Abstract
The aim of this study was to test the hypothesis that prenatal hypoxia in rats might lead to consistent changes in the entrainment of the circadian clock by light. Pregnant female rats were placed in a chamber provided with hypoxic gas (10 % O2-90 % N2) at gestational day 5 and returned to normoxia before delivery. Once adult, rats born to hypoxic mothers had significant alterations in their circadian rhythm of locomotor activity (recorded in freely accessible running wheels). Under a regular 12/12 light/dark (LD) cycle, they showed a phase advance of their rhythm of activity (mean phase advance of 87 min) and were less active than control rats. After an abrupt 6 h phase delay in the LD cycle, rats from the prenatal hypoxic group (PNH) took significantly more time to resynchronise to the new LD cycle compared to controls (+53 %; 6.0 ± 1.5 vs. 9.2 ± 0.5 days respectively). Under constant darkness, PNH and control rats had a similar period of activity (24.27 ± 0.20 vs. 24.40 ± 0.13) but the response of PNH rats to a light pulse in the early subjective night was less marked than that of control rats (101 ± 9 vs. 158 ± 13 min). When submitted to acute restraint stress, PNH rats had a prolonged secretion of corticosterone compared to controls. These results indicate that prenatal hypoxia is a factor that has long lasting consequences for the functional output of the biological clock and the hormonal response to stress.
Daily rhythms of activity, mainly represented by sleep/wake alternations, are of great importance in living organisms and allow them to be in phase with the natural changes occurring throughout the 24 h rotation of the earth on its axis. In mammals, the main biological clock is located in the suprachiasmatic nuclei (SCN – lying just above the optical chiasma in the ventral hypothalamus); its destruction leads to the abolition of many physiological rhythms such as feeding, drinking, locomotor activity, body temperature, sleep-wake, corticosterone, melatonin and growth hormone secretion (Ruzak & Zucker, 1979; Meijer & Rietveld, 1989; Turek & Van Reeth, 1995). Transplantation of neural grafts containing SCN tissue is able to restore rhythms to arrhythmic animals whose own nucleus has been ablated (Lehman et al. 1987; Ralph et al. 1990). Under laboratory conditions devoid of any environmental time cues (also termed ‘free-running’ conditions; i.e. constant light or constant darkness), the clock has its proper period, that is generally found to be slightly more than 24 h and thus referred to as ‘circadian', i.e. about one day. To avoid a mismatch between internal and external rhythmicity, which may have dramatic consequences for an organism living under natural conditions, the rhythm of the internal clock is subjected to environmental influences, light being one of the most important external factors known to synchronise and entrain the rhythm of the biological clock on a 24 h basis in animals (Takahashi & Menaker, 1982; Turek & Van Reeth, 1995). This paradigm is very important in chronobiology, and is also clinically relevant as mood disorders are generally associated with alterations of the circadian rhythmicity and SCN functional output (Koehl et al. 1999; Maccari & Van Reeth, 2000; Maccari et al. 2001; Taillard et al. 2001; Zhou et al. 2001).
Previous studies reported that sustained hypoxia in adults affects the functioning of the internal clock located in the suprachiasmatic nucleus (Poncet et al. 1999). The fetal suprachiasmatic nucleus is known to be entrained by signals related to the maternal circadian system (Davis, 1997; Davis & Mannion, 1988), however a noxious maternal environment, such as repeated stress during gestation, also appears to disturb the circadian rhythmicity in adult offspring (Koehl et al. 1999; Maccari & Van Reeth, 2000; Maccari et al. 2001). In the present study, we hypothesised that gestational hypoxia, a condition found in many pathological conditions in pregnant women and naturally associated with life at high altitude, can cause long lasting consequences for the synchronisation of the circadian clock to light.
Methods
All experiments were carried out according to the ethical principles laid down by the French (Ministére de l'Agriculture) and EU Council directives for care of laboratory animals (No. 02889). At the end of the experiments, all animals were killed by exposure to a rising concentration of CO2. All the animals used in this study were sibling pups from a previously published study (Mamet et al. 2002).
Gestational hypoxia
Sixty pregnant Sprague-Dawley rats (IFFA-CREDO, l'Arbresle, France) were housed in a temperature-controlled room (26 ± 1 °C) with a 12/12 light/dark cycle and allowed free access to food and water. The first day of gestation was determined on the basis of a vaginal smear. Pregnant rats were first exposed to hypoxia on the fifth day of gestation so as not to disturb implantation of the embryos. From the 5th to 20th day of gestation, the experimental group was housed in a Plexiglas chamber, with a normobaric hypoxic atmosphere consisting of 10 % O2-90 % N2. The O2 and CO2 contents of this atmosphere were monitored twice daily using Servomex analysers (Servomex Co., Norwood, MA, USA). The CO2 expired by the rats was eliminated by circulating the atmosphere through soda lime, so that the atmospheric content of this gas never exceeded 0.1 %. The water contained in the expired gas was trapped in a chilled glass tank. On the 20th day of gestation, hypoxic pregnant rats were returned to normoxia (21 % O2), under which they gave birth. Immediately after birth, the male pups were grouped together, and then randomly redistributed to nursing females (n = 60) which had never been exposed to hypoxic conditions (10-12 pups per female). This procedure was followed to avoid sex differences, eliminate variations between litters, ensure a standard nutritional status, and avoid any possible effect of hypoxia on the nutritional quality of the milk. These offspring were designated as the prenatal hypoxic (PNH) group. Pups from another 60 pregnant Sprague-Dawley rats, whose gestation occurred under normoxic conditions (21 % O2), but were otherwise treated in the same manner and placed in a similar Plexiglas chamber, were designated as the control (cont) group.
Randomly selected pups from different litters were weighed at birth and during growth. After weaning (at postnatal day 21), one pup from each litter was chosen as a candidate for the recording of its running wheel activity. Pups were grouped according to their status (4-6 animals/cage) and maintained under regular housing conditions.
Maternal hormones during hypoxia
Blood samples of normoxic and hypoxic mothers were taken from the tail vein in heparanised microtubes at gestational days 12 and 19. After centrifugation (5000 g for 15 min), the plasma was collected, aliquoted and kept at −80 °C for subsequent measurements of catecholamines (noradrenaline and adrenaline-HPLC) and corticosterone (radioimmunoassay; see below).
Running wheel activity
All rats were 7–9 months old at the time of experiments. Rats were housed in light tight chambers equipped with continuously operating ventilating fans and placed in individual cages equipped with a running wheel that allowed continuous recording of locomotor activity via an on-line computer (Chronobiology kit, Stanford Software System, CA, USA) under a regular 12/12 LD cycle (light intensity was set at 20–30 lx at cage floor level). During the course of the experiments, food and water were provided ad libitum, room temperature (22 °C) and humidity (60 %) were kept constant. A first group of rats (n = 8 for each group) was used to analyse the rhythm of circadian activity under a regular 12/12 LD cycle, the response to an abrupt phase shift of the LD cycle, and the hormonal response to acute restraint stress. A second group of rats (n = 8 for each group) was used to analyse their rhythmicity in constant darkness and their response to an acute light pulse during the early subjective night, 3 h after the onset of activity (circadian time 15 h).
Activity under a regular 12/12 LD cycle and abrupt phase shift
After 10–15 days of adaptation to the running wheels, the rhythms of activity were individually analysed over six consecutive days. The onset of activity was identified with a 5 min resolution and was defined as the first time point at which the mean intensity of activity was above 10 % of the maximum and remained above that point for at least 50 % of the time during the following 30 min. The reversed procedure was used for the cessation (offset) of activity (first time point below 10 % of maximum and activity remained below that point for at least 50 % of the time during the following 30 min). The time elapsing between the onset and offset of activity was defined as the total time of nocturnal activity, the peak value of activity and peak hour of activity were directly determined on the actogram for each animal. The mean 24 h integrated activity was determined by adding minute-by-minute the number of revolutions in the wheel for each animal. The data were then plotted hour-by-hour; this represented the mean distance run by the animals.
At the end of the recording of normal activity, rats were exposed to an abrupt 6 h delay of the LD cycle: on day zero (D0) of the experiment, lights were turned off 6 h later than previous days and the new LD (12/12) cycle was maintained for several days. The time to resynchronise to the new LD cycle was defined as the number of days for the animal to exhibit a regular activity for at least three following days. The first day of regular activity respective to D0 was defined as the day of full resynchronisation.
Hormonal response to acute stress in hypoxic offspring
Following recording of running wheel activity, rats were returned to normal housing conditions (individually housed, without wheels) for two to three weeks before testing their hormonal response to acute stress. Acute restraint stress was induced by placing rats in individual transparent plastic tubes (7 cm diameter, 19 cm long) under bright light (1500 lx). At the onset of stress, blood was rapidly collected (2 to 3 min) in heparinised tubes via the tail vein to determine basal corticosterone levels. The second sampling was performed 20 min after restraint stress was initiated. The rats were then returned to their home cages until and between the next blood samplings that were performed 60 and 90 min after the initiation of stress. Blood corticosterone levels were determined by radioimmunoassay (see below).
Activity in constant darkness and light-pulse-induced phase shift
After habituation to the cage with the running wheel and entrainment under 12/12 LD cycle for 7–10 days, PNH and control rats were transferred into constant darkness (DD) and remained undisturbed until a steady-state phase of free-running activity was achieved. After 7 days of stable activity in DD, rats were exposed to a 10 min monochromatic light pulse (20-30 lx at floor level) at circadian time 15:00 h (CT15, i.e. 3 h after the onset of locomotor activity) and then returned to DD for continuous recording of activity. The period of activity in DD (τ) was calculated for the 7 days preceding light stimulation by the χ2 periodogram function included in the chronobiology kit software. The number of days that elapsed from the light pulse until new regular activity was achieved was calculated for each animal by visual examination of the actogram.
Hormonal assays
Catecholamines
Blood samples were centrifuged (5000 g for 15 min) and plasma noradrenaline (NA) and adrenaline (A) were extracted on alumina and detected using high-performance liquid chromatography (HPLC) assay with electrochemical detection. 100 μl of plasma was transferred into a 1.5 ml polyethylene tube containing 7 mg acid-washed alumina, 13 mm sodium metabisulfite (125 μl), 2 m Tris-HCl buffer pH 8.8 containing 27 mm ethylenediaminetetracetate (250 μl), bi-distilled water (500 μl) and internal standard dihydroxybenzylamine (2.3 pmol). After agitation on an Eppendorf mixer for 15 min, the supernatant was removed by vacuum aspiration and the alumina washed three times with 600 μl 5 mm Tris-HCl containing 2.7 mm EDTA (pH 8.8). Finally the catecholamines were eluted from the alumina with 60 μl 0.25 m acetic acid-0.15 mm sodium bisulfite-0.6 mm EDTA on an Eppendorf mixer for 15 min. A 16 μl sample of the eluate was injected into the HPLC column. The mobile phase consisted of 30 mm citric acid, 50 mm sodium acetate, 1 mm EDTA, 1.7 mm heptane sulfonate, 60 ml l−1 methanol-500 mm acetic acid. The flow rate was 0.5 ml min−1. The potential was set at 0.68 V and the sensitivity range at 0.5 pmol NA. The detection limits were 0.03 and 0.05 pmol for NA and A, respectively.
Corticosterone
Blood samples were centrifuged (5000g for 15 min) and plasma corticosterone was assayed by radioimmunoassay using a highly specific corticosterone antiserum (ICN Biomedicals, Cleveland, OH, USA) as previously described (Dugovic et al. 1999). The detection threshold was 1 ng ml−1. The interassay and intra-assay variations were, respectively, 6 and 3.5 % at a mean value of 1.5 ng tube−1 and 6.8 and 4 % at a mean value of 10 ng tube−1.
Statistical analyses
All analyses were done with the Statview software (Abacus Concepts, Berkeley, CA, USA). For single measurements, data were analysed by a simple ANOVA. For more complex data (mean integrated activity and corticosterone response to acute restraint stress) data were analysed by ANOVA for repeated measures then for factorial measures by a simple ANOVA and Fisher's protected least significant difference (PLSD) post-ANOVA test for each time point. Statistical values of the PLSD are indicated by symbols in the figures. All data are presented as means ± s.e.m. The level of significance for all analyses was set at 0.05.
Results
Maternal plasma catecholamines and corticosterone during hypoxia
Gestational hypoxia produced a marked increase of plasma noradrenaline at embryonic day 12 (E12; +38 %) and E19 (+97 %) compared to control mothers and increased adrenaline at E19 only (+72 %). Plasma corticosterone levels measured 3 h after lights-off were not significantly altered by gestational hypoxia (Table 1).
Table 1.
Plasma noradrenaline, adrenaline and cortkosterone at embryonic days 12 and 19 in pregnant control and hypoxic pregnant rats
| E12 | E19 | |||
|---|---|---|---|---|
| Cont(10) | PNH(11) | Cont(11) | PNH(11) | |
| NA (pmol ml−1) | 6.41 ± 0.56 | 8.87 ± 0.73* | 8.25 ± 0.40 | 16.3 ± 1.70*† |
| A(pmol ml−1) | 2.12 ± 0.31 | 1.57 ± 0.32 | 1.53 ± 0.19 | 2.64 ± 0.45* |
| Cort (ng ml−1) | 236 ± 21 | 227 ± 40 | 195 ± 28 | 186 ± 28 |
NA, plasma nor adrenaline; A, adrenaline; Cort, corticosterone; E12, embryonic day 12; E19, embryonic day 19; Cont, control rats; PNH, hypoxic pregnant rats. All values are mean ± S.E.M., the number of animals in each group is indicated in column headings (n).
P < 0.05 PNH vs. Cont for same age
E19 vs. E12 for same group.
Body weight at birth and during growth
As previously described, exposure of pregnant female rats to hypoxia resulted in decreased litter size (from a mean of 13 ± 1.3 pups to 6.7 ± 1.5; P < 0.05). Prenatal hypoxia also significantly reduced the body weight of the offspring at birth and during the first postnatal week, while in older animals there was no apparent effect of prenatal hypoxia on body weight (Table 2). Similarly, in the 7-9-month-old animals used for the running wheel activity, there was no apparent effect of prenatal hypoxia on body weight (control, 515 ± 14 g, n = 16; PNH, 536 ± 17 g; n = 16; P = 0.36).
Table 2.
Body weight of control and prenatal hypoxic pups at birth and until weaning (at postnatal day 21)
| Age (days) | 0 | 7 | 14 | 21 | |
|---|---|---|---|---|---|
| BW(g) | Cont | 6.20 ± 0.05 (18) | 17.7 ± 0.4 (23) | 34.2 ± 0.4 (20) | 55.8 ± 0.9 (15) |
| PNH | 5.88 ± 0.09* (20) | 16.0 ± 0.4* (23) | 32.8 ± 1.0(20) | 56.0 ± 1.3(14) |
Cont, control rats; PNH, prenatal hypoxic rats. All values are mean ± s.e.m.
P < 0.05 vs. Cont. The number of animals is indicated for each age and group (n).
Activity in running wheels under regular light/dark cycle and time to resynchronise to a new LD
From the eight rats used in each group, two control and one PNH rat showed marked irregularities in their circadian rhythmicity, and were discarded from subsequent analyses. Under a regular LD cycle (12/12) control rats began to be active 83 ± 22 min (n = 6) after lights-off, while hypoxic rats began to be active 4.3 ± 15 min (n = 7; P < 0, 01) before lights-off (Fig. 1). Hypoxic rats were less active than normoxic rats as shown by the clear difference of the mean integrated wheel revolution (Fig. 2). There was no difference in the timing of cessation of activity at the end of the dark phase (5.00 ± 32 min after lights-on for control vs. 7.14 ± 40 min in PNH), in the peak value of activity (13.2 ± 3.4 wheel revolutions min−1 in control vs. 8.69 ± 2.8 in PNH), in the timing of the maximum of activity during the dark phase (17:40 ± 1:00 h, circadian time for control, vs. 16:45 ± 1:30 for PNH), or in the total time of activity between onset and cessation of activity (10:42 ± 00:15 h in control, vs. 12:11 ± 00:28 in PNH).
Figure 1. Activity of PNH and control rats under regular LD cycle.
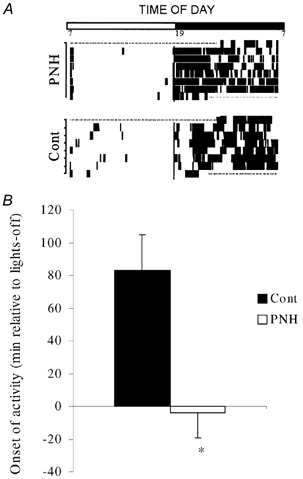
A, representative actograms showing the phase advance in one PNH compared to one control (Cont) rat during six consecutive days under regular (12/12) LD cycle (see LD bar above graph). B, group mean for the onset of activity (minutes relative to lights-off, see details in text) in PNH and control rats. Values are means ± s.e.m. * P < 0.05, PNH vs. Cont.
Figure 2. Mean integrated daily activity recorded under regular LD cycle during 6 consecutive days for controls (Cont) and prenatally hypoxic rats (PNH).
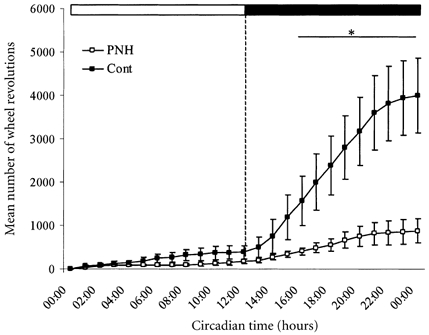
Abscissa, light hours elapsed from circadian time 00:00 to 12:00 h and dark from 12:00 to 00:00 h (see LD bar above graph). Mean integrated activity (mean number of wheel revolutions; ordinate) of PNH rats was significantly lower than control from circadian time 16:00 to 24:00 h. * P < 0.05, PNH vs. Cont. Values are means ± s.e.m.
When exposed to an abrupt shift of the LD cycle, hypoxic rats needed more time than control rats to resynchronise to the new cycle (9.2 ± 0.5 days vs. 6.0 ± 1.5 days; P < 0.05; Fig. 3).
Figure 3. Activity of PNH and control rats during 6 h phase shift.
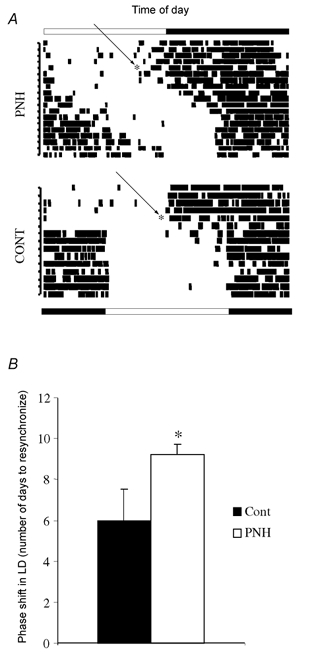
A, representative actograms recorded during the abrupt 6 h phase shift (D0 of experiment indicated by arrowed *; see text) in one PNH compared to one control (Cont) rat. LD cycles before and after phase shift are respectively indicated above and below the actograms. B, mean number of days to resynchronise to the new LD cycle after an abrupt 6 h phase delay in control and PNH rats. Values are means ± s.e.m. * P < 0.05, PNH vs. Cont.
Light-pulse induced phase delays in constant darkness
Under free-running conditions, there was no difference between groups in the period of circadian activity (control, 24.40 ± 0.13 h; PNH, 24.27 ± 0.20 h; P = 0.59; n = 8 for each group). After exposure to the light pulse at CT15, PNH rats showed a shorter phase delay of their rhythm of activity (101 ± 9 min) than control rats (158 ± 13 min; P < 0.005; Fig. 4).
Figure 4. Activity of PNH and control rats during constant darkness.
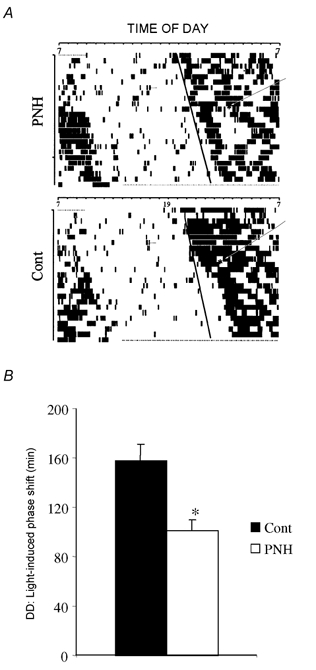
A, representative actograms recorded in constant darkness (DD) before and after a single light pulse at circadian time 15:00 h (indicated by arrrowed * in each actogram) in one PNH compared to one control (Cont) rat. Continuous line in each actogram shows the regular shift of circadian activity before the light pulse. For each animal this line was the reference to calculate the effect of the light pulse. B, mean values of phase shift (min) induced by a pulse of light at circadian time 15:00 h, under constant darkness in control and PNH rats. Values are means ± s.e.m. * P < 0.05, PNH vs. Cont.
Plasma corticosterone stress response
There was no difference between control and PNH rats in the basal level of corticosterone (control, 37.8 ± 20 ng ml−1vs. PNH, 32.5 ± 7.3 ng ml−1; not significant) and the early stress-induced increase of corticosterone (Fig. 5). PNH rats clearly showed a prolonged response to stress compared to control animals that was evidenced by a sustained elevation of plasma corticosterone 90 min after the onset of stress (control, 185 ± 22 ng ml−1vs. PNH, 334 ± 34 ng ml−1; P = 0.002; Fig. 5).
Figure 5. Hormonal response to acute restraint stress in adult PNH and control rats.
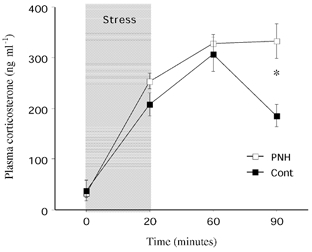
Plasma corticosterone (ng ml−1) before (0), 20, 60 and 90 min after the onset of 20 min of acute restraint stress (shaded area). All values are means ± s.e.m. * P < 0.05 PNH, vs. Cont.
Discussion
The present results show that prenatal hypoxia induced, in the adult offspring, marked alterations of the functional organisation of the circadian rhythm of activity associated with decreased sensitivity of the biological clock to light. Under a regular LD cycle, PNH rats had a phase advance of the onset of activity and were less active than control rats. After an abrupt phase shift of the LD cycle, they needed more time to resynchronise to the new LD cycle. Under free-running conditions (constant darkness) hypoxic rats had a similar period of activity, but their response to a single light pulse at CT15 was blunted compared to control rats. As previously reported by others in aged rats (28-month-old), prenatal hypoxic rats had a prolonged hormonal response to acute stress (Nyakas et al. 1994), that may suggest an impaired feedback response of the neuro-hormonal axis controlling corticosterone secretion (Koehl et al. 1999). Additionally, prenatal hypoxia induced a marked elevation of plasma catecholamines in pregnant mothers, but failed to induce any changes in plasma corticosterone, thus indicating that the effects of prenatal hypoxia are unlikely to be the consequences of maternal corticosterone exposure.
Methodological considerations
In this model of prenatal hypoxia, birth occurs in normoxia and we used normoxic nursing females in order to eliminate possible noxious effects of hypoxia on the nutritional quality of milk. Birth weight is clearly reduced by prenatal hypoxic exposure and the increased placental weight and placental-to-body weight ratio in PNH pups (Mamet et al. 2002) is a specific index of maternal hypoxia rather than low protein diet (de Grauw et al. 1986).
As classically done in chronobiology, we used the circadian rhythm of activity as a general output of the internal clock, this being recognised as a useful tool to assess the functionality of the clock which is located within the suprachiasmatic nuclei. The internal timing system that regulates the circadian rhythm of activity also regulates most if not all 24-hour rhythms within the organism, thus leading to the generally accepted view that when alterations of the circadian clock regulating the rhythm of locomotor activity can be monitored by running wheel activity, one can be confident that the other rhythms regulated by the clock (such as endocrine and neural rhythms) are also altered (Turek & Van Reeth, 1995).
Animal models of prenatal hypoxia
One of the pending questions about animal models of prenatal hypoxia, such as the one used in this study, is to assess their relevance for clinical cases of fetal hypoxaemia. Although we did not perform maternal or fetal sampling to measure blood gases, indirect comparisons may help to address this point. In clinical cases of pre-eclampsia, fetal arterial PO2 is decreased to levels between 15 and 20 mmHg and in most severe cases fetal arterial PO2 can be reduced to almost 10 mmHg, i.e. more than 20 mmHg below the mean normal expected value (Okamura et al. 1992). In fetal lambs, decreasing the maternal inspired fraction of O2 to 12–13 % (PO2 around 90–100 mmHg – similar to what is found in major high altitude cities) for 28 days decreases fetal arterial PO2 from 29.7 to 19.1 mmHg (Kitanaka et al. 1989). In a model of acute hypoxia in fetal sheep, lowering the inspired fraction of oxygen to 8 % (PO2 around 60 mmHg) decreases fetal arterial PO2 from 23.8 to 14.6 mmHg (Adams et al. 2001). The level of hypoxia used in our model (10 % O2 or PO2 around 75–80 mmHg) should therefore reduce fetal arterial PO2 to levels between 20 and 15 mmHg, i.e. similar to what is observed in regular cases of pre-eclampsia. On the other hand it seems appropriate to mention that the level of oxygen pressure used in our study is similar to that observed at an altitude between 5500 to 6000 m above sea level. Given that the highest permanent high altitude inhabitants are believed to live at between 5000 and 5500 m (Wu, 2001), and that most high altitude residents are found between 3000 and 4000 m (with PO2 around 100 mmHg), the model used in this study appears to be relevant only for the highest altitude residents (and only if pregnant women stay at those very high altitudes) otherwise our model would be slightly more severe than what would occur in high altitude residents, and its relevance for this situation may be questioned. Nevertheless, one study reported an increased incidence of pre-eclampsia from 3 % at low altitude to 16 % at a moderate altitude of 3100 m in Colorado, USA (Palmer et al. 1999), leading to enhanced pre-term deliveries, marked reduction of birth weight and very high incidence of small-for-gestational-age babies in pre-eclamptic mothers living at 3100 m (Zamudio et al. 1993; Zamudio et al. 1995b). We are not aware of any report of fetal arterial oxygen tension in such cases, but low to very low levels may be expected.
Another question regarding our model of prenatal hypoxia was to assess whether it may be a factor of non-specific maternal stress increasing the release of stress hormones. Indeed we found similar levels of corticosterone between control and PNH mothers, while plasma adrenaline and noradrenaline were markedly increased during PNH. Similar increases of sympatho-adrenal activity were previously reported in adult rats exposed to long-term hypoxia (Dalmaz, 1987, 1994).Thus specific activation of the maternal sympathetic system is apparent during prenatal hypoxia, which may be a contributing factor in the increased systemic vascular resistance and reduction of utero-placental blood flow during gestation at high altitude in humans or experimental animals (Harrison & Moore, 1990; Zamudio et al. 1995a).
Apart from pre-eclampsia, anaemia, which may be of fetal or maternal origin, also leads to fetal hypoxaemia (Dallman & Mentzer, 1996), and is particularly important in African populations in which the occurrence of sickle cell disease is high and clearly compromises fetal growth (Leborgne-Samuel et al. 2000). Iron deficiency during gestation is found in as many as 20 % of women in industrialised countries (Haram et al. 2001) and is also a factor that clearly impairs fetal oxygenation if not recognised and treated.
Fetal responses to hypoxia
The fetal response to acute hypoxia includes a redistribution of blood flow from peripheral to vital organs such as the brain, heart and adrenals, which allows proper oxygen delivery to these organs despite low oxygen content in fetal blood. The blood flow redistribution is mediated by various mechanisms including peripheral chemoreceptor activation, hormonal responses, and local vascular reactivity to low oxygen (Richardson & Bocking, 1998). Growth restriction occurs in fetuses in response to chronic hypoxaemia, with an associated decrease of protein synthesis and related oxygen consumption, which provides some protection against low PO2, and may even result in normalised arterial oxygenation (Richardson & Bocking, 1998). Nevertheless, chronic hypoxaemia in ovine foetuses induces an increased expression of both protein and mRNA of the neural subtype of nitric oxyde synthase (Aguan et al. 1998) and causes increased extracellular glutamate concentration in the cerebral cortex in the near-term fetal sheep (Henderson et al. 1998); both of these responses may contribute to the neural damage induced by chronic fetal hypoxaemia. Other studies reported that hypoxaemia prolonged for 48 h delays or inhibits the cell migration rate in the hippocampus in near-term ovine fetuses (Braaksma et al. 1999). In our model of chronic prenatal hypoxia in rats, an increase of the hypoxia inducible factor-1 αmRNA – a transcription factor exclusively activated by cellular hypoxia – and glucose transporters in the brain of fetuses has been reported as a result of PNH (Royer et al. 2000), thus showing selective hypoxic responses and direct effects of hypoxaemia in the fetal brain. Given the intrinsic complexity of the fetal response to chronic hypoxaemia, it still appears difficult to clearly underline a possible mechanism for the reported circadian alterations, nevertheless direct or indirect neural damage and/or alterations in the architectural development of neural networks within the suprachiamstic nuclei may be hypothesised.
Prenatal hypoxia impairs the ability of the circadian clock to be properly entrained by light
As the present results clearly show that PNH alters the SCN response to light rather than the time-counting mechanism itself (unaltered periodicity of running wheel activity in constant darkness) the discussion will focus on the SCN responses to light.
The main biological clock located within the SCN is a complex neural network and molecular machinery with specific genes being activated in a time-based pattern within SCN neurones (King & Takahashi, 2000). The SCN receives retinal projections through the retino-hypothalamic tract (RHT) and light pulses during the early night, such as used in this study, induce glutamate release from RHT terminals, which activates numerous types of postsynaptic glutamate receptors, including the NMDA receptors which play a prominent role in light-induced phase delay. Intracellular light/glutamate signalling during the early night implicates the activation of nitric oxide synthase (NOS) and the release of NO, and requires the activation of neuronal ryanodine receptors to release intracellular stores of Ca2+ that finally induces activation of target genes including the non-specific c-fos protooncogene and specific genes involved in generating and setting the circadian time (Ding et al. 1998; Tischkau et al. 2000; van Esseveldt et al. 2000). Accordingly a hypothetical mechanism of impaired SCN response to light would include impaired glutamate release from RHT projections to the SCN upon retinal light stimulation, desensitisation or a decreased number of NMDA glutamate receptors and/or an impaired intracellular response to glutamate receptor activation.
Prenatal hypoxia leads to prolonged hormonal response to acute stress
Specific tests of emotional reactivity and the behavioural response to novelty show that rats made hypoxaemic during gestation have a longer latency for the start of ambulating in an open-field test and are less active than control rats throughout the test, thus showing that PNH leads to increased emotionality (Nyakas et al. 1994). Additionally, in a conditioned fear situation, vocalisation of aged (28 months-old) PNH rats is increased compared to controls and PNH rats display a prolonged plasma corticosterone stress response and have a higher adrenal weight than their controls (Nyakas et al. 1994, 1996). All these features are considered to be consistent signs of anxiety-like behaviour. Our own results showing that PNH markedly reduces the spontaneous activity in freely moving rats, and induces a prolonged plasma corticosterone response to stress are consistent with the general hypothesis that prenatal hypoxia might trigger long-term behavioural consequences.
Concluding remarks
Mood disorders, such as depression, are generally associated with marked alterations of the circadian rhythmicity and complex relationships between mood disorders and the biological clock have been postulated many years ago (Van Cauter & Turek, 1986). In animal models of mood disorders the organisation of the sleep/wake cycle and/or the ability of the internal clock to be correctly entrained by light is clearly impaired (Koehl et al. 1999; Maccari & Van Reeth, 2000; Maccari et al. 2001). Sleep alterations and a prolonged response to stress in rats born to mothers submitted to repeated stress during gestation have also been reported and strongly suggest that these rats exhibit clinical signs of depression- or anxiety-like behaviour (Dugovic et al. 1999; Maccari & Van Reeth, 2000; Maccari et al. 2001). In humans, recent studies showed that the circadian clock can have a major impact on self-reported morbidity and health (Taillard et al. 2001) and that severe clinical depression is associated with consistent neurochemical changes within the SCN (Zhou et al. 2001). To date the causal relationship between mood disorders and disturbances of circadian rhythm (i.e. altered rhythm may participate in the onset of depression or depression may cause rhythm alterations) is still unknown. The importance of the present findings for human health remain to be questioned, but the demonstration that prenatal events associated with fetal hypoxaemia will later compromise the functional ability of the circadian clock to be properly entrained by light appears to be of clinical significance.
Acknowledgments
This work was supported by the Université Libre de Bruxelles, Belgium, Région Rhône-Alpes (grant ‘Souffrance foetale et Maturation neuronale'), the Centre National de la Recherche Scientifique and the Université Claude Bernard Lyon 1, France. V.J. and F.L. held a fellowship from the Janssen Research Foundation. J.M. held a fellowship from the Ministére de l'Enseignement Supérieur et de la Recherche, France.
References
- Adams MB, Brown RE, Gibson C, Coulter CL, McMillen IC. Tyrosine hydroxylase protein content in the medulla oblongata of the foetal sheep brain increases in response to acute but not chronic hypoxia. Neuroscience Letters. 2001;316:63–66. doi: 10.1016/s0304-3940(01)02381-3. [DOI] [PubMed] [Google Scholar]
- Aguan K, Murotsuki J, Gagnon R, Thompson LP, Weiner CP. Effect of chronic hypoxemia on the regulation of nitric-oxide synthase in the fetal sheep brain. Brain Research. Developmental Brain Research. 1998;111:271–277. doi: 10.1016/s0165-3806(98)00145-x. [DOI] [PubMed] [Google Scholar]
- Braaksma MA, Douma BR, Nyakas C, Luiten PG, Aarnoudse JG. Delayed neuronal migration of protein kinase Cgamma immunoreactive cells in hippocampal CA1 area after 48 h of moderate hypoxemia in the near term ovine fetus. Brain Research. Developmental Brain Research. 1999;114:253–260. doi: 10.1016/s0165-3806(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Dallman PR, Mentzer WC. Blood and blood forming tissues: anemia. In: Rudolph AM, Hofman JIE, Rudolph CD, editors. Rudolph's Pediatrics. 20. Stamford, Connecticut: Appleton and Lange; 1996. pp. 1172–1220. [Google Scholar]
- Dalmaz Y, Pequignot JM, Cottet-Emard JM, Peyrin L. Adrenal response to long-term hypoxia is still increased after carotid body denervation in rat. Journal of Applied Physiology. 1994;76:1049–1054. doi: 10.1152/jappl.1994.76.3.1049. [DOI] [PubMed] [Google Scholar]
- Dalmaz Y, Pequignot JM, Cottet-Emard JM, Tavitian E, Peyrin L. Sustained enhancement of the catecholamine dynamics in rat carotid bodies, adrenals, sympathetic ganglia and target organs under long-term moderate hypoxia. Biomedica Biochimica Acta. 1987;46:899–902. [PubMed] [Google Scholar]
- Davis FC. Melatonin: role in development. Journal of Biological Rhythms. 1997;12:498–508. doi: 10.1177/074873049701200603. [DOI] [PubMed] [Google Scholar]
- Davis FC, Mannion J. Entrainment of hamster pup circadian rhythms by prenatal melatonin injections to the mother. American Journal of Physiology. 1988;255:R439–448. doi: 10.1152/ajpregu.1988.255.3.R439. [DOI] [PubMed] [Google Scholar]
- De Grauw TJ, Myers RE, Scott WJ. Fetal growth retardation in rats from different levels of hypoxia. Biology of the Neonate. 1986;49:85–89. doi: 10.1159/000242515. [DOI] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, Mcpherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Maccari S, Weibel L, Turek FW, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. Journal of Neuroscience. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haram K, Nilsen ST, Ulvik RJ. Iron supplementation in pregnancy-evidence and controversies. Acta Obstetricia et Gynecologica Scandinavica. 2001;80:683–688. doi: 10.1034/j.1600-0412.2001.080008683.x. [DOI] [PubMed] [Google Scholar]
- Harrison GL, Moore LG. Systemic vascular reactivity during high-altitude pregnancy. Journal of Applied Physiology. 1990;69:201–206. doi: 10.1152/jappl.1990.69.1.201. [DOI] [PubMed] [Google Scholar]
- Henderson JL, Reynolds JD, Dexter F, Atkins B, Hrdy J, Poduska D, Penning DH. Chronic hypoxemia causes extracellular glutamate concentration to increase in the cerebral cortex of the near-term fetal sheep. Brain Research. Developmental Brain Research. 1998;105:287–293. doi: 10.1016/s0165-3806(97)00192-2. [DOI] [PubMed] [Google Scholar]
- King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annual Review of Neuroscience. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. American Journal of Physiology. 1989;256:R1348–1354. doi: 10.1152/ajpregu.1989.256.6.R1348. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary- adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. Journal of Neurobiology. 1999;40:302–315. [PubMed] [Google Scholar]
- Leborgne-Samuel Y, Janky E, Venditelli F, Salin J, Daijardin JB, Couchy B, Etienne-Julan M, Berchel C. Sickle cell anemia and pregnancy: review of 68 cases in Guadeloupe. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 2000;29:86–93. [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. Journal of Neuroscience. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Rettori MC, Van Reeth O. Hormonal and behavioral abnormalities induced by stress in utero: an animal model of depression. Stress. 2001;4:169–181. doi: 10.3109/10253890109035016. [DOI] [PubMed] [Google Scholar]
- Maccari S, Van Reeth O. Circadian rhythms, effects of prenatal stress in rodents: An animal model for human depression. In: Fink G, editor. Encyclopedia of Stress. Vol. 1. San Diego: Academic Press; 2000. pp. 467–470. [Google Scholar]
- Mamet J, Peyronnet J, Roux JC, Perrin D, Cottet-Emard JM, Pequignot JM, LagercrantZ H, DalmaZ Y. Long-term prenatal hypoxia alters maturation of adrenal medulla in rats. Pediatric Research. 2002;51:1–8. doi: 10.1203/00006450-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Rietveld WJ. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiological Reviews. 1989;69:671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Buwalda B, Luiten PGM. Hypoxia and brain development. Progress in Neurobiology. 1996;49:1–51. doi: 10.1016/0301-0082(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Buwalda B, Markel E, Korte SM, Luiten PG. Life-spanning behavioural and adrenal dysfunction induced by prenatal hypoxia in the rat is prevented by the calcium antagonist nimodipine. European Journal of Neuroscience. 1994;6:746–753. doi: 10.1111/j.1460-9568.1994.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Okamura K, Murotsuki J, Watanabe T, Tanigawara S, Uehara S, Yano M, Yajima A, Sakai T. Relation between fetal blood gas levels and the outcome of babies in severe preeclampsia. Tohuku Journal of Experimental Medicine. 1992;167:279–285. doi: 10.1620/tjem.167.279. [DOI] [PubMed] [Google Scholar]
- Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. American Journal of Obstetrics and Gynecology. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- Poncet L, Pequignot JM, Cottet-Emard JM, DalmaZ Y, Denoroy L. Altered daily rhythms of brain and pituitary indolamines and neuropeptides in long-term hypoxic rats. American Journal of Physiology. 1999;277:R66, R75. doi: 10.1152/ajpregu.1999.277.1.R66. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comparative Biochemistry and Physiology. Molecular and Integrative Physiology. 1998;119:717–723. doi: 10.1016/s1095-6433(98)01010-1. [DOI] [PubMed] [Google Scholar]
- Royer C, Lachuer J, Crouzoulon G, Roux J, Peyronnet J, Mamet J, Pequignot J, DalmaZ Y. Effects of gestational hypoxia on mRNA levels of Glut3 and Glut4 transporters, hypoxia inducible factor-1 and thyroid hormone receptors in developing rat brain. Brain Research. 2000;856:119–128. doi: 10.1016/s0006-8993(99)02365-3. [DOI] [PubMed] [Google Scholar]
- Ruzak B, Zucker I. Neural regulation of circadian rhythms. Journal of Physiology. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Chastang JF, Diefenbach K, Bioulac B. Is self-reported morbidity related to the circadian clock? Journal of Biological Rhythms. 2001;16:183–190. doi: 10.1177/074873001129001764. [DOI] [PubMed] [Google Scholar]
- Tischkau SA, Gallman EA, Buchanan GF, Gillette MU. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. Journal of Neuroscience. 2000;20:7830–7837. doi: 10.1523/JNEUROSCI.20-20-07830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Van Reeth O. Circadian Rhythms. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. New York: Oxford University Press; 1995. pp. 1329–1359. [Google Scholar]
- Van Cauter E, Turek FW. Depression: a disorder of timekeeping? Perspectives in Biology and Medicine. 1986;29:510–519. doi: 10.1353/pbm.1986.0033. [DOI] [PubMed] [Google Scholar]
- Van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Research. Brain Research Reviews. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Wu T. The Qinghai-Tibetan plateau: how high do Tibetans live? High Altitude Medicine and Biology. 2001;2:489–499. doi: 10.1089/152702901753397054. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Palmer SK, Dahms TE, Berman JC, McCullough RG, McCullough RE, Moore LG. Blood volume expansion, preeclampsia, and infant birth weight at high altitude. Journal of Applied Physiology. 1993;75:1566–1573. doi: 10.1152/jappl.1993.75.4.1566. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. Journal of Applied Physiology. 1995a;79:7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Palmer SK, Stamm E, Coffin C, Moore LG. Uterine blood flow at high altitude. In: Sutton JR, Houston CS, Coates G, editors. Hypoxia and the Brain (Proceedings of the 9th International Hypoxia Symposium, Lake Louise, Canada) Burlington, VT, USA: Queen City Printers; 1995b. pp. 112–124. [Google Scholar]
- Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, Van Heerikhuize JJ, Hofman MA, Swaab DF. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Archives of General Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]


