Abstract
Bradykinin is known to cause vasodilatation in resistance vessels and may, together with adenosine, be an important regulator of tissue blood flow during exercise. Whether tissue concentrations of bradykinin change with exercise in skeletal muscle and tendon-related connective tissue has not yet been established. Microdialysis (molecular mass cut-off 5 kDa) was performed simultaneously in calf muscle and peritendinous Achilles tissue at rest and during 10 min periods of incremental (0.75 W, 2 W, 3.5 W and 4.75 W) dynamic plantar flexion exercise in 10 healthy individuals (mean age 27 years, range 22–33 years). Interstitial bradykinin and adenosine concentrations were determined using an internal reference to determine relative recovery ([2,3,prolyl-3,4-3H(N)]-bradykinin and [2-3H]-adenosine). Bradykinin and adenosine recovery were closely related and in the range of 30–50 %. The interstitial concentration of bradykinin rose in response to exercise both in skeletal muscle (from 23.1 ± 4.9 nmol l−1 to 110.5 ± 37.9 nmol l−1; P < 0.05) and in the peritendinous tissue (from 27.7 ± 7.8 nmol l−1 to 105.0 ± 37.9 nmol l−1; P < 0.05). In parallel, the adenosine concentration increased both in muscle (from 0.48 ± 0.07 μmol l−1 to 1.59 ± 0.35 μmol l−1; P < 0.05) and around the tendon (from 0.33 ± 0.03 μmol l−1 to 0.86 ± 0.16 μmol l−1; P < 0.05). In conclusion, the data show that muscular activity increases the interstitial concentrations of bradykinin and adenosine in both skeletal muscle and the connective tissue around its adjacent tendon. These findings support a role for bradykinin and adenosine in exercise-induced hyperaemia in skeletal muscle and suggest that bradykinin and adenosine are potential regulators of blood flow in peritendinous tissue.
Blood flow to skeletal muscle increases with exercise in an intensity-dependent manner (Andersen & Saltin, 1985). Despite the demonstration of vasodilatation in active skeletal muscle, the identification and respective roles of vasodilatory substances is far from being solved. Recently, it was demonstrated that blood flow to tendon regions - similar to skeletal muscle - increases with exercise intensity, with peritendinous blood flow increasing most markedly at low workloads, whereas muscle blood flow increased linearly (Langberg et al. 1998; Boushel et al. 2000b). Furthermore, blood flow is close to its absolute maximum in skeletal muscle during intense exercise, whereas the increase in tendon blood flow with intense exercise only represents around 20 % of that attained maximally during reactive hyperaemia (Boushel et al. 2000a). This raises the possibility of regional differences in the relative role of vasoactive substances contributing to the regulation of blood flow during physical activity in skeletal muscle and its adjacent tendon. The vasoactive peptide bradykinin is known to result in vasodilatation in human resistance vessels (Fox, 1961; Collier et al. 1972; Cockcroft et al. 1994; Honing et al. 2000), and it has been demonstrated that bradykinin-generating proteases (e.g. kallikrein) and precursor proteins for bradykinin production (such as kininogen) are present in skeletal muscle (Shimojo et al. 1987) and vascular smooth muscle (Oza et al. 1990). In addition, specific bradykinin receptors have been revealed in both skeletal and cardiac muscle (Figueroa et al. 1996; Rabito et al. 1996) as well as in endothelial tissue (Regoli et al. 1977; Busse & Fleming, 1996). Despite the indication of bradykinin release from human skeletal muscle (Rett et al. 1989), and the demonstration of bradykinin release from cat skeletal muscle (Stebbins et al. 1990), there have been no attempts to study the local concentration of bradykinin in skeletal muscle or other tissues of relevance to mechanical loading.
In the present study, interstitial bradykinin and adenosine concentrations were measured both in skeletal muscle and peritendinous tissue, with the hypothesis that interstitial concentrations would increase in response to exercise in both regions, thus playing a possible role in the regulation of regional blood flow during exercise.
Methods
Subjects
Ten healthy and physically active students (nine men and one woman) participated in the study, which was approved by the Ethical Committee of Copenhagen ((KF) 01-257/98) and conformed to the Declaration of Helsinki. All subjects gave their informed consent to participate. The mean age was 27 years (range 22–33 years), height 180 cm (range 170–191 cm) and weight 79 kg (range 70–103 kg). The participants were all involved in different sporting activities, had no previous history of Achilles tendon injury or took any form of medication prior to the experiment (> 2 months). The subjects were asked not to participate in any kind of heavy exercise during the last week before the experiment.
Microdialysis
Microdialysis was carried out in both calf skeletal muscle and in the peritendinous Achilles tissue, as described in detail previously (Langberg et al. 1999). The internal reference technique was used to determine the relative recovery of adenosine and bradykinin (Scheller & Kolb, 1991; Lonnroth & Strindberg, 1995). The microdialysis probes used in the present study were custom made from single, hollow plasmaphoresis fibres (0.4 mm in diameter, membrane length 30 mm, molecular mass cut-off 5 kDa; Alwall, GFE 11, Gambro Dialysatoren, Hechingen, Germany) glued to a suture thread (Johnson & Johnson, Brussels, Belgium) to provide mechanical stability, and glued to gas-tight nylon inlet and outlet tubing (Protex Autoclavable Nylon Tubing, Smiths Industries, Belgium) before sterilisation (ETO-sterilisation; Mærsk Medical, Denmark). After anaesthetizing the skin with lidocaine (0.1 ml; 1 %) four microdialysis fibres were inserted into the right calf of each subject, two into the gastrocnemius medialis muscle and two into the peritendinous space just ventral to the Achilles tendon. Ultrasound screening was used during the positioning of the two fibres in the gastrocnemius muscle, placed parallel to the muscle fibres to minimize the insertion trauma, with an inter-fibre distance of 25 mm. In the peritendinous space the active part of the membrane covered the area 30–60 mm proximal to the point of insertion of the Achilles tendon into the calcaneus bone. The microdialysis fibres were perfused at a rate of 5 μl min−1 (Karamouzis et al. 2001) with a Ringer-acetate solution (Frescenius Kabi, Norge) containing 3 mmol glucose and 0.5 mmol lactate, and either radioactively labelled bradykinin ([2,3,prolyl-3,4-3H(N)]: 3.4 TBq mmol−1 or 90.6 Ci mmol−1, 1 μl per 10 ml; NEN, Boston, USA) or adenosine ([2-3H]: 740 GBq mmol−1 or 20 Ci mmol−1, 2 μl per 10 ml; Amersham, UK).
In vitro recovery
The relative recovery of adenosine and bradykinin was determined as described previously (Scheller & Kolb, 1991; Langberg et al. 1999). Pilot studies showed that the analysis of interstitial bradykinin was influenced significantly by the presence of radioactively labelled bradykinin, thus obviating the use of radioactively labelled bradykinin in the fibre used for bradykinin analysis. From a series of in vitro experiments where the recovery of adenosine and subsequently bradykinin was determined in the same microdialysis fibre over a broad range of perfusions rates and membrane lengths, it was found that the recovery for adenosine (40-50 %) was systematically higher than that for bradykinin (30-40 %). Based on these experiments, a fixed relationship between adenosine and bradykinin could be determined, giving a conversion factor of 1.19 (range: 1.1-1.36) when calculating bradykinin recovery from the determined recovery of adenosine, and of 0.84 (range: 0.77-0.96) when converting bradykinin recovery to adenosine recovery (Fig. 1).
Figure 1. In vitro experiments.
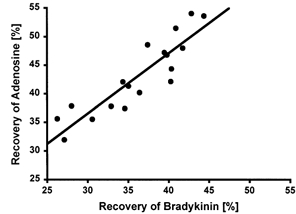
Relationship between the relative recovery of bradykinin and adenosine measured in the same fibres. The fibres were tested in a bath, three at a time. The bath contained Ringer-acetate and different concentrations of bradykinin, adenosine and glucose (R = 0.903; P < 0.01).
In vivo recovery
As a result of the in vitro experiments, a crossover design was used where the microdialysis probes designated for bradykinin determination were initially perfused with bradykinin-labelled perfusate for 45 min and thereafter switched to adenosine-labelled perfusate for 45 min (Fig. 2). This in vivo calibration procedure revealed a good relationship between the recovery of both bradykinin and adenosine in muscle and tendon (Table 1), and was used to calculate a specific correlation between adenosine and bradykinin for each microdialysis fibre. During the in vivo experiment, the dialysate samples were collected in capped microvials (CMA/Microdialysis, Stockholm, Sweden) and immediately frozen at −80 °C until analysed. Neither during rest nor with exercise did the microdialysis volumes obtained from skeletal muscle and peritendinous tissue differ by more than 5 % from the expected values based on the pre-set infusion rates.
Figure 2. Crossover design.
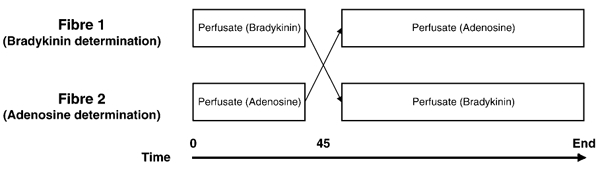
The microdialysis probes designated for bradykinin determination were initially perfused with bradykinin-labelled perfusate for 45 min and thereafter switched to adenosine-labelled perfusate for 45 min.
Table 1.
Relative recovery for bradykinin and adenosine
| Bradykinin | Adenosine | |||
|---|---|---|---|---|
| Muscle | Peritendon | Muscle | Peritendon | |
| Baseline | 28.8 ± 1.8 | 30.6 ± 1.6 | 35.9 ± 4.6 | 42.3 ± 1.9 |
| 0.75W | 41.0 ± 3.6* | 33.4 ± 1.9 | 44.7 ± 4.5* | 45.7 ± 1.2 |
| Rest 1 | 30.0 ± 3.1 | 32.3 ± 1.5 | 36.9 ± 5.3 | 41.7 ± 2.0 |
| 2 W | 36.9 ± 3.4* | 35.2 ± 2.1* | 42.8 ± 4.5* | 46.6 ± 1.7* |
| Rest 2 | 31.0 ± 3.9 | 36.0 ± 1.9* | 38.5 ± 2.7 | 43.1 ± 1.8 |
| 3.5 W | 35.6 ± 2.6* | 38.0 ± 1.6* | 45.0 ± 2.6* | 46.3 ± 3.3 |
| Rest 3 | 31.2 ± 3.2 | 35.1 ± 2.6* | 39.7 ± 3.8 | 44.3 ± 2.2 |
| 4.75W | 36.1 ± 2.9* | 36.1 ± 2.1* | 46.8 ± 2.9* | 47.7 ± 1.3* |
Relative recovery for bradykinin and adenosine in muscle (n = 8) and in the peritendinous region (n = 8). Values are means ±s.e.m.
Significantly different from baseline, P < 0.05.
The adenosine concentrations were determined quantitatively by reverse-phase high-performance liquid chromatography, as described previously (Hellsten & Frandsen, 1997). The analysis of bradykinin was performed by radioimmunoassay analysis (RIK-7051, Peninsula Laboratories, CA, USA).
Experimental protocol
All experiments were performed in the morning and the microdialysis probes were inserted as described above. The subjects rested in horizontal posture for 90 min, and the dialysate collected during this period (6 × 15 min) was used for calculation of the adenosine:bradykinin recovery-rate ratio (Table 1). This procedure also ensured a time delay between the insertion of the fibres and the sampling of at least 100 min in the resting state, sufficient to minimize any tissue response to the insertion trauma (Langberg et al. 1999). Following this rest period, the subjects were seated in a set-up that had been custom made for plantar flexion exercise (Boushel et al. 2000a; Green et al. 2000) and performed dynamic plantar flexion contractions (10 min exercise followed by 10 min rest, 30 contractions min−1 metronome pace). The incremental loads of the triceps surae in plantar flexion were gradually increased to levels corresponding to 0.75 W, 2 W, 3.5 W and 4.75 W. On average, these workloads represented 15 %, 40 %, 70 % and 95 %, respectively of the maximal workload that could be achieved by this type of exercise. Microdialysis samples were collected every 10 min.
Statistical analysis
All values are presented as means ± s.e.m. or, if indicated, as the mean and range. To investigate whether a change appeared with increasing workload, a Friedman test with repeated measures and subsequent Wilcoxon signed-rank tests were used (SPSS; standard version 7.5). P < 0.05 (two-tailed testing) was considered significant. Non-parametric statistics were used due to small sample size.
Results
Bradykinin
The baseline concentration of bradykinin in the gastrocnemius medialis was 23.1 ± 4.9 nmol l−1 and was not significantly different from the other determinations made prior to exercise (Fig. 3). However, a significant fall in bradykinin concentration was observed from the initial measurements after insertion of the fibres (131.5 ± 34.2 nmol l−1) to baseline values prior to exercise (P < 0.05). All exercise periods except for the first load (0.75 W: 54.3 ± 19.5 nmol l−1) resulted in significantly higher interstitial concentrations than measured during baseline (2 W: 110.6 ± 27.0 nmol l−1; 3.5 W: 97.3 ± 23.7 nmol l−1; 4.75 W: 110.5 ± 43.8 nmol l−1; P < 0.05; Fig. 3). The bradykinin concentration tended to increase from baseline during the rest periods, and was significantly increased during the third rest period (79.6 ± 23.5 nmol l−1; P < 0.05; Fig. 3).
Figure 3. Interstitial bradykinin.
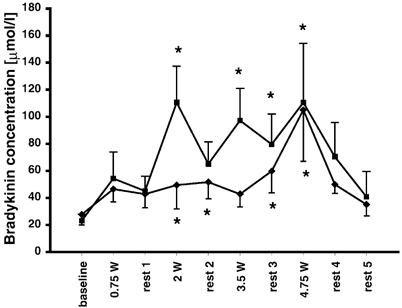
Concentrations of interstitial bradykinin in the medial part of the gastrocnemius muscle (▪, n = 7) and in the peritendinous space of the human Achilles tendon (♦, n = 6). Measurements made at rest, during dynamic incremental exercise (0.75 W, 2 W, 3.5 W and 4.75 W) and in recovery were carried out using microdialysis. *Significantly different from baseline level (P < 0.05). Values are presented as means ± s.e.m.
In the peritendinous space of the Achilles tendon, as in muscle, the interstitial concentration of bradykinin fell significantly from insertion of catheters (102.0 ± 33.3 nmol l−1) to baseline prior to exercise (baseline: 27.7 ± 7.8 nmol l−1; P < 0.05; Fig. 3). During exercise the peritendinous bradykinin concentration rose for 2 W and 4.75 W (2 W: 49.4 ± 17.6 nmol l−1 and 4.75 W: 105.0 ± 37.9 nmol l−1; P < 0.05; Fig. 3) and tended to rise for 0.75 W and 3.5 W (46.5 ± 9.4 nmol l−1 and 42.8 ± 9.4 nmol l−1, respectively; P < 0.12).
Adenosine
The interstitial concentration of adenosine in the interstitium of the gastrocnemius medialis showed no significant difference between the six pre-baseline measurements, the baseline measurements and the resting values (baseline: 0.5 ± 0.1 μmol l−1; P < 0.05; Fig. 4). During all of the exercise periods, except for 2 W, the concentration of adenosine was significantly elevated compared to baseline (0.75 W: 1.0 ± 0.2; 2 W: 1.0 ± 0.3; 3.5 W: 1.1 ± 0.3; 4.75 W: 1.6 ± 0.4 μmol l−1; P < 0.05; Fig. 4). In all except the second rest period, the concentration of adenosine was lower compared to during the period immediately preceding the exercise period (rest 1: 0.7 ± 0.2; rest 3: 0.8 ± 0.3; rest 4: 0.6 ± 0.1; rest 5: 0.5 ± 0.1 μmol l−1; P < 0.05). Apart from the concentrations determined following the last exercising period (rests 4 and 5), a trend towards higher adenosine levels in rest periods was observed as exercise intensities progressed (P < 0.1).
Figure 4. Interstitial adenosine.
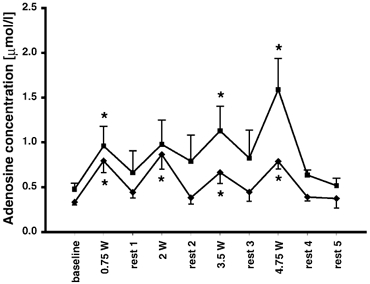
The concentration of interstitial adenosine in the medial part of the gastrocnemius muscle (▪, n = 6) and in the peritendinous space of the human Achilles tendon (♦, n = 7). Measured at rest, during dynamic incremental exercise (0.75 W, 2 W, 3.5 W and 4.75 W) and during recovery, using microdialysis. *Significantly different from baseline concentration (P < 0.05). Values are means ± s.e.m.
In the peritendinous space of the Achilles tendon, no difference was observed between pre-baseline, baseline and resting values of adenosine concentration (baseline: 0.3 ± 0.0 μmol l−1; P < 0.05; Fig. 4). Adenosine concentrations during all exercise periods, however, were higher than the baseline concentration (0.75 W: 0.8 ± 0.1; 2 W: 0.9 ± 0.2; 3.5 W: 0.7 ± 0.1; 4.75 W: 0.8 ± 0.1 μmol l−1; P < 0.05; Fig. 4), but no intensity-dependent increase was observed (P > 0.05; Fig. 4).
Discussion
The present study demonstrates the occurrence of muscle-contraction-induced increases in interstitial concentrations of bradykinin in both skeletal muscle and the extracellular matrix around the Achilles tendon. No clear intensity-dependent increases in bradykinin concentration were found either in the muscle or around the tendon (Fig. 3). Furthermore, the concentration of another vasodilatory agent, adenosine, was found to increase the most at the highest work intensity in muscle tissue, whereas the exercise-induced increase in peritendinous bradykinin was not influenced by the intensity of the workload. These findings favour the view that both bradykinin and adenosine contribute to the regulation of blood flow in contracting muscle as well as in the area along its adjacent tendon. The fact that in skeletal muscle even moderate exercise loads result in a marked bradykinin release with no further increase in interstitial concentrations at higher exercise loads could indicate that bradykinin contributes to increases muscle blood flow mainly during lower workloads (Boushel et al. 2000b). Although such findings do not prove a causal relationship, they are compatible with a role for both bradykinin and adenosine in exercise-induced hyperaemia in both muscle and peritendinous tissue (Boushel et al. 2000b). Unfortunately no skeletal muscle blood flow measurements were performed in the present study.
The magnitude of changes in tissue concentrations of bradykinin in the present study was from 2 × 10−8m at rest and up to 10 × 10−8m during exercise. It is interesting, that in an isolated perfused rat heart model, which, in contrast to in vitro models of skeletal muscle, contains an intact endothelium, bradykinin has been shown to cause a vasodilatory effect at concentrations of 5 × 10−8m (Rosen et al. 1983). The extent to which a vasodilatory effect of bradykinin would be exerted directly on the vasculature (Busse & Fleming, 1996) or indirectly via release of other substances such as nitric oxide (O'Kane et al. 1994), prostaglandins (PGs; Barrow et al. 1986) or endothelium-derived hyperpolarizing factor (Mombouli et al. 1992; Honing et al. 2000) has yet to be established. The mechanism may vary between species and between regions of the studied vasculature (Nagao & Vanhoutte, 1992). The present study does not provide direct evidence for the underlying mechanism; however, it has been shown that both in skeletal muscle and peritendinous tissue, intense exercise results in an immediate increase in tissue prostanoid (PGE2 and PGI2) concentration (Langberg et al. 1999; Frandsen et al. 2000; Karamouzis et al. 2001). More recently, indomethacin-induced blockade of PG synthesis was found to cause a reduction in the exercise-induced increase in peritendinous tissue blood flow (Langberg et al. unpublished observations).
The exercise-induced increase in tissue bradykinin is likely to result from an increased synthesis, but the exact location of this increase cannot be stated on the basis of the present study. However, levels of bradykinin obtained in our study were several-fold higher than concentrations found previously in mixed venous blood before and after exercise (Marceau, 1995; Blais et al. 1999) as well as in effluent venous blood from contracting human forearm muscle (Rett et al. 1989). This would be in accordance with the hypothesis that during exercise the major source of bradykinin is the muscular tissue, either via endothelial or skeletal muscle cells. Finally, the pattern of increased concentrations of bradykinin in the peritendinous area indicates that this tissue also generates bradykinin during exercise.
In a previous study it was shown that intermittent forearm exercise for 3 min caused an increased release of bradykinin to the blood, especially in the 3 min immediately following exercise (Rett et al. 1989). In the study reported herein, bradykinin was also significantly elevated above baseline levels during rest periods. This implies that most likely an increased bradykinin synthesis could have taken place not only during exercise but also during at least a part of our 10 min post-exercise rest periods. Although the present study design did not allow for any detailed investigation of interstitial bradykinin concentrations during rest periods following exercise, due to their relatively short duration, it is a fact that any increased release in the tissue even for a part of the post-exercise rest periods would result in a gradual accumulation of bradykinin from rest-period to rest-period. Finally, it cannot be excluded that the presence of the microdialysis fibre within the tissue results in an inflammatory reaction in the contracting muscle. However, in a previous study we have shown that the presence of the microdialysis catheter in muscle or peritendinous tissue does not trigger an inflammatory response during muscular contraction, as determined by the concentration of the inflammatory mediator PGE2 (R. Boushel, H. Langberg, C. Gemmer, J. Olesen, R. Cremari, C. Scheede, M. Sander & M. Kjær, unpublished obervations). In addition, data from a recent unpublished study of the present authors on PG concentrations in exercising skeletal muscles using comparable methods and protocols as in the present study did not reveal any increase in resting values of this inflammatory marker over time. This reduces the possibility that the released bradykinin is the result of inflammation due to the presence of the microdialysis fibres within the tissue (Radegran et al. unpublished observations).
Using exactly the same experimental set-up and exercise protocol, we have already shown that regional blood flow increases in skeletal muscle, as determined with radiolabelled xenon washout, dye-dilution and indocyanine green near-infrared spectroscopy (Langberg et al. 1998; Boushel et al. 2000b). Taken together with the exercise-induced increase in interstitial bradykinin concentration in both skeletal muscle and tendon-near tissue observed in the present study, these data suggest a possible role of bradykinin in the stimulation of blood flow in skeletal muscle and around the tendon, although no measurements of skeletal muscle blood flow were undertaken in the present study.
Adenosine concentrations were found to increase in muscle with exercise in accordance with findings by Hellsten et al. (1998). Somewhat in contrast to previous studies, we found baseline levels of adenosine of 0.48 ± 0.07 μmol l−1 in the gastrocnemius muscle, while Hellsten et al. (1998) found 0.22 ± 0.10 μmol l−1 in the vastus lateralis, and Costa et al. (2000) found 0.29 ± 0.06 μmol l−1 in the digitorum superficialis. The discrepancy in rest values between the present findings and the study of Hellsten et al. (1998) may be due to the different muscles examined or differences in the properties of the microdialysis probe. It is unlikely that the high basal concentration measured in our study is related to insertion trauma, as the determination of adenosine concentrations revealed stable values over the six pre-exercising measurements. It is interesting, however, that levels of tissue adenosine during exercise are fully comparable with those found by others (Hellsten et al. 1998). Whether the effective tissue concentration of adenosine in exercising muscle is uniform independent of muscle region can only be hypothesized.
In addition to the increase in adenosine concentration in muscle, the interstitial adenosine concentration around the adjacent tendon was found to follow the same pattern. This supports a role for adenosine in exercise-induced hyperaemia in both skeletal muscle and its adjacent tendon.
In conclusion, the present study demonstrated that in humans, muscular contractions result in increases in the tissue concentrations of bradykinin and adenosine in both skeletal muscle and peritendinous tissue. Such changes could be important for vasodilatation, formation of inflammatory mediators and glucose transport in relation to muscular activity, may help to explain tissue-specific reactions between musculature and connective tissue, and provide insight into the relationships between the vascular regulation of blood supply and the oxidative status of the tissue in vivo.
Acknowledgments
We thank Birgitte Lillethorup, Anni Høy and Karina Olsen for skilled technical assistance. This study was supported by the Team Denmark Research Council, the Danish Sports Science Foundation, the Novo Nordisk Foundation, the Danish Medical Research Council (22-01-0154), Copenhagen University Hospital Research Foundation, the Danish National Research Foundation (504-14) and Ministry of Culture Sports Research Council.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow SE, Dollery CT, Heavey DJ, Hickling NE, Ritter JM, Vial J. Effect of vasoactive peptides on prostacyclin synthesis in man. British Journal of Pharmacology. 1986;87:243–247. doi: 10.1111/j.1476-5381.1986.tb10177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais C, Adam A, Massicotte D, Peronnet F. Increase in blood bradykinin concentration after eccentric weight-training exercise in men. Journal of Applied Physiology. 1999;87:1197–1201. doi: 10.1152/jappl.1999.87.3.1197. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Green S, Skovgaard D, Bulow J, Kjær M. Blood flow and oxygenation in peritendinous tissue and calf muscle during dynamic exercise in humans. Journal of Physiology. 2000a;524:305–313. doi: 10.1111/j.1469-7793.2000.t01-2-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen J, Nowak M, Simonsen L, Bulow J, Kjær M. Regional blood flow during exercise in humans measured by near-infrared spectroscopy and indocyanine green. Journal of Applied Physiology. 2000b;89:1868–1878. doi: 10.1152/jappl.2000.89.5.1868. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Molecular responses of endothelial tissue to kinins. Diabetes. 1996;45:S8–13. doi: 10.2337/diab.45.1.s8. [DOI] [PubMed] [Google Scholar]
- Cockcroft JR, Chowienczyk PJ, Brett SE, Bender N, Ritter JM. Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent B2-receptor antagonist. British Journal of Clinical Pharmacology. 1994;38:317–321. doi: 10.1111/j.1365-2125.1994.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier JG, Nachev C, Robinson BF. Effect of catecholamines and other vasoactive substances on superficial hand veins in man. Clinical Science. 1972;43:455–467. doi: 10.1042/cs0430455. [DOI] [PubMed] [Google Scholar]
- Costa F, Heusinkveld J, Ballog R, Davis S, Biaggioni I. Estimation of skeletal muscle interstitial adenosine during forearm dynamic exercise in humans. Hypertension. 2000;35:1124–1128. doi: 10.1161/01.hyp.35.5.1124. [DOI] [PubMed] [Google Scholar]
- Figueroa CD, Dietze G, Muller-Esterl W. Immunolocalization of bradykinin B2 receptors on skeletal muscle cells. Diabetes. 1996;45:S24, S28. doi: 10.2337/diab.45.1.s24. [DOI] [PubMed] [Google Scholar]
- Fox RH. Bradykinin as a vasodilator in man. Journal of Physiology. 1961;157:589–602. doi: 10.1113/jphysiol.1961.sp006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Langberg H, Saltin B, Hellsten Y. Inhibition of nitric oxide synthesis by systemic N(G)-monomethyl-l-arginine administration in humans: effects on interstitial adenosine, prostacyclin and potassium concentrations in resting and contracting skeletal muscle. Journal of Vascular Research. 2000;37:297–302. doi: 10.1159/000025743. [DOI] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bulow J, Kjær M. Effects of exercise intensity and ischaemia on muscle interstitial K+ and pain in humans. Journal of Physiology. 2000;529:849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. Journal of Physiology. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Honing ML, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- Karamouzis M, Langberg H, Skovgaard D, Bulow J, Kjær M, Saltin B. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiologica Scandinavica. 2001;171:71–76. doi: 10.1046/j.1365-201X.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bulow J, Kjær M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiologica Scandinavica. 1998;163:149–153. doi: 10.1046/j.1365-201X.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjær M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. Journal of Physiology. 1999;515:919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnroth P, Strindberg L. Validation of the ‘internal reference technique’ for calibrating microdialysis catheters in situ. Acta Physiologica Scandinavica. 1995;153:375–380. doi: 10.1111/j.1748-1716.1995.tb09875.x. [DOI] [PubMed] [Google Scholar]
- Marceau F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Illiano S, Nagao T, Scott-Burden T, Vanhoutte PM. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circulatory Research. 1992;71:137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- Nagao T, Vanhoutte PM. Characterization of endothelium-dependent relaxations resistant to nitro-l-arginine in the porcine coronary artery. British Journal of Pharmacology. 1992;107:1102–1107. doi: 10.1111/j.1476-5381.1992.tb13414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane KP, Webb DJ, Collier JG, Vallance PJ. Local l-NG-monomethyl-arginine attenuates the vasodilator action of bradykinin in the human forearm. British Journal of Clinical Pharmacology. 1994;38:311–315. doi: 10.1111/j.1365-2125.1994.tb04359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza NB, SchwartZ JH, Goud HD, Levinsky NG. Rat aortic smooth muscle cells in culture express kallikrein, kininogen, and bradykininase activity. Journal of Clinical Investigation. 1990;85:597–600. doi: 10.1172/JCI114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabito SF, Minshall RD, Nakamura F, Wang LX. Bradykinin B2 receptors on skeletal muscle are coupled to inositol 1,4,5-trisphosphate formation. Diabetes. 1996;45:S29–33. doi: 10.2337/diab.45.1.s29. [DOI] [PubMed] [Google Scholar]
- Regoli D, Barabe J, Theriault B. Does indomethacin antagonize the effects of peptides and other agents on the coronary circulation of rabbit isolated hearts. Canadian Journal of Physiology and Pharmacology. 1977;55:307–310. doi: 10.1139/y77-044. [DOI] [PubMed] [Google Scholar]
- Rett K, Wicklmayr M, Fink E, Maerker E, Dietze G, Mehnert H. Local generation of kinins in working skeletal muscle tissue in man. Biological Chemistry. 1989;370:445–449. doi: 10.1515/bchm3.1989.370.1.445. [DOI] [PubMed] [Google Scholar]
- Rosen P, Eckel J, Reinauer H. Influence of bradykinin on glucose uptake and metabolism studied in isolated cardiac myocytes and isolated perfused rat hearts. Zeitzchrift für Physiologi und Chemi. 1983;364:1431–1438. doi: 10.1515/bchm2.1983.364.2.1431. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscientific Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Shimojo N, Chao J, Chao L, Margolius HS, Mayfield RK. Identification and characterization of a tissue kallikrein in rat skeletal muscles. Biochemical Journal. 1987;243:773–778. doi: 10.1042/bj2430773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. Journal of Applied Physiology. 1990;69:1225–1230. doi: 10.1152/jappl.1990.69.4.1225. [DOI] [PubMed] [Google Scholar]


