Abstract
A recent study on malaria-infected human red blood cells (RBCs) has shown induced ion channel activity in the host cell membrane, but the questions of whether they are host- or parasite-derived and their molecular nature have not been resolved. Here we report a comparison of a malaria-induced anion channel with an endogenous anion channel in Plasmodium falciparum-infected human RBCs. Ion channel activity was measured using the whole-cell, cell-attached and excised inside-out configurations of the patch-clamp method. Parasitised RBCs were cultured in vitro, using co-cultured uninfected RBCs as controls. Unstimulated uninfected RBCs possessed negligible numbers of active anion channels. However, anion channels could be activated in the presence of protein kinase A (PKA) and ATP in the pipette solution or by membrane deformation. These channels displayed linear conductance (∼15 pS), were blocked by known anion channel inhibitors and showed the permeability sequence I− > Br− > Cl−. In addition, in less than 5 % of excised patches, an outwardly rectifying anion channel (∼80 pS, outward conductance) was spontaneously active. The host membrane of malaria-infected RBCs possessed spontaneously active anion channel activity, with identical conductances, pharmacology and selectivity to the linear conductance channel measured in stimulated uninfected RBCs. Furthermore, the channels measured in malaria-infected RBCs were shown to have a low open-state probability (Po) at positive potentials, which explains the inward rectification of membrane conductance observed when using the whole-cell configuration. The data are consistent with the presence of two endogenous anion channels in human RBCs, of which one (the linear conductance channel) is up-regulated by the malaria parasite P. falciparum.
Until recently, electrophysiological studies on human RBCs have been difficult, and little is known of the anionic conductive pathways present in the RBC membrane. Anion permeability is particularly relevant in the context of malaria where a wealth of transport studies have demonstrated the importance of parasite-induced transport pathways for anions and other solutes in infected RBCs (Ginsburg, 1999; Kirk, 2001).
Recently, it has been reported that human RBCs infected by Plasmodium falciparum exhibit novel parasite-induced voltage-dependent anion channels (Desai et al. 2000) but the questions of whether they are host- or parasite-derived and their molecular nature were not resolved. Using the patch-clamp method on avian RBCs infected by P. gallinaceum, we were unable to identify novel parasite-induced pathways in infected chicken RBCs (Thomas et al. 2001), rather the avian malaria parasite up-regulates existing pathways in the host RBC membrane. This observation raises the possibility that the small conductance anion channels identified in infected human RBCs (Desai et al. 2000) are also endogenous components of the RBC membrane, and there are some indications in the literature (Freedman et al. 1994; Schwartz et al. 1997) that this may be so.
In the light of this evidence, we used various configurations of the patch-clamp technique and conditions that are known to activate quiescent channels to produce the first electrophysiological evidence at the single channel level for two anionic channels in human RBCs. In addition, we have demonstrated that the membrane current measured in human RBCs, infected by P. falciparum, could be accounted for by up-regulation of one of these anion channel types.
Methods
Preparation of cells
RBCs were obtained from healthy volunteers, who gave informed, written consent, in accordance with the Declaration of Helsinki. The RBCs were washed three times in a saline solution containing (mm): 150 NaCl, 5 KCl, 1.4 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose, pH 7.4, 320 ± 5 mosmol (kg H2O)−1. P. falciparum-infected human RBCs, using clone 3D7, were cultured as described previously (Doerig et al. 1995) and washed into the same saline as uninfected RBCs prior to experimentation.
Current recordings
Patch pipettes (tip resistance 10–20 MΩ) were prepared from borosilicate glass capillaries pulled and polished on a Werner Zeitz DMZ programmable puller (Augsburg, Germany). Pipette and bath solutions contained (mm): 155 NMDG-Cl, 1 MgCl2, 10 Hepes, 10 glucose, pH 7.4, 320 ± 5 mosmol (kg H2O)−1. The Ca2+ concentrations were adjusted to different pCa (-log[Ca2+]) in the pipette and bathing solutions (pCa 7 on the cytosolic side and pCa 3 on the extracellular side).
Single channel current and voltage recordings were made as described previously (Egée et al. 2000), using cell-attached and excised inside-out configurations. The ruptured patch whole-cell configuration was used to record whole-cell currents. All experiments were performed at 37 °C. Whole-cell current-voltage (I-V) relationships were run and analysed using WCP V3.2 software (Strathclyde, UK). Seal resistances were 4–20 GΩ. Data are given as mean values ± s.e.m. Significance was assessed using the Fisher F test and Student's t test.
Materials
ATP (as Mg2+ or Na+ salts), 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS), furosemide (frusemide), niflumic acid, glibenclamide, N-methyl-d-glucamine chloride (NMDG), the catalytic subunit of protein kinase A (PKA) and anthracene-9-carboxylic acid (9-AC) were purchased from Sigma (Saint Quentin Fallavier, France). 5-Nitro-2-(3-phenylpropylamino)-benzoate (NPPB) and diphenylamine-2-carboxylic acid (DPC) were obtained from Research Biochemicals International (Saint Quentin Fallavier, France). Tamoxifen was obtained from Calbiochem (Fontenay-sous-Bois, France).
Results
Whole-cell patch clamp of uninfected and malaria-infected RBCs
Using the whole-cell patch-clamp method, with the impermeable monovalent cation NMDG in all pipette and bathing solutions (to prevent cationic currents), uninfected RBCs displayed negligible conductance with linear I-V relationships and reversal potentials (Er) close to 0 mV (Fig. 1A and D). The membrane conductance (Gm) was measured to be only 44 ± 1 pS (n = 32).
Figure 1. Whole-cell and cell-attached single channel recordings of uninfected and infected RBCs.
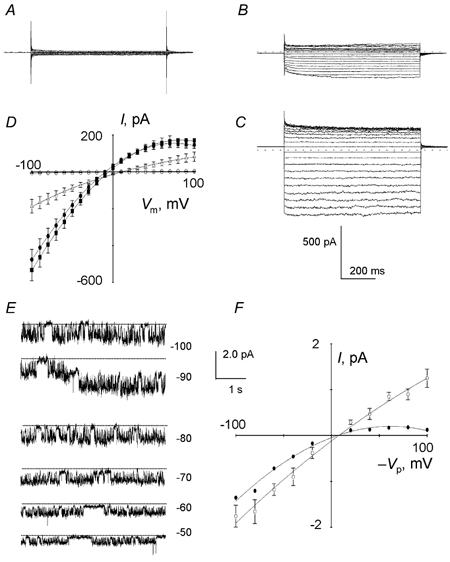
Current traces in uninfected RBCs in control (A) or after addition of PKA catalytic subunit (100 nm) to the pipette solution (B). Current traces in infected RBCs (C) bathing in NMDG-Cl solution (voltage pulses between −100 and +100 mV, 10 mV increments, 700 ms). D, corresponding I-V plots (mean ± s.e.m.) in control (○, n = 32), after addition of PKA (▵, n = 58), in infected cells with NMDG-Cl (•, n = 24) or NaCl (▪, n = 12) in the bath. E, typical current recordings at different potentials (-Vp) from infected RBCs. F, corresponding I-V plot (mean ± s.e.m., ○, n = 25). •, I-V plot obtained after multiplication of the I value by the corresponding mean Po.
It is known that phosphorylation of a consensus site by PKA in the presence of ATP can activate ion channels (e.g. cAMP-regulated CFTR channels; Tabcharani et al. 1991). We therefore investigated whether the addition of the catalytic subunit of PKA (100 nm) and ATP (10 mm) to the pipette solution, in the presence of theophylline (100 μM), could enhance membrane conductance. Under these conditions, the membrane current was 40 times larger than in unstimulated RBCs (Fig. 1B and D) and showed inward rectification. ATP or theophylline alone did not induce channel activity. The conductance between −100 mV and Er was 1700 ± 40 pS (n = 58). Px/PCl permeability ratios estimated from the shift in Er after replacement of Cl− ions in the bath solution for anions were calculated to be I− (2.8 ± 0.3; n = 3) > Br− (1.2 ± 0.5; n = 3) ≥ Cl− (1) > F− (0.4 ± 0.2; n = 3). In addition, the increased conductance did not occur in the presence of the anion channel blockers NPPB (100 μM), glibenclamide (100 μM) and DPC (1 mm; pre-incubated for at least 5 min) in the bath solution.
Whole-cell experiments on malaria-infected cells showed a membrane current 100- to 150-fold larger than in unstimulated uninfected cells (Fig. 1C and D) with the corresponding I-V curves showing inward rectification. Addition of PKA and ATP to the patch pipettes had no additive effect. The membrane current reversed polarity at −9 ± 1 mV and the Gm, calculated between −100 mV and Er, was 5300 ± 140 pS (n = 24). No significant change in the I-V relationship was observed when NMDG was replaced by Na+ (Fig. 1D), which indicates further an anionic conductance. Px/PCl ratios for anions were calculated to be I− (3.4 ± 0.4, n = 4) > NO3− (1.6 ± 0.2, n = 4) > Br− (1.3 ± 0.3, n = 5) ≥ Cl− (1.00) > F− (0.4 ± 0.2, n = 5) > gluconate (0.2 ± 0.1, n = 3). NPPB, niflumic acid and glibenclamide, added in the bathing solution, reduced immediately the membrane conductance of infected cells in a dose-related manner. The IC50 values (i.e. the concentration at which the inward current is reduced by half) were 0.8 ± 0.3 μM (n = 4), 2.5 ± 0.3 μM (n = 4), 35.0 ± 8.7 μM (n = 3), respectively. Furosemide (200 μM), known as a potent blocker of malaria-induced solute transport via the infected RBC membrane (Kirk et al. 1994), reduced the membrane conductance by 45 ± 1 % (n = 3). By contrast, 100 μM DIDS produced no significant inhibition.
Cell-attached patch clamp of uninfected and malaria-infected RBCs
To elucidate the exact nature of the channels responsible for the whole-cell membrane conductance of intact normal and infected RBCs, we recorded single channel activity in the cell-attached configuration. As implied by the whole-cell data, negligible channel activity was detected in uninfected cells.
In contrast to uninfected RBCs, spontaneous channel activity was observed in infected RBCs, as bursts of channel openings separated by short closures (Fig. 1E). All successful patches contained one or more spontaneously active channels. These channels showed slight inward rectification at negative potentials (cubic polynomial regression, P < 0.005), with an average conductance of 18.0 ± 1.1 pS (82 data points from 25 experiments) between −100 and −20 mV. In the majority of cases, the estimated Er was twelve millivolts more positive than the resting membrane potential (Fig. 1F), which indicates that intracellular chloride was slightly above electrochemical equilibrium at the spontaneous membrane potential.
These channels exhibited voltage-dependent gating, with low Po between +50 and +100 mV, and increasing Po between +50 and −100 mV. In addition, for a given membrane patch, the number of simultaneously active channels was directly proportional to the membrane potential (Vm), with the maximum number (3) activated at negative potentials.
A dwell time analysis was performed on patches containing only one channel, and the kinetics analysis, made at holding potentials (-Vp) of +80 and −80 mV, showed that within bursts the open-time and closed-time durations were fitted best by single exponential distributions. The kinetic constants, τo and τc, were 19.4 ± 2.4 ms and 9.9 ± 2.3 ms (n = 5), respectively, at −80 mV and 9.4 ± 2.9 ms and 7.9 ± 1.6 ms (n = 6), respectively, at +80 mV. Using the data presented above, it was possible to calculate that approximately 250–300 copies of the 18 pS channel could be responsible for the measured whole-cell currents in infected RBCs (if we assume that the whole-cell current can be accounted for totally by this channel type).
Excised inside-out patch clamp of uninfected and malaria-infected RBCs
Finally, we used the excised inside-out configuration to identify further the different channel types present in the plasma membranes of uninfected and malaria-infected RBCs. After excision, one type of channel, showing linear conductance (12-15 pS), was observed in more than 80 % of membrane patches from both uninfected and malaria-infected RBCs. In addition, another channel type, which displayed outward rectification, was present in less than 5 % of uninfected cell patches but was never observed in infected cell patches.
In uninfected membrane patches, the linear conductance channel was not spontaneously active but could be activated by exposure of the cytosolic side of the membrane to the catalytic subunit of PKA (100 nm) in the presence of ATP (1 mm), after a lag period of 1–3 min (Fig. 2A). The mean I-V relationship was linear (P < 0.001) and the unit conductance was 12.3 ± 0.5 pS (62 data points from 8 experiments) (Fig. 2D). In the majority of cases, a progressive run-down of channel activity was observed within the 5 min period after activation, which was probably due to endogenous protein phosphatase activity. ATP alone could not induce channel activity.
Figure 2. Excised inside-out single channel recordings of linear conductance anion channels in uninfected and infected RBCs.
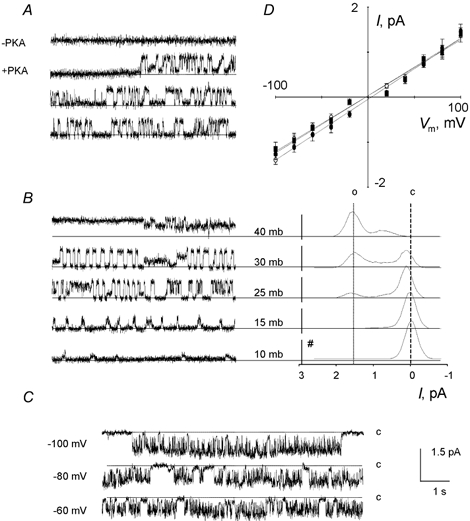
A, activation of an anion channel by exposure of the cytosolic side of the membrane to PKA (100 nm) and ATP (1 mm), after a 1–3 min lag period, at Vm of +100 mV. B, activation of the same channel by membrane deformation upon application of calibrated depression in the pipette (Vm of +100 mV). Substates corresponding to 1/3 and 2/3 of full amplitude are clearly visible. The right panel shows the distribution of current amplitude from 3 min recordings (#, number of events; o, open state; c, closed state; mb, millibars). C, examples of current recordings from infected RBCs showing fast gating at negative potentials. D, mean I-V plots (± s.e.m.) corresponding to A (○, n = 8), B (▪, n = 10) and C (•, n = 7).
Similar channel activity could also be observed, in the absence of PKA, by the application of suction to the pipette of quiescent patches. Under this condition, the Po values estimated for the activated channels, measured at a given membrane potential, were related to the degree of depression imposed on the membrane patch (Fig. 2B). There was no run-down of the activity during this procedure and its effect was reversible. Using this mode of activation, the I-V curves were linear and the average conductance (13.4 ± 0.5 pS, 55 data points from 10 experiments) was not statistically different (P < 0.001) from the value calculated by PKA activation (Fig. 2D).
The linear conductance channel exhibited two characteristic substates in most membrane patches, corresponding to 1/3 and 2/3 of the full amplitude. These substates were more visible after stretch activation than after PKA-activation (Fig. 2B). After stretch activation, the distribution of relative amplitudes was clearly related to the degree of depression imposed on the membrane patches (i.e. longer periods of full opening increased with pressure). In the case of activation by PKA, full channel openings were obtained at high voltages (< −60 mV and > +60 mV) for brief durations and were usually preceded by channel openings at the 1/3 substate. At low voltages (-60 to +60 mV), amplitude distributions oscillated in a non-discrete manner between the two substates (1/3 and 2/3) with few full channel openings.
In infected cells, a single channel type was always spontaneously active after excision (Fig. 2C) and displayed linear conductance, with a mean unit conductance of 15.7 ± 0.8 pS (60 data points from 7 experiments) (Fig. 2D). The channel exhibited voltage-dependent gating, with a low Po between +50 and +100 mV and an increasing Po between +50 and −100 mV (consistent with the observations in the cell-attached configuration). Run-down of channel activity was never observed after excision.
The linear conductance channels present in both uninfected (produced via both modes of activation) and infected membrane patches displayed the same halide selectivity (I− > Br− > Cl−). Furthermore, they also shared the same pharmacology. Total inhibition of channel activity was obtained by treatment with 100 μM NPPB, 100 μM niflumic acid, 1 mm DPC and 10 μM tamoxifen. DIDS, at a concentration of 100 μM, induced a variable partial block in all cases.
In addition to the linear conductance channel, after excision into the inside-out configuration, an outwardly rectifying channel was identified in only 10 of 243 membrane patches. As shown in Fig. 3, the current-voltage relationship showed strong outward rectification. The channel slope conductance was 11.5 ± 1.3 pS (n = 10) between −100 and 0 mV, and it was 79.9 ± 4.1 pS (n = 10) for positive membrane potentials. Addition of the chloride channel blockers NPPB (n = 4), DIDS (n = 4) and 9-AC (n = 3) on the cytosolic face of excised patches (100 μM) led, within seconds, to 90–100 % inhibition of channel activity. The presence of PKA and ATP never induced activity of this channel type.
Figure 3. Excised inside-out single channel recordings of outwardly rectifying anion channels in uninfected RBCs.
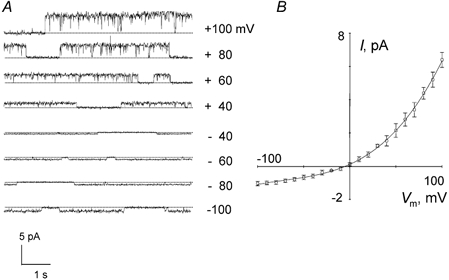
A, example of current traces from uninfected RBCs showing outward rectification. B, mean I-V plots (± s.e.m.) corresponding to 10 recordings.
Discussion
Endogenous anion channels in uninfected RBCs
The data presented in this report show that unstimulated, uninfected human RBCs have an almost total absence of anionic channel activity. However, the uninfected RBC membrane is not devoid of anionic channels. In the present study, we have identified a small (∼15 pS), linear conductance, stretch-activated Cl− channel in uninfected RBCs. This channel can be induced via two modes of activation (PKA/ATP and membrane deformation). The two modes of activation produce slightly different channel gating activity but the activated channels have the same number of substates, pharmacology, conductance and selectivity, which indicate that they are the same channel type. Furthermore, we have also identified an outward rectifying anion channel in uninfected RBCs. However, due to the infrequent observation of this channel in single channel recordings and the absence of outward rectification in the whole-cell configuration, we have been unable to characterise this channel type in detail.
The molecular nature of these two anion channels is as yet unknown and might represent novel classes of anion channels. However, they may also be related to several highly characterised anion channels, which are found in a variety of cell types, including the cystic fibrosis transmembrane regulator protein (CFTR), the volume-regulated anion channel (VRAC) and the ClC chloride channel family (see Table 1 for comparisons).
Table 1.
An electrophysiological comparison of a malaria-induced small, linear conductance anion channel with several known chloride channel types (compiled from the review by Jentsch et al. 2002)
| Channel type | |||||||
|---|---|---|---|---|---|---|---|
| Malaria induced | CFTR | ClC(1–7) | VRAC | Ca2+-activated | Ligand-gated | Maxi | |
| Conductance | 12–18 pS | 5–9 pS | 1–9 pS | 40–50 pS(+Vm) | 1–10 pS | 10–90 pS | 200–400 pS |
| I–V | Linear | Linear | Subtype dependent | Outward rectiflication | [Ca2+]dependent | Ligand-type dependeent | Linear |
| Permeation | I−>Br−>Cl− | Br−>Cl−>I− | Cl−>Br−>I− | I−>Br−>Cl− | I−>Br−>Cl− | I−>Br−>Cl− | Cl−>acetate |
| Inhibitors | NPPB niflumate, glibenclamide, tamoxifen, DPC | NPPB, 9-AC, glibenclamide DPC, DIDS*, suramin* | Subtype dependent but including: DPC, 9-AC | NPPB, DIDS, tamoxifen, niflumate, 1,9-dideoxyforskolin | NPPB, DIDS, niflumate | Ligand-type dependent but including: picrotoxin, cyanotriphenylborate | Phalloidin, DIDS, NPPB, pertussis toxin |
| Modulators | Unknown | Phosphorylation by PKA | Subtype dependent but including: cell swelling hyperpolarisation, internal PH, internal [Ca2+], depolarisation | Cell swelling | Internal[Ca2+] | Specific ligands (e.g.GABA, glycine) | Cell swelling, phorbol esters, PKC |
CFTR, cystic fibrosis transmembrane regulator protein; VRAC, volume-regulated anion channel.
Intracellular application
The mechanosensitivity of the linear conductance channel type is very interesting and may relate to the ATP transport pathway reported by Sprague and co-workers (1998) in human RBCs, which is activated by mechanical deformation and reduced in RBCs from patients with cystic fibrosis. This would support a model in which mechanical deformation of RBCs in the vasculature would result in the activation of anionic channels. These channels would then provide a pathway for ATP release and, thereby, play an important role in regulation of vascular resistance in vivo (by the activation of purinergic receptors and synthesis of nitric oxide; Busse et al. 1988).
Malaria-induced anion channels in infected RBCs
The data show that the dramatic increase in the membrane conductance of malaria-infected human RBCs results from the activation of anionic channels of a single type. Their general pharmacology, selectivity and electrophysiological characteristics are in good agreement with the previous electrophysiological report, in malaria-infected human RBCs, of small conductance anion channels (Desai et al. 2000). However, the channel conductance measured by Desai and co-workers, using the cell-attached configuration with 1150 mm Cl− in the bath and pipette solutions, was only 20 pS. This approximates to a channel conductance of only 3 pS (under the conditions used during this study (i.e. 155 mm Cl−)) compared to a conductance of 12–18 pS presented here. The reason for the higher conductance observed in the present work compared to that of Desai and co-workers is not known, but may be related to the different experimental conditions used. The present study was carried out at 37 °C with glucose in the bath, conditions that may affect (directly or indirectly) the particular anion channel or conductance substate observed in malaria-infected RBCs. Desai and co-workers also reported a greater effect of furosemide on the malaria-induced anion channel than reported here (for which we are unable to provide an explanation).
Extending this work, we have shown here that the single channel conductance of the malaria-induced channel is linear with a low Po at positive potentials. Together with the voltage-dependent open-time duration and number of active channels, it provides an explanation for the inward rectification of the whole-cell I-V curves (see Fig. 1D) observed in malaria-infected RBCs.
Furthermore, the conductance, substrate selectivity and pharmacology of the observed malaria-induced channel, using the excised inside-out configuration of the patch-clamp method, are identical to the endogenous linear conductance anion channel measured in uninfected RBCs. The finding that P. falciparum activates an endogenous ion channel is similar to the action of P. gallinaceum in infected chicken RBCs (Thomas et al. 2001) and is consistent with the hypothesis that P. falciparum up-regulates native anion channels in the host plasma membrane.
If this is the case, the observed differences in gating and Po between the anion channel and the malaria-induced channel may well be due to the mode of action by which the parasite up-regulates the channels. This hypothesis is reinforced by the differences in the gating of the anion channel measured, using two different modes of activation (PKA/ATP and deformation). The present observation that it is possible to induce, in uninfected RBCs, a whole-cell current by the addition of PKA and ATP, which mimics, albeit to a lower extent, the membrane current observed in infected cells suggests that the mechanism of up-regulation used by the parasite may involve phosphorylation steps. It is known that the malaria parasite produces many kinases (Kappes et al. 1999) and parasite-dependent kinase activities have been detected at the membrane of the host RBC (Chishti et al. 1994). Furthermore, the host PKA present in the erythrocyte appears to be modified following infection with P. falciparum, which produces its own PKA molecule (Syin et al. 2001). A better understanding of a possible role for these kinases in activating anion channels could be important in developing strategies for future malarial chemotherapies.
Recently, in a similar study to our present work, Huber and co-workers (2002) have also reported that native anion channels are up-regulated in malaria-infected human RBCs. However, they found that two endogenous channel types are activated by the parasite. One channel produces inward rectification of membrane conductance while the other produces outward rectification and, although sharing functional and pharmacological characteristics, neither is identical to the channels reported here and by Desai and co-workers (2000). Paradoxically, Huber and co-workers reported that the whole-cell conductance in infected RBCs, which has the closest pharmacological profile to the inwardly rectifying malaria-induced conductance reported here and by Desai and co-workers, is the outwardly rectifying conductance and that it is this conductance that mediates malaria-induced transport predominantly.
While our findings, like Huber and co-workers, demonstrate the presence of a second endogenous anion channel in the host RBC membrane, we have never observed its activation in infected RBCs. Furthermore, Huber and co-workers show evidence that these channels are activated by oxidation of the host membrane. It is impossible to rule out this mechanism of activation from the data presented here but it is important to note that neither Huber and co-workers (2002) nor Desai and co-workers (2000) used glucose in their solutions. This will have a major impact on the ATP levels present in the infected RBC and the consequent important physiological changes that low ATP levels produce (e.g. depletion of ATP by the removal of glucose from the medium in malaria-infected RBCs induces a novel Ca2+ permeation route; Staines et al. 1999).
In conclusion, using the patch-clamp technique, we have identified two quiescent anion channels in uninfected human RBCs (a small conductance, stretch-activated channel and an outwardly rectifying channel). The data are consistent with the hypothesis that the linear conductance anion channel is activated by the malaria parasite P. falciparum during its intraerythrocytic phase.
Acknowledgments
This work was supported by the French Ministry of Research (PAL+ and PRFMMIP programmes), the French Ministry of Defence (DGA), the WHO/UNDP/World Bank TDR programme, the Wellcome Trust (Grant No. 058230 and 066067) and the Fondation Langlois.
References
- Busse R, Ogilvie A, Pohl U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. American Journal of Physiology. 1988;254:H823–828. doi: 10.1152/ajpheart.1988.254.5.H828. [DOI] [PubMed] [Google Scholar]
- Chishti AH, Maalouf GJ, Marfatia S, Palek J, Wang W, Fisher D, Liu S. Phosphorylation of Protein 4. 1 in. Plasmodium falciparum-infected human red blood cells. Blood. 1994;83:3339–3345. [PubMed] [Google Scholar]
- Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- Doerig CD, Doerig CM, Horrocks P, Carlton J, Sultan A, Arnot D, Carter R. Pfcrk-1, a developmentally regulated cdc2-related protein kinase of Plasmodium falciparum. Molecular and Biochemical Parasitology. 1995;70:167–174. doi: 10.1016/0166-6851(95)00033-w. [DOI] [PubMed] [Google Scholar]
- Egée S, Lapaix F, Cossins AR, Thomas SLY. The role of anion and cation channels in volume regulatory responses in trout red blood cells. Bioelectrochemistry. 2000;52:133–149. doi: 10.1016/s0302-4598(00)00096-9. [DOI] [PubMed] [Google Scholar]
- Freedman JC, Novac TS, Bisognano JD, Pratap PR. Voltage dependence of DIDS-insensitive chloride conductance in human red blood cells treated with valinomycin or gramicidin. Journal of General Physiology. 1994;104:961–983. doi: 10.1085/jgp.104.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H. The permeability properties of the parasite cell membrane. Novartis Foundation Symposium. 1999;226:99–108. doi: 10.1002/9780470515730.ch8. [DOI] [PubMed] [Google Scholar]
- Huber SM, Uhlemann AC, Gamper NL, Duranton C, Kremsner PG, Lang F. Plasmodium falciparum activates endogenous Cl− channels of human erythrocytes by membrane oxidation. EMBO Journal. 2002;21:22–30. doi: 10.1093/emboj/21.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiological Reviews. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Kappes B, Doerig CD, Graeser R. An overview of Plasmodium protein kinases. Parasitology Today. 1999;15:449–454. doi: 10.1016/s0169-4758(99)01527-6. [DOI] [PubMed] [Google Scholar]
- Kirk K. Membrane transport in the malaria-infected erythrocyte. Physiological Reviews. 2001;81:495–537. doi: 10.1152/physrev.2001.81.2.495. [DOI] [PubMed] [Google Scholar]
- Kirk K, Horner HA, Elford BC, Ellory JC, Newbold CI. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. Journal of Biological Chemistry. 1994;269:3339–3347. [PubMed] [Google Scholar]
- SchwartZ RS, Rybicki A, Nagel RL. Molecular cloning and expression of a chloride channel-associated protein pl Cln in human young red blood cells: association with actin. Biochemical Journal. 1997;327:609–616. doi: 10.1042/bj3270609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, KleinhenZ ME, Lonigro AJ. Deformation-induced ATP release from red blood cell requires CFTR activity. American Journal of Physiology. 1998;275:H1726–1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Staines HM, Chang W, Ellory JC, Tiffert T, Kirk K, Lew VL. Passive Ca2+ transport and Ca2+-dependent K+ transport in Plasmodium falciparum-infected red cells. Journal of Membrane Biology. 1999;172:13–24. doi: 10.1007/s002329900579. [DOI] [PubMed] [Google Scholar]
- Syin C, Parzy D, Traincard F, Boccaccio I, Joshi MB, Lin DT, Yang XM, Assemat K, Doerig C, Langsley G. The H89 cAMP-dependent protein kinase inhibitor blocks Plasmodium falciparum development in infected erythrocytes. European Journal of Biochemistry. 2001;268:4842–4849. doi: 10.1046/j.1432-1327.2001.02403.x. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Thomas SLY, Egée S, Lapaix F, Kaestner L, Staines HM, Ellory JC. Malaria parasite Plasmodium gallinaceum up-regulates host red blood cell channels. FEBS Letters. 2001;500:45–51. doi: 10.1016/s0014-5793(01)02579-0. [DOI] [PubMed] [Google Scholar]


