission in inhibitory interneurones was facilitated by the summation of inputs elicited by the femoral and conditioning volleys.
It is therefore concluded that the suppression involved facilitation by the conditioning volley of oligosynaptic non-reciprocal inhibition due to afferents in the femoral volley itself.
Facilitation of non-reciprocal group I inhibition by conditioning volleys
The conclusion that the suppression of the homonymous femoral group Ia excitation involved non-reciprocal group I inhibitory interneurones (see the wiring diagram in Fig. 7) is supported by the finding that the peripheral afferent inputs eliciting this suppression were mainly articular and cutaneous (see Figs 2-3, 5-6). This is consistent with data from the cat in which the two afferent modalities have potent projections to these interneurones. In addition, the duration of the suppression (≈5-10 ms) both of the H reflex (Fig. 1A) and of the peak of monosynaptic excitation in single units (Fig. 4G) is consistent with the time course of the cutaneous or articular facilitation of the disynaptic non-reciprocal group I IPSP described in cat MNs (see Lundberg et al. 1977, 1978). A potent facilitation of heteronymous non-reciprocal group I inhibition from gastrocnemius-soleus to Q MNs by cutaneous (Pierrot-Deseilligny et al. 1982) and articular (Iles et al. 1990) afferents has also been documented in human subjects.
Figure 7. Wiring diagram of possible connections investigated in the present studies.
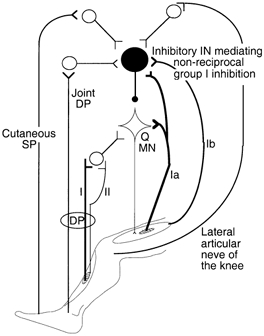
The large filled circle shows the interneurones (IN) mediating disynaptic autogenetic non-reciprocal group I inhibition of quadriceps motoneurones (Q MN). Homonymous Ia and Ib afferents converge onto these inhibitory INs. Joint afferents in the lateral articular nerve of the knee joint and the deep peroneal nerve (DP, presumably from ankle and foot joints), and cutaneous afferents in the superficial peroneal nerve (SP) also converge onto these inhibitory INs. Group I and group II afferents from pretibial flexors excite Q MNs through a distinct interneuronal pathway (‘group I-group II INs’, Jankowska, 1992). Excitatory synapses are represented by Y-shaped bars, inhibitory synapse by a small filled circle, and excitatory and inhibitory INs by open and filled circles, respectively. As sketched, the different joint and cutaneous excitatory effects are mediated on last-order inhibitory INs through separate first-order excitatory INs, although it is possible that they converge on first-order excitatory INs (see text).
Convergence of the test FN volley and the conditioning volleys on common inhibitory interneurones was apparent in the PSTHs, but was not demonstrated unequivocally in the H reflex experiments. Nevertheless, the DP- (or articular-) induced reflex suppression during strong contractions is consistent with potentiation of the transmission in disynaptic autogenetic inhibitory pathways activated by the test volley itself. The different findings can all be explained by the convergence onto non-reciprocal group I interneurones of three inputs : (i) group I afferents in the test volley, (ii) the ‘natural’ peripheral and/or descending inputs associated with ‘strong’ contractions, and (iii) the conditioning volleys (see Fig. 7). When there are only two of these inputs (e.g. test and one conditioning volley at rest or during ‘weak’ contraction; ‘natural’ inputs and one conditioning volley when measuring ongoing EMG) there is usually no suppression of the DP- (or joint-) induced facilitation of Q MNs. It is likely that excitation of non-reciprocal group I interneurones by the peripheral and/or descending inputs associated with relatively strong contractions is necessary for the combined test and conditioning volleys to inhibit MNs. Inversely, the finding that increasing the FN test volley did not increase the suppression of the reflex (Fig. 1E) or of the peak of Ia excitation in single MUs might indicate occlusion in the relevant interneurones between the three inputs.
At rest or during weak contraction, suppression of the homonymous Ia excitation can be disclosed provided that two conditioning volleys (articular and cutaneous) are combined.
It should be noted that, while the studies on convergence between conditioning and test volleys indicate a projection to common last-order inhibitory interneurones, convergence between the different conditioning volleys may also have occurred at first-order interneurones.
Significance of the findings
Methodological considerations
This is the first investigation providing experimental, rather than theoretical, evidence for a disynaptic limitation of the group Ia excitation that is the basis of the H reflex. The present studies were designed to increase an existing limitation on the size of the H reflex by potentiating an inhibitory effect of afferents in the test volley. The complementary experiment would involve a reverse approach, i.e. facilitating the H reflex by suppressing non-reciprocal group I inhibition to Q MNs, but we are unaware of a peripheral input that can demonstrably suppress transmission across the interneurones mediating presumed non-reciprocal group I inhibition to Q MNs. The potential for such a limitation on the size of the H reflex must always be considered when interpreting alterations in the size of the H reflex, because small reflexes elicited by weak stimulus intensities are equally contaminated by this disynaptic inhibition. This is not surprising, given (i) the close electrical thresholds of Ia and Ib afferents in the femoral nerve (see Hultborn et al. 1987a); and (ii) the convergence, described in the cat, of Ia and Ib afferents onto interneurones of non-reciprocal group I inhibition (see Jankowska, 1992).
The potent descending control of the transmission in the pathway of non-reciprocal group I inhibition and the wide range of peripheral afferents that can excite them, as described in the cat (see Jankowska, 1992), provide ample scope for reflex alterations independent of changes in the group Ia monosynaptic projection (i.e. independent of alterations in MN excitability and/or presynaptic inhibition of Ia terminals).
Can these results be generalized?
The question arises of the extent to which the results described here with the Q H reflex may be transposed to the soleus H reflex, which is more commonly used in human experiments. The Q was chosen for the present study because the experimental circumstances are particularly favourable: (i) the DP conditioning volley elicits a large group I-group II facilitation at rest, and this is not contaminated by recurrent inhibition; and (ii) cutaneous and articular facilitation of interneurones mediating non-reciprocal group I inhibition have so far been described in pathways to Q (see above) but not to soleus MNs. On the other hand, we are unaware of a peripheral input that can demonstrably increase (or decrease) transmission across the interneurones mediating presumed non-reciprocal group I inhibition to soleus MNs.
The possibility that afferents in the group I test volley could impose a limitation on the size of the H reflex was first raised for the soleus H reflex. Indeed, it was argued that the duration of the compound group I EPSP underlying the soleus H reflex (some 1–2 ms) is so short that the monosynaptic Ia component of the EPSP must be curtailed by oligosynaptic inhibition activated by the test volley (Burke et al. 1984). In addition, because the degree of desynchronisation of the reflex discharge allows monosynaptic Ia excitation of the MNs to be curtailed by disynaptic inhibition, the limitation should be more pronounced for the soleus (≈2 ms, Burke et al. 1984) than for the Q (≈1.5 ms, Fournier et al. 1986). Finally, the longer afferent pathway of the soleus H reflex would allow greater dispersion of the afferent volley and thereby a greater influence on the reflex discharge from Ib afferents activated by the test stimulus. For these reasons, it is probable that soleus H reflexes are also truncated by disynaptic inhibitory activity. This limitation could contribute to the common absence of H reflex at rest in muscles, such as tibialis anterior and extensor carpi radialis. In these muscles, the appearance of an H reflex during a tonic voluntary contraction may be due, in part, to the depression of non-reciprocal group I inhibition to the active MN pool (Fournier et al. 1983), as well as the increased excitability of the MN pool.
Functional considerations
In so far as data obtained with electrically evoked synchronised volleys during isometric contractions may be transposed to the normal modus operandi of the spinal cord, the present results suggest two functional considerations. (i) During voluntary contractions, there is a descending control which decreases non-reciprocal group I inhibition directed to the active MN pool (Fournier et al. 1983). Presumably this descending control is sufficient to suppress ‘natural’ autogenetic group I inhibition during voluntary contractions, but the present findings suggest that peripheral afferent inputs would be able to reverse any such suppression. The ability of peripheral afferents to restore transmission through interneurones mediating non-reciprocal group I inhibition would have functional significance in allowing autogenetic inhibition to reappear when necessary to modulate contractions. As hypothesised by Lundberg et al. (1977), cutaneous facilitation of non-reciprocal group I inhibition could be used to curtail a movement when it was unexpectedly obstructed, and facilitation of Ib inhibitory interneurones by joint afferents would prevent an excessive contraction from damaging ligaments and capsule of the joint (the finding that this facilitation was only disclosed during strong contractions could provide some support to the latter). (ii) It may seem surprising, functionally speaking, that the same DPN volley may have opposite effects on Q MNs: facilitation observed at rest or during ‘weak’ contractions involving some MUs, and inhibition during stronger voluntary contractions. In fact, these opposite effects are due to stimulation of different afferents activating distinct interneuronal pools (see the wiring diagram in Fig. 7). These afferents are not necessarily recruited in parallel under natural conditions, and their interneuronal projections have different roles in natural movement: interneurones mediating non-monosynaptic group I-group II excitation from pretibial flexors onto Q MNs help secure the contraction of the Q during the stance phase of gait (Marchand-Pauvert & Nielsen, 2002), while, as discussed above, interneurones mediating non-reciprocal group I inhibition from femoral nerve may be excited by joint and cutaneous afferents to restore non-reciprocal group I inhibition when necessary.
Acknowledgments
Our thanks are due to A. Rigaudie and M. Dodo for excellent technical assistance. This work was supported by grants from Assistance Publique-Hôpitaux de Paris, Ministère de la Recherche (UPRES EA 2393), Institut pour la Recherche sur la Moelle Épiniére (IRME) and the National Health and Research Council of Australia. Véronique Marchand-Pauvert was supported by grants from IRME and Fondation Singer-Polignac.
References
- Araki T, Eccles JC, Ito M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. Journal of Physiology. 1960;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal F, Schmalbruch H. Contraction times of twitches evoked by H reflexes. Acta Physiologica Scandinavica. 1970;80:378–382. doi: 10.1111/j.1748-1716.1970.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size : a study in man and the cat. Experimental Brain Research. 1990;81:33–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. Journal of Physiology. 1957;138:227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget R, Hultborn H, Meunier S, Pantieri R, Pierrot-Deseilligny E. Facilitation of quadriceps motoneurones by group I afferents from pretibial flexors in man. 2. Changes occurring during voluntary contraction. Experimental Brain Research. 1989;78:21–27. doi: 10.1007/BF00230682. [DOI] [PubMed] [Google Scholar]
- Fournier E, KatZ R, Pierrot-Deseilligny E. Descending control of reflex pathways in the production of voluntary isolated movements in man. Brain Research. 1983;288:375–377. doi: 10.1016/0006-8993(83)90122-1. [DOI] [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. Journal of Physiology. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C, Haase J, Wolf E. Depression of recurrent inhibition of extensor motoneurones by the action of group II afferents. Brain Research. 1977;120:459–468. doi: 10.1016/0006-8993(77)90399-7. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneurone pool and its inputs. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, Motor Control, II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 423–507. part 1. [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. Journal of Physiology. 1987a;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. Journal of Physiology. 1987b;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. Journal of Physiology. 1979;297:229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Pierrot-Deseilligny E, Wigström H. Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. Journal of Physiology. 1979;297:253–266. doi: 10.1113/jphysiol.1979.sp013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Roberts RC. Inhibition of monosynaptic reflexes in the human lower limb. Journal of Physiology. 1987;385:69–87. doi: 10.1113/jphysiol.1987.sp016484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Stokes M, Young A. Reflex actions of knee joint afferents during contraction of the human quadriceps. Clinical Physiology. 1990;10:489–500. doi: 10.1111/j.1475-097x.1990.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- KatZ R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988;111:417–437. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC. Conduction and synaptic transmission of the reflex response to stretch in spinal cats. Journal of Neurophysiology. 1943;6:317–326. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. Journal of Physiology. 1977;265:763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. Journal of Physiology. 1978;284:327–343. doi: 10.1113/jphysiol.1978.sp012543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan C. The behaviour of receptors of extramuscular and muscular origin with afferent fibres contributing to the group I and group II of the cat tibialis anterior muscle nerve. Journal of Physiology. 1972;222:90–91P. [PubMed] [Google Scholar]
- Magladery JW, Porter WE, Park AM, Teasdall RD. Electrophysiological studies on nerve and reflex activity in normal man. IV. Two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bulletin of Johns Hopkins Hospital. 1951;88:499–519. [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nielsen JB. Modulation of non-monosynaptic excitation from ankle dorsiflexor afferents to quadriceps motoneurones during human walking. Journal of Physiology. 2002;538:647–657. doi: 10.1113/jphysiol.2001.012675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to thigh motoneurones. Journal of Physiology. 1999;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Experimental Brain Research. 1996;109:357–360. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Spindles and their Central Action. London: Edward Arnold; 1972. p. 630. [Google Scholar]
- Meunier S, Pénicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. Journal of Physiology. 1990;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. Journal of Physiology. 1989;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of Ia afferents in humans. Experimental Brain Research. 1998;119:415–426. doi: 10.1007/s002210050357. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Pattern of heteronymous recurrent inhibition in the human lower limb. Experimental Brain Research. 1994;102:149–159. doi: 10.1007/BF00232447. [DOI] [PubMed] [Google Scholar]
- Milner-Brown SH, Stein RE, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. Journal of Physiology. 1973;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard J. Réflexes et Régulations d'Origine Proprioceptive chez l'Homme. Paris: Arnette; 1955. p. 293. [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, KatZ R. Reversal in cutaneous control of Ib pathways during human voluntary contraction. Brain Research. 1982;233:400–403. doi: 10.1016/0006-8993(82)91213-6. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Experimental Brain Research. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. Journal of Physiology. 1999;517:287–300. doi: 10.1111/j.1469-7793.1999.0287z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Talbot WH, Kato M. Inhibitory convergence upon Renshaw cells. Journal of Neurophysiology. 1964;27:1063–1079. doi: 10.1152/jn.1964.27.6.1063. [DOI] [PubMed] [Google Scholar]


