Abstract
Intracellular electrical activity was recorded from smooth muscle tissues of the mouse proximal colon, and the impaled cells were visualized by injection of neurobiotin. Slow potentials with initial fast and subsequent plateau components (plateau potentials), generated at a frequency of 14.8 min−1, were recorded from oval-shaped cells with bipolar processes. Periodic bursts of spike potentials (4.6 min−1) and bursts of oscillatory potentials (4.3 min−1) were recorded in circular and longitudinal smooth muscle cells, respectively. Nifedipine (0.1 μm) abolished the bursts of spike and oscillatory potentials and reduced the duration of plateau potentials. The plateau potentials were abolished by 1 μm nifedipine. The plateau potentials were also abolished by cyclopiazonic acid (an inhibitor of Ca2+ uptake into internal stores) or 2-aminoethoxydiphenyl borate (an inhibitor of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release), and were inhibited by bis-(aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (a chelator of intracellular Ca2+). Carbonyl cyanide m-chlorophenylhydrazone (a mitochondrial protonophore) abolished plateau potentials, and its action was not mimicked by oligomycin (an inhibitor of mitochondrial ATPase). It is concluded that in mouse proximal colon, submucosal c-kit-positive bipolar cells spontaneously generate plateau potentials with rhythms different from those generated by smooth muscle cells. The plateau potentials are generated through activation of voltage-gated Ca2+ channels, which are coupled to the release of Ca2+ from the internal stores and the handling of Ca2+ in mitochondria.
Gastrointestinal tissues are spontaneously active, and the cellular basis underlying this activity is the rhythmic generation of electrical activity such as slow waves and spike potentials in smooth muscles (Tomita, 1981). The interstitial cells of Cajal (ICC), which are distributed in the myenteric layer between the circular and longitudinal muscle layers, may be the pacemaker cells of gastrointestinal activity. Impairment of the development of ICC by inhibiting the expression of c-kit receptor proteins induces gastrointestinal disorders including the disappearance of rhythmic activity of smooth muscles (Huizinga et al. 1995; Sanders, 1996; Sanders et al. 1999). Excitation of smooth muscle cells follows that of ICC (Dickens et al. 1999), suggesting that ICC are indeed driving the activity of smooth muscles in the guinea-pig stomach.
The cellular mechanisms that underlie spontaneous activity in gastrointestinal tissues remain unclear. The spontaneous activity is not abolished by several types of organic Ca2+ antagonists such as verapamil (Golenhofen & Lammel, 1972), diltiazem (Ishikawa et al. 1985) and nifedipine (Liu et al. 1995; Dickens et al. 1999). These results indicate that the influx of Ca2+ through voltage-gated Ca2+ channels may not be the main factor in the initiation of this activity. In mouse antrum, the lack of expression of inositol 1,4,5-trisphosphate (IP3) receptors inhibits the generation of slow waves (Suzuki, 2000; Suzuki et al. 2000). In cultured ICC from mouse intestine, the spontaneous activity is inhibited by carbonyl cyanide m-chlorophenylhydrazone or carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (both mitochondrial protonophores that prevent Ca2+ uptake into mitochondria), by xestospongin (an inhibitor of IP3 receptors) or by cyclopiazonic acid (an inhibitor of Ca2+-ATPase at the internal stores; Ward et al. 2000). These observations suggest that elevated Ca2+ uptake into mitochondria coupled with IP3 receptor-mediated Ca2+ release from internal stores is involved in the initial process of spontaneous activity. It has also been suggested that IP3 receptor-mediated Ca2+ release from internal stores is involved in the generation of spontaneous activity in the murine small intestine (Malysz et al. 2001) and the guinea-pig stomach (Hirst & Edwards, 2001).
In some laboratory animals, antiperistaltic activity is generated in a special region of the proximal colon, termed the ‘pacemaker area’ (Hukuhara & Neya, 1968). A similar area in the guinea-pig colon, the pacemaker nodule, produces rhythmic contractions at a frequency of 10–12 cycles min−1 (Kobayashi et al. 1996). In addition to the myenteric ICC, this area contains bipolar or multipolar cells that express c-kit proteins in the submucosal layer (Nahar et al. 1998), suggesting the possibility that the antiperistaltic activities present in the proximal colon are generated by these submucosal c-kit-positive cells.
In the present study, the properties of spontaneous electrical activities recorded from tissues isolated from mouse proximal colon were investigated. We recorded three types of electrical activities from the tissue and localization of the recorded cells with neurobiotin injection revealed that submucosal bipolar cells were spontaneously active with rhythms which differed from those of circular and longitudinal smooth muscle cells. Immunohistochemical observations revealed that neurobiotin-filled cells and c-kit-positive cells have a similar morphology. We found that these submucosal cells generate plateau-type potentials by activation of L-type Ca2+ channels. A part of these experimental data was reported briefly in the 43rd Annual Meeting of The Japanese Smooth Muscle Society (Yoneda et al. 2001).
Methods
Male mice (BALB/C strain), weighing 20–25 g, were anaesthetized with fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether (sevoflurane; Maruishi Pharmaceutical, Osaka, Japan), and then decapitated. The animals were treated humanely according to the guidelines for the Care and Use of Animals approved by the Physiological Society of Japan. The proximal colon was isolated, opened along the mesenteric border and the mucosal layer was removed. Tissues both with, and without, the submucosal layer were prepared. In the latter tissues, the submucosal layer was peeled off completely using fine forceps.
Small rectangular pieces of tissue (2 mm × 3 mm) were isolated and mounted on a silicone rubber plate that was fixed to the bottom of a recording chamber, either with the submucosal layer side uppermost or the longitudinal muscle side uppermost, and immobilized using tiny pins (diameter, 0.1 mm). The recording chamber (8.0 mm wide × 20.0 mm long × 5.0 mm deep, with a capacity of about 1.0 ml) was made from Lucite plate, and the tissue segment was superfused with warmed (35 °C) Krebs solution at a constant flow rate of about 3 ml min−1. Conventional microelectrode techniques were used to record electrical responses from single cells from each tissue. Glass capillary microelectrodes (borosilicate glass tube, 1.2 mm o.d.) filled with 3 m KCl had tip resistances ranging between 50 and 80 MΩ. The intracellular potentials thus recorded were displayed on a cathode ray oscilloscope (SS-7602, Iwatsu, Tokyo, Japan). The data were also saved onto a personal computer (Dell Computer, Kawasaki, Japan) through an A/D converter (Axon Instruments, CA, USA) at 500 Hz, filtered at 100 Hz, and analysed using Axoscope 7 (Axon Instruments).
The impaled cells were visualized by injection of neurobiotin, using the methods reported by Klemm et al. (1999). Briefly, the recording electrode was filled to the tip with a solution of neurobiotin (Vector Laboratories, Burlingame, CA, USA) dissolved in 3 m KCl solution at a concentration of 4 %. After electrical activity had been recorded, a train of depolarizing current pulses (0.1 nA, 1 s duration, 0.2 Hz frequency for 3 min) was applied to the electrode to inject neurobiotin into the cell. Tissues containing neurobiotin-filled cells were pinned on a waxed plate and fixed overnight at 4 °C with fresh paraformaldehyde (4 % w/v) dissolved in 0.1 % phosphate buffer, pH 7.2. The tissues were washed three times each for 10 min with 0.1 % phosphate buffered saline (PBS) containing 0.3 % Triton X-100 (PBS/Triton) and then incubated with PBS/Triton containing streptavidin-CY3 (Jackson Immunoresearch, PA, USA; dilution, 1:500) for 24 h at 4 °C.
Interstitial cells were visualized by staining c-kit proteins with anti-c-kit antibody (ACK-2), according to the method reported by Torihashi et al. (1997). Briefly, whole-mount preparations of colonic segments with the submucosal layer attached were fixed with acetone for 10 min at 4 °C. Sections were incubated with ACK-2 (a rat monoclonal antibody raised against the c-kit protein, diluted to 5 mg ml−1; Gibco BRL, Gaithsberg, MD, USA) overnight at 4 °C to stain c-kit proteins. The immunoreactivity was detected by an Alexa-conjugated IgG (1:400). The preparations were washed three times with PBS, mounted on glass slides with Dako mounting medium (Dako, Carpinteria, CA, USA), coverslipped and then examined with the aid of a confocal microscope (Biorad MRC-1000; BioRad, CA, USA). The confocal microscope, using a krypton/argon laser, allowed the visualization of CY3 (568 nm excitation filter and 605–632 nm emission filter) and Alexa (488 nm excitation filter).
The ionic composition of the Krebs solution was as follows (mm): Na+ 137.4, K+ 5.9, Mg2+ 1.2, Ca2+ 2.5, HCO3− 15.5, H2PO4− 1.2, Cl− 134, glucose 11.5. The solution was aerated with O2 containing 5 % CO2, and the pH of the solution was 7.1-7.2. Solutions containing high concentrations of K+ were prepared by replacing NaCl with KCl.
Drugs used were as follows: atropine sulfate, bis-(aminophenoxy) ethane-N,N,N‘,N‘-tetraacetic acid acetoxymethyl ester (BAPTA AM), cyclopiazonic acid (CPA), carbonyl cyanide m-chlorophenylhydrazone (CCCP), 18β-glycyrrhetinic acid (18β-GA), nifedipine, oligomycin, glibenclamide (glybenclamide) and tetrodotoxin (TTX) were purchased from Sigma (MO, USA), and 2-aminoethoxydiphenyl borate (2-APB) was purchased from Calbiochem (CA, USA). 2-APB, BAPTA AM, 18β-GA, CPA, CCCP, oligomycin, glibenclamide and nifedipine were dissolved in dimethyl sulfoxide at concentrations of 5–10 mm. Other chemicals were dissolved in distilled water as a stock solution, and diluted further with Krebs solution to the desired concentration (the ratios of the dilution were over 1:1000). The dilution procedures did not alter the pH of the Krebs solution.
Values measured are expressed as the mean ± s.d., with the n value representing the number of preparations taken from different animals. Differences between values were tested using Student's paired t test, and probabilities less than 5 % (P < 0.05) were considered significant.
Results
Electrical activities recorded from smooth muscle tissues of the proximal colon
Intracellular recordings were made from different types of smooth muscle tissues prepared from the proximal colon of mice. In the uppermost tissues of the submucosal layer, with circular and longitudinal smooth muscles attached, square-shaped potential changes consisting of initial fast rising and subsequent plateau components (plateau potentials) were recorded (Fig. 1A). The plateau potentials were generated at frequencies ranging between 10 and 22 min−1 (mean, 14.8 ± 3.4 min−1, n = 83), with peak amplitudes ranging between 10 and 40 mV (mean, 19.6 ± 6.9 mV, n = 83). The rates of rise of the initial potentials and the duration of plateau potentials measured at the foot ranged between 68 and 331 mV s−1 (mean, 148 ± 61 mV s−1, n = 32) and between 2.1 and 3.1 s (mean, 2.6 ± 0.3 s, n = 32), respectively. The resting membrane potential of cells generating plateau potentials ranged between −41 and −58 mV (mean, −48.2 ± 5.9 mV, n = 83). TTX (1 μm) did not alter the resting membrane potential (control, −48.4 ± 4.1 mV; in TTX, −48.6 ± 2.6 mV; n = 6; P > 0.05), the amplitude (control, 17.2 ± 3.5 mV; in TTX, 17.6 ± 3.9 mV; n = 6; P > 0.05) or the frequency (control, 13.6 ± 2.0 min−1; in TTX, 13.8 ± 3.0 min−1; n = 6; P > 0.05) of plateau potentials. Atropine (1 μm) again did not alter the resting membrane potential (control, −49.8 ± 0.4 mV; in atropine, −49.3 ± 0.4 mV; n = 3; P > 0.05), the amplitude (control, 14.8 ± 4.4 mV; in atropine, 15.9 ± 5.4 mV; n = 3; P > 0.05) or the frequency (control, 14.9 ± 0.6 min−1; in atropine, 15.0 ± 0.4 min−1; n = 3; P > 0.05) of plateau potentials.
Figure 1. Spontaneous activity recorded from mouse proximal colon.
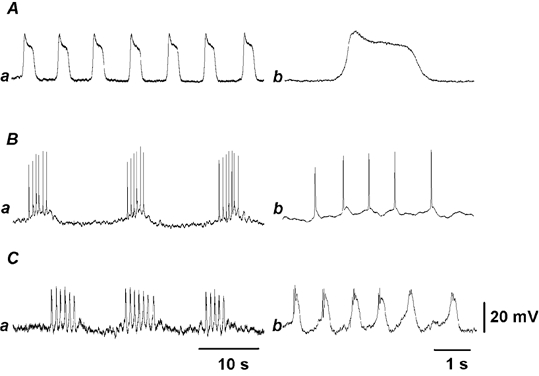
Representative electrical activity recorded from submucosal interstitial cells (A), circular (B) and longitudinal smooth muscle cells (C). Responses recorded from tissue with mucosal layer uppermost and intact submucosal layer attached (A), tissue with circular smooth muscle layer uppermost and no submucosal layer (submucosal side uppermost, B), and tissue with longitudinal muscle uppermost, without the submucosal layer (C) are shown on different time scales: a, slow speed; b, fast speed. The resting membrane potentials in A, B and C were −50, −45 and −50 mV, respectively. Each pair of responses was recorded from the same cell.
In tissues with the circular muscle layer exposed uppermost after removal of the submucosal layer, the rhythmic generation of bursts of spike potentials was recorded (Fig. 1B). These cells had resting membrane potentials ranging between −40 and −55 mV (mean, −44.9 ± 5.5 mV, n = 10). Bursts of spike potentials were generated with frequencies ranging between 2 and 6 min−1 (mean, 4.6 ± 1.1 min−1, n = 10). The duration of individual spike potentials measured at the middle amplitude ranged between 56 and 106 ms (mean, 79 ± 19 ms, n = 10). The number of spike potentials generated in each burst was 5.5 ± 1.1 (n = 10). TTX (1 μm) did not alter the resting membrane potential (control, −52.4 ± 1.8 mV; in TTX, −53.6 ± 1.7 mV; n = 3; P > 0.05), the number of spike potentials generated in each burst (control, 4.3 ± 0.6 spikes; in TTX, 4.4 ± 0.4 spikes; n = 3; P > 0.05), but increased the frequency of spike potentials within bursts (control, 3.5 ± 1.2 min−1; in TTX, 8.3 ± 1.1 min−1; n = 3; P < 0.05). Atropine (1 μm) did not alter the resting membrane potential (control, −45.5 ± 3.2 mV; in atropine, −45.3 ± 3.2 mV; n = 3; P > 0.05), the frequency of bursts (control, 4.5 ± 0.8 min−1; in atropine, 4.6 ± 0.8 min−1; n = 3; P > 0.05) or the number of spike potentials generated in each burst (control, 3.9 ± 0.6 spikes; in atropine, 3.9 ± 0.4 spikes; n = 3; P > 0.05).
In tissues with the longitudinal muscle layer uppermost and with no attached submucosal layers, periodic bursts of oscillatory potentials were recorded (Fig. 1C). Although the duration of individual oscillatory potentials varied between 0.62 and 1.14 s (mean, 0.83 ± 0.16 s, n = 7), all oscillatory potentials recorded were similar in nature. The form of oscillatory potentials differed from plateau potentials and spike potentials. The duration of oscillatory potentials was much longer than the spike potentials and much shorter than the plateau potentials (fast traces in Fig. 1A–C). The generation of several spike-like potentials in the initial phase was also one of the characteristics of the oscillatory potentials (Fig. 1Cb). In seven cells that exhibited these activities, the resting membrane potential ranged between −40 and −57 mV (mean, −48.1 ± 7.2 mV). Bursts of oscillatory potentials were generated at frequencies ranging between 2 and 6 min−1 (mean, 4.3 ± 1.3 min−1, n = 7). The number of oscillatory potentials in each burst was 6.0 ± 0.9 potentials (n = 7), with a mean amplitude of 17.8 ± 3.3 mV (n = 7). TTX (1 μm) did not alter the resting membrane potential (control, −50.2 ± 4.6 mV; in TTX, −50.3 ± 6.3 mV; n = 3; P > 0.05) or the number of oscillatory potentials generated in each burst (control, 4.5 ± 0.4 spikes; in TTX, 4.3 ± 0.3 spikes; n = 3; P > 0.05), but increased the frequency of spike potentials in each burst (control, 3.9 ± 1.2 min−1; in TTX, 6.6 ± 1.3 min−1; n = 3; P < 0.05). Atropine (1 μm) did not alter the resting membrane potential (control, −44.7 ± 3.3 mV; in atropine, −44.5 ± 2.6 mV; n = 4; P > 0.05), the frequency of bursts of spike potentials (control, 4.45 ± 0.6 min−1; in atropine, 4.48 ± 0.7 min−1; n = 4; P > 0.05) or the number of spike potentials generated in each burst (control, 3.7 ± 0.6 spikes; in atropine, 3.7 ± 0.7 spikes; n = 4; P > 0.05).
In order to determine whether the spontaneously active cells in the submucosal layer drive electrical activities in circular smooth muscle cells, recordings were made from tissues with intact submucosal and circular muscle layers but without the longitudinal muscle layers. The circular smooth muscle cells of such tissues again generated bursts of spikes periodically with frequencies similar to those seen in tissues with no attached submucosal layers (4.3 ± 1.0 min−1; n = 7; P > 0.05, data not shown). Similarly, bursts of oscillatory potentials were generated periodically in longitudinal muscle cells of the tissues with attached submucosal layers (3.7 ± 0.8 min−1; n = 6; P > 0.05, data not shown). Thus, the periodic generation of bursts of spike potentials or oscillatory potentials in smooth muscles was not causally related to the submucosal layers. As none of these electrical activities were blocked by TTX or atropine, the activities were not considered to be triggered by excitation of cholinergic nerves.
Morphology of spontaneously active cells in the proximal colon
In the uppermost tissues of the submucosal layer with circular and longitudinal muscle layers attached, neurobiotin was injected into three cells after generation of plateau potentials had been confirmed. Examination with a confocal microscope revealed that cells with low-intensity fluorescence were distributed widely, with no clearly stained site. This was considered to be due to the diffusion of neurobiotin to surrounding cells through gap junctions, as reported to occur in guinea-pig stomach muscles (Dickens et al. 1999). Attempts were made to inject neurobiotin in the presence of 18β-GA (20 μm), a known inhibitor of gap junctions in smooth muscle tissues (Yamamoto et al. 1998). In six tissues examined, application of 18β-GA depolarized the membrane (control, −47.8 ± 6.5 mV; in 18β-GA, −40.8 ± 5.4 mV, P < 0.01), reduced the amplitude (control, 21.1 ± 7.8 mV; in 18β-GA, 6.2 ± 5.4 mV, P < 0.01) and increased the frequency of plateau potentials (control, 14.9 ± 2.9 min−1; in 18β-GA, 18.1 ± 1.7 min−1, P < 0.05). In all tissues, cells in the submucosal layer were stained. In five out of six tissues, the stained cells had oval-shaped cell bodies, with the longer diameter ranging between 20 and 30 μm and the thickness ranging between 5 and 8 μm (Fig. 2Aa). Each cell had bipolar processes of 50–150 μm, with the periphery of the process often being bifurcated. These cells ran parallel to the circular muscle bundles. In one out of six tissues, the depolarization elicited by 18β-GA was apparently weak (about 2 mV), and a group of cells was stained with different intensities, all distributed in the submucosal layer, and running along the direction of circular muscle bundles (Fig. 2Ab). Immunohistochemical examination revealed the existence of a dense network of c-kit-positive interstitial cells in the submucosal layer at the boundary of the circular muscle layer (Fig. 2B), as had been reported by Vanderwinden et al. (2000). The c-kit-positive cells were distributed in the same layer as the neurobiotin-filled cells, and were of a similar shape and size (Fig. 2Ba,b). Thus, the plateau potentials could have been recorded from the c-kit-positive cells.
Figure 2. Morphology of spontaneously active cells in the submucosal layer.
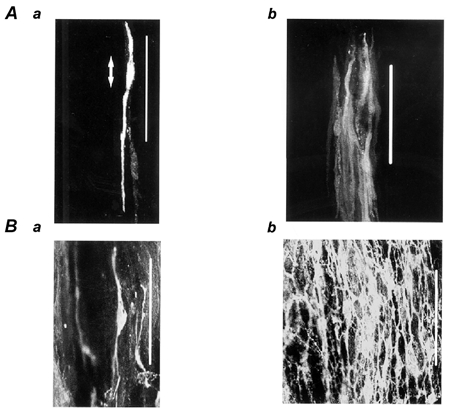
A, neurobiotin-filled cells distributed in the submucosal layer (a and b are from different pieces of tissue). Membrane potentials were recorded from tissues of the mouse proximal colon, in the presence of 20 μm 18β-glycyrrhetinic acid for 15 min, and then neurobiotin was injected into the recorded cell by applying a depolarizing current for 5 min. B, c-kit protein-positive cells distributed in the submucosal layer (a and b are from different regions in the same preparation). The preparation was viewed with the aid of a confocal microscope (magnification × 630). Circular muscle bundles run in a vertical direction. Calibration bar / 100 μm. In Aa, the two-headed arrow indicates the length of the cell body.
In the uppermost tissues of the circular muscle layer with no submucosal layers attached, injected neurobiotin was again not restricted to the impaled cells. Therefore, neurobiotin was injected in the presence of 20 μm 18β-GA. 18β-GA depolarized the membrane (7.5 ± 2.0 mV, n = 10) and abolished spontaneous bursts of spike potentials. In six tissues where cells generating bursts of spike potentials were successfully injected with neurobiotin, all stained cells were found to be distributed in the circular muscle bundle, having a length of 300–500 μm and diameter at the widest part of about 10 μm (Fig. 3A). In the uppermost tissues of the longitudinal muscle layer with attached submucosal layers, four cells were successfully injected with neurobiotin after bursts of oscillatory potentials had been recorded from them. The stained cells were all distributed in the longitudinal muscle bundle, displaying a length of 100–200 μm and a thickness of 10–20 μm at the widest point (Fig. 3B). These results suggest that the observed plateau potentials were most likely to have originated in submucosal c-kit-positive cells, whereas the spike potentials and oscillatory potentials were generated in the circular and longitudinal smooth muscle cells, respectively.
Figure 3. Morphology of spontaneously active cells in the circular and longitudinal muscle layers.
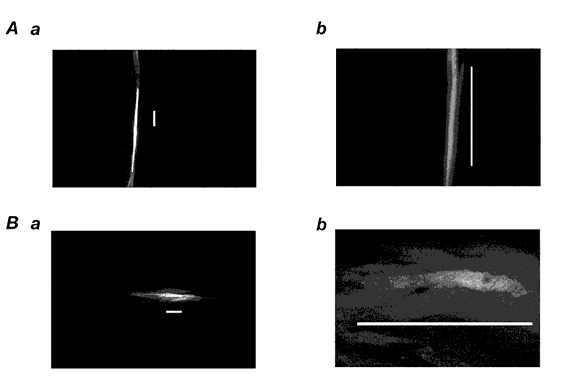
Membrane potentials were recorded from smooth muscle tissues of mouse proximal colon in the presence of 20 μm 18β-glycyrrhetinic acid for 15 min. Neurobiotin was then injected into the recorded cell by applying a depolarizing current for 5 min. Panels show neurobiotin-injected cells located in the circular smooth muscle bundles (A), and longitudinal smooth muscle bundles (B) of mouse proximal colon. Circular muscle bundles run in the vertical direction. Calibration bar / 100 μm. The preparations were viewed with the aid of a confocal microscope at different magnifications (Aa and Ba, × 200; Ab and Bb, × 630).
Effects of nifedipine on spontaneous activity
Experiments were carried out to test the effects of nifedipine, an inhibitor of the voltage-gated L-type Ca+ channels (Nelson et al. 1990), on spontaneously generated potentials in colonic tissues. Application of 0.01 μm nifedipine did not have any effect on these electrical activities (n = 3, data not shown). Increasing the concentration of nifedipine to 0.1 μm caused the disappearance of spike potentials (Fig. 4Ca and b), with no alteration of the resting membrane potential (control, −50.7 ± 8.0 mV; in nifedipine, −49.6 ± 8.3 mV; n = 11; P > 0.05). The oscillatory potentials recorded from longitudinal muscles were also abolished by 0.1 μm nifedipine (Fig. 4Cc and d), again with no alteration of the resting membrane potential (control, −48.2 ± 2.5 mV; in nifedipine, −48.0 ± 2.1 mV; n = 8; P > 0.05). Plateau potentials, however, were generated in the presence of 0.1 μm nifedipine, with frequencies similar to those recorded in the absence of nifedipine (control, 15.8 ± 5.4 min−1; in nifedipine, 15.3 ± 4.3 min−1; n = 10; P > 0.05), but with a significant reduction in the duration of plateau component (control, 2.6 ± 0.3 s; in nifedipine, 2.0 ± 0.3 3 s; n = 10; P < 0.05; Fig. 4A). Recordings shown on an expanded time scale indicated that the amplitude (control, 19.9 ± 6.6 mV; in nifedipine, 19.7 ± 6.0 mV; n = 10; P > 0.05) and the rate of rise of the initial component of plateau potentials (control, 155 ± 65 mV s−1; in nifedipine, 148 ± 69 mV s−1, n = 10; P > 0.05) were not altered by 0.1 μm nifedipine (Fig. 4B). Thus, the inhibitory actions of 0.1 μm nifedipine on plateau potentials appeared mainly as a shortening of the duration of the plateau component. Plateau potentials were abolished when the tissues were exposed to 1 μm nifedipine (Fig. 4Ac). The resting membrane potential was reduced slightly in the presence of 1 μm nifedipine (control, −49.3 ± 3.2 mV; in nifedipine, −44.0 ± 2.2 mV; n = 4; P < 0.05). These observations indicate that spike potentials, the oscillatory potentials and the plateau potentials are all dependent upon activation of voltage-gated L-type Ca2+ channels.
Figure 4. Effect of nifedipine on plateau potentials, spike potentials and oscillatory potentials.
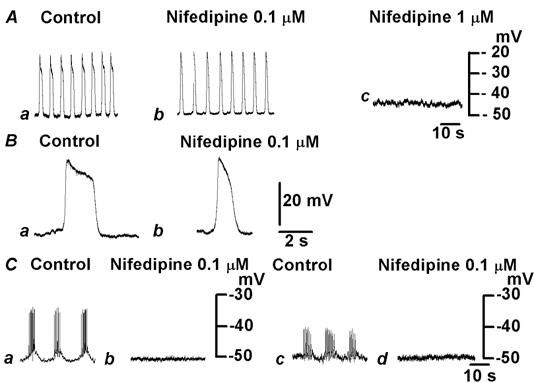
A, plateau potentials were recorded in the absence (Aa and Ba) and presence of nifedipine (Ab and Bb, 0.1 μm; Ac, 1 μm) from the uppermost tissue of the submucosal layer. Records in B are shown on an expanded time scale. C, bursts of spike potentials (a and b) or oscillatory potentials (c and d) were recorded from tissues in which the circular muscle or longitudinal muscle was uppermost, respectively, in the absence (a and c) and presence of nifedipine (b and d; 0.1 μm). Responses in A and B, and Cab and Ccd were obtained from different pieces of tissue. The resting membrane potentials were: A and B, −50 mV; Cab, −50 mV; Ccd, −51 mV.
Role of internal Ca2+ stores in the generation of plateau potentials
Experiments were carried out to investigate the cellular mechanisms underlying the generation of plateau potentials in the proximal colon using chemicals known to modulate intracellular Ca2+ handling, including the function of internal Ca2+ stores. Chemicals tested included CPA, an inhibitor of Ca-ATPase at the internal Ca2+ stores (Uyama et al. 1992), BAPTA AM, an intracellular Ca2+ chelator (Michelangeli et al. 1989), and 2-APB, an inhibitor of IP3-receptor-mediated release of Ca2+ from internal stores (Maruyama et al. 1997; Cui & Kanno, 1997).
Plateau potentials were abolished by exposing tissues for over 10 min to a solution containing 1 μm CPA, which depolarized the membrane by about 15 mV (control, −51.2 ± 6.0 mV, in CPA, −36.1 ± 5.6 mV; n = 6; P < 0.05; Fig. 5A). The effects of CPA were reversible, and washing for 20 min was required for recovery (data not shown). The effects of increasing extracellular K+ ([K+]o) concentrations by a factor of 4.2 ([K+]o = 24.7 mm) on plateau potentials were tested to determine whether the depolarization was causally related to the inhibition of these potentials. The high [K+]o solution depolarized the membrane by about 25 mV (control, −51.5 ± 3.5 mV; in high [K+]o solution, −35.1 ± 7.3 mV, n = 4; P < 0.05), but did not abolish plateau potentials (data not shown). Thus, the inhibition of plateau potentials by CPA was not due to depolarization of the membrane. In the presence of 20 μm BAPTA AM for 15 min, the amplitude of plateau potentials was reduced to less than half of control levels (control, 18.7 ± 5.7 mV; in BAPTA AM, 8.3 ± 5.9 mV; n = 6; P < 0.05), with significant reduction of the frequency (control, 12.7 ± 1.6 min−1; in BAPTA AM, 3.3 ± 3.6 min−1; n = 6; P < 0.01), but with no alteration of the membrane potential (control, −49.5 ± 6.0 mV; in BAPTA, −49.4 ± 7.4 mV; n = 8; P > 0.05; Fig. 5B). The inhibitory effects of BAPTA AM persisted for up to 2 h in washing solution (n = 5). 2-APB (10 μm) abolished plateau potentials, with no alteration of the membrane potential (control, −52.4 ± 4.6 mV; 2-APB, −53.0 ± 5.4 mV; n = 6; P > 0.05; Fig. 5C). The effects of 2-APB on plateau potentials were reversible, and washing for 20 min was required for the recovery (data not shown).
Figure 5. Modulation of internal Ca2+ stores and plateau potentials.
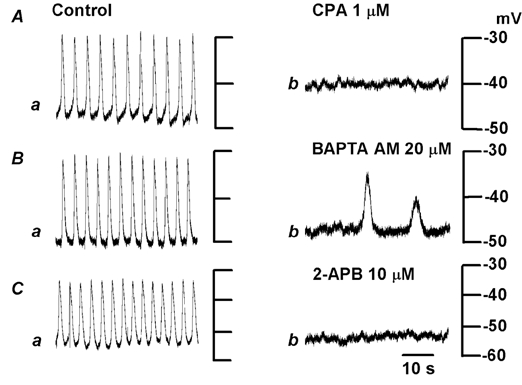
Plateau potentials were recorded from tissues in which the submucosal layer was uppermost, in the control solution (a) and after exposure to solution containing 1 μm cyclopiazonic acid (CPA) for 10 min (Ab), 20 μm bis-(aminophenoxy) ethane-N,N,N‘,N‘-tetraacetic acid acetoxymethyl ester (BAPTA AM) for 15 min (Bb) or 10 μm 2-aminoethoxydiphenyl borate (2-APB) for 10 min (Cb). Each pair of responses in A-C was recorded from single cells in different pieces of tissue.
Role of mitochondrial functions in the plateau potential
A mitochondrial protonophore, CCCP (1 μm; Duchen, 1999), hyperpolarized the membrane (control, −46.9 ± 7.3 mV; in CCCP, −56.2 ± 7.3 mV; n = 6; P < 0.05) and reduced the frequency of plateau potentials, and within 8 min they were abolished (Fig. 6A). Removal of CCCP from the superfusate allowed a slow recovery of activity, and complete recovery took about 30 min (data not shown). Glibenclamide (10 μm), an inhibitor of ATP-sensitive K+ channels (Cook, 1988), did not alter the resting membrane potential (control, −46.2 ± 2.5 mV; in glibenclamide, −45.5 ± 3.1 mV; n = 8; P > 0.05), the amplitude (control, 19.0 ± 6.3 mV; in glibenclamide, 20.8 ± 3.6 mV; n = 8; P > 0.05) or the frequency (control, 15.0 ± 3.3 min−1; in glibenclamide, 14.0 ± 4.4 min−1; n = 8; P > 0.05) of plateau potentials (Fig. 6Bb). However in the presence of glibenclamide, CCCP depolarized the membrane (glibenclamide alone, −46.1 ± 2.9 mV; CCCP in glibenclamide, −36.3 ± 3.2 mV; n = 4; P < 0.01) and abolished plateau potentials (Fig. 6Bc). These results suggest that the CCCP-induced hyperpolarization is produced by an activation of ATP-sensitive K+ channels, and that the inhibition of plateau potentials by CCCP is not causally related to the hyperpolarization of the membrane.
Figure 6. Effects of CCCP on plateau potentials.
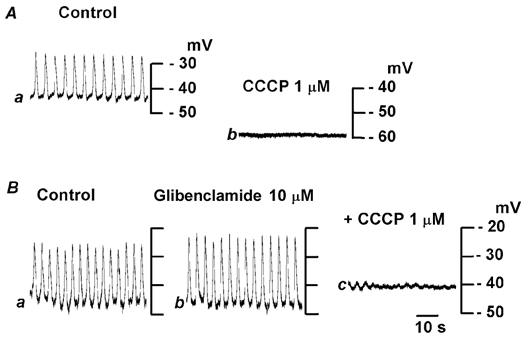
In the uppermost layer of the submucosal layer, plateau potentials were recorded in control solution (Aa) and after 10 min exposure to a solution containing 1 μm carbonyl cyanide m-chlorophenylhydrazone (CCCP; Ab). In different pieces of tissue, plateau potentials were recorded in control solution (Ba) and after 10 min exposure to solution containing 10 μm glibenclamide (Bb) and 10 μm glibenclamide with 1 μm CCCP for 10 min (Bc). All responses in A and B were obtained from different single cells.
Possible involvement of the inhibition of ATP production in the effects of CCCP was tested by applying oligomycin, an inhibitor of mitochondrial ATPase (Nagamune et al. 1993). Oligomycin (10 μm) did not alter the resting membrane potential (control, −48.4 ± 3.3 mV; in oligomycin, −48.8 ± 1.7 mV; n = 4; P > 0.05), the amplitude (control, 15.3 ± 6.3 mV; in oligomycin, 14.8 ± 6.5 mV; n = 4; P > 0.05) or the frequency (control, 12.3 ± 3.3 min−1; in oligomycin, 12.4 ± 4.4 min−1; n = 8; P > 0.05) of plateau potentials. These results indicate that the abolition of slow potentials by CCCP was not causally related to the reduced production of ATP.
Discussion
In smooth muscle tissues of the mouse proximal colon, we found three types of spontaneously active cells, plateau potentials and a burst of spike potentials or oscillatory potentials. Visualization of the recorded cells following neurobiotin injection indicated that plateau potentials, spike potentials and oscillatory potentials were recorded from cells distributed in the submucosal layers, circular muscles and longitudinal muscles, respectively. Immunohistochemical examination indicated that the submucosal layer contained c-kit-positive interstitial cells, and the form of individual cells and their networks were similar to those of the neurobiotin-filled cells, suggesting that the plateau potentials were recorded from c-kit-positive interstitial cells distributed in the submucosal layers.
Similar c-kit-positive cells were noted in the submucosal layers of the colon in mouse (Vanderwinden et al. 2000), guinea-pig (Kobayashi et al. 1996; Nahar et al. 1998) and dog (Kobayashi et al. 1995), and they are considered to be the pacemaker cells of colonic activity. The plateau potentials recorded from the submucosal cells, however, differed from the spontaneous activity recorded from circular and longitudinal muscles, in both form and frequency. The frequency of plateau potentials was much higher than that of the burst excitation generated in circular and longitudinal smooth muscle cells, although the frequency of the latter two was similar. Moreover, the spontaneous electrical activity of circular and longitudinal muscles remained unaltered, irrespective of the presence or absence of attached submucosal layers. Thus, the activities of smooth muscle cells do not seem to be causally related to the plateau potentials generated in submucosal cells, which differs from the generation mechanism of slow waves in the stomach and small intestine (Sanders et al. 1999; Suzuki, 2000).
In the rat colon there are two distinct pacemakers, and slow waves derived from submucosal interstitial cells (IC-SM) induce contractions of circular smooth muscle cells, while the cyclic oscillatory potentials derived from the myenteric interstitial cells (IC-MY) trigger contractions of both circular and longitudinal smooth muscle cells (Plujà et al. 2001). In the canine colon, IC-SM and smooth muscle cells form gap-junctional networks (Smith et al. 1987; Berezin et al. 1988; Sanders & Smith, 1989; Kobayashi et al. 1995), and smooth muscle cells are also excited by signals from IC-SM, in addition to those from IC-MY (Keef et al. 1997). The present experiments show that neurobiotin-injected cells are only visualized successfully in the presence of 18β-GA, an inhibitor of gap-junctional communication (Yamamoto et al. 1998), suggesting that spontaneously active cells form gap-junctional networks with surrounding cells. Nevertheless, the frequencies of spontaneous electrical activity differed between submucosal cells and smooth muscle cells. No clear explanation is available, and it is speculated that the mouse proximal colon may have systems which differ from other parts of the colon, stomach or small intestine, in which IC-MY are driving smooth muscle activities (Sanders, 1996; Huizinga et al. 1997; Sanders et al. 1999). The proximal colon differs from other parts of the gastrointestinal tract, and ‘antiperistaltic’ movement appears first in this region (Hukuhara & Neya, 1968). The present experiments provide no insight into how this antiperistaltic movement is controlled, and further study is required. The ionic mechanisms giving rise to electrical activity in gastrointestinal smooth muscle tissues remain unclear. In general, spike potentials appearing in smooth muscles are inhibited by organic Ca2+ antagonists, suggesting that the potentials are produced by activation of voltage-gated L-type Ca2+ channels (Tomita, 1981). In the antrum smooth muscle of the dog, the plateau components, but not the initial fast components, of the slow action potentials are inhibited by organic Ca2+ antagonists (El-Sharkawy et al. 1978; Fujii et al. 1985), suggesting an involvement of voltage-gated L-type Ca2+ channels for generation of the plateau component potential. Slow waves generated in the guinea-pig stomach are not inhibited by organic Ca2+ antagonists, suggesting that the potentials are not produced by activation of voltage-gated L-type Ca2+ channels (Golenhofen & Lammel, 1972; Ishikawa et al. 1985; Dickens et al. 1999). The present experiments demonstrate that the plateau potentials recorded from submucosal layers are abolished by nifedipine in a manner similar to the spontaneous activity recorded from circular and longitudinal muscles. Thus, the voltage-gated L-type Ca2+ channels play a major role in the generation of plateau potentials in the mouse proximal colon. The threshold concentration of nifedipine required to inhibit plateau potentials was about 10 times higher than that required to inhibit the spike or oscillatory potentials generated in smooth muscles, suggesting that the pharmacological identities of these nifedipine-sensitive channels are different, although they might be categorized as voltage-gated Ca2+ channels.
In the canine colon, reducing the uptake of Ca2+ into internal IP3-sensitive stores by CPA or chelating intracellular Ca2+ by BAPTA AM inhibits pacemaker activity, suggesting that the Ca2+ refilling cycle determines the frequency of pacemaker activity (Liu et al. 1995). Inhibition of slow waves by 2-APB in the guinea-pig stomach (Hirst & Edwards, 2001) and the absence of slow waves in the gastric smooth muscles of mice lacking expression of the IP3 receptor (Suzuki et al. 2000) suggest that IP3 is involved in the process of generation of spontaneous activity. In cultured ICC of mouse jejunum, spontaneous activity is coupled to the elevation of Ca2+ concentration in mitochondria and is inhibited by preventing the release of Ca2+ from IP3-sensitive internal stores, suggesting that it is the intracellular Ca2+ handling between an internal Ca2+ store and the mitochondria that produces the rhythmic activity (Ward et al. 2000). The present experiments indicate that the plateau potentials are inhibited or abolished by CPA or 2-APB, and all these results agree with the cellular mechanisms proposed in the cultured myenteric ICC of the murine jejunum (Ward et al. 2000).
The importance of mitochondrial Ca2+ function in the generation of pacemaker potentials is also suggested in submucosal cells of the mouse proximal colon. In these cells, CCCP, a mitochondrial protonophore (Duchen, 1999), abolished slow potentials and hyperpolarized the membrane. The inhibition of CCCP-induced hyperpolarization by glibenclamide appeared to be causally related to the activation of ATP-sensitive K+ channels due to reduced production of ATP. However, oligomycin did not modulate the plateau potentials, suggesting that the actions of CCCP are not due simply to the reduction of ATP production. As the plateau potentials are abolished by CCCP in the absence of hyperpolarization by glibenclamide blockade, the hyperpolarization itself may not be an essential factor in the inhibition of plateau potentials.
It is concluded that in the mouse proximal colon, submucosal oval-shaped cells, which correspond to c-kit-positive interstitial cells, are spontaneously active and generate plateau potentials. The plateau potentials are produced by activation of voltage-gated L-type Ca2+ channels, and initiation of the potentials may be coupled with IP3-mediated Ca2+ release from an internal store and Ca2+ uptake into the mitochondria. The generation of plateau potentials is not synchronous with the rhythmic activity of smooth muscles, which generate bursts of spikes or oscillatory potentials with slower frequencies than plateau potentials.
Acknowledgments
The authors are grateful to Dr Frank R. Edwards of the University of Melbourne, for critical reading of the manuscript. Dr S. Torihashi, The Nagoya University, facilitated the immunohistochemical examination of tissues with ACK-2. Part of the present experiments was supported by Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports Science and Technology (MEXT) of Japan to M. T. (no. 14370189).
References
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: special communication network at the inner border of the circular muscle. Journal of Comparative Neurology. 1988;273:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Cook NS. The pharmacology of potassium channels and their therapeutic potential. Trends in Pharmacological Sciences. 1988;9:21–27. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Cui ZJ, Kanno T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. Journal of Physiology. 1997;290:11–20. doi: 10.1111/j.1469-7793.1997.047bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. Journal of Physiology. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Contribution of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. Journal of Physiology. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy TY, Morgan KG, Szurszewski JH. Intracellular electrical activity of canine and human gastric smooth muscle. Journal of Physiology. 1978;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Inoue R, Yamanaka K, Yoshitomi T. Effects of calcium antagonists on smooth muscle membrane of the canine stomach. General Pharmacology. 1985;16:217–221. doi: 10.1016/0306-3623(85)90072-2. [DOI] [PubMed] [Google Scholar]
- Golenhofen K, Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (verapamil) Pflügers Archiv. 1972;331:233–243. [PubMed] [Google Scholar]
- Hirst GDS, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. Journal of Physiology. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, MalysZ J, Mikkelesen H, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Vanderwinden J-M, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends in Pharmacological Sciences. 1997;18:393–402. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- Hukuhara T, Neya T. The movements of the colon of rats and guinea pigs. Japanese Journal of Physiology. 1968;18:551–562. doi: 10.2170/jjphysiol.18.551. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Komori K, Nagao T, Suzuki H. Effects of diltiazem on electrical responses evoked spontaneously or by electrical stimulation in the antrum smooth muscle cells of the guinea-pig stomach. British Journal of Pharmacology. 1985;86:789–797. doi: 10.1111/j.1476-5381.1985.tb11100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef KD, Murray DC, Sanders KM, Smith TK. Basal release of nitric oxide induces an oscillatory motor pattern in canine colon. Journal of Physiology. 1997;499:773–786. doi: 10.1113/jphysiol.1997.sp021968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm MF, Exintaris B, Lang RJ. Identification of the cells underlying pacemaker activity in the guinea-pig upper urinary tract. Journal of Physiology. 1999;519:867–884. doi: 10.1111/j.1469-7793.1999.0867n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Chowdhury JU, Tokuno H, Nahar S, Iino S. A smooth muscle nodule producing 10–12 cycles min−1 regular contractions at the mesenteric border of the pacemaker area in the guinea-pig colon. Archives of Histology and Cytology. 1996;59:159–168. doi: 10.1679/aohc.59.159. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Torihashi S, Iino S, Pang YW, Chowdhury JU, Tomita T. The inner sublayer of the circular muscle coat in the canine proximal colon: origins of spontaneous electrical and mechanical activity. Archives of Histology and Cytology. 1995;58:45–63. doi: 10.1679/aohc.58.45. [DOI] [PubMed] [Google Scholar]
- Liu LWC, Thuneberg L, Huizinga JD. Cyclopiazonic acid, inhibiting the endoplasmic reticulum calcium pump, reduces the canine colonic pacemaker frequency. Journal of Pharmacology and Experimental Therapeutics. 1995;275:1058–1068. [PubMed] [Google Scholar]
- MalysZ J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP3-sensitive calcium release in the murine small intestine. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2001;280:G439–448. doi: 10.1152/ajpgi.2001.280.3.G439. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins (1,4,5)-induced Ca2+ release. Journal of Biochemistry. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, RuiZ MC, FernandeZ E, Ciarrocchi A. Role of Ca2+ in H+ transport by rabbit gastric glands studied with A23187 and BAPTA, an incorporated Ca2+ chelator. Biochimica et Biophysica Acta. 1989;983:82–90. doi: 10.1016/0005-2736(89)90383-0. [DOI] [PubMed] [Google Scholar]
- Nagamune H, Fukushima Y, Takada J, Yoshida K, Unami A, Shimooka T, Terada H. The lipophilic weak base (Z)-5-methyl-2-[2-(1-naphthyl) ethenyl]-4-piperidinopyridine (AU-1421). is a potent protonophore type cationic uncoupler of oxidative phosphorylation in mitochondria. Biochimica et Biophysica Acta. 1993;1141:231–237. doi: 10.1016/0005-2728(93)90047-j. [DOI] [PubMed] [Google Scholar]
- Nahar NS, Torihashi S, Iino S, Senda T, Chowdhury JU, Kobayashi S. Special smooth muscle cells along the submucosal surface of the guinea pig colon with reference to its spontaneous contractions. Cell and Tissue Research. 1998;293:143–154. doi: 10.1007/s004410051106. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. American Journal of Physiology. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Plujà L, Alberti E, FernandeZ E, Mikkelsen HB, Thuneberg L, JimeneZ M. Evidence supporting presence of two pacemakers in rat colon. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2001;281:G255–266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ördög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterology and Motility. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Smith TK. Electrophysiology of colonic smooth muscle. In: SchultZ SG, Wood JD, editors. The Gastrointestinal System (Handbook of Physiology) Bethesda, MD: American Physiological Society; 1989. pp. 251–271. [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in the muscularis of the canine proximal colon. American Journal of Physiology. 1987;252:C290–299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Cellular mechanisms of myogenic activity in gastric smooth muscle. Japanese Journal of Physiology. 2000;50:289–301. doi: 10.2170/jjphysiol.50.289. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol triphosphate receptor. Journal of Physiology. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle. London: Edward Arnold; 1981. pp. 127–156. [Google Scholar]
- Torihashi S, Ward SM, Sanders KM. Development of c-kit positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- Uyama Y, Imaizumi Y, Watanabe M. Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. British Journal of Pharmacology. 1992;106:208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, Bernex F, Schiffmann SN, Panthier JJ. Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and Kit(W-lacZ) mice. Cell and Tissue Research. 2000;302:155–170. doi: 10.1007/s004419900170. [DOI] [PubMed] [Google Scholar]
- Ward SM, Ördög T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. Journal of Physiology. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Fukuta H, Nakahira Y, Suzuki H. Blockade by 18β-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. Journal of Physiology. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Takano H, Kadowaki M, Takaki M, Suzuki H. Electrical activity of submucosal pacemaker cells in the mouse proximal colon. Journal of Smooth Muscle Research (Japanese Section) 2001;4:J–28. [Google Scholar]


