Abstract
Interleukin (IL)-6 is a pleiotropic cytokine, which has a variety of physiological roles including functions within the central nervous system. Circulating IL-6 increases markedly during exercise, partly due to the release of IL-6 from the contracting skeletal muscles, and exercise-induced IL-6 may be linked with central fatigue, which is enhanced by hyperthermia. Exercise-induced IL-6 may also stimulate hepatic glycogenolysis, which is important during prolonged and repeated exercise. Thus, in a randomised order and separated by 60 min of rest, eight young male subjects completed two 60 min exercise bouts: one bout with a normal (38 °C) and the other with an elevated (39.5 °C) core temperature. The cerebral IL-6 response was determined on the basis of internal jugular venous to arterial IL-6 differences and global cerebral blood flow. There was no net release or uptake of IL-6 in the brain at rest or after 15 min of exercise, but a small release of IL-6 was observed after 60 min of exercise in the first bout (0.06 ± 0.03 ng min−1). This release of IL-6 from the brain was five-fold greater at the end of the second bout (0.30 ± 0.08 ng min−1; P < 0.05) with no separate influence of hyperthermia. In conclusion, IL-6 is released from the brain during prolonged exercise in humans and it appears that the duration of the exercise rather than the increase in body temperature dictates the cerebral IL-6 response.
The plasma concentration of interleukin-6 (IL-6) increases markedly in response to exercise (Northoff & Berg, 1991; Nehlsen-Cannarella et al. 1997; Nieman et al. 1998; Pedersen et al. 2001) and it appears that IL-6 production in working skeletal muscles is a major source of the exercise-induced increase in arterial IL-6 (Bartoccioni et al. 1994; Steensberg et al. 2000). In the muscles the stimuli for IL-6 production are contractions per se and a low muscle glycogen content (Keller et al. 2001; Steensberg et al. 2001a) and the IL-6 produced by the active skeletal muscles may partly mediate the hepatic glucose output necessary to maintain blood glucose homeostasis when the uptake of glucose by the muscles is increased during prolonged exercise (Pedersen et al. 2001). However, other tissues also rely on blood glucose as metabolic fuel and blood glucose homeostasis is important especially for the metabolic function of the central nervous system (CNS) (Sokoloff, 1981; Boyle et al. 1994). Within the CNS IL-6 levels remain low under normal conditions, but during brain injury, inflammation, hypoxia and certain diseases, the IL-6 level becomes elevated, and the predominant source of IL-6 production in CNS appears to be activated astrocytes (Van Wagoner & Benveniste, 1999). IL-6 is also expressed in hypothalamic nuclei, where its synthesis and secretion may be enhanced after long-term stress (Schobitz et al. 1993; Shizuya et al. 1998). It furthermore appears that centrally acting IL-6 plays a role in the regulation of appetite, energy expenditure and body composition. Thus, IL-6-deficient mice increase their food intake and develop mature-onset obesity, while intracerebroventricular injection of IL-6 in rats induces an acute increase in whole body oxygen consumption (Wallenius et al. 2002). Gleeson (2000) speculated that IL-6 may be taken up by the brain during exercise and that the large release of IL-6 from the skeletal muscles during prolonged exercise could act as a feedback mechanism contributing to the development of so called ‘central fatigue’. Alternatively, IL-6 could be released from the brain as a stimulus for increased hepatic glucose output, similar to the observation from the skeletal muscles, or IL-6 could be released during exercise as a consequence of an increased production in brain regions activated during exercise.
This study investigated whether prolonged exercise is associated with an altered cerebral IL-6 response. Cerebral IL-6 kinetics were evaluated during both normothermic and hyperthermic exercise conditions. The latter exercise condition is associated with central fatigue (Nybo & Nielsen, 2001a), a slowing of the electroencephalogram and an increased perception of exertion (Nybo & Nielsen, 2001b). We hypothesised that during exercise the cerebral IL-6 response could be influenced by the combined effect of the exercise duration and heat stress and especially so if the cerebral IL-6 response is linked with central fatigue.
Methods
Eight health endurance-trained males participated in the study (mean age 27 ± 2 years (± s.e.m.), height 1.82 ± 0.02 m, mass 73 ± 3 kg and maximal oxygen uptake (V̇O2,max) 63 ± 2 ml kg−1 min−1). The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethical Committee of Copenhagen and Frederiksberg (KF 01-135/00).
Experimental set-up
All subjects completed two 1 h exercise bouts on a cycle ergometer (Monark 829E, Sweden) at a power output (170 ± 4 W) that corresponded to ≈50 % V̇O2,max. In one trial, exercise was carried out in a thermoneutral environment, whereas an external heat stress was superimposed in the other trial (peak oesophageal temperature, Toes = 39.5 ± 0.2 °C at the end of the trial). The environmental temperature was 20 °C in both trials and the hyperthermic exercise condition was generated by dressing the subjects in waterproof clothing, plastic hood and rubber gloves (Nybo et al. 2002). The two trials were separated by 1 h recovery and the treatment order was randomly assigned and counterbalanced across subjects. The subjects had water freely available to them during the trials and were asked to drink at least 500 ml water in the recovery period between the two trials. Hydration status was thereby maintained from the first to the second exercise bout as indicated by similar arterial concentrations of haemoglobin and sodium. Also the haemoglobin concentration was similar in the jugular venous (14.5 ± 0.2 g l−1) and arterial blood (14.6 ± 0.2 g l−1; exercise values), indicating that haemoconcentration did not take place across the brain.
The subjects were instructed to consume a carbohydrate-rich breakfast plus ≈400 ml water 1–2 h before arrival at the laboratory. The subjects were furthermore requested to abstain from coffee, tea or other caffeine-containing items on the day of the experiment. Upon arrival, an oesophageal thermocouple was inserted via the nasal passage at a distance equal to one-fourth of the subject's standing height and a heart rate (HR) monitor was attached to the subject. The subject then rested on a couch while catheters were inserted into the radial artery of the non-dominant arm and into the bulb of the right internal jugular vein. An antecubital venous catheter for infusion of 133Xe was placed contralaterally to the arterial catheter. The subject then rested for half an hour before baseline measurements of core temperature, HR and blood samples were obtained. Toes and HR were measured every 5 min during the exercise bouts. The global cerebral blood flow (CBF) was determined after 15 min and 60 min of exercise in both trials and blood samples were drawn at the same time.
Blood sample analyses and calculations
Arterial and jugular venous values of haemoglobin, haematocrit, sodium, glucose and lactate were determined on ABL 615 apparatus (Radiometer, Copenhagen, Denmark) with an accuracy of 0.1 mm. Blood samples for determination of IL-6, adrenaline and noradrenaline were drawn into glass tubes containing EDTA. These samples were immediately spun at 2200 g for 5 min at 4 °C. Plasma was divided into fractions and stored at −80 °C until the analyses were performed. IL-6 was measured with a high-sensitivity ELISA kit (R&D Systems, Minneapolis, USA) and the concentrations of adrenaline and noradrenaline were determined with a RIA kit (Biotech-IgG, Copenhagen, Denmark).
CBF was measured in millilitres per minute per gram of cerebral tissue by the Kety-Schmidt technique in the desaturation mode with xenon as the radioactive tracer (Kety & Schmidt, 1948; Madsen et al. 1993) and global CBF was estimated assuming an average brain mass of 1400 g (Miller & Corsellis, 1977; Dekaban, 1978). Cerebral plasma flow was calculated on basis of the global CBF and the corresponding haematocrit value, and the cerebral balance of IL-6 was calculated as cerebral plasma flow multiplied by the arteriovenous difference (a-vDIL-6).
Statistical analysis
One- and two-way (time-by-trial) repeated measures analyses of variance (ANOVA) were performed to evaluate differences between and within trials. Following a significant F test, pair-wise differences were identified using Tukey's post hoc procedure. ANOVA was also performed to test if the order of the trials (i.e. first vs. second exercise bout) had an effect on the physiological responses. Data are presented as means ± s.e.m. unless otherwise indicated. The level of significance was set at P < 0.05.
Results
The arterial IL-6 concentration increased during both the first and the second bout of exercise. Thus, the arterial IL-6 concentration was 0.3 ± 0.1 ng l−1 at rest and increased to 2.8 ± 0.4 ng l−1 at the end of the first trial and to 5.3 ± 1.1 ng l−1 at the end of the second trial (P < 0.05). There was no net release or uptake of IL-6 in the brain at rest or after 15 min exercise, but a small release of IL-6 from the brain was observed at the end of the first exercise period (Fig. 1). However, the v-aDIL-6 increased significantly during the second bout of exercise whereas the CBF did not differ between the first (47 ± 5 ml (100 g)−1 min−1) and the second bout of exercise (48 ± 4 ml (100 g)−1 min−1). The cerebral release of IL-6 was therefore higher at the end of the second compared with the first exercise period (Fig. 2).
Figure 1. Jugular venous to arterial IL-6 differences (upper panel) and arterial IL-6 concentrations (lower panel) at rest and during exercise.
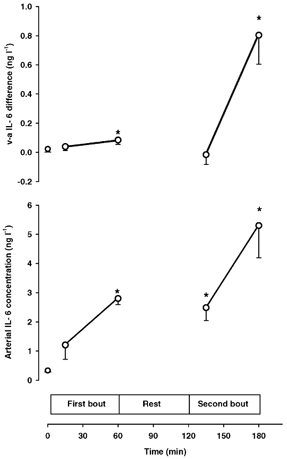
Both exercise trials were completed at a workload (170 ± 4 W) that corresponded to 50 % V̇O2,max and interspaced with 1 h of rest. Data are mean values of 8 subjects. * Significantly different from the resting value (P < 0.05).
Figure 2. Mean and individual values of IL-6 release from the brain at the end of the first and the second exercise bout.
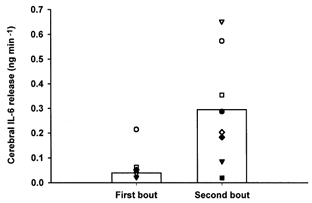
Both exercise trials were completed at a workload (170 ± 4 W) that corresponded to 50 % V̇O2,max and interspaced with 1 h of rest. The mean value represented by the vertical bar is significantly higher in the second compared with the first exercise bout (P < 0.01).
The arterial blood glucose concentration was 5.2 ± 0.2 mm at rest and similar at the end of the first exercise bout (5.0 ± 0.2 mm) and at the end of the second bout (4.9 ± 0.2 mm), demonstrating that blood glucose homeostasis was maintained throughout the exercise protocol. The arterial blood glucose concentration was also similar when the hyperthermic trial (5.0 ± 0.2 mm) was compared with the normothermic trial (4.8 ± 0.2 mm). The core temperature was not different after 15 min exercise in the two trials (37.8 ± 0.1 vs. 37.9 ± 0.1 °C), but in the hyperthermic bout, the core temperature kept increasing throughout exercise, to reach a peak value of 39.5 ± 0.2 °C, whereas it stabilised at 37.9 ± 0.1 °C for the remaining exercise period in the normothermic trial. However, the significantly elevated core temperature did not affect the cerebral IL-6 response, which was similar when the normothermic and hyperthermic trials were compared (Fig. 3).
Figure 3. Jugular venous to arterial IL-6 differences at rest and during exercise with normothermic (Toes ≈38 °C) and hyperthermic (Toes ≈39.5 °C) conditions.
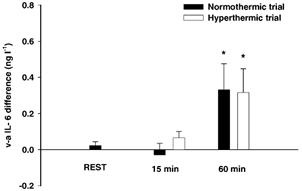
Data are mean values of 8 subjects. * Significantly different from the resting value (P < 0.05).
The arterial adrenaline concentration was similar in the normothermic and hyperthermic trials, but it tended (P = 0.07) to be higher in the second (3.5 ± 1.0 nm) compared to the first exercise bout (1.8 ± 0.3 nm). The arterial concentration of noradrenaline was higher at 60 min in the hyperthermic trial (16.2 ± 4.01 nm) than at the corresponding time in the normothermic trial (6.2 ± 1.0 nm; P < 0.05). The jugular venous plasma concentrations of catecholamines were similar to the arterial concentrations.
Discussion
The major finding of the present study is that the brain releases IL-6 during prolonged exercise, independent of whether the exercise is carried out under normothermic or hyperthermic conditions. Although the net release of IL-6 from the brain is manyfold lower than that released from an exercising limb (≈0.3 ng min−1 from the brain vs. a peak value of ≈30 ng min−1 from an exercising leg), cerebral IL-6 kinetics during the two exercise bouts followed the same pattern of response as that of the exercising muscles (Steensberg et al. 2001a). During exercise the release of IL-6 from the brain would be expected to have the same biological effect as that from the exercising muscles. An effect which may include stimulation of hepatic glycogenolysis and glucose release, thereby contributing to maintain blood glucose homeostasis (Gleeson, 2000; Steensberg et al. 2000; Pedersen et al. 2001). The finding that the cerebral release of IL-6 and the arterial IL-6 concentration were higher during the second compared with the first exercise bout may support this idea, as the oxidation of blood glucose is expected to increase when an exercise bout is repeated after a relatively short recovery period (Steensberg et al. 2001a; Ronsen et al. 2002). However, the cerebral uptake of blood glucose is much smaller than that of the exercising skeletal muscles (Steensberg et al. 2001a; Nybo et al. 2002) and the contribution from cerebrally derived IL-6 on the overall glucose homeostasis may be considered to be less than that of muscle-derived IL-6. Furthermore, the similarity in IL-6 kinetics between the brain and an exercising limb does not necessary implicate that the biological role is the same and the functional importance of the cerebral IL-6 release during exercise awaits further investigation.
The augmented IL-6 release from the brain during the second exercise bout may be ascribed to the elevated arterial adrenaline concentration, as IL-6 production by astrocytes is stimulated by adrenaline (Van Wagoner & Benveniste, 1999). However, the influence from catecholamines is ambiguous as the cerebral IL-6 release was not different in the hyperthermic compared with the normothermic trial, although noradrenaline is known to induce IL-6 in astrocytes (Norris & Benveniste, 1993) and hyperthermia markedly increased the arterial and jugular venous concentrations of noradrenaline. Also, the exercise-induced increase in systemic IL-6 cannot be mimicked by adrenaline infusion, suggesting that adrenaline plays only a minor role in IL-6 production during exercise (Steensberg et al. 2001b).
IL-6 is known as a centrally produced and centrally acting endogenous pyrogen and it is essential in tumour necrosis factor-induced fever (Rothwell et al. 1991; Sundgren-Andersson et al. 1998). However, the elevated core temperature during the hyperthermic trial was not associated with an altered cerebral IL-6 release compared with the control trial which supports the notion that fever and hyperthermia are distinct physiological responses. During prolonged exercise the development of hyperthermia reduces CBF (Nybo & Nielsen, 2001c; Nybo et al. 2002), but the unaltered cerebral IL-6 kinetics in response to hyperthermia indicate that oxygen delivery to the brain was not critically impaired, as CNS ischaemia results in an increased IL-6 production (Clark et al. 1999).
That IL-6 is released rather than taken up by the brain during prolonged exercise does not support the idea that IL-6 output from the exercising muscles acts as a feedback neuromodulator, which contributes to central fatigue (Gleeson, 2000). However, the increased cerebral release of IL-6 may result from an elevated expression in the brain and an increased IL-6 level in the brain could affect the sensation of fatigue during prolonged exercise (Davis & Bailey, 1997). On the other hand, hyperthermia is associated with central fatigue (Nybo & Nielsen, 2001a) and increased perception of effort (Nybo & Nielsen, 2001b) and the cerebral IL-6 response would be expected to be influenced by the elevated core temperature during the hyperthermic trial if IL-6 and central fatigue were connected. However, the observed net release of IL-6 from the brain does not exclude the possibility that there may have been both an uptake and a release occurring simultaneously. Furthermore, we cannot exclude the possibility that circadian rhythms in IL-6 influenced the cerebral IL-6 changes during the protocol, since a resting control group was omitted in the study design due to the invasive nature of the experiment. Yet the observation that IL-6 release from the brain had returned to the baseline level (no release) at the beginning of the second exercise bout indicates that the changes in cerebral IL-6 release during the exercise protocol are dependent on the exercise time rather than a simple diurnal effect.
Another crucial assumption for the determination of the cerebral IL-6 kinetics is that the blood sampled from the catheter in the internal jugular vein represents cerebral venous blood. Although the catheter was placed with the tip positioned in the bulb of the internal jugular vein, a slight admixture of extracranial origin can be expected, but the amount of extracranial contamination is usually small (< 2.6 %; Shenkin et al. 1948) and the influence of extracranially derived IL-6 is expected to be negligible.
Wallenius et al. (2002) demonstrated that centrally acting IL-6 exerts anti-obesity effects in rodents, and it is tempting to suggest that cerebral IL-6 to some extent is involved in mediating the effect of physical activity on the balance between energy expenditure and food intake (van Baak, 1999). Furthermore, in rats the basal oxygen consumption was increased for 3 h after a cerebroventricular injection of IL-6 in the lateral ventricle (Wallenius et al. 2002), and an increased IL-6 level in the brain at the end of a prolonged exercise bout may contribute to the excess post-exercise oxygen consumption that persists for hours after a prolonged exercise bout (Maehlum et al. 1986; Gore & Withers, 1990). However, these suggestions remain speculative and the complex functions of IL-6 within the CNS are far from understood, and we can only speculate that the cerebral IL-6 release during exercise may be somehow connected to the effect of physical activity on the balance between energy expenditure and energy intake.
In conclusion, IL-6 is released from the brain during prolonged exercise in humans and the release appears to be influenced by the duration of exercise rather than by the increase in body temperature. A role for IL-6 in central fatigue is not supported by the present results as the arterial and cerebral IL-6 responses were similar during normothermia and hyperthermia, although hyperthermia is associated with central fatigue.
References
- Bartoccioni E, Michaelis D, Hohlfeld R. Constitutive and cytokine-induced production of interleukin-6 by human myoblasts. Immunology Letters. 1994;42:135–138. doi: 10.1016/0165-2478(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Nagy RJ, O'Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proceedings of the National Academy of Sciences of the USA. 1994;91:9352–9456. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WM, Rinker LG, Lessov NS, Hazel K, Eckenstein F. Time course of IL-6 expression in experimental CNS ischemia. Neurological Research. 1999;21:287–292. doi: 10.1080/01616412.1999.11740933. [DOI] [PubMed] [Google Scholar]
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Medicine and Science in Sports and Exercise. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Interleukins and exercise. Journal of Physiology. 2000;529:1. doi: 10.1111/j.1469-7793.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore CJ, Withers RT. The effect of exercise intensity and duration on the oxygen deficit and excess post-exercise oxygen consumption. European Journal of Applied Physiology. 1990;60:169–174. doi: 10.1007/BF00839153. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle – influence of muscle glycogen content. FASEB Journal. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. Journal of Clinical Investigation. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiødtc G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. Journal of Applied Physiology. 1993;74:245–250. doi: 10.1152/jappl.1993.74.1.245. [DOI] [PubMed] [Google Scholar]
- Maehlum S, Grandmontagne M, Newsholme EA, Sejersted OM. Magnitude and duration of excess post exercise oxygen consumption in healthy young subjects. Metabolism. 1986;35:425–429. doi: 10.1016/0026-0495(86)90132-0. [DOI] [PubMed] [Google Scholar]
- Miller AK, Corsellis JA. Evidence for a secular increase in human brain weight during the past century. Annals of Human Biology. 1977;4:253–257. doi: 10.1080/03014467700007142. [DOI] [PubMed] [Google Scholar]
- Nehlsen-Cannarella S, Fagoaga O, Nieman D, Henson D, Butterworth D, Schmitt R, Bailey E, Warren B, Utter A, Davis J. Carbohydrate and the cytokine response to 2. 5 h of running. Journal of Applied Physiology. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Nieman D, Nehlsen-Cannarella S, Fagoaga O, Henson D, Utter A, Davis JM, Williams F, Butterworth D. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Medicine and Science in Sports and Exercise. 1998;30:671–678. doi: 10.1097/00005768-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Norris JG, Benveniste EN. Interleukin-6 production by astrocytes: induction by the neurotransmitter norepinephrine. Journal of Neuroimmunology. 1993;45:137–146. doi: 10.1016/0165-5728(93)90174-w. [DOI] [PubMed] [Google Scholar]
- Northoff H, Berg A. Immunologic mediators as parameters of the reaction to strenuous exercise. International Journal of Sports Medicine. 1991;12:S9–15. doi: 10.1055/s-2007-1024743. [DOI] [PubMed] [Google Scholar]
- Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. Journal of Applied Physiology. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. Journal of Applied Physiology. 2001a;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Perceived exertion during prolonged exercise with progressive hyperthermia is associated with an altered electrical activity of the brain. Journal of Applied Physiology. 2001b;91:2017–2023. doi: 10.1152/jappl.2001.91.5.2017. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Middle cerebral artery blood flow velocity is reduced with hyperthermia during prolonged exercise in humans. Journal of Physiology. 2001c;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. Journal of Physiology. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsen O, Lea T, Bahr R, Pedersen BK. Enhanced plasma IL-6 and IL-1ra responses to repeated versus single bout of prolonged cycling in endurance athletes. Journal of Applied Physiology. 2002;92:2547–2553. doi: 10.1152/japplphysiol.01263.2001. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Busbridge NJ, Lefeuvre RA, Hardwick J, Gauldie J, Hopkins SJ. Interleukin-6 is a centrally acting endogenous pyrogen in the rat. Canadian Journal of Physiology and Pharmacology. 1991;69:1465–1469. doi: 10.1139/y91-219. [DOI] [PubMed] [Google Scholar]
- Schobitz B, De Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. European Journal of Neuroscience. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Shenkin HA, Harmel MH, Kety SS. Dynamic anatomy of the cerebral circulation. Archives of Neurological Psychiatry. 1948;60:240–252. doi: 10.1001/archneurpsyc.1948.02310030021002. [DOI] [PubMed] [Google Scholar]
- Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J. The expressions of mRNAs for interleukin-6 (IL-6) and the IL-6 receptor (IL-6R). in the rat hypothalamus and midbrain during restraint stress. Life Science. 1998;62:2315–2320. doi: 10.1016/s0024-3205(98)00212-4. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. Journal of Cerebral Blood Flow and Metabolism. 1981;1:7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, Van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. Journal of Physiology. 2001a;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Toft AD, Schjerling P, Halkjæber-kristensen A, Pedersen BK. Plasma interleukin-6 during strenuous exercise – role of adrenaline. American Journal of Physiology – Cell Physiology. 2001b;281:C1001–1004. doi: 10.1152/ajpcell.2001.281.3.C1001. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscle can account for the exercise-induced increase in plasma interleukin-6. Journal of Physiology. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundgren-Andersson AK, Östlund P, Bartfai T. IL-6 is essential in TNF-α-induced fever. American Journal of Physiology. 1998;275:R2028–2034. doi: 10.1152/ajpregu.1998.275.6.R2028. [DOI] [PubMed] [Google Scholar]
- van Baak MA. Physical activity and energy balance. Public Health Nutrition. 1999;2:335–339. doi: 10.1017/s1368980099000452. [DOI] [PubMed] [Google Scholar]
- Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. Journal of Neuroimmunology. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6 deficient mice develop mature-onset obesity. Nature Medicine. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]


