Abstract
The molecular events by which eccentric muscle contractions induce muscle damage and remodelling remain largely unknown. We assessed whether eccentric exercise modulates the expression of proteinases (calpains 1, 2 and 3, proteasome, cathepsin B+L), muscle structural proteins (α-sarcoglycan and desmin), and the expression of the heat shock proteins Hsp27 and αB-crystallin. Vastus lateralis muscle biopsies from twelve healthy male volunteers were obtained before, immediately after, and 1 and 14 days after a 30 min downhill treadmill running exercise. Eccentric exercise induced muscle damage as evidenced by the analysis of muscle pain and weakness, creatine kinase serum activity, myoglobinaemia and ultrastructural analysis of muscle biopsies. The calpain 3 mRNA level was decreased immediately after exercise whereas calpain 2 mRNA level was increased at day 1. Both mRNA levels returned to control values by day 14. By contrast, cathepsin B+L and proteasome enzyme activities were increased at day 14. The α-sarcoglycan protein level was decreased immediately after exercise and at day 1, whereas the desmin level peaked at day 14. αB-crystallin and Hsp27 protein levels were increased at days 1 and 14. Our results suggest that the differential expression of calpain 2 and 3 mRNA levels may be important in the process of exercise-induced muscle damage, whereas expression of α-sarcoglycan, desmin, αB-crystallin and Hsp27 may be essentially involved in the subsequent remodelling of myofibrillar structure. This remodelling response may limit the extent of muscle damage upon a subsequent mechanical stress.
Contractile filaments of skeletal muscle are maintained in a highly ordered structure by a set of specialized proteins. This structural organization allows the generation of a rapid and synchronized movement and force in a specific direction. The physiological importance of this highly ordered structure is emphasized by the deleterious effects of the absence and/or abnormal expression of some of these proteins in progressive muscular dystrophies and myopathies (Campbell, 1995; Dalakas et al. 2000).
Some physiological conditions can also lead to the destabilization of this molecular architecture. Unaccustomed or high-intensity exercise may induce muscle damage, especially when it involves eccentric contractions. Everyone has experienced delayed-onset muscle soreness (DOMS) and the functional consequences for muscle capability. Originally described by Hough (1902), the effects of eccentric exercise on human muscle performance have subsequently been extensively studied. Muscle strength can be reduced by 50 % or more during the 24 h following exercise, and it gradually recovers over 5–10 days depending on the intensity of the damaging exercise (Sargeant & Dolan, 1987; Gibala et al. 1995). Eccentric exercise also produces protein leakage from skeletal muscle as illustrated by elevated serum levels of myoglobin and plasma levels of creatine phosphokinase (CK) activity (Balnave & Thompson, 1993; Rodenburg et al. 1993). The most striking morphological feature of muscle damage is probably the extensive sarcomeric disruption and Z-disk streaming observed in longitudinal sections of electron micrographs from muscle samples (Fridén et al. 1981, 1983, 1984). Using animal models, this ultrastructural evidence of muscle damage has been related to a loss of desmin (Lieber & Fridén, 1996; Fridén & Lieber, 1998), suggesting that intermediate filament integrity during eccentric exercise is essential for the maintenance of intermyofibrillar organization.
The molecular mechanisms involved in DOMS may be directly linked to the activation of proteases, and more specifically to the calcium-activated proteases (calpains). Indeed, exercise-induced muscle damage produces an early activation of the ubiquitous calpains 1 and 2 (CAPN1 and CAPN2; Belcastro, 1993), whose in vitro substrates include desmin, titin (Huang & Forsberg, 1998; for review see Belcastro et al. 1998), and the transcription factors c-fos and c-jun (Hirai et al. 1991). Calpain 3 (CAPN3), mutations of which are responsible for a recessive limb girdle muscular dystrophy (LGMD2A; Richard et al. 1995), can also potentially cleave proteins involved in muscle structure (Kinbara et al. 1997), and/or myonuclear death (Baghdiguian et al. 1999). Therefore a wide spectrum of biological effects of calpain activation can be expected in response to eccentric exercise, including Z-disk streaming (Lieber & Fridén, 1996), A-band disruption and gene expression. However, nothing is known about the effects of eccentric exercise on CAPN expression and its relation to muscle damage.
When a second bout of exercise is performed several weeks later, most of the muscle damage, previously observed after the first bout, is attenuated or does not appear (Clarkson & Tremblay, 1988; Clarkson et al. 1992; Mair et al. 1995). Specific adaptations must have thus occurred in muscle cells following the first bout of exercise to decrease muscle fibre susceptibility to subsequent mechanical stress (Gibala et al. 1995). Therefore, eccentric exercise can also potentially lead to a remodelling of skeletal muscle. Little is known about the mechanisms involved in the remodelling of muscle cells. However, there is evidence to support the contribution of the small heat shock proteins (Hsp), Hsp27 and αB-crystalline in this process, particularly in the assembly and maintenance of the intermediate filament network (Djabali et al. 1997; Sugiyama et al. 2000). Furthermore, the expression of numerous Hsps is increased in response to exercise and endurance training (Mattson et al. 2000).
The purpose of the present study was to determine the proteolytic and remodelling responses of human skeletal muscle at the molecular level to a single bout of downhill treadmill running exercise. We report that this moderate eccentric exercise induces (i) an early opposing response of CAPN2 versus CAPN1 and 3 mRNA levels, and a delayed increase in proteasome and cathepsin enzyme activities, (ii) an immediate and persistent decrease in α-sarcoglycan expression associated with a delayed increase in desmin expression, and (iii) an early and persistent increase in Hsp27 and αB-crystallin expression. We suggest that the molecular remodelling which occurs in response to moderate eccentric exercise may have profound effects on muscle fibre response to subsequent mechanical stress. The physiological relevance of these results will also be discussed with special reference to muscular diseases.
Methods
Subjects and preliminary testing
Twelve male students aged 20.3 ± 0.8 years (mean ± s.e.m.) were selected by screening which included a health history questionnaire and physical examination. Exclusion criteria included clinical evidence of cardiac, pulmonary or orthopaedic abnormalities. Body mass and height were 69.9 ± 0.4 kg and 178 ± 7 cm, respectively. Two weeks before the downhill running experiment, the subjects performed an incremental 0 % gradient test until exhaustion. The mean maximal O2 uptake (V̇O2max), maximal heart rate and maximal lactate concentration were respectively: 52.1 ± 1.1 ml min−1 kg−1, 207.9 ± 2.3 pulses min−1 and 12.4 ± 0.5 mmol l−1. The subjects were instructed not to perform any exercise for 1 month prior to the experiment and during the entire duration of the protocol. All subjects were fully informed of the nature and possible risks of the various procedures prior to giving their written consent. This investigation was approved by the Consultant Committee on Human Protection from Medical Research of Rhône-Alpes-Loire Region in accordance with the Declaration of Helsinki.
Eccentric exercise
Subjects performed an eccentric exercise consisting of a treadmill run at a −12 % gradient for 30 min. The running speed corresponded to the one sustained at 80 % of a 0 % gradient. Heart rate, pulmonary ventilation and oxygen uptake were continuously monitored.
Testing of the muscle function
Maximal power of the lower limbs was measured using a test performed on a cycle ergometer (Linossier et al. 1996) 1 week before, immediately at the end of exercise, and 1, 7 and 14 days post exercise. This test was chosen as a criterion of dynamic quadriceps muscle function, and allows calculation of muscle power with reference to the pedalling rate, the tension of the mechanical brake and the inertia of the flywheel of the ergometer. Several bouts (4-6) of acceleration (6-10 s duration) were performed until the maximal pedalling rate was reached. Each bout was performed at a given mechanical brake value and they were separated by 2 min of rest. This allowed the maximal power (W) to be assessed.
Pain assessment
Subjects completed a muscle soreness questionnaire rated on an eleven-point scale ranging from zero (normal: without pain or stiffness) to ten (unendurably painful) for the anterior and posterior muscle groups of the upper and lower legs and for gluteus muscle groups, on the day of the experiment (before and after exercise) and on the following days until the total disappearance of pain.
Blood sample analyses
Blood samples were withdrawn from the median cubital vein before, and at the end of exercise, and 1 and 14 days post exercise. Creatine phosphokinase (CK) activity in plasma was spectrophotometrically determined (Roche Diagnostics, France). Myoglobin concentration in serum was determined using a radioimmunoassay (Ria-mat, Byk-Sangtec Diagnostica, Germany).
Muscle biopsies
Muscle biopsy samples (about 100 mg) were taken under local anaesthesia from the superficial portion of the vastus lateralis muscle using a Weil-Blakesley conchotome and a percutaneous technique (Henriksson, 1979). Biopsy specimens were taken before and immediately after completion of the exercise. Two additional biopsies were taken 1 and 14 days post exercise. Muscle samples were extemporaneously divided into four pieces under a binocular lens at 4 °C. Two pieces were immediately frozen in liquid nitrogen for mRNA and protein analyses. A third large group of well-organized fascicles of fibres was oriented, placed in Tissue-Tek, frozen in isopentane cooled by liquid nitrogen and used for histochemical analyses. A fourth fascicle of muscle fibres was fixed in a 2.5 % glutaraldehyde-0.2 m cacodylate buffer (pH = 7.2) and used for ultrastructural analyses. In order to assess possible damage due to repetitive biopsies on the same muscle, half of the subjects underwent the first and third biopsies on the right leg, and the second and fourth biopsies on the left leg. The other subjects underwent the first and second biopsies on the right leg and the third and fourth biopsies on the left leg. When performed on the same muscle, the sites of the biopsies were at least 2 cm apart. No differences were observed between these two subgroups.
Histomorphological analyses
Transverse serial sections 10 μm thick were cut in a cryotome at −20 °C and stained by haematoxylin and eosin (H&E). For electron microscopy, muscles were then rinsed, post fixed in osmium tetroxide, dehydrated and embedded in Epon and Araldite. Ultrathin longitudinal muscle sections (60-90 nm) were cut, mounted on copper grids, contrasted with uranyl acetate and lead citrate and examined in a Hitachi H800 electron microscope. Four to six grids (4-8 sections per grid) were analysed for each muscle sample. Approximately 1500 sections were observed.
Total RNA extraction and real-time quantitative RT-PCR
Muscle samples (20-25 mg) were homogenized in a FastPrep (Bio101) instrument, RNA was extracted using a Trizol extraction kit (Gibco-BRL) and cDNA was synthesized using the Superscript II reverse transcriptase kit with random hexamers (Gibco-BRL) as described by Stockholm et al. (1999, 2001). Quantitation of reverse-transcription of calpain 1, calpain 2 and calpain 3 mRNA and TBP-TFIID mRNA was performed as a real-time measurement in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems Inc.), according to the manufacturer's instructions. Taqman probes were used for detection of the calpain 3 sequence and TBP-TFIID. SYBR Green I, a dye that fluoresces only in the presence of double stranded DNA was used for the detection of the calpain 1 and calpain 2 sequences. The sequences of the primers and probes used are shown in Table 1. For each primer set, a standard curve was generated by serial dilution of a cDNA from a reference sample control (skeletal muscle biopsy). The data were calculated using the standard curve method giving PCR efficiency for each primer set and attributing values for each sample relative to the reference sample control. All samples and serial dilutions were run in duplicate. Results were expressed as ratios to the TBP-TFIID, which was considered as an endogenous mRNA control allowing variations due to RNA extraction as well as RT efficiencies to be taken into account. Final normalizing attributed the value 100 % to the average of the samples corresponding to biopsies taken before the exercise.
Table 1.
Sequences of the primers and probes used for real-time quantitative RT-PCR
| Gene name | Accession no. | Primer name | Sequence |
|---|---|---|---|
| Calpain 1 | NM_005186 | CAPN1. forward | 5′-GAAGGAGTTGCGGACAATCC-3′ |
| Calpain 1 | NM_005186 | CAPN1. reverse | 5′-CGGCACGACTCTAGGCTGA-3′ |
| Calpain 2 | NM_001748 | CAPN2. forward | 5′-CAAGATGCCCTGTCAACTCCA-3′ |
| Calpain 2 | NM_001748 | CAPN2. reverse | 5′-CGAACCAAACACCGAACAAA-3′ |
| Calpain 3 | NM_000070 | CAPN3. forward | 5′-GCCAGAAGTTCCCCATCCA-3′ |
| Calpain 3 | NM_000070 | CAPN3. reverse | 5′-TTCTGTTGGCTCCATCAATGATA-3′ |
| Calpain 3 | NM_000070 | CAPN3. probe | 5′(FAM)-CCGGAAATTTGCGAGAATCCCCG-3′(TAMRA) |
| TBP-TFIID | X54993 | TBP. forward | 5′-GAGAGCCACGAACCACGG-3′ |
| TBP-TFIID | X54993 | TBP. reverse | 5′-ACATCACAGCTCCCCACCAT-3′ |
| TBP-TFIID | X54993 | TBP. probe | 5′(FAM)-TGTGCACAGGAGCCAAGAGTGAAGA-3′ (TAMRA) |
Protein extraction
Frozen muscles were weighed and suspended in a 0.1 m Na/KH2PO4 buffer containing 2 mm EDTA (1/20, w/v). Muscles were then minced with scissors, crushed in a frosted mortar, stirred for 15 min, and sonicated (4 × 10 s on ice. Homogenates were then centrifuged at 15 000 g for 10 min at 4 °C. The supernatants were removed and the pellets resuspended at a 1/20 dilution (w/v) and processed as described above. Both supernatants were then combined, aliquoted and stored at −80 °C. Protein concentrations of muscle homogenates were measured spectrophotometrically using the Bio-Rad protein assay at 750 nm.
Enzyme assays
All enzyme activities were measured by using a SFM25 fluorometer (Kontron Instruments) at λexc = 380 nm and λem = 460 nm. The chymotrypsin-like enzyme activity of the ubiquitin-proteasome pathway was measured with 10 μl of protein extract in 980 μl of 60 mm imidazole buffer pH = 7.4. The reaction was started by the addition of the fluorogenic peptide SLLVY-MCA (100 μM) (Bachem catalogue no. I-1395). Specificity of the reaction was checked by preincubating muscle protein extract with 20 μM Z-IE(OtBu)Al-CHO (Bachem catalogue no. C-3900). Proteolytic activity of cathepsin B+L was measured as follows: 10 μl of protein extract was incubated with 980 μl of 100 mm sodium acetate buffer (pH = 6.0) containing 1 mm EDTA. The assay was started by the addition of 10 mm Z-FR-MCA (Bachem I-1160). To ensure specificity of the reaction, 50 μM of E-64 (Sigma catalogue no. E-3132) was added to the assay buffer before the addition of the substrate. Standard AMC (amido-4-methylcoumarin) concentrations of 20, 40, 60, 80 and 100 nm were used to quantify enzyme activities. Rates were expressed as mmoles per minute per gram of wet muscle.
Immunoblotting
Aliquots of protein (20 μg lane−1 for α-actin and desmin, 30 μg lane−1 for Hsp27 and αB-crystallin and 60 μg lane−1 for α-sarcoglycan) were mixed with a loading buffer (62.5 mm Tris pH = 6.8, 5 % glycerol (v/v), 1 % SDS (v/v), 2.5 % β-mercaptoethanol (v/v)), boiled for 3 min, applied to a 12.5 % (w/v) SDS-PAGE gel, and electrophorezed at 115 V for 4 h at 4 °C. Muscle samples of a given subject were run on the same gel. Separated proteins were electrotransferred (200 mA) for 1 h at 4 °C onto nitrocellulose membranes. The membranes were then blocked in Tris phosphate saline buffer (TBS)-5 % milk solution (α-actin and desmin) or in TBS-Tween-5 % milk solution (α-sarcoglycan, Hsp27 and αB-crystallin) at room temperature for 1 h. The following primary antibodies were used for immunoblotting: α-actin, 1/3000 dilution (AC-40; Sigma); desmin, 1/500 dilution (D33, DAKO); α-sarcoglycan, 1/60 dilution (Ad1/20A6; Novocastra); Hsp27, 1/1000 dilution (G3.1; StressGen); αB-crystalline, 1/1000 dilution (Novocastra). Primary antibodies were incubated for 2 h at room temperature. Rabbit anti-mouse IgG (1/4000 (v/v) TBS-1 % milk; P0161, DAKO) conjugated to horseradish peroxidase was used for chemiluminescent detection of proteins (ECL, Amersham). The films were scanned and quantified using NIH image 1.61 software. Briefly, bands on blots to be densitometrically analysed were verified to be within the linear range of the primary antibody using our scanning method. Protein level quantification was assessed by integrating band area with band density for each band, and normalizing to the pre-exercise band quantification on the same blot.
Statistical analysis
Data are expressed as mean ± s.e.m. One-way ANOVA was used to evaluate the effects of eccentric exercise as a function of time, with post hoc comparisons performed with Fisher's protected least significance difference test. Differences were considered to be statistically significant at the 0.05 confidence level.
Results
Eccentric exercise induces DOMS
Subjects ran down a 12 % gradient at 11.1 ± 0.4 km h−1 on a treadmill at 53.9 ± 1.5 % of V̇O2,max for 30 min. The mean oxygen uptake, heart rate and lactate concentration of the subjects during eccentric exercise were 28.1 ± 0.7 ml min−1 kg−1, 179.4 ± 1.9 pulse min−1 and 3.9 ± 0.5 mmol l−1, respectively. The characteristics of the exercise were chosen to elicit eccentric contractions of the extensor muscles of lower limbs while inducing a moderate increase in energy metabolism. As expected, this exercise intensity generated a modest increase in lactate level. Despite the moderate intensity of the exercise, the maximal power of the lower limbs was still significantly lowered on day 1 post exercise (Fig. 1A). Based on a muscle soreness questionnaire, all subjects reported subjective pain consistent with the appearance of DOMS after the eccentric exercise. As expected, the extensor muscles (quadriceps, calf, gluteus) were prominently affected by the exercise. The perceived soreness had entirely disappeared by day 6 post exercise. Myoglobinaemia was significantly increased immediately after eccentric exercise and had returned to control level on day 1 (Fig. 1B). In contrast, serum creatine kinase level was increased only at day 1 after exercise.
Figure 1. Eccentric exercise induces delayed onset muscle soreness.
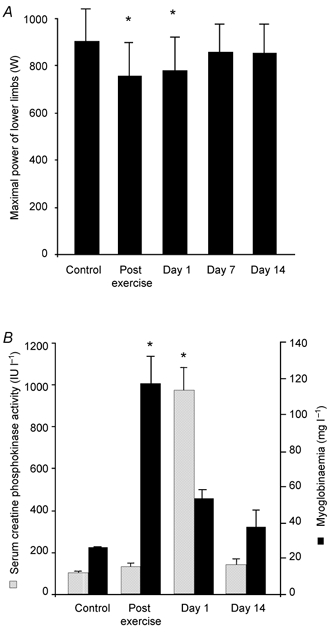
A, maximal power of lower limbs. At the indicated time points, subjects performed a force velocity test (see ‘Testing of the muscle function’ in Methods for details). B, serum creatine kinase activity and myoglobinaemia. Values are means ± s.e.m. (n = 12). Asterisks denote statistical difference from control values: * P < 0.05.
Histomorphological analyses
Inspections of H&E-stained sections did not show any morphological changes to muscle fibres, or the presence of necrotic fibres or inflammatory cells (data not shown). However, eccentric muscular contractions gave rise to extensive disorganization of the myofibrillar ultrastructure. Streaming, disruption and dissolution of the Z-disk were frequently observed (Figs 2c-f). The most severe Z-disk damage was associated with A-band disruption and misalignment of myofibrils. Disturbances of the cross-striated band pattern were focalized along the longitudinal sections. These alterations varied in size and frequency within and between fibres. Examination of survey sections also showed the presence of normal muscle fibres adjacent to damaged fibres. Overall, these ultrastructural injuries were observed in eight subjects (n = 2, post exercise; n = 8, day 1). Normal ultrastructural profiles were always observed in controls and day 14 muscle biopsies (Fig 2a and b).
Figure 2. Electron micrographs of longitudinal sections illustrating muscle damage following eccentric exercise in human skeletal muscle.
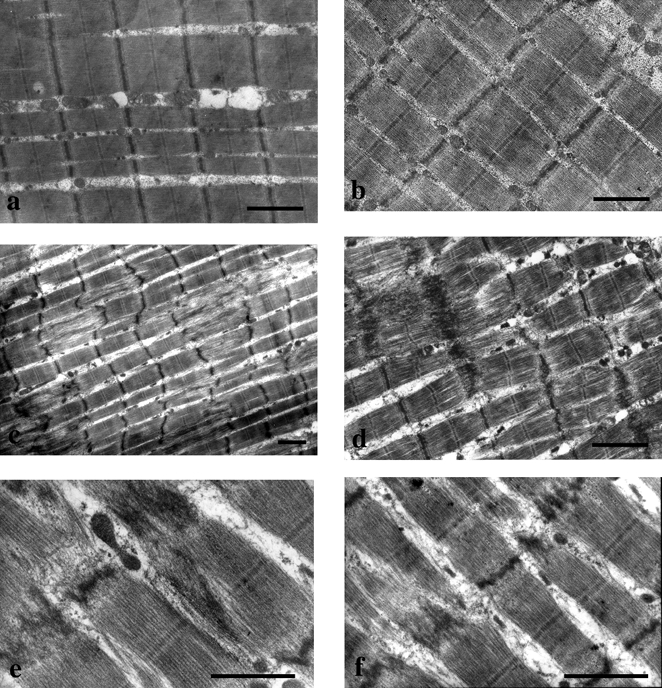
All muscle sections were obtained from the same subject. Normal ultrastructural profiles were observed in control (a) and in day 14 muscle biopsies (b). Eccentric muscular contractions gave rise to extensive disorganization of the myofibrillar ultrastructure immediately after exercise (c) and at day 1 post exercise (d, e and f). Overall, eight subjects experienced muscle damage (n = 2, post exercise; n = 8, day 1). Streaming, disruption and dissolution of the Z-disk were frequently observed. Severe Z-disk damage was associated with A-band disruption and misalignment of myofibrils (c, d, e and f). Disturbances of the cross-striated band pattern were focalized along the longitudinal sections (c). Scale bars: 1 μm.
Eccentric exercise differentially regulates the mRNA levels of CAPN1, 2 and 3
To investigate the potential role of proteolytic systems in exercise-induced muscle damage, we first determined the mRNA levels of CAPN1, 2 and 3 by RT-PCR analysis. The main feature of the data presented in Fig. 3 was the early and differential regulation of calpain mRNA levels in response to eccentric exercise. On day 1 post exercise, CAPN1 and 3 mRNA levels were significantly downregulated by about 25 and 40 %, respectively, whereas CAPN2 mRNA level was dramatically increased (220 %; P < 0.001; Fig. 3). On day 14, mRNA levels were not significantly different from control values.
Figure 3. Eccentric exercise differentially regulates the mRNA levels of calpains 1, 2 and 3.
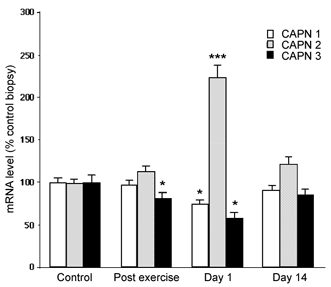
RT-PCR was used to quantify mRNA levels of calpains 1, 2 and 3 (see ‘Total RNA extraction and real-time quantitative RT-PCR’ in Methods for details). Values are means ± s.e.m. (n = 9) and are expressed relative to control mRNA level. * P < 0.05; *** P < 0.001 vs. control.
Eccentric exercise induces an increase in lysosomal and proteasome enzyme activities
To investigate further the possible involvement of proteolytic systems in exercise-induced muscle damage, we next determined the lysosomal and ATP-ubiquitin-dependent proteinase activities by fluorometric analysis (Table 2). In contrast to the calpain system, cathepsin B+L and proteasome activities were significantly increased on day 14, by about 50 % and 60 %, respectively.
Table 2.
Effects of eccentric exercise on muscle proteolytic enzyme activities
| Enzyme activities | Control | Post exercise | Day 1 | Day 14 |
|---|---|---|---|---|
| Cathepsin B+L | 13.7 ± 1.7 | 13.8 ± 1.7 | 15.5 ± 1.3 | 20.6 ± 1.4** |
| Proteasome | 12.6 ± 1.6 | 12.3 ± 1.7 | 15.3 ± 1.5 | 19.8 ± 1** |
Rates of enzyme activities were fluorometrically determined and are expressed as nmol min−1 (g muscle)−1. Values are means ± s.e.m.(n = 12).
P < 0.01 : significantly different from control values.
Effects of eccentric exercise on α-actin, desmin and α-sarcoglycan protein levels
In the light of the effects of exercise on muscle structure, we then attempted to identify whether expression of components of the myofibrillar network could be altered in response to mechanical stress (Fig. 4). We first determined the expression of desmin, a key component of the Z-disk structure and potential substrate of the calpain system. Immunoblotting analysis indicated that eccentric exercise did not statistically affect the desmin level immediately and 1 day after exercise. A closer examination of the band pattern did not show any cleavage. However, the desmin level was increased about 2.5-fold (P < 0.001) on day 14 post exercise. In contrast, the level of α-actin remained unchanged. Expression of α-sarcoglycan, a component of the dystrophin glycoprotein complex (DGC), was significantly decreased immediately and 1 day after exercise by 58 and 54 %, respectively. On day 14 post exercise, the α-sarcoglycan level was not significantly different from control levels.
Figure 4. Effects of eccentric exercise on α-actin, α-sarcoglycan and desmin protein levels.
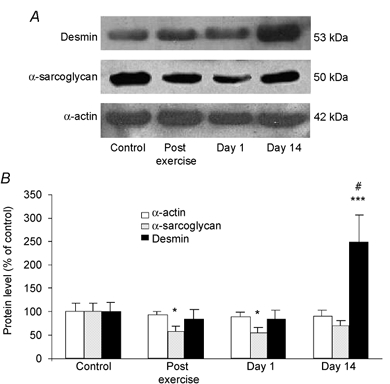
A, representative immunoblots of α-actin, α-sarcoglycan and desmin protein levels in control, post exercise, day 1 and day 14 muscle biopsies. B, immunoblotting quantification. Values are means ± s.e.m. (n = 8-12) and are expressed relative to control protein level. * P < 0.05 and *** P < 0.001 vs. control, # P < 0.001 vs. post exercise and day 1.
Effects of eccentric exercise on Hsp27 and αB-crystallin protein levels
On the basis of the ability of mechanical stress to increase desmin expression, we investigated whether the expression of the small Hsps, Hsp27 and αB-crystallin, was altered by the exercise. Immunoblotting analyses demonstrated a progressive increase in protein chaperone expression, which reached significant levels on day 1 post exercise (Fig. 5). At this time, Hsp27 and αB-crystallin were increased about 2.8- and 2.2-fold, respectively. On day 14, protein levels remained significantly elevated above control levels.
Figure 5. Effects of eccentric exercise on αB-crystallin and Hsp 27 protein levels.
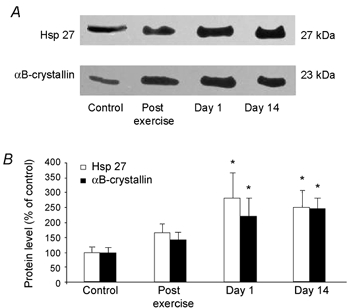
A, representative immunoblots of the small heat shock proteins, Hsp27 and αB-crystallin protein levels in control, post exercise, day 1 and day 14 muscle biopsies. B, immunoblotting quantification. Values are means ± s.e.m. (n = 9-10) and are expressed relative to control protein level. * P < 0.05.
Discussion
Intense and strenuous exercise is generally used to study skeletal muscle damage in humans (Fridén et al. 1983; Sargeant & Dolan, 1987; Gibala et al. 1995). In the present study, a moderate downhill running exercise was performed to elicit eccentric contractions of extensor muscles of the lower limbs, and simulate a physiological mechanical stress that could occur during everyday life. The mild increase in oxygen uptake and the low lactate levels observed in response to exercise clearly indicated a moderate increase in energy metabolism. In addition, our data showed that this exercise paradigm was sufficient to induce DOMS symptoms, as demonstrated by prolonged maximal power loss and muscle pain, increased release or leakage of muscle proteins, and disturbances of the myofibrillar network. Biopsies taken on day 1 post exercise showed an increased proportion of subjects with myofibrillar disruption compared to the corresponding samples taken immediately post exercise, suggesting that a progressive extension of the damaged area occurs in response to eccentric exercise. Furthermore, this may imply that muscle damage is not only induced by mechanical stress, but also depends on post-exercise events (Armstrong et al. 1991). On day 14 post exercise, examination of muscle samples showed that normal ultrastructural myofibrillar profiles had been restored, indicating the existence of a remodelling response after exercise-induced damage.
The disturbances of the cross-striated band pattern observed in the present study may be linked to the activation of proteases. We first focused our attention on the response of the calpain system. Since discrimination and quantification of the three calpain gene products is difficult to perform at the protein level (Kinbara et al. 1998), we used quantitative RT-PCR analysis to determine the mRNA level of calpains 1, 2, and 3. Although we cannot infer enzyme activity or protein level from our mRNA measurements, previous studies have shown that calpain mRNA level correlates at first approximation with protein level (Kumamoto et al. 1995) or enzyme activity (Spencer et al. 1997). One striking feature observed in this study was the differential expression pattern of calpain 2 mRNA level compared to calpain 3 and calpain 1 mRNA levels. Calpain 3 was downregulated immediately and on day 1 after exercise, while calpain 2 was upregulated on day 1. Such opposing changes between calpain 2 and 3 mRNA levels have been already reported in Yoshida AH-130 rat ascites hepatoma-related cachexia (Busquets et al. 2000), and in human neuromuscular disorders (progressive muscular dystrophy and amyotrophic lateral sclerosis; Ueyama et al. 1998), suggesting that calpain 3 could negatively regulate calpain 2. The mechanism underlying the counter-regulatory role of calpain 3 is currently unknown, but several arguments suggest that calpain 3 may act at the transcriptional level through the regulation of transcription factor activity (Baghdiguian et al. 1999). Ubiquitous calpains initiate the degradation of muscle-specific proteins in vitro including components of the myofibrillar network such as desmin, titin, nebulin, vimentin, α-actinin and talin (Saido et al. 1994; Huang & Forsberg, 1998). In the present study, the expression of calpain 2 peaked when most of the subjects experienced ultrastructural muscle damage. In agreement with others (Belcastro, 1993; Lieber & Fridén, 1996), our data suggest that calpain 2 may be involved in the early cleavage of muscle-specific proteins in response to exercise. However, the mechanical effect of eccentric contractions on myofibrillar structure may also contribute to the observed damage. These data may also be of importance in the understanding of the pathophysiological processes of LGMD2A. In skeletal muscle of patients deficient in calpain 3, the counter-regulatory function of calpain 3 on calpain 2 would not be effective, therefore leading to uncontrolled expression of calpain 2 and extensive, progressively irreversible, muscle damage.
In order to further explore the role of proteases in exercise-induced muscle damage, we next investigated the response of lysosomal and ubiquitin-proteasome pathways to eccentric exercise. In contrast to the calpain system, our results showed an increase in cathepsin B+L and ubiquitin-proteasome capacities at day 14. The present results add a new example of conditions under which Ca2+-independent proteolytic pathways are altered in human skeletal muscle. For ethical reasons, only four muscle biopsies were performed per subject in the present study. The time points chosen for muscle sampling did not permit determination of whether the increase in proteasome and cathepsin activities reported here may account for an early catabolic response. However, our data corroborate previous experiments carried out on rabbit skeletal muscle in which proteasome activity peaked after 14 days of chronic contractile activity (Ordway et al. 2000). Our data suggest that the observed increase in the ubiquitin-proteasome pathway may participate in the remodelling response of skeletal muscle to eccentric exercise. Regarding the activation of proteolysis in response to eccentric exercise, interleukin-6 (IL-6) appears to be a key factor (Ostrowski et al. 1998). IL-6 is known to induce proteolysis by activating cathepsins B and L and proteasome activities in skeletal muscle cells (Ebisui et al. 1995; Tsujinaka et al. 1996). Furthermore, contracting skeletal muscle is one of the major sites of IL-6 production. Interestingly, calpain 3 gene expression is also decreased in skeletal muscle of IL-6 transgenic mice (Tsujinaka et al. 1996). Therefore, IL-6 may be an overall regulatory factor for both Ca2+-dependent and Ca2+-independent proteolysis responses to eccentric exercise.
Our results showed that eccentric exercise had major effects on muscle protein leakage, myofibrillar organization and proteolysis pathways. We therefore investigated whether the expression of some structural proteins would be modified in response to exercise. Sarcoglycans belong to the dystrophin glycoprotein complex (DGC). Pathological studies suggest that the DGC may protect the sarcolemma from mechanical stress by linking the contractile apparatus to the extracellular matrix (ECM; Petrof et al. 1993; Holt & Campbell, 1998; Cohn & Campbell, 2000). In the present study, eccentric exercise markedly reduced the expression of α-sarcoglycan. Although the precise significance of this decrease is presently unknown, it correlates with the leakage of CK and myoglobin into the circulating blood. Thus, the loss of α-sarcoglycan may destabilize the sarcolemma leading to modifications of membrane permeability. In agreement with this hypothesis, α-sarcoglycan-deficient mice show loss of membrane integrity, changes in muscle force and profound destabilization of the DGC (Duclos et al. 1998). This decrease in the level of α-sarcoglycan protein may also regulate the intracellular calcium concentration. Indeed, α-sarcoglycan has an ecto-ATPase activity, which modulates the P2X receptor, a non-specific cationic channel (Betto et al. 1999). A reduction in α-sarcoglycan protein level may therefore lead to the persistent activation of P2X receptors, resulting in intracellular Ca2+ overload in muscle fibres (Betto et al. 1999). This may ultimately activate Ca2+-dependent proteolytic pathways. Desmin is a muscle-specific cytoskeletal protein, which synchronizes the contraction of myofibrils by connecting adjacent myofibrils at the Z-disk level, and the Z-disk to the sarcolemma (Tokayasu et al. 1982). Here, we report for the first time a dramatic increase in desmin protein level 14 days after cessation of exercise. This finding suggests that eccentric exercise elicits a remodelling response, which could reinforce the Z-disk through an increased resistance to mechanical constraints. Therefore, this may attenuate the deleterious effects of a second bout of eccentric exercise on muscle myofibrillar structure. Further experiments will be necessary to determine the physiological significance of this increase in desmin level. In contrast, the α-actin protein level remained unchanged in response to exercise, illustrating that molecular signals triggered by eccentric exercise differentially regulate muscle protein expression.
Little is known about the mechanisms elicited by eccentric exercise in the remodelling of myofibrillar structure. However, recent evidence suggests that the molecular chaperones, αB-crystallin and Hsp27, may have important roles. αB-crystallin interacts with desmin intermediate filaments and may prevent stress-induced protein aggregation (Djabali et al. 1997). Similarly, Hsp27, together with αB-crystallin, has been suggested to interact with microfilaments (Sugiyama et al. 2000; for review see Liu & Steinacker, 2001). A mutation in the αB-crystallin gene is a cause of desmin-related myopathy characterized by an accumulation of desmin aggregates (Vicart et al. 1998; Fardeau et al. 2000). These data strongly support the idea that αB-crystallin and Hsp27 are crucial for the assembly/maintenance and remodelling of myofibrillar structures. In the present study, αB-crystallin and Hsp27 were both markedly increased at day 1 post exercise, and remained elevated 14 days later. This is in agreement with Thompson et al. (2001), who showed an increase in Hsp27 2 days post eccentric exercise. Although not conclusive, our data, together with the above considerations, support the idea that αB-crystallin and Hsp27 may regulate intermediate filament and microfilament dynamics following eccentric exercise (1 and 2 days post exercise) and protect myofibrillar structures against a subsequent bout of eccentric exercise (day 14).
Altogether, the molecular adaptations reported in the present study may thus potentially decrease muscle fibre susceptibility to mechanical stress in particular by the reinforcement of myofibrillar structures. Although, it is unknown whether similar adaptations occur under non-eccentric exercise paradigms, one may suggest that active contractions of skeletal muscle from patients with neuromuscular disorders may potentially lead to a reinforcement of the myobrillar network. Further experiments will be necessary to test the therapeutic relevance of this hypothesis.
In summary, our data suggest that a single bout of moderate eccentric exercise elicits profound adaptations in human skeletal muscle. Although the specific mechanisms involved in this response remain to be determined, our results suggest that a proteolytic response, differential expression of structural proteins and induction of molecular chaperones are involved in this damage-repair process.
Acknowledgments
The authors thank M.-P. Blanc for her helpful technical assistance in micron electronic process, J. Castells, D. Dormois and M.-T. Linossier for technical assistance in subject testing. Dr M. Herrasse is also acknowledged for her helpful comments on the manuscript and Dr S. Cure for reviewing the English version of the manuscript (Genethon, Evry, France). This research was supported by grants from the Association Française contre les Myopathies. L. Féasson and D. Stockholm were recipients of a grant from the Association Française contre les Myopathies.
References
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. Sports Medicine. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N, Hay RT, Chemaly R, Halaby G, Loiselet J, Anderson LVB, De Munain AL, Fardeau M, Mangeat P, Beckmann JS, Lefranc G. Calpain3 deficiency is associated with myonuclear apoptosis and profound perturbation of the Iκα/NF-κB pathway in limb-girdle muscular dystrophy type A. Nature Medicine. 1999;5:503–511. doi: 10.1038/8385. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Thompson MW. Effect of training on eccentric exercise-induced muscle damage. Journal of Applied Physiology. 1993;75:1545–1551. doi: 10.1152/jappl.1993.75.4.1545. [DOI] [PubMed] [Google Scholar]
- Belcastro AN. Skeletal muscle calcium-activated neutral protease (calpain) with exercise. Journal of Applied Physiology. 1993;74:1381–1386. doi: 10.1152/jappl.1993.74.3.1381. [DOI] [PubMed] [Google Scholar]
- Belcastro AN, Shewchuk LD, Raj DA. Exercise-induced muscle injury: A calpain hypothesis. Molecular and Cellular Biochemistry. 1998;179:135–145. doi: 10.1023/a:1006816123601. [DOI] [PubMed] [Google Scholar]
- Betto R, Senter L, Ceoldo S, Tarricone E, Biral D, Salviati G. Ecto-ATPase activity of sarcoglycan (Adhalin) Journal of Biological Chemistry. 1999;274:7907–7912. doi: 10.1074/jbc.274.12.7907. [DOI] [PubMed] [Google Scholar]
- Busquets S, Garcia-MartineZ C, AlvareZ B, Carbo N, LopeZ-Soriano F, Argiles J. Calpain-3 gene expression is decreased during experimental cancer cachexia. Biochimica et Biophysica Acta. 2000;1475:5–9. doi: 10.1016/s0304-4165(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Clarkson MP, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. Journal of Applied Physiology. 1988;65:1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Medicine and Science in Sports and Exercise. 1992;24:512–520. [PubMed] [Google Scholar]
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle and Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Park K-Y, Semino-Mora C, Lee HS, Sivakumar K, Goldfarb LG. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. New England Journal of Medicine. 2000;342:770–780. doi: 10.1056/NEJM200003163421104. [DOI] [PubMed] [Google Scholar]
- Djabali K, De NÉCHAUD B, Landon F, Portier M-M. αB-crystallin interacts with intermediate filaments in response to stress. Journal of Cell Science. 1997;110:2759–2769. doi: 10.1242/jcs.110.21.2759. [DOI] [PubMed] [Google Scholar]
- Duclos F, Straub V, Moore S, Venzke D, Hrstka R, Crosbie R, Durbeej M, Lebakken C, Ettinger A, Van Der Meulen J, Holt K, Lim L, Sanes J, Davidson B, Faulkner J, Williamson R, Campbell K. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. Journal of Cell Biology. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clinical Science. 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- Fardeau M, Vicart P, Caron A, Chateau D, Chevallay M, Collin H, Chapon F, Duboc D, Eymard B, Tome FMS, Dupret JM, Paulin D, Guicheney P. Myopathie familiale avec surcharge en desmine, sous forme de matériel granulo-filamentaire dense en microscopie électronique, avec mutation dans le géne de l'αB-cristalline. Revue Neurologique. 2000;156:497–504. [PubMed] [Google Scholar]
- Fridén J, Kjörell U, Thornell L-E. Delayed muscle soreness and cytoskeletal alterations: an immunocytological study in man. International Journal of Sports Medicine. 1984;5:15–18. doi: 10.1055/s-2008-1025873. [DOI] [PubMed] [Google Scholar]
- Fridén J, Lieber RL. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Research. 1998;293:165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- Fridén J, Sjöström M, Ekblom B. A morphological study of delayed muscle soreness. Experientia. 1981;37:506–507. doi: 10.1007/BF01986165. [DOI] [PubMed] [Google Scholar]
- Fridén J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. International Journal of Sports Medicine. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, MacDougall JD, Tarnopolsky MA, Stauber WT, Elorriaga A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. Journal of Applied Physiology. 1995;78:702–708. doi: 10.1152/jappl.1995.78.2.702. [DOI] [PubMed] [Google Scholar]
- Henriksson KG. Semi-open muscle biopsy technique: A simple outpatient procedure. Acta Neurologica Scandinavica. 1979;59:317–323. [PubMed] [Google Scholar]
- Hirai S-I, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Letters. 1991;287:57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- Holt KH, Campbell KP. Assembly of the sarcoglycan complex. Journal of Biological Chemistry. 1998;273:34667–34670. doi: 10.1074/jbc.273.52.34667. [DOI] [PubMed] [Google Scholar]
- Hough T. Ergographic studies in muscle soreness. American Journal of Physiology. 1902;7:76–92. [Google Scholar]
- Huang J, Forsberg NE. Role of calpain in skeletal-muscle protein degradation. Proceedings of the National Academy of Sciences of the USA. 1998;95:12100–12105. doi: 10.1073/pnas.95.21.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinbara K, Ishiura S, Tomioka S, Sorimachi H, Jeong S-Y, Amano S, Kawasaki H, Kolmerer B, Kimuras S, Labeit S, Suzuki K. Purification of native p94, a muscle-specific calpain, and characterization of its autolysis. Biochemical Journal. 1998;335:589–596. doi: 10.1042/bj3350589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinbara K, Sorimachi H, Ishiura S, Suzuki K. Muscle-specific calpain, p94, interacts with the extreme C-terminal region of connectin, a unique region flanked by two immunoglobulin C2 motifs. Archives of Biochemistry and Biophysics. 1997;342:99–107. doi: 10.1006/abbi.1997.0108. [DOI] [PubMed] [Google Scholar]
- Kumamoto T, Ueyama H, Watanabe S, Yoshioka K, Miike T, Goll DE, Ando M, Tsuda T. Immunohistochemical study of calpain and its endogenous inhibitor in the skeletal muscle of muscular dystrophy. Acta Neuropathologica. 1995;89:399–403. doi: 10.1007/BF00307642. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fridén J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. Journal of Applied Physiology. 1996;80:278–284. doi: 10.1152/jappl.1996.80.1.278. [DOI] [PubMed] [Google Scholar]
- Linossier M-T, Dormois D, Fouquet R, Geyssant A, Denis C. Use of the force-velocity test to determine the optimal braking force for a sprint exercise on a friction-loaded cycle ergometer. European Journal of Applied Physiology. 1996;74:420–427. doi: 10.1007/BF02337722. [DOI] [PubMed] [Google Scholar]
- Liu Y, Steinacker JM. Changes in skeletal muscle heat shock proteins: pathological significance. Frontiers in Bioscience. 2001;6:12–25. doi: 10.2741/liu. [DOI] [PubMed] [Google Scholar]
- Mair J, Mayr M, MÜLLER E, Koller A, Haid C, Artner-Dworzak E, Calzolari C, Larue C, Puschendorf B. Rapid adaptation to eccentric exercise-induced muscle damage. International Journal of Sports Medicine. 1995;16:352–356. doi: 10.1055/s-2007-973019. [DOI] [PubMed] [Google Scholar]
- Mattson JP, Ross CR, Kilgore JL, Musch TI. Induction of mitochondrial stress proteins following treadmill running. Medicine and Science in Sports and Exercise. 2000;32:365–369. doi: 10.1097/00005768-200002000-00016. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Neufer PD, Chin ER, Demartino G. Chronic contractile activity upregulates the proteasome system in rabbit skeletal muscle. Journal of Applied Physiology. 2000;88:1134–1141. doi: 10.1152/jappl.2000.88.3.1134. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Hermann C, Banagash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in human in response to treadmill running. Journal of Physiology. 1998;513:889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, Hillaire D, Passos-Bueno M-R, ZatZ M, Tishfield JA, Fardeau M, Jackson CE, Cohen D, Beckmann JSR. Mutations in the proteolytic enzyme calpain 3 cause limb girdle muscular dystrophy type 2A. Cell. 1995;81:21–47. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- Rodenburg JB, BÄR PR, De Boer RW. Relations between muscle soreness and biochemical and functional outcomes of eccentric exercise. Journal of Applied Physiology. 1993;74:2976–2983. doi: 10.1152/jappl.1993.74.6.2976. [DOI] [PubMed] [Google Scholar]
- Saido TC, Sorimachi H, Suzuki K. Calpain : new perspectives in molecular diversity and physiological-pathological involvement. FASEB Journal. 1994;8:814–822. [PubMed] [Google Scholar]
- Sargeant AJ, Dolan P. Human muscle function following prolonged eccentric exercise. European Journal of Applied Physiology. 1987;56:704–711. doi: 10.1007/BF00424814. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Lu B, Tidball JG. Calpain II expression is increased by changes in mechanical loading of muscle in vivo. Journal of Cellular Biochemistry. 1997;64:55–66. doi: 10.1002/(sici)1097-4644(199701)64:1<55::aid-jcb9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Stockholm D, Barbaud C, Marchand S, Ammarguellat F, Barritault D, Richard I, Beckmann J, Martelly I. Studies on calpain expression during differentiation of rat satellite cells in primary cultures in the presence of heparin or a mimic compound. Experimental Cell Research. 1999;252:393–400. doi: 10.1006/excr.1999.4628. [DOI] [PubMed] [Google Scholar]
- Stockholm D, Herasse M, Marchand S, Praud C, Roudaut C, Richard I, Sebille A, Beckmann J. Calpain 3 mRNA expression in mice after denervation and during muscle regeneration. American Journal of Physiology – Cell Physiology. 2001;280:C1561–1569. doi: 10.1152/ajpcell.2001.280.6.C1561. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MMY, Tsui SKW, Yoshida S, Ohno S. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. Journal of Biological Chemistry. 2000;275:1095–1104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- Thompson H, Scordilis S, Clarkson P, Lohrer W. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiologica Scandinavica. 2001;171:187–193. doi: 10.1046/j.1365-201x.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- Tokayasu KT, Dutton AH, Singer SJ. Immunoelectron microscopic studies of desmin (skeletin). localization and intermediate filament organization in chicken skeletal muscle. Journal of Cell Biology. 1982;96:1727–1735. doi: 10.1083/jcb.96.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H, Monden M. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. Journal of Clinical Investigation. 1996;97:244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama H, Kumamoto T, Fujimoto S, Murakami T, Tsuda T. Expression of three calpain isoform genes in human skeletal muscles. Journal of Neurological Sciences. 1998;155:163–169. doi: 10.1016/s0022-510x(97)00309-2. [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nature Genetics. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]


