Abstract
Research into cardiovascular variabilities intersects both human physiology and quantitative modelling. This is because respiratory and Mayer wave (or 10 s) cardiovascular oscillations represent the integrated control of a system through both autonomic branches by systemic haemodynamic changes within a fluid-filled, physical system. However, our current precise measurement of short-term cardiovascular fluctuations does not necessarily mean we have an adequate understanding of them. Empirical observation suggests that both respiratory and Mayer wave fluctuations derive from mutable autonomic and haemodynamic inputs. Evidence strongly suggests that respiratory sinus arrhythmia both contributes to and buffers respiratory arterial pressure fluctuations. Moreover, even though virtual abolition of all R-R interval variability by cholinergic blockade suggests that parasympathetic stimulation is essential for expression of these variabilities, respiratory sinus arrhythmia does not always reflect a purely vagal phenomenon. The arterial baroreflex has been cited as the mechanism for both respiratory and Mayer wave frequency fluctuations. However, data suggest that both cardiac vagal and vascular sympathetic fluctuations at these frequencies are independent of baroreflex mechanisms and, in fact, contribute to pressure fluctuations. Results from cardiovascular modelling can suggest possible sources for these rhythms. For example, modelling originally suggested low frequency cardiovascular rhythms derived from intrinsic delays in baroreceptor control, and experimental evidence subsequently corroborated this possibility. However, the complex stochastic relations between and variabilities in these rhythms indicate no single mechanism is responsible. If future study of cardiovascular variabilities is to move beyond qualitative suggestions of determinants to quantitative elucidation of critical physical mechanisms, both experimental design and model construction will have to be more trenchant.
Periodicities in cardiovascular variables were recognized long before the ability to adequately probe them, as early as 1733 by Stephen Hales (Hales, 1733). Although our measurement of short-term cardiovascular fluctuations is now more precise, our understanding of them may be inadequate for their widespread application. Numerous origins have been proposed for cardiovascular oscillations, including irradiation from respiratory centres (Hering, 1869; Mayer, 1877; Fredericq, 1882), autochthonous central rhythms (Kruger, 1933; Preiss & Polosa, 1974), negative feedback system engagement (Guyton et al. 1951; Sagawa et al. 1962) and vascular autorhythmicities (Rothlin & Cerletti, 1950). Research into these oscillations has ranged from simple inferential data to intricate theoretical models. Although inferential data can provide strong evidence that an association exists between fluctuations, it is generally limited to a single input and a single output, ignoring countervailing influences. Alternatively, mathematical models can provide potential mechanistic insight to the links between fluctuations, but proposed models which account for multiple cardiovascular influences have generally been too complex for direct validation. Thus, despite their apparent simplicity, spontaneous cardiovascular oscillations have not been explained adequately by previous mathematical models or empirical data.
This review will provide a brief historical context, summary of experimental approaches to and mathematical models of the physiology, and some suggestions for future work in cardiovascular variabilities. We limit this review to slow wave oscillations at respiratory and ≈0.10 Hz frequencies for reasons of tractability and direct major application to normal human physiology.
Historical background
During the last three centuries, studies of cardiovascular haemodynamics have provided critical data for both understanding the physiology and developing the continuum mechanics necessary for insight into a complex, multiple-component system. Original explorations of cardiovascular oscillations relied upon classical scientists educated as both physician and physicist. When, in 1628, Harvey was among the first to propose quantitating fluid flow, his interest was in understanding reciprocal flow from the heart to the lungs. A century later, Steven Hales published a book authorized by Sir Isaac Newton, then President of the Royal Society, wherein he described arterial pressure fluctuations, explaining them in part via recent studies of viscosity. Subsequently, Poiseuille (1799-1829) experimentally derived the law for laminar fluid flow by relating it to blood viscosity, artery radius and pressure change; and Young (1773-1829) derived a basic elasticity law from pulsatile velocity propagation in blood vessels and displacement of the arterial wall. After Ludwig's kymograph (1847) produced the first recordings of pressure oscillations, subsequent studies identified slow rhythmic blood pressure waves independent of respiration (Mayer, 1877; Fredericq, 1882), as well as waves around the respiratory frequency (Traube, 1865; Hering, 1869). When fluctuations with these same frequencies were also found in cardiac interval, they were thought by some investigators to be the cause of arterial pressure oscillations (Fredericq, 1882; Morawitz, 1903).
The development of sophisticated neurophysiological techniques allowed more precise observation of concurrent fluctuations in haemodynamics and nervous outflow. Guyton et al. (1951) observed large ‘vasomotor waves’ in anaesthetized, hypovolaemic dogs and concluded that these blood pressure fluctuations were autonomically mediated (Guyton et al. 1951; Guyton & Harris, 1951). Indeed, congruent temporal patterns in sympathetic preganglionic activity and arterial pressure Mayer waves were subsequently observed by Preiss & Polosa (1974). Moreover, temporal associations were also reported to exist between respiratory patterns and arterial pressure and sympathetic outflow (Preiss et al. 1975). As a result of these animal studies it was commonly accepted that respiratory periodicities must encompass irradiation from respiratory to cardiovascular centres in the brain and that arterial pressure Mayer waves derive from sympathetically mediated vascular resistance fluctuations.
The advent of greater computing power brought a more refined mathematical approach to quantifying and modelling cardiovascular oscillations. Moreover, the development of non-invasive measures of beat-by-beat arterial pressure allowed more extensive work in humans. Frequency domain research into slow wave cardiovascular oscillations in humans started in the late 1970s (Penaz et al. 1978) and early 1980s (Rompelman et al. 1982; Taguchi, 1983). In fact, from then on, there has been an explosion in the use of frequency domain methods to study slow cardiovascular variabilities, with an excess of 1500 papers in the last 10 years alone.
Physiological basis of cardiovascular rhythms
The dynamic moment-by-moment responses of heart rate and arterial pressure to internal and external perturbations ensure sustained and appropriate perfusion of all tissues. Beat-by-beat time series show the manifold nature of these physiological functions in humans, and have been described as complex time series (Kaplan et al. 1991). Underlying the apparent complexity are two rhythms. The first, occurring over several cardiac cycles, is respiratory related. Both heart rate and the entire arterial pressure waveform rise and fall at the frequency of respiration. In humans, these oscillations represent both autonomic neural fluctuations and mechanically induced central blood volume changes in synchrony with respiration. However, the contributions of these two mechanisms to both heart rate and arterial pressure fluctuations remain equivocal. The second rhythm occurs over an approximate 10 s cycle, and in arterial pressure, has been termed Mayer waves. This rhythm has been attributed to various sources, but most recently has been presumed to reflect reflex-mediated fluctuations in sympathetic outflow to the vasculature and in parasympathetic and sympathetic outflows to the heart. Although fluctuations at the respiratory and Mayer wave frequencies have been studied most, there is significant variability at longer time scales. Cardiovascular variability at frequencies well below 0.03 Hz, or period longer than 30 s, have been variously termed very low frequency and ultra-low frequency spectral fluctuations (Bigger et al. 1992a), β and α anticorrelations (Peng et al. 1993) and the fractal dimension and approximate entropy (Yeragani et al. 1993). These have been interpreted as secondary to the renin-angiotensin system (Brandenberger et al. 1985), as indicative of ‘system decoupling’ (Pincus & Goldberger, 1994) and as diagnostic instruments with well-behaved mathematical properties (Bigger et al. 1996). However, it remains unclear whether variabilities below ≈0.03 Hz represent primary cardiovascular oscillations or are epiphenomena of long-term cardiovascular recordings. Nonetheless, most interpretations and uses of all cardiovascular variabilities assume a simple, linear input-output model of cardiovascular regulation, ignoring data clearly suggesting that cardiovascular oscillations are a complex interplay of effectors.
Respiratory frequency oscillations
Respiratory frequency cardiac interval oscillations, termed respiratory sinus arrhythmia, could be generated by a number of scenarios coupling haemodynamic and reflex responses. One line of reasoning is that respiration-synchronous fluctuations in intrathoracic pressure provoke fluctuations in stroke volume (Innes et al. 1993) which contribute to respiratory frequency arterial pressure variability in humans (Dornhorst et al. 1952; Toska & Eriksen, 1993; Triedman & Saul, 1994). Presumably, this arterial pressure fluctuation provokes parallel changes in arterial baroreflex afferent activity, appropriately increasing and decreasing cardiac vagal outflow and generating respiratory sinus arrhythmia (de Boer et al. 1987; Guzzetti et al. 1994; Scheffer et al. 1994). By this reasoning, respiratory sinus arrhythmia arises from a baroreflex mechanism that should counteract stroke volume fluctuations and reduce arterial pressure fluctuations (Toska & Eriksen, 1993). However, there are also data suggesting that central vagal outflow can be directly inhibited by respiratory neuron firing (Gilbey et al. 1984) and that respiratory sinus arrhythmia and respiratory frequency arterial pressure variability occur in synchrony without any appreciable phase lag (de Boer et al. 1987; Taylor & Eckberg, 1996). From this, it would seem that respiratory sinus arrhythmia arises from an efferent vagal oscillation that contributes to arterial pressure fluctuations (Akselrod et al. 1985; Taylor & Eckberg, 1996).
If respiratory sinus arrhythmia is arterial baroreflex buffering of respiration-induced pressure fluctuations, then the simplest hypothesis suggests that elimination of R-R interval fluctuations should increase respiratory arterial pressure fluctuations since the buffering component has been removed. However, parasympathetic blockade which eliminates sinus arrhythmia decreases respiratory-induced arterial pressure fluctuations in supine, but not upright, humans (Taylor et al. 1998a). (The decrease may not be observed in the absence of paced breathing (Wray et al. 2001); however, paced breathing is critical for interpreting power spectra in humans, since respiration frequency and depth can vary widely and strongly determine fluctuation amplitude (Brown et al. 1993; Laude et al. 1995).) Similar to parasympathetic blockade, fixed rate atrial pacing that eliminates R-R interval variability significantly reduces pressure oscillations at the respiratory frequency in supine humans, but increases them in the 40 deg tilt position (shown in Fig. 1). This corroborates the data from parasympathetic blockade, and indicates that the effect is probably the direct physiological result of the removal of R-R interval oscillations. These state differences may be explained by the shift in cross-spectral phase relation between systolic pressure and R-R interval. In the supine position, the R-R interval is in phase with or slightly leads arterial pressure changes (de Boer et al. 1987), and in the upright position, follows pressure changes (see Fig. 2). Thus, respiratory sinus arrhythmia can actually contribute to respiratory arterial pressure fluctuations and does not always represent simple baroreflex buffering.
Figure 1. R-R interval and systolic pressure power in 20 healthy young subjects in the supine and 40 deg tilt positions with and without fixed rate atrial pacing.
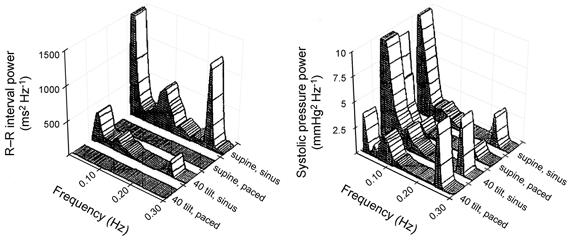
Elimination of R-R interval variability significantly reduces pressure oscillations at the respiratory frequency in supine humans, but increases them in the 40 deg tilt position. Moreover, it has no effect on Mayer wave (≈0.1 Hz) pressure oscillations in supine humans, but increases them in the 40 deg tilt position. This demonstrates the frequency and state dependence of cardiovascular oscillations (modified from Taylor & Eckberg, 1996).
Figure 2. The cross-spectral phase and coherence between R-R interval and systolic pressure in 20 healthy young subjects in the supine and 40 deg tilt positions with normal sinus rhythm.
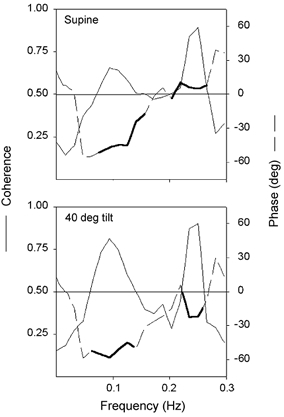
Thick lines indicate phase where coherence is significant. Phase at the respiratory frequency shifts from R-R interval in phase with or slightly leading arterial pressure changes in supine humans to R-R interval following pressure changes in the upright position. This may reveal differential baroreflex engagement and explain contrasting effects of atrial pacing in supine and tilt positions. However, the phase relation at the respiratory frequency is consistently negative, and therefore provides no insight to the contrasting effects of atrial pacing the two positions (modified from Taylor & Eckberg, 1996).
The view of respiratory sinus arrhythmia as a vagal phenomenon is widely held, and is supported by substantial published data (Katona et al. 1970; Katona & Jih, 1975; Kollai & Koizumi, 1979; Raczkowska et al. 1983; Fouad et al. 1984; Koizumi et al. 1985). However, despite physiological studies questioning respiratory sinus arrhythmia as a valid and reliable cardiac parasympathetic index (Kollai & Mizsei, 1990; Hedman et al. 1995), this interpretation of respiratory sinus arrhythmia holds such sway that amplitude increases in response to cardiac sympathetic blocking drugs have been construed as evidence of a heretofore unrecognized vagotonic property of the drugs (Grossman & Kollai, 1993). Even though ‘high frequency’ heart rate variability (i.e. respiratory sinus arrhythmia as measured by spectral power, the squared amplitude of the input; Laude et al. 1995) is increased equally by β-blockers that do (lipophilic) and do not (hydrophilic) cross the blood-brain barrier (Pitzalis et al. 1998), the prevailing notion is that β-blockers can increase cardiac vagal modulation of heart rate (Coker et al. 1984; Vaile et al. 1999). However, cardioselective β-adrenergic blockade enhances respiratory sinus arrhythmia at all breathing frequencies, not just at higher breathing frequencies (Taylor et al. 2001) (shown in Fig. 3). If cardiac sympathetic effects are truly constrained to frequencies lower than ≈0.15 Hz, opposing sympatholytic and vagotonic actions should not increase variability across all frequencies. Therefore, a more reasonable physiological interpretation that does not rely on newfound pharmacological properties is that cardiac sympathetic outflow can oppose vagally mediated R-R interval oscillations and sympathetic blockade removes this effect. This might be analogous to the large mutual interactive effect of vagal and sympathetic inputs to the heart in rabbits. Direct white noise stimulation shows that input from one system is antagonistic to the other (Sunagawa et al. 1998). If these strong effects are similar in humans, it could explain these sympathetic effects. Most importantly, if sympathetic influences significantly impact respiratory sinus arrhythmia, this challenges the notion that respiratory sinus arrhythmia represents a purely vagal mechanism.
Figure 3. Effects of cardioselective β-adrenergic blockade (0.2 mg kg−1 atenolol) and double autonomic blockade (atenolol + 0.04 mg kg−1 atropine) on respiratory sinus arrhythmia during controlled frequency breathing from 0.25 to 0.05 Hz in the 40 deg tilt position.
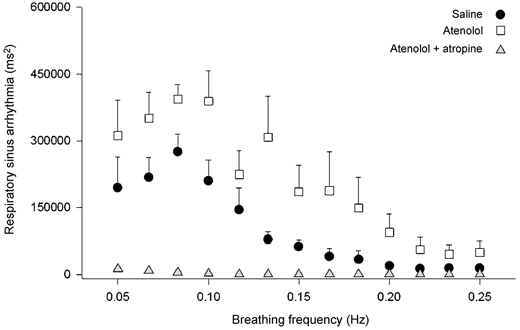
n = 10. β-blockade enhances respiratory sinus arrhythmia at all breathing frequencies, not just at higher breathing frequencies. This undermines the contention that respiratory sinus arrhythmia is a purely vagal phenomenon (redrawn from Taylor et al. 2001).
There does appear to be a purely mechanical portion of respiratory sinus arrhythmia. For example, heart transplant patients can show a small (2-8 % of normal) respiratory sinus arrhythmia. Since rapid re-innervation of donor hearts seems unlikely, this is probably due to the mechanical effects of breathing (Bernardi et al. 1989). This finding was further corroborated by Conci et al. (2001) in individuals before and after brain death. Moreover, virtually all low frequency power is lost and only a very narrow, small respiratory peak remains after brain death, underscoring the neural mediation of cardiovascular rhythms. Nonetheless, the effect of atrial stretch on R-R interval variability in humans can be substantial; after complete autonomic blockade, slow deep breathing can provoke R-R interval oscillations of ≈120 ms (Taylor et al. 2001). If this effect remains in the absence of autonomic blockade, it would account for as much as 20 % of respiratory sinus arrhythmia, indicating that atrial stretch may make an important contribution to respiratory sinus arrhythmia. Nonetheless, the effects of muscarinic blockade show that vagal expression is necessary for normal amplitude respiratory sinus arrhythmia.
Respiratory-related fluctuations in vascular sympathetic outflow also present interpretive dilemmas. If these oscillations derive from a baroreflex mechanism, both arterial and cardiopulmonary baroreceptors may be involved. The arterial baroreflex is a primary controller of vascular sympathetic nerve firing, and with breathing, blood pressure falls during expiration and rises during inspiration. Cardiopulmonary receptors also influence vascular sympathetic outflow but in response to changes in central venous pressure. It remains unclear whether the arterial baroreflex responds to respiratory-related blood pressure fluctuations by generating inversely proportional changes in vascular sympathetic nerve firing (Eckberg et al. 1985; Seals et al. 1990, 1993; St Croix et al. 2000) or whether respiration-related fluctuations in venous return to the heart mediate respiratory frequency sympathetic oscillations (Gootman & Cohen, 1974). Whether either or both are active in respiratory sympathetic fluctuations, the reflex pattern should reduce arterial pressure oscillations with breathing. However, published data on blockade of sympathetic effects via α-adrenergic antagonism provide no clear insight; only overall variance (Clement et al. 1985) or ‘relative contribution’ (Nakata et al. 1998) or ‘normalized’ changes (van de Borne et al. 2001) have been presented. Thus, no published data present the possible blood pressure buffering role for respiratory-related vascular sympathetic oscillations. Moreover, as with respiratory sinus arrhythmia, there is evidence that factors independent of reflex mechanisms generate sympathetic fluctuations with respiration. Lung volume may modulate sympathetic firing through pulmonary afferents (Seals et al. 1990) and inspiratory motor neurons may modulate sympathetic activity (Seals et al. 1993). Thus, the respiratory phase influence on vascular sympathetic outflow may be independent of baroreflex mechanisms and, in reality, be a contributor to arterial pressure fluctuations.
Mayer wave frequency oscillations
The hypothesis that arterial pressure Mayer waves result from rhythmic, sympathetic vasomotor activity (Fredericq, 1882; Guyton & Harris, 1951; Dornhorst et al. 1952; Preiss & Polosa, 1974; Madwed et al. 1989) and generate R-R interval oscillations at this same frequency through the baroreflex (Akselrod et al. 1985; de Boer et al. 1987; Scheffer et al. 1994) holds especial currency of late. The spectral estimate of Mayer wave amplitude is commonly used to measure vascular sympathetic activity (Pagani et al. 1986, 1997; Furlan et al. 1990; Radaelli et al. 1994; Parati et al. 1995; van de Borne et al. 1997; Montano et al. 1998) and the cross-spectral magnitude of pressure and R-R interval to measure baroreflex gain (Honzikova et al. 1992; Hughson et al. 1993; Cerutti et al. 1994; Scheffer et al. 1994; Sleight et al. 1995). However, these simple interpretations are inadequate for the complex interactions that appear to underlie Mayer wave fluctuations.
Unlike respiratory frequency oscillations, Mayer wave fluctuations appear spontaneously and inconsistently across a broad frequency range, as low as 0.03 Hz and up to 0.15 Hz, although generally close to 0.10 Hz. Since Mayer waves are most apparent in response to sympatho-excitatory stimuli (Pagani et al. 1986), it has been presumed that arterial pressure Mayer waves are closely linked to vascular sympathetic outflow, either tonic or oscillatory. In fact, it may be reasonable to conclude that waxing and waning vascular resistance due to sympathetic neural oscillations generates arterial pressure Mayer waves. For example, some spinal cord injury patients demonstrate no measurable arterial pressure Mayer waves (Inoue et al. 1991), while others demonstrate clearly identifiable Mayer waves (Koh et al. 1994). In healthy humans, the exact relation between mean sympathetic outflow and Mayer wave amplitude is unclear. Subjects with characteristically different levels of muscle sympathetic outflow show no difference in Mayer wave amplitude (Taylor et al. 1998b). Older healthy males demonstrate arterial pressure Mayer waves comparable to those seen in young females despite the striking difference in resting sympathetic outflow (see Fig. 4). As with respiratory-related fluctuations, pharmacological blockade in humans has provided no clear information on the dependence of Mayer waves on sympathetic outflow. Moreover, even though some evidence does suggest that Mayer waves depend upon intact arterial baroreflex function (Sleight et al. 1995), baroreflex dependence does not require proportionality between arterial pressure Mayer wave amplitude and sympathetic outflow.
Figure 4. Relations between resting peroneal nerve muscle sympathetic activity and arterial pressure Mayer wave amplitude in 10 young females (18-28 years old), 11 young males (18-29 years old) and 13 older males (60-72 years old).
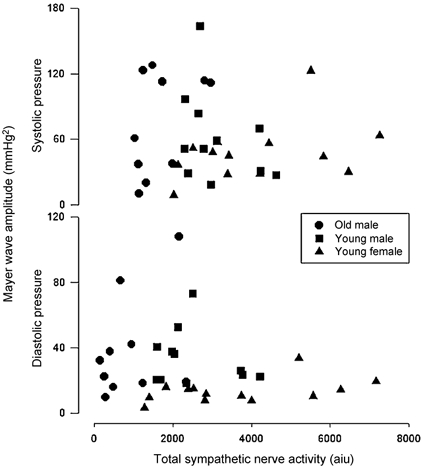
There were no consistent relations among arterial pressure Mayer wave amplitude and vascular sympathetic outflow. Despite striking differences in resting sympathetic outflow, young females and older males have comparable Mayer wave amplitude (redrawn from Taylor et al. 1998b). aiu, arbitrary integration units.
Cardiac interval oscillations at the Mayer wave frequency are mediated primarily by cardiac vagal but also cardiac sympathetic outflows and are thought to represent arterial baroreflex responses to pressure oscillations (de Boer et al. 1987; Scheffer et al. 1994). One popular theoretical and practical explanation is a resonant response in the baroreflex due to feedback delays (de Boer et al. 1987; Ottesen, 1997; Bertram et al. 1998). However, the link between oscillations in cardiac interval and arterial pressure may not be consistent. In supine humans, monotonic cardiac pacing (elimination of cardiac interval variability) does not increase low frequency arterial pressure oscillations, indicating that pressure is not damped by cardiac interval oscillations (Taylor & Eckberg, 1996). This may mean that a sympathetic mechanism is responsible, perhaps a latency in baroreflex-induced changes in vascular sympathetic outflow, in other words, a pressure-pressure feedback loop (Baselli et al. 1988). However, when vascular sympathetic outflow is high (e.g. head-up tilt), elimination of cardiac interval variability augments Mayer waves (Taylor & Eckberg, 1996). This suggests that the arterial baroreflex link between Mayer wave oscillations in pressure and cardiac interval may be evident only when sympathetic outflow is augmented. However, when low frequency pressure oscillations are augmented artificially, a highly variable relation between Mayer wave frequency oscillations in arterial pressure and cardiac interval is revealed (Hamner et al. 2001). Oscillatory lower body negative pressure at 0.10 Hz increases both low frequency blood pressure and R-R interval oscillations, but with considerable inconsistencies in their spectral correlation (i.e. coherence). Although forced oscillations in pressure can be large, and those in cardiac interval large apparently in response, the coherence between them can be highly variable, both among subjects and across conditions (see Fig. 5). Thus, short-term fluctuations in cardiac interval do not appear to be linked inextricably to those in arterial pressure. Cardiovascular oscillations at the Mayer wave frequency may be generated and influenced by factors other than simply sympathetically mediated vasoconstriction and/or baroreflex gain.
Figure 5. Coherence between systolic pressure and R-R interval in 18 young males during 5 min of supine rest, and of 0.1 Hz oscillatory lower body suction at 10 and 30 mmHg.
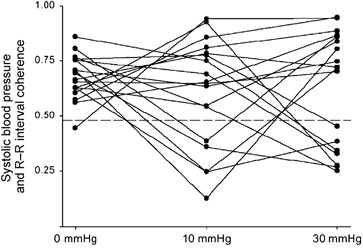
The oscillatory suction significantly augments arterial pressure oscillations and cardiac interval oscillations at the Mayer wave frequency; however, the correlation (i.e. coherence) between the oscillations is highly variable, both among subjects and across levels of suction (modified from Hamner et al. 2001).
State and trait determinants for mechanisms
Both respiratory and Mayer wave frequency oscillations may be influenced by age and sex. The nature of these differences underscores the variability in physiological strategies that underlie these cardiovascular periodicities. For example, muscle sympathetic oscillations at the respiratory frequency decline with age (Taylor et al. 1998b), yet respiratory frequency pressure oscillations are unchanged (Veerman et al. 1994; Taylor et al. 1998b). This could reflect age-related baroreflex gain reductions (Gribbin et al. 1971; Ebert et al. 1992), if arterial baroreflex afferent activity drives vascular sympathetic oscillations (Gootman & Cohen, 1974; de Boer et al. 1987). However, reduced baroreflex gain with age cannot explain the parallel age-related declines in arterial pressure Mayer waves and both cardiac interval and vascular sympathetic oscillations at this frequency (Veerman et al. 1994; Taylor et al. 1998b; Barnett et al. 1999). Sex-related differences also demonstrate that there is no simple relation among cardiovascular variabilities. Respiratory frequency arterial pressure oscillations are accompanied by vascular sympathetic oscillations which are lower in women than in men (Taylor et al. 1998b). At the Mayer wave frequency, cardiac interval oscillations are no different (Murata et al. 1992; Taylor et al. 1998b), while both arterial pressure and sympathetic oscillations are lower in women (Ryan et al. 1994; Taylor et al. 1998b; Barnett et al. 1999). These inconsistent sex-related differences cannot simply reflect lower baroreflex gain in women than men (Abdel-Rahman et al. 1994). It could be postulated that, for example, arterial baroreflex engagement is frequency specific. However, data suggest that baroreflex buffering of arterial pressure in young males is similar at both the respiratory and Mayer wave frequencies (Taylor & Eckberg, 1996). Another possibility is different vascular α-receptor responsiveness to sympathetic nervous outflow. Both older (Sugiyama et al. 1996; Davy et al. 1998) and female (Luzier et al. 1998) humans demonstrate lesser resistance responses to sympathetic activation than young men. This could produce differential effects at the respiratory and Mayer wave frequencies and thereby confound simple interpretation of discrepancies. It is apparent that the simultaneous cardiac, vascular and respiratory influences integrate to produce cardiovascular fluctuations and must be examined jointly if we are to understand possible differences in their regulation.
Mathematical attempts to model cardiovascular oscillations
Current mathematical study of cardiovascular variabilities follows three main approaches. First, attempts have been made to construct simple, generally novel quantitative indices to classify broad physiological or pathophysiological conditions. Ratios of low to high frequency spectral power have been used to gauge ‘sympathovagal balance’ (Malliani et al. 1994), Mayer wave frequency power has been used to classify congestive heart failure patients (Teich et al. 2000) or predict death after heart attacks (Bigger et al. 1992a, b), and the slope of very low frequency blood pressure oscillations has been used as a correlate for cardiovascular mortality (Makikallio et al. 1999). When successful, this work produces useful classification tools for clinical populations. However, to produce physiologically insightful models of these variabilities, a set of equations to explain the relations between them is necessary. These more complete explanations form the latter two approaches. One approach uses principles of applied mathematics and physics to describe cardiovascular rhythms via complicated, generally non-linear models, for example, non-linear delay differential equations (Cavalcanti & Belardinelli, 1996; Ottesen, 1997), difference equations (de Boer et al. 1987), non-linear oscillators (Abbiw-Jackson & Langford, 1998), regularization theory (Seydnejad & Kitney, 2001) and non-linear fluid dynamics (Olufsen, 1999; Olufsen et al. 2000). Since these models are based on simple physical principles, they can explain the physical origins of cardiovascular oscillations and could lead to unique insight. However, current application of these mathematical approaches produces models that are quite difficult to evaluate statistically and provide at best a theoretical framework for possible mechanisms. The other approach uses application of linear time series analysis and systems identification to study the connection between these variabilities (Pagani et al. 1986; Cerutti et al. 1996; Chon et al. 1997; Mullen et al. 1997). In many cases, these are misapplied to construct generally improperly validated linear stationary models of cardiovascular variables such as heart rate, blood pressure, lung volume and sympathetic nerve activity. Moreover, although easily interpreted, these models have been limited largely to single-input relations for mechanistic inferences (Baselli et al. 1988; Parati et al. 1995; Kocsis et al. 1999) and routinely ignore differences across multiple subjects. Only these latter two approaches will be considered here; although simple indices may have clinical utility and may even lead to more complete formulation of potential models, an explanation of the relation between physiological variables requires a set of equations that specify possible inter-relations.
Model criteria
Any model applied to cardiovascular variabilities must meet certain criteria. Of course, what is paramount is whether the model makes an accurate prediction. Generally, this accuracy must be more than a minimal, statistically significant relation, since it should capture a large portion of the true relation between variables (Box et al. 1994). The validity of the model should be assessed by simulations and goodness of fit statistics, whereas reliability of the posited parameters can be assessed easily from their confidence intervals; it is best if the model is simple, having few parameters and postulating simple quantitative relations (Box et al. 1994). The idealizations and assumptions should have a natural physical interpretation; only then will the model will be useful for further modelling and experimentation (Koopmans, 1995). Moreover, the equation terms should represent physiologically meaningful variables expected to relate to one another and interindividual variability should be expressed and explained, since humans are not phenotypically homogeneous. Lastly, it is useful if the model can be compared with alternative models of the same phenomena (Kaplan et al. 1991).
It should be noted that there is generally a tradeoff between accuracy and simplicity in applied science (Koopmans, 1995). A model that provides highly accurate predictions is usually neither simple nor physically interpretable. However, simplicity and interpretability are the more important criteria for mathematical models of human physiology. There is little economic, social or military need to have highly accurate predictions for the timing of the next heartbeat, the amplitude of the next blood pressure wave or the size of the next lung volume. The need is for explanatory, generalizable models of cardiovascular function rather than purely predictive machines of cardiovascular variability.
Models deriving from principles of applied mathematics and physics
Generally, although theoretically possible, statistical validation or analysis is rarely attempted for models deriving from principles of applied mathematics and physics because of the complexity in the transfer between input and output and the often large number of implicit parameters. However, despite inadequate empirical validation, these models can provide a rich source of physical intuitions due to their dependence upon known physiological or physical principles. Most are constructed from principles of hydrodynamics and hydrostatics.
Most of these models, realized as differential or delay differential equations, lump the entire cardiovascular system into a small number of variables for simulation and analysis. A routine starting point for many of these models is the Windkessel model of cardiovascular fluid flow that consists of resistors, a capacitor and a non-linear function. For example, Cavalcanti & Belardinelli (1996) used this to provide an oscillator that can be tuned to produce spectra mimicking typical Mayer waves. An idealized model of the Windkessel equations was also used by de Boer et al. (1985) to produce time series, power spectra, and cross-spectra that were comparable to those observed in human subjects. Further, Abbiw-Jackson & Langford (1998) modified the Windkessel system by incorporating two pumps, one for each side of the heart, and adding the baroreflex via a non-linear transfer from pressure to heart rate. This model displayed persistent oscillations that could be abolished by a decrease in the modelled baroreflex gain or an increase in venous volume, presumably reflecting the effect of ageing.
Although these physical approaches produced seemingly useful models, most of those assessing cardiovascular variability suffer serious limitations. All have lacked quantitative or statistical validation; the implicit assumption seems to be that if it produces physiological-looking waveforms, the model must be physiological. It is also unclear why diastolic or systolic pressure are usually considered; for these type models, they are merely arbitrary local maxima and minima on the continuous analogue pressure waveform that should be in the model. Generally, no noise is input to these models so that the entire trajectories of the waveforms produced by it are completely predictable. The above models are also open to the criticism that lumping cardiovascular variables, while simplifying the model, can invalidate the underlying physical assumptions. These limitations can be avoided. For example, although not directly examining slow wave oscillations, Olufsen and colleagues (Olufsen, 1999; Olufsen et al. 2000) successfully applied a full set of hydrodynamic equations to predict fluid and pressure waveforms in the entire arterial tree. Even though extensive validation is not physically possible, they did provide some quantitative validation from excellent fits to MRI flow data. Therefore the strength of these approaches, the application of physical principles, can be realized, but in most applications to cardiovascular variability this strength has actually proved illusory.
Time and frequency domain models
Spectral analysis has yielded important insights, including delimiting frequency responses of the cardiovascular system and potential causal relations among cardiovascular and respiratory variables. Spectral analysis can be accomplished directly by non-parametric fast Fourier transform (FFT) analysis and windowing in the frequency domain (Priestley, 1994) or indirectly by autoregressive multi-parameter modelling of the time series (Barbieri et al. 1997). Rather than reproducing the power spectrum, simplified autoregressive models have the potential of quantifying essential delays and gains by which blood pressure, cardiac interval, sympathetic activity and lung volume affect each other. Moreover, non-stationary variants of these autoregressive models have the capacity to treat trends in the data and can be extended to certain classes of non-linear models. However, the spectral models used to date have either only assessed a single input-single output relation or have been too complex (i.e. over-parameterized) for direct validation.
In general, these models posit a linear and stationary relation between independent and dependent variables. Throughout this literature it is generally further assumed that a linear relation holds between the directly measured variables. However, it is possible that suitable, non-linear transforms of the input data should be used; non-linear effects are amply documented in cardiovascular control systems, for example, saturation and threshold elements (Eckberg, 1980), respiratory gating (Seals et al. 1993), hysteresis (Rudas et al. 1999), sinoatrial node transduction (Michaels et al. 1987), CO2 effects on arterial diameter (Ursino & Lodi, 1998) and mechanical saturation of aortic diameter (Wesseling et al. 1993). Nonetheless, this approach has provided some information on the characteristics of cardiovascular oscillations. For example, it is clear that cardiac interval spectral power is inversely proportional to respiratory frequency (Hirsch & Bishop, 1981; Brown et al. 1993). Moreover, cross-spectral derived phase relations indicate that cardiac interval is in phase with arterial pressure in supine humans, but lags pressure in upright humans (Saul et al. 1991; Taylor & Eckberg, 1996). A close coherence between arterial pressure Mayer waves and both cardiac interval and sympathetic nervous oscillations is usually shown; however, cross-spectral phase between arterial pressure and sympathetic activity at the Mayer wave frequency provides no firm support for either a predominant feedback or feedforward mechanism (Taylor et al. 1998b). Thus, simple spectral models provide the characteristics of the oscillations, but do not provide insight as to whence they derive.
In contrast, autoregressive linear stationary time series modelling imposes causality upon the model and hence the power spectrum estimate. In addition, the model selection is, in principle, an objective procedure. This provides the advantage of a methodology which can identify the feedforward and feedback links between variabilities. One example is the autoregressive model proposed by Nakata et al. (1998) to explore the links among heart rate, systolic pressure and sympathetic activity at the Mayer wave frequency. This novel approach suggested that sympathetic oscillations predict ≈70 % of the power in systolic Mayer waves, an effect partly abolished by α-receptor blockade. Ironically, the novelty of this approach prevents direct comparison to previous work, all of which used cross-spectral and coherence analysis generally at fixed temporal frequencies. Moreover, confidence intervals for each subject's relative power contributions were not provided and interindividual and intergroup variability in the relative contributions were not assessed. Thus, the estimates could intersect with zero and vary widely from subject to subject, limiting the usefulness of this model. This has been the case with other similar autoregressive models examining syncope (Di Virgilio et al. 1997; Mainardi et al. 1997) and interactions between lung volume and heart rate (Mullen et al. 1997).
One evident problem of autoregressive models is the potentially large number of parameters. Since the model selection procedure defines lags iteratively from zero, a model that includes a coefficient with lag t typically includes all coefficients with lags less than t (Box et al. 1994; Ljung, 1999) As a result, not only are the parameters for an adequate fit reached quickly within a small lag range, but also the number of parameters are inflated if all lags less than t are included. It is not uncommon for standard autoregressive models of 4 Hz cardiovascular time series to include in the order of 40–100 parameters (Barbieri et al. 1997). Although effectively increasing the variance that the model can predict (Penm & Terrell, 1982; Duong, 1984), it excludes interpretation of the model's significance to the physiology. That is, the existence of 40 different time relations between heart rate and arterial pressure changes is difficult to reconcile with accepted concepts of haemodynamic control. In addition, even more parameters may be introduced because autoregressive models allow for arbitrary feedback interactions between independent and dependent variables. For example, a 1000 time point series of lung volume, heart rate, blood pressure and sympathetic activity will exhaust the degrees of freedom with time lags representing only 1.2 % of the entire data length, if allowed full interactions (Box et al. 1994). One major reason for this lack of parsimony is the dominant use of standard scalar or vector autoregressive models rather than the more parsimonious transfer function models introduced by Box and Jenkins over 30 years ago (Box et al. 1994), perhaps due to the relative unavailability of software. Thus, the standard approach generally leads to many more estimated parameters and obscures the relation between input and output. Not only are longer (and, perhaps, more meaningful) lags not considered, but the parameters can be capricious. For example, current blood pressure could be linked causally to future lung volumes. Nonetheless, a large number of closely spaced, commensurately sized parameters are common in linear autoregressive models of cardiovascular variabilities (Di Virgilio et al. 1997; Korhonen, 1997; Mainardi et al. 1997). This suggests that currently proposed models have poor predictive reliability and limited physiological generalizability and that models with few, sparse parameters might improve our understanding of cardiovascular oscillations.
Although simplified autoregressive models can potentially quantify essential delays and gains that define interactions among variabilities, they generally assume that the cardiovascular rhythms are stationary. This may mean that they only partially model the data due to the presence of significant non-linearities and non-stationarities (Hayano et al. 1993; Jasson et al. 1997; Badra et al. 2001; Mangin et al. 2001). The attempt to fit non-linear data to a linear model can result in model parameters that depend largely on the distribution of the input and output, not on the actual causal relation between them. As a result, the goodness of fit would be highly variable. Such effects have been documented in frequency domain estimates of baroreflex gain (Badra et al. 2001). Therefore, it would seem that non-stationary variants are the most advantageous autoregressive models for cardiovascular time series. They have the capacity to treat trends in the data and can be extended to certain classes of non-linear models. Although such autoregressive modelling techniques have been used (Chon et al. 1996; Di Virgilio et al. 1997), they generally have had limited validity since they usually fit only small sections of the data with the same parameters. This effectively represents applying sequential stationary models, thereby limiting their predictive accuracy.
A simple model
Although linear models have limitations, a conservative starting point for understanding cardiovascular variabilities is to force a linear model that is as simple as possible. If at least a partial linear relation exists between the input and output variables, then positing the simplest possible relation will probably reveal it. The simplest of linear relations are easily related to physical principles of fluid dynamics. For example, a simple model expressing arterial pressure Mayer waves as a linear combination of heart rate and sympathetic activity can be derived from Poiseuille's law. This model can be limited to two weighted inputs, each with a single time lead, allowing direct assessment of the time relations and the relative contributions of heart rate and sympathetic activity to Mayer wave amplitude. In addition, the simple model facilitates characterization of both trait (e.g. intersubject) and state (e.g. high sympathetic outflow) differences in Mayer wave genesis. An application of this model was able to account for approximately half the variance in Mayer wave amplitude (Myers et al. 2001). Moreover, it showed that sympathetic activity contributes to Mayer wave amplitude most when sympathetic activity is high, but that heart rate was a much more potent contributor, regardless of state differences (see Fig. 6). This simple model suggests that Mayer waves can be generated by mechanisms other than sympathetically mediated vasoconstriction, such as autochthonous vascular smooth muscle contractions (Siegel et al. 1976). Moreover, this demonstrates that the simplest possible bivariate linear autoregressive model can lead to testable hypotheses to guide further experimental work.
Figure 6. Contribution to explained variance in arterial pressure Mayer waves from preceding heart rate and sympathetic nerve activity waves for each of 8 subjects in two conditions, basal and stimulated (i.e. heightened) sympathetic nervous outflow.
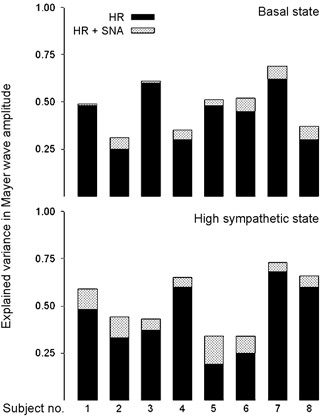
This simple model of arterial pressure Mayer wave amplitude suggests that sympathetic outflow contributes more when sympathetic activity is high. However, heart rate consistently appears to contribute much more to arterial pressure oscillations regardless of the sympathetic state (modified from Myers et al. 2001). HR, heart rate; SNA, sympathetic nerve activity.
Other mathematical explanations and characterizations
The bulk of this review concerned modelling with regression, providing fits to physiologically observed quantities to predict their values. However, there are attempts to characterize time series with intrinsic scaling relations, fractal analysis, or series entropy. Under many conditions heart rate and blood pressure variability have a relatively large DC component, even when the signal is detrended. Some investigators have examined this and related measures (de Boer et al. 1985; Yeragani et al. 1993; Cerutti et al. 1996; Ivanov et al. 1999; Heneghan & McDarby, 2000; Mateo & Laguna, 2000; Teich et al. 2000), often with an interest to use these relations to assess pathophysiological conditions (Turcott & Teich, 1996; Makikallio et al. 1999; Porta et al. 2000, 2001). However, these applications can be highly problematical for both practical and theoretical reasons. Simple spectral power may provide predictors as good as (Bigger et al. 1993) or even better than (Teich et al. 2000) more complicated analyses. Moreover, slope estimates over very slow frequencies are, in fact, unstable, and pure white noise switching with Brownian motion at relatively long intervals can generate similar results (Pilgram & Kaplan, 1999). Thus, many of these metrics may simply be epiphenomena of non-stationarity.
Suggestions for future work
Empirical observation suggests that cardiovascular oscillations are complex and mutable, yet may provide unique insight into the integrated control of the closed loop cardiovascular system. For example, respiratory sinus arrhythmia could be generated by a number of cardiovascular control scenarios, and yet, teleologically may improve pulmonary gas exchange (Hayano et al. 1996) and clinically may predict cardiovascular outcomes (Bigger et al. 1992a, b). However, current mathematical models have limited interpretability. This is, in part, because they usually are not combined with experimental interventions designed to soundly test the models. The current literature is characterized by approaches that simply observe the behaviour of the system and impose broad assumptions to derive mechanistic conclusions. Future advances in understanding the physiology of cardiovascular oscillations will require more rigorous approaches; models constructed from experimental data should be subsequently validated by further experiments explicitly designed to test the veracity of the original model. Moreover, there are methods of non-linear systems identification which might have utility in studying cardiovascular variabilities. Bilinear models (Christini et al. 1995) seem to fit heart rate time series well, but require more extensive investigation; non-parametric autoregressive time series (Fan & Gijbels, 1996) or threshold autoregressive (Tong, 1990) models can be used as exploratory tools to suggest parametric models; and, wavelet non-stationary time series identification (Mallat et al. 1998) can sharply delineate temporal variations. However, careful statistical analysis of models should be routine and alternative models should be fitted to the same data set for comparison.
The historical record demonstrates the importance of the study of cardiovascular oscillations for providing unique insight to fluid dynamics, vessel elasticity, integrative cardiovascular physiology and medical technology. Relatively low frequency cardiovascular oscillations represent a window into the control by two separate nervous system branches (parasympathetic and sympathetic) of a biochemically active fluid (blood) bounded by an elastic medium (arteries). Furthering our understanding of cardiovascular interactions that determine the frequency and amplitude of spontaneous oscillations should continue to advance the range of disciplines these phenomena intersect.
Acknowledgments
This work was supported by the National Institute on Aging (grant AG14376), Washington, DC, USA, and a generous contribution from the Hinda and Fred Shuman Charitable Foundation, Boston, MA, USA.
References
- Abbiw-Jackson RM, Langford WF. Gain-induced oscillations in blood pressure. Journal of Mathematical Biology. 1998;37:203–234. doi: 10.1007/s002850050126. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman AR, Merrill RH, Wooles WR. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. Journal of Applied Physiology. 1994;77:606–613. doi: 10.1152/jappl.1994.77.2.606. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: Investigation by spectral analysis. American Journal of Physiology. 1985;249:H867–875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. American Journal of Physiology – Heart and Circulatory Physiology. 2001;280:H2674–2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- Barbieri R, Bianchi AM, Triedman JK, Mainardi LT, Cerutti S, Saul JP. Model dependency of multivariate autoregressive spectral analysis. IEEE Engineering in Medicine and Biology. 1997;16:74–85. doi: 10.1109/51.620498. [DOI] [PubMed] [Google Scholar]
- Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC, LipsitZ LA. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension. 1999;33:1195–1200. doi: 10.1161/01.hyp.33.5.1195. [DOI] [PubMed] [Google Scholar]
- Baselli G, Cerutti S, Civardi S, Malliani A, Pagani M. Cardiovascular variability signals: Towards the identification of a closed-loop model of the neural control mechanisms. IEEE Transactions on Biomedical Engineering. 1988;35:1033–1046. doi: 10.1109/10.8688. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, Pinsky MR. Respiratory sinus arrhythmia in the denervated human heart. Journal of Applied Physiology. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- Bertram D, Barres C, Cuisinaud G, Julien C. The arterial baroreceptor reflex of the rat exhibits positive feedback properties at the frequency of Mayer waves. Journal of Physiology. 1998;513:251–261. doi: 10.1111/j.1469-7793.1998.251by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992a;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. American Journal of Cardiology. 1992b;69:891–898. doi: 10.1016/0002-9149(92)90788-z. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Steinman RC, Rolnitzky LM, Fleiss JL, Albrecht P, Cohen RJ. Power law behavior of RR-interval variability in healthy middle-aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation. 1996;93:2142–2151. doi: 10.1161/01.cir.93.12.2142. [DOI] [PubMed] [Google Scholar]
- Box GE, Jenkins GM, Reinsel GC. Time Series Analysis: Forecasting and Control. Englewood Cliffs: Prentice Hall; 1994. [Google Scholar]
- Brandenberger G, Follenius M, Muzet A, Ehrhart J, Schieber JP. Ultradian oscillations in plasma renin activity: Their relationships to meals and sleep stages. Journal of Clinical Endocrinology and Metabolism. 1985;61:280–284. doi: 10.1210/jcem-61-2-280. [DOI] [PubMed] [Google Scholar]
- Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. Journal of Applied Physiology. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- Cavalcanti S, Belardinelli E. Modeling of cardiovascular variability using a differential delay equation. IEEE Transactions on Biomedical Engineering. 1996;43:982–989. doi: 10.1109/10.536899. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: Assessment by spectral analysis. American Journal of Physiology. 1994;266:H1993–2000. doi: 10.1152/ajpheart.1994.266.5.H1993. [DOI] [PubMed] [Google Scholar]
- Cerutti S, Carrault G, Cluitmans PJ, Kinie A, Lipping T, Nikolaidis N, Pitas I, Signorini MG. Non-linear algorithms for processing biological signals. Computer Methods and Programs in Biomedicine. 1996;51:51–73. doi: 10.1016/0169-2607(96)01762-2. [DOI] [PubMed] [Google Scholar]
- Chon KH, Mukkamala R, Toska K, Mullen TJ, Armoundas AA, Cohen RJ. Linear and nonlinear system identification of autonomic heart-rate modulation. IEEE Engineering in Medicine and Biology. 1997;16:96–105. doi: 10.1109/51.620500. [DOI] [PubMed] [Google Scholar]
- Chon KH, Mullen TJ, Cohen RJ. A dual-input nonlinear system analysis of autonomic modulation of heart rate. IEEE Transactions on Biomedical Engineering. 1996;43:530–544. doi: 10.1109/10.488800. [DOI] [PubMed] [Google Scholar]
- Christini DJ, Bennett FM, Lutchen KR, Ahmed HM, Hausdorff JM, Oriol N. Application of linear and nonlinear time series modeling to heart rate dynamics analysis. IEEE Transactions on Biomedical Engineering. 1995;42:411–415. doi: 10.1109/10.376135. [DOI] [PubMed] [Google Scholar]
- Clement DL, De Pue N, Jordaens LJ, Packet L. Adrenergic and vagal influences on blood pressure variability. Clinical and Experimental Hypertension. 1985;7:159–166. doi: 10.3109/10641968509073535. [DOI] [PubMed] [Google Scholar]
- Coker R, Koziell A, Oliver C, Smith SE. Does the sympathetic nervous system influence sinus arrhythmia in man? Evidence from combined autonomic blockade. Journal of Physiology. 1984;356:459–464. doi: 10.1113/jphysiol.1984.sp015476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conci F, Di Rienzo M, Castiglioni P. Blood pressure and heart rate variability and baroreflex sensitivity before and after brain death. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:621–631. doi: 10.1136/jnnp.71.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Spectrum of a series of point events, generated by the integral pulse frequency modulation model. Medicine and Biology in Engineering and Computing. 1985;23:138–142. doi: 10.1007/BF02456750. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: A beat-to-beat model. American Journal of Physiology. 1987;253:H680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- di Virgilio V, Barbieri R, Mainardi L, Strano S, Cerutti S. A multivariate time-variant AR method for the analysis of heart rate and arterial blood pressure. Medical Engineering and Physics. 1997;19:109–124. doi: 10.1016/s1350-4533(96)00058-6. [DOI] [PubMed] [Google Scholar]
- Dornhorst AC, Howard P, Leathart GL. Respiratory variations in blood pressure. Circulation. 1952;6:553–558. doi: 10.1161/01.cir.6.4.553. [DOI] [PubMed] [Google Scholar]
- Duong QP. On the choice of the order of autoregressive models: A ranking and selection approach. Journal of Time Series Analysis. 1984;5:145–157. [Google Scholar]
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. American Journal of Physiology. 1992;263:H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Nonlinearities of the human carotid baroreceptor-cardiac reflex. Circulation Research. 1980;47:208–216. doi: 10.1161/01.res.47.2.208. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. Journal of Physiology. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Gijbels I. Monographs on Statistics and Applied Probability. Vol. 66. New York: Chapman & Hall; 1996. Local polynomial modelling and its applications; pp. 341–356. [Google Scholar]
- Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. American Journal of Physiology. 1984;246:H838–842. doi: 10.1152/ajpheart.1984.246.6.H838. [DOI] [PubMed] [Google Scholar]
- Fredericq L. De l'influence de la respiration sur la circulation. Les oscillations respiratoires de la pression arterielle chez le chien. Archives de Biologie (Paris) 1882;3:55–100. [Google Scholar]
- Furlan R, GuETTIZZ S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. Journal of Physiology. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootman PM, Cohen MI. The interrelationships between sympathetic discharge and central respiratory drive. In: Umbach W, Koepchen HP, editors. Central Rhythms and Regulation. Stuttgart: Hippokrates Verlag; 1974. pp. 127–144. [Google Scholar]
- Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circulation Research. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: Within- and between-individual relations. Psychophysiology. 1993;30:486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Batson HM, Smith CM, Armstrong GG. Method for studying the competence of the body's blood pressure regulatory mechanisms and effect of pressoreceptor denervation. American Journal of Physiology. 1951;164:360–368. doi: 10.1152/ajplegacy.1951.164.2.360. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Harris JW. Pressoreceptor-autonomic oscillation: A probable cause of vasomotor waves. American Journal of Physiology. 1951;165:158–166. doi: 10.1152/ajplegacy.1951.165.1.158. [DOI] [PubMed] [Google Scholar]
- GuETTIZZ S, Cogliati C, Broggi C, CaroIZZ C, Caldiroli D, Lombardi F, Malliani A. Influences of neural mechanisms on heart period and arterial pressure variabilities in quadriplegic patients. American Journal of Physiology. 1994;266:H1112–1120. doi: 10.1152/ajpheart.1994.266.3.H1112. [DOI] [PubMed] [Google Scholar]
- Hales S. Statical Essays: concerning Haemastaticks; or, An Account of some Hydraulick and Hydrostatical Experiments made on the Blood and Blood-vessels of Animals. London: W. Innys and R. Manby; 1733. [Google Scholar]
- Hamner JW, Morin RJ, Rudolph JL, Taylor JA. Inconsistent link between low-frequency oscillations: R-R interval responses to augmented Mayer waves. Journal of Applied Physiology. 2001;90:1559–1564. doi: 10.1152/jappl.2001.90.4.1559. [DOI] [PubMed] [Google Scholar]
- Hayano J, Taylor JA, Yamada A, Mukai S, Hori R, Asakawa T, Yokoyama K, Watanabe Y, Takata K, Fujinami T. Continuous assessment of hemodynamic control by complex demodulation of cardiovascular variability. American Journal of Physiology. 1993;264:H1229–1238. doi: 10.1152/ajpheart.1993.264.4.H1229. [DOI] [PubMed] [Google Scholar]
- Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. 1996;94:842–847. doi: 10.1161/01.cir.94.4.842. [DOI] [PubMed] [Google Scholar]
- Hedman AE, Hartikainen JE, Tahvanainen KU, Hakumaki MO. The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic ‘tone’. Acta Physiologica Scandinavica. 1995;155:267–273. doi: 10.1111/j.1748-1716.1995.tb09973.x. [DOI] [PubMed] [Google Scholar]
- Heneghan C, McDarby G. Establishing the relation between detrended fluctuation analysis and power spectral density analysis for stochastic processes. Physical Review. E, Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics. 2000;62:6103–6110. doi: 10.1103/physreve.62.6103. [DOI] [PubMed] [Google Scholar]
- Hering E. Uber den einfluss der atmung auf den kreislauf i. Uber athenbewegungen des gefasssystems. Sitzungsberichte Kaiserlich Akad Wissenschaft Mathemat-Naturwissenschaft Classe. 1869;60:829–856. [Google Scholar]
- Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. American Journal of Physiology. 1981;241:H620–629. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- Honzikova N, Fiser B, Honzik J. Noninvasive determination of baroreflex sensitivity in man by means of spectral analysis. Physiological Research. 1992;41:31–37. [PubMed] [Google Scholar]
- Hughson RL, Quintin L, Annat G, Yamamoto Y, Gharib C. Spontaneous baroreflex by sequence and power spectral methods in humans. Clinical Physiology. 1993;13:663–676. doi: 10.1111/j.1475-097x.1993.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Innes JA, De Cort SC, Kox W, GuZ A. Within-breath modulation of left ventricular function during normal breathing and positive-pressure ventilation in man. Journal of Physiology. 1993;460:487–502. doi: 10.1113/jphysiol.1993.sp019483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Miyake S, Kumashiro M, Ogata H, Ueta T, Akatsu T. Power spectral analysis of blood pressure variability in traumatic quadriplegic humans. American Journal of Physiology. 1991;260:H842–847. doi: 10.1152/ajpheart.1991.260.3.H842. [DOI] [PubMed] [Google Scholar]
- Ivanov PC, Amaral LA, Goldberger AL, Havlin S, Rosenblum MG, Struzik ZR, Stanley HE. Multifractality in human heartbeat dynamics. Nature. 1999;399:461–465. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- Jasson S, Medigue C, Maison-Blanche P, Montano N, Meyer L, Vermeiren C, Mansier P, Coumel P, Malliani A, Swynghedauw B. Instant power spectrum analysis of heart rate variability during orthostatic tilt using a time-/frequency-domain method. Circulation. 1997;96:3521–3526. doi: 10.1161/01.cir.96.10.3521. [DOI] [PubMed] [Google Scholar]
- Kaplan DT, Furman MI, Pincus SM, Ryan SM, LipsitZ LA, Goldberger AL. Aging and the complexity of cardiovascular dynamics. Biophysical Journal. 1991;59:945–949. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: Noninvasive measure of parasympathetic cardiac control. Journal of Applied Physiology. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. American Journal of Physiology. 1970;218:1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Karlsson T, Wallin BG. Cardiac- and noncardiac-related coherence between sympathetic drives to muscles of different human limbs. American Journal of Physiology. 1999;276:R1608–1616. doi: 10.1152/ajpregu.1999.276.6.R1608. [DOI] [PubMed] [Google Scholar]
- Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. Journal of Physiology. 1994;474:483–495. doi: 10.1113/jphysiol.1994.sp020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Terui N, Kollai M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. Journal of the Autonomic Nervous System. 1985;12:251–259. doi: 10.1016/0165-1838(85)90065-7. [DOI] [PubMed] [Google Scholar]
- Kollai M, Koizumi K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. Journal of the Autonomic Nervous System. 1979;1:33–52. doi: 10.1016/0165-1838(79)90004-3. [DOI] [PubMed] [Google Scholar]
- Kollai M, Mizsei G. Repiratory sinus arrhythmia is a limited measure of cardiac parasympathetic control in man. Journal of Physiology. 1990;424:329–342. doi: 10.1113/jphysiol.1990.sp018070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen I. Multivariate closed-loop model for analysis of cardiovascular dynamics. Methods of Information in Medicine. 1997;36:264–267. [PubMed] [Google Scholar]
- Kruger K. Ist der sinus caroticus bei der entstehung der blutdruckwellen hoherer ordnung beteiligt? Zietschrifte Biologie. 1933;94:135–149. [Google Scholar]
- Ljung L. Prentice Hall Information and System Sciences Series. Upper Saddle River, NJ, USA: Prentice Hall; 1999. System Identification: Theory for the User. [Google Scholar]
- Luzier AB, Nawarskas JJ, Anonuevo J, Wilson MF, Kazierad DJ. The effects of gender on adrenergic receptor responsiveness. Journal of Clinical Pharmacology. 1998;38:618–624. doi: 10.1002/j.1552-4604.1998.tb04468.x. [DOI] [PubMed] [Google Scholar]
- Madwed JB, Albrecht P, Mark RG, Cohen RJ. Low-frequency oscillations in arterial pressure and heart rate: A simple computer model. American Journal of Physiology. 1989;256:H1573–1579. doi: 10.1152/ajpheart.1989.256.6.H1573. [DOI] [PubMed] [Google Scholar]
- Mainardi LT, Bianchi AM, Furlan R, PiaAZZ S, Barbieri R, Di Virgilio V, Malliani A, Cerutti S. Multivariate time-variant identification of cardiovascular variability signals: A beat-to-beat spectral parameter estimation in vasovagal syncope. IEEE Transactions on Biomedical Engineering. 1997;44:978–989. doi: 10.1109/10.634650. [DOI] [PubMed] [Google Scholar]
- Makikallio TH, Hoiber S, Kober L, Torp-Pedersen C, Peng CK, Goldberger AL, Huikuri HV. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. Trace investigators. Trandolapril cardiac evaluation. American Journal of Cardiology. 1999;83:836–839. doi: 10.1016/s0002-9149(98)01076-5. [DOI] [PubMed] [Google Scholar]
- Mallat S, Papanicolaou G, Zhang ZF. Adaptive covariance estimation of locally stationary processes. Annals of Statistics. 1998;26:1–47. [Google Scholar]
- Malliani A, Pagani M, Lombardi F. Physiology and clinical implications of variability of cardiovascular parameters with focus on heart rate and blood pressure. American Journal of Cardiology. 1994;73:3–9C. doi: 10.1016/0002-9149(94)90617-3. [DOI] [PubMed] [Google Scholar]
- Mangin L, Monti A, Medigue C, Macquin-Mavier I, Lopes M, Gueret P, Castaigne A, Swynghedauw B, Mansier P. Altered baroreflex gain during voluntary breathing in chronic heart failure. European Journal of Heart Failure. 2001;3:189–195. doi: 10.1016/s1388-9842(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Mateo J, Laguna P. Improved heart rate variability signal analysis from the beat occurrence times according to the ipfm model. IEEE Transactions on Biomedical Engineering. 2000;47:985–996. doi: 10.1109/10.855925. [DOI] [PubMed] [Google Scholar]
- Mayer S. Studien zur physiologie des herzens und der blutgefasse. V. Uber spontane blutdruckschwankungen. Sitzungsberichte Kaiserlich Akad Wissenschaft Mathemat-Naturwissenschaft Classe. 1877;74:281–307. [Google Scholar]
- Michaels DC, Matyas EP, Jalife J. Mechanisms of sinoatrial pacemaker synchronization: A new hypothesis. Circulation Research. 1987;61:704–714. doi: 10.1161/01.res.61.5.704. [DOI] [PubMed] [Google Scholar]
- Montano N, Cogliati C, Porta A, Pagani M, Malliani A, NarkiewicZ K, Abboud FM, Birkett C, Somers VK. Central vagotonic effects of atropine modulate spectral oscillations of sympathetic nerve activity. Circulation. 1998;98:1394–1399. doi: 10.1161/01.cir.98.14.1394. [DOI] [PubMed] [Google Scholar]
- MorawitZ P. Zur differenzierung rhythmischer blutdruckschwankungen. Archives of Anatomy and Physiology. 1903;1:82–99. [Google Scholar]
- Mullen TJ, Appel ML, Mukkamala R, Mathias JM, Cohen RJ. System identification of closed-loop cardiovascular control: Effects of posture and autonomic blockade. American Journal of Physiology. 1997;272:H448–461. doi: 10.1152/ajpheart.1997.272.1.H448. [DOI] [PubMed] [Google Scholar]
- Murata K, Landrigan PJ, Araki S. Effects of age, heart rate, gender, tobacco and alcohol ingestion on R-R interval variability in human ECG. Journal of the Autonomic Nervous System. 1992;37:199–206. doi: 10.1016/0165-1838(92)90041-e. [DOI] [PubMed] [Google Scholar]
- Myers CW, Cohen MA, Eckberg DL, Taylor JA. A model for the genesis of arterial pressure Mayer waves from heart rate and sympathetic activity. Autonomic Neuroscience. 2001;91:62–75. doi: 10.1016/S1566-0702(01)00289-2. [DOI] [PubMed] [Google Scholar]
- Nakata A, Takata S, Yuasa T, Shimakura A, Maruyama M, Nagai H, Sakagami S, Kobayashi K. Spectral analysis of heart rate, arterial pressure, and muscle sympathetic nerve activity in normal humans. American Journal of Physiology. 1998;274:H1211–1217. doi: 10.1152/ajpheart.1998.274.4.H1211. [DOI] [PubMed] [Google Scholar]
- Olufsen MS. Structured tree outflow condition for blood flow in larger systemic arteries. American Journal of Physiology. 1999;276:H257–268. doi: 10.1152/ajpheart.1999.276.1.H257. [DOI] [PubMed] [Google Scholar]
- Olufsen MS, Peskin CS, Kim WY, Pedersen EM, Nadim A, Larsen J. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Annals of Biomedical Engineering. 2000;28:1281–1299. doi: 10.1114/1.1326031. [DOI] [PubMed] [Google Scholar]
- Ottesen JT. Modelling of the baroreflex-feedback mechanism with time-delay. Journal of Mathematical Biology. 1997;36:41–63. doi: 10.1007/s002850050089. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, GuETTIZZ S, Rimoldi O, Furlan R, PiINELLIZZ P, Sandrone G, Malfatto G, Dell'orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286. doi: 10.1161/01.hyp.25.6.1276. [DOI] [PubMed] [Google Scholar]
- PenaZ J, Honzikova N, Fiser B. Spectral analysis of resting variability of some circulatory parameters in man. Physiologia Bohemoslovaca. 1978;27:349–357. [PubMed] [Google Scholar]
- Peng C-K, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Physical Review Letters. 1993;70:1343–1346. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- Penm JHW, Terrell RD. On the recursive fitting of subset autoregressions. Journal of Time Series Analysis. 1982;3:43–59. [Google Scholar]
- Pilgram B, Kaplan DT. Nonstationarity and 1/f noise characteristics in heart rate. American Journal of Physiology. 1999;276:R1–9. doi: 10.1152/ajpregu.1999.276.1.R1. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: What does regularity quantify? American Journal of Physiology. 1994;266:H1643–1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Porta A, GuETTIZZ S, Montano N, Furlan R, Pagani M, Malliani A, Cerutti S. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Transactions on Biomedical Engineering. 2001;48:1282–1291. doi: 10.1109/10.959324. [DOI] [PubMed] [Google Scholar]
- Porta A, GuETTIZZ S, Montano N, Pagani M, Somers V, Malliani A, Baselli G, Cerutti S. Information domain analysis of cardiovascular variability signals: Evaluation of regularity, synchronisation and co-ordination. Medicine and Biology in Engineering and Computing. 2000;38:180–188. doi: 10.1007/BF02344774. [DOI] [PubMed] [Google Scholar]
- Preiss G, Iscoe S, Polosa C. Analysis of a periodic breathing pattern associated with Mayer waves. American Journal of Physiology. 1975;228:768–774. doi: 10.1152/ajplegacy.1975.228.3.768. [DOI] [PubMed] [Google Scholar]
- Preiss G, Polosa C. Patterns of sympathetic neuron activity associated with Mayer waves. American Journal of Physiology. 1974;226:724–730. doi: 10.1152/ajplegacy.1974.226.3.724. [DOI] [PubMed] [Google Scholar]
- Raczkowska M, Eckberg DL, Ebert TJ. Muscarinic cholinergic receptors modulate vagal cardiac responses in man. Journal of the Autonomic Nervous System. 1983;7:271–278. doi: 10.1016/0165-1838(83)90080-2. [DOI] [PubMed] [Google Scholar]
- Radaelli A, Bernardi L, Valle F, LeuIZZ S, Salvucci F, Pedrotti L, Marchesi E, Finardi G, Sleight P. Cardiovascular autonomic modulation in essential hypertension. Effect of tilting. Hypertension. 1994;24:556–563. doi: 10.1161/01.hyp.24.5.556. [DOI] [PubMed] [Google Scholar]
- Rompelman O, Snijders JB, Van Spronsen CJ. The measurement of heart rate variability spectra with the help of a personal computer. IEEE Transactions on Biomedical Engineering. 1982;29:503–510. doi: 10.1109/TBME.1982.324922. [DOI] [PubMed] [Google Scholar]
- Rothlin E, Cerletti A. Uber die koppelung von atmung und kreislauf. Schweizerische Medizinische Wochenschrift. 1950;80:1394–1399. [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. American Journal of Physiology. 1999;276:H1691–1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Goldberger AL, Pincus SM, Mietus J, LipsitZ LA. Gender- and age-related differences in heart rate dynamics: Are women more complex than men? Journal of the American College of Cardiology. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. Journal of Physiology. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: Unique insights into cardiovascular regulation. American Journal of Physiology. 1991;261:H1231–1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Scheffer GJ, Tenvoorde BJ, Karemaker JM, Ros HH. Effects of epidural analgesia and atropine on heart rate and blood pressure variability: Implications for the interpretation of beat-to-beat fluctuations. European Journal of Anaesthesiology. 1994;11:75–80. [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circulation Research. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circulation Research. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- Seydnejad SR, Kitney RI. Time-varying threshold integral pulse frequency modulation. IEEE Transactions on Biomedical Engineering. 2001;48:949–962. doi: 10.1109/10.942584. [DOI] [PubMed] [Google Scholar]
- Siegel G, Roedel H, Hofer HW. Basic rhythms in vascular smooth muscle. In: Worcel M, Vassort G, editors. Smooth Muscle Pharmacology and Physiology. Paris: INSERM; 1976. pp. 215–231. [Google Scholar]
- Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, LeuIZZ S, Bianchini B, TavaIZZ L, Bernardi L. Physiology and pathophysiology of heart rate and blood pressure variability in humans: Is power spectral analysis largely an index of baroreflex gain? Clinical Science. 1995;88:103–109. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- Sunagawa K, Kawada T, Nakahara T. Dynamic nonlinear vago-sympathetic interaction in regulating heart rate. Heart Vessels. 1998;13:157–174. doi: 10.1007/BF01745040. [DOI] [PubMed] [Google Scholar]
- Taguchi T. Studies on the periodicity of fetal and neonatal heart rate variability by fast fourier transform analysis. Nippon Sanka Fujinka Gakkai Zasshi. 1983;35:53–60. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998a;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation. 1996;93:1527–1532. doi: 10.1161/01.cir.93.8.1527. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: Implications for vagal-cardiac tone assessment in humans. American Journal of Physiology – Heart and Circulatory Physiology. 2001;280:H2804–2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Williams TD, Seals DR, Davy KP. Low-frequency arterial pressure fluctuations do not reflect sympathetic outflow: gender and age differences. American Journal of Physiology. 1998b;274:H1194–1201. doi: 10.1152/ajpheart.1998.274.4.H1194. [DOI] [PubMed] [Google Scholar]
- Teich MC, Lowen SB, Jost BM, Vibe-Rheymer K, Heneghan C. Heart rate variability: measures and models. In: Akay M, editor. Nonlinear Biomedical Signal Processing. New York: IEEE Press; 2000. pp. 159–213. [Google Scholar]
- Tong H. Oxford Statistical Science Series. Vol. 6. UK: Oxford University Press; 1990. Non-linear time series: a dynamical system approach; pp. 564–580. [Google Scholar]
- Toska K, Eriksen M. Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. Journal of Physiology. 1993;472:501–512. doi: 10.1113/jphysiol.1993.sp019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traube L. Ueber periodische thatigkeits-aeusserungen des vasomotorischen und hemmungs-nervencentrums. Medizin Wissenschaft. 1865;56:881–885. [Google Scholar]
- Triedman JK, Saul JP. Blood pressure modulation by central venous pressure and respiration. Buffering effects of the heart rate reflexes. Circulation. 1994;89:169–179. doi: 10.1161/01.cir.89.1.169. [DOI] [PubMed] [Google Scholar]
- Turcott RG, Teich MC. Fractal character of the electrocardiogram: Distinguishing heart-failure and normal patients. Annals of Biomedical Engineering. 1996;24:269–293. doi: 10.1007/BF02667355. [DOI] [PubMed] [Google Scholar]
- Ursino M, Lodi CA. Interaction among autoregulation, CO2 reactivity, and intracranial pressure: A mathematical model. American Journal of Physiology. 1998;274:H1715–1728. doi: 10.1152/ajpheart.1998.274.5.H1715. [DOI] [PubMed] [Google Scholar]
- Vaile JC, Fletcher J, Al-Ani M, Ross HF, Littler WA, Coote JH, Townend JN. Use of opposing reflex stimuli and heart rate variability to examine the effects of lipophilic and hydrophilic beta-blockers on human cardiac vagal control. Clinical Science. 1999;97:585–593. [PubMed] [Google Scholar]
- van de Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation. 1997;95:1449–1454. doi: 10.1161/01.cir.95.6.1449. [DOI] [PubMed] [Google Scholar]
- van de Borne P, Rahnama M, MeETTIZZ S, Montano N, Porta A, Degaute JP, Somers VK. Contrasting effects of phentolamine and nitroprusside on neural and cardiovascular variability. American Journal of Physiology – Heart and Circulatory Physiology. 2001;281:H559–565. doi: 10.1152/ajpheart.2001.281.2.H559. [DOI] [PubMed] [Google Scholar]
- Veerman DP, ImholZ BP, Wieling W, Karemaker JM, Van Montfrans GA. Effects of aging on blood pressure variability in resting conditions. Hypertension. 1994;24:120–130. doi: 10.1161/01.hyp.24.1.120. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. Journal of Applied Physiology. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- Wray DW, Formes KJ, Weiss MS, Ah OY, Raven PB, Zhang R, Shi X. Vagal cardiac function and arterial blood pressure stability. American Journal of Physiology – Heart and Circulatory Physiology. 2001;281:H1870–1880. doi: 10.1152/ajpheart.2001.281.5.H1870. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Srinivasan K, Vempati S, Pohl R, Balon R. Fractal dimension of heart rate time series: An effective measure of autonomic function. Journal of Applied Physiology. 1993;75:2429–2438. doi: 10.1152/jappl.1993.75.6.2429. [DOI] [PubMed] [Google Scholar]


