Abstract
Neuropeptide Y (NPY) plays a modulatory role in processing nociceptive information. The present study investigated the effects of NPY on axonal transport of particles in neurites of cultured adult dorsal root ganglion (DRG) cells using video-enhanced microscopy. Application of NPY decreased the number of particles transported in both the anterograde and retrograde directions. This effect was persistently observed during NPY application and was reversed after washout. The inhibitory effect of NPY was concentration dependent between 10−9m and 10−6m. The instantaneous velocity of individual particles moving in anterograde and retrograde directions was also reduced by NPY. Both the NPY Y1 receptor agonist [Leu31,Pro34]-NPY and NPY Y2 receptor agonist NPY13-36 mimicked the effect of NPY on the number of transported particles. An immunocytochemical study using an antiserum against the NPY Y1 receptor protein revealed that the Y1 receptor was expressed in the majority (85.9 %) of cultured adult mouse DRG cells. Pre-treatment of cells with pertussis toxin, a GTP-binding protein (G protein) inhibitor, completely blocked the inhibitory effect of NPY. Each application of SQ-22536, an adenylate cyclase inhibitor, and H-89, a protein kinase A inhibitor, mimicked and occluded the effect of NPY. In contrast, dibutyryl cAMP (dbcAMP), a membrane permeable cAMP analogue, and forskolin, an activator of adenylate cyclase, produced a transient increase in axonal transport. The application of dbcAMP and forskolin in combination with NPY negated the effect of NPY alone. These results suggest that NPY, acting at Y1 and Y2 receptors, inhibits axonal transport of particles in sensory neurones. The effect seems to be mediated by a pertussis toxin-sensitive G protein, adenylate cyclase, and protein kinase A pathway. Therefore, NPY may be a modulatory factor for axonal transport in sensory neurones.
Neuropeptide Y (NPY), a 36-amino acid peptide, is widely distributed in the central and peripheral nervous systems to control a variety of biological events. In the somatosensory system, NPY has been suggested to play an important role in the modulation of nociceptive information. Receptors for NPY are expressed in cell bodies and fibres in dorsal root ganglion (DRG) neurones (Mantyh et al. 1994; Zhang et al. 1994a, 1994b, 1995, 1997; Marchand et al. 1999). This peptide produces antinociceptive effects (Duggan et al. 1991; Hua et al. 1991; Broqua et al. 1996; Naveilhan et al. 2001) by inhibiting the release of neurotransmitters from central terminals of primary afferent neurones (Duggan et al. 1991) and by directly inhibiting primary afferent nociceptors (Mantyh et al. 1994).
Intracellular transport systems, e.g. axonal transport, are essential for expression and maintenance of neuronal cell function. Sensory neuropeptides such as substance P and calcitonin gene-related peptide (CGRP), transmitting nociceptive information, are conveyed from the cell body to axon terminals by an axonal transport system (Brimijoin et al. 1980; Harmar & Keen, 1982; Keen et al. 1982; Kashihara et al. 1989, Fernandez & Hodges-Savola, 1994). Receptors expressed in DRG neurones are also transported within axons (Zarbin et al. 1990; Hökfelt et al. 1998). Thus, axonal transport is important for the regulation of sensory nervous system function. However, it is not known how the regulatory peptide NPY affects axonal transport in sensory neurones. The object of the present study was to examine the effect of NPY on axonal transport in neurites of cultured mouse DRG cells. We used video-enhanced microscopy, which can detect a quick response to stimuli, to observe in real-time the particles moving within neurites.
Methods
Cell culture
The experimental protocol was approved by the Animal Experimentation and Ethics Committee of Kitasato University School of Medicine. Adult male C57BL/6 mice (8 weeks old) were killed with ether and the dorsal root ganglia were removed. The ganglia were immersed immediately in Ham's F-12 culture medium (Gibco BRL, Grand Island, NY, USA) and incubated for 90 min at 37 °C in Ham's F-12 medium containing 2 mg ml−1 collagenase (Worthington Biochemical, Freehold, NJ, USA). The ganglia were then incubated for 15 min at 37 °C in Ca2+- and Mg2+-free Hanks' balanced salt solution containing 2.5 mg ml−1 trypsin (Sigma Chemical Co., St Louis, MO, USA). Immediately after incubation, trypsin activity was inhibited by the addition of 0.125 mg ml−1 trypsin inhibitor (Sigma). After a rinse with enzyme-free Ham's F-12 medium, the ganglia were triturated using fire-polished pipettes (inner diameter: 0.2-0.5 mm). The cells were plated onto polylysine-coated coverslips and cultured for 48 h at 37 °C under 5 % CO2 (pH 7.4) in Ham's F-12 medium containing 10 % fetal bovine serum and penicillin (100 units ml−1) – streptomycin (100 μg ml−1).
Experimental cell preparation
The coverslip on which cells were cultured was attached with waterproof tape to the underside of a 0.5 mm-thick stainless-steel chamber (50 × 80 mm) with a lozenge-shaped hole (25 × 35 mm). The topside of the stainless-steel chamber was covered with another coverslip, leaving a small opening on each side to perfuse solutions. The volume of the chamber was about 0.45 ml. The culture medium was then replaced with Hepes-buffered salt solution (135 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm Hepes and 5.5 mm glucose, pH 7.4, 37 °C). The chamber was mounted onto the stage of an inverted Zeiss Axiomat microscope equipped with an oil-immersed planapochromat ×64 objective (Carl Zeiss, Oberkochen, Germany). The stage was maintained at 37 °C by a thermocontroller. Experiments commenced 30 min after changing the extracellular medium from culture medium to Hepes-buffered solution. The drug-containing solution (3 ml) was injected into one side opening using a Pasteur pipette, and the solution spilling from the other opening was removed by an infusion pump.
Drugs
Neuropeptide Y (NPY, Sigma), NPY receptor agonists ([Leu31,Pro34]-NPY and NPY13-36; both from Sigma), the guanosine triphosphate (GTP)-binding protein (G protein) inhibitor pertussis toxin (List Biological Laboratories, Inc., Campbell, CA, USA), and the membrane permeable cAMP analogue dibutyryl cAMP (Sigma) were dissolved directly in Hepes-buffered salt solution. The adenylate cyclase inhibitor SQ-22536 (Biomol Research Labs, Inc., Plymouth Meeting, PA, USA), the adenylate cyclase activator forskolin (RBI, Natick, MA, USA), and the protein kinase A inhibitor H-89 (Biomol) were each dissolved in dimethyl sulfoxide (DMSO, Wako Pure Chemical, Osaka, Japan) and then diluted with aqueous solution. The DMSO concentration was 0.01 % and at this concentration DMSO had no effect on axonal transport. Pertussis toxin was applied to cultured cells in the medium 4 h prior to the start of recording, and the cells were incubated again at 37 °C. Other drugs were injected into the chamber (37 °C) during video-enhanced microscopic recordings.
Video-enhanced microscopic recording
Nomarski images obtained by an inverted microscope were transformed into video signals by a video camera (Harpicon, Hamamatsu Photonics, Hamamatsu, Japan) and a camera controller (C2741, Hamamatsu Photonics). The signals were processed to digital video images with enhanced contrast by a video image enhancement system (DVS-20, Hamamatsu Photonics). Video images were displayed in real-time on a video monitor (C1864, Hamamatsu Photonics), and stored on a video recorder (PVW-2800, Sony, Tokyo, Japan). This processing provided a 10 000-fold final magnification on the video monitor.
Analysis of axonal transport
Axonal transport was analysed from the video replay. The number and instantaneous velocity of particles (diameter > 50 nm) moving toward the axon terminal (anterograde) and back to the cell body (retrograde) were measured. The number of particles was counted for 2 min at 3 min intervals before, during and, when necessary, after application of the drug. Averaged data are expressed as means (± s.d.) percentage of the control value that was obtained before the drug application. Analysis of variance (ANOVA) was used to evaluate the statistical significance of fluctuations over time. Differences between the control and test conditions were examined for statistical significance using Student's paired t test. Instantaneous velocity of individual moving particles was analysed as follows. Serial video images were digitised at 5 ms intervals to a Macintosh computer (Power Macintosh, 7600/200) equipped with an LG-3 video capture board (Scion Co., Frederick, MD, USA) using Scion image software (Scion). The instantaneous velocity was determined using the formula: velocity = distance/time.
Immunocytochemistry
Mouse DRG cells cultured for 48 h on coverslips were fixed with 4 % paraformaldehyde for 5 min at room temperature. After fixation, they were washed for 3 min with 0.025 m phosphate-buffered saline (PBS) containing 0.3 % Triton X-100 (PBST), and were treated for 10 min with protein blocking agent (Immunon, Pittsburgh, PA, USA) at room temperature to block non-specific protein sites. The cells were incubated overnight at 4 °C with rabbit anti-NPY Y1 receptor serum (1:500, DiaSorin Inc, Stillwater, MN, USA). The antiserum was diluted with 0.2 % bovine serum albumin, 1 % normal goat serum, and 0.1 % sodium azide in PBST. After washing with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:100, Organon Teknika Corp., Durham, NC, USA) for 30 min at room temperature. The immunostained cells were examined with an inverted Zeiss Axiomat microscope equipped with a 485 nm excitation filter and a 520 ± 15 nm emission filter (Carl Zeiss, Oberkochen, Germany). The number of immunoreactive neurones was determined under microscopic observation and also by examining microphotographs.
Results
Isolated and cultured DRG neurones that made no contact with other cells were used for the experiment. Video-enhanced microscopy displayed the movement of particles in anterograde and retrograde directions (Fig. 1). Some of moving particles appeared to be mitochondria according to their microscopic morphology (Forman et al. 1987; Takenaka et al. 1990; Okada et al. 1995; Fig. 1). In the control extracellular medium (Hepes-buffered salt solution, pH 7.4, 37 °C), the mean number of particles (min−1) transported in anterograde and retrograde directions were 71.3 ± 17.4 (mean ± s.d., n = 75) and 71.9 ± 16.5 (n = 75), respectively. The mean instantaneous velocities of moving particles in anterograde and retrograde directions under the control condition were 0.66 ± 0.42 μm s−1 (n = 178) and 0.63 ± 0.39 μm s−1 (n = 159), respectively.
Figure 1. Particles moving within a neurite of the cultured adult mouse dorsal root ganglion (DRG) cell, visualised by video-enhanced microscopy.
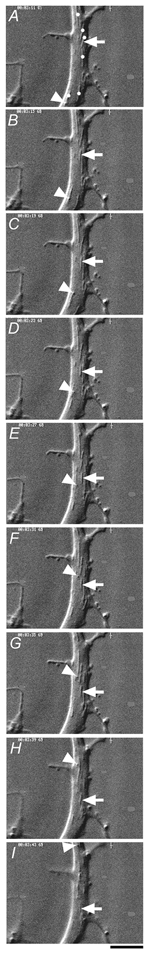
Serial images obtained at 4 s intervals indicate particles moving in anterograde (arrow) and retrograde (arrowhead) directions. Mitochondria (dots in A) were identified by their long-shape, large-size and saltatory movement. Scale bar, 2 μm.
Effects of NPY on axonal transport
Application of NPY (100 nm) for 10 min resulted in a rapid but reversible decrease in the number of particles transported in both anterograde and retrograde directions (Fig. 2). Application of NPY (1 μM) for a longer period (26 min) resulted in a significant decrease in the number of transported particles during the application in each of five neurones tested (Fig. 3). Maximum inhibition of particle transport in both directions amounted to 60 % of control at 14 min after the start of application, reaching a plateau for the remaining period of the experiment (Fig. 3). Comparison of the values measured before and 15 min after each application indicated that NPY reduced the number of particles in a concentration-dependent manner between 10−9m and 10−6m (Fig. 4). The median inhibitory concentrations (IC50) in anterograde and retrograde directions were 7.5 × 10−9m and 9 × 10−9m, respectively (Fig. 4). When the instantaneous velocities of particles were analysed in one of the tested neurones, NPY (1 μM) was found to reduce the instantaneous velocities of particles moving in both anterograde and retrograde directions (Fig. 5).
Figure 2. Effects of a brief application of neuropeptide Y (NPY) on the number of particles transported in neurites of cultured DRG cells.
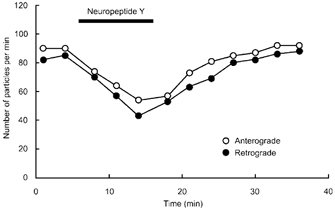
Changes in the number of transported particles in both anterograde (○) and retrograde (•) directions before, during, and after a brief (10 min) application of 100 nm NPY.
Figure 3. Effects of a prolonged application of NPY on the number of particles transported in neurites of cultured DRG cells.
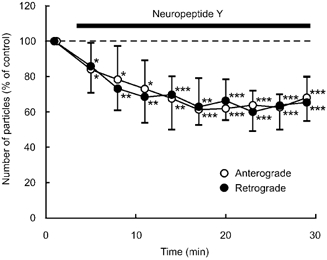
Percentage changes in the number of transported particles of control (the value before application) in anterograde (○) and retrograde (•) directions induced by application of 1 μM NPY. Each point indicates the mean (± s.d.) of the values obtained from five DRG cells. * P < 0.05, ** P < 0.005, *** P < 0.0005 compared to the value before application.
Figure 4. Concentration dependence of NPY-induced reduction in the number of particles transported in anterograde and retrograde directions.
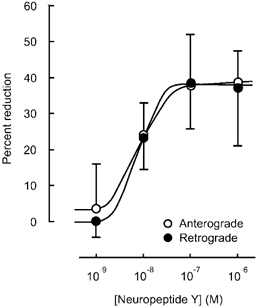
Percentage reduction in the number of transported particles at various concentrations of NPY. The value obtained 15 min after each application of NPY was compared to the value before the application (control), and is expressed as a percentage. Each point indicates the mean (± s.d.) of the values obtained from five DRG cells.
Figure 5. Histogram of instantaneous velocity of individual particles moving in anterograde (A) and retrograde (B) directions.
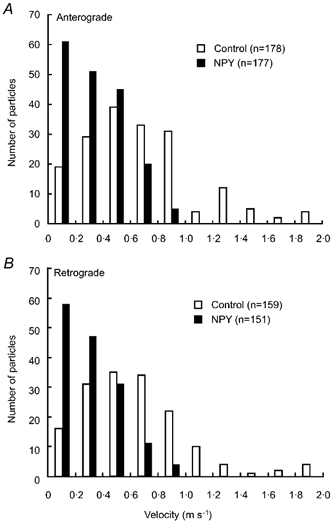
Data were obtained before (control) and during application of 1 μM NPY.
Effects of NPY receptor agonists
In DRG neurones, NPY Y1 and Y2 receptors and their mRNAs are expressed (Mantyh et al. 1994; Zhang et al. 1994a, 1995, 1997; Hökfelt et al. 1998; Naveilhan et al. 1998; Marchand et al. 1999; Zhang et al. 1999). We therefore investigated whether these receptor subtypes are involved in the inhibitory response to NPY. Application of the NPY Y1 receptor agonist [Leu31,Pro34]-NPY (1 μM) significantly reduced the number of particles in anterograde and retrograde axonal transport in a similar manner to NPY in each of five neurones tested (Fig. 6A). The number decreased to 45 % of control for both directions (Fig. 6A). The Y2 receptor agonist fragment NPY13-36 (1 μM) also decreased the number of particles to 60 % of control in both anterograde and retrograde directions in each of the five neurones tested (Fig. 6B). These results suggest that both Y1 and Y2 receptors probably participate in mediating the NPY-induced inhibition of axonal transport.
Figure 6. Effects of NPY Y1 and Y2 receptor agonists on axonal transport.
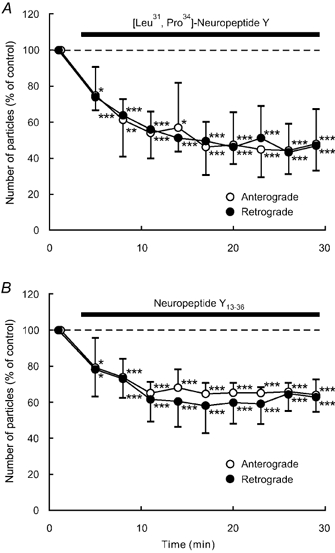
Percentage changes in the number of transported particles of control (the value before the application) in anterograde (open circles) and retrograde (closed circles) directions induced by the Y1 receptor agonist [Leu31,Pro34]-NPY (1 μM) (A) and Y2 receptor agonist NP13-36 (1 μM; B). Each data point represents the mean (± s.d.) of the values obtained from five DRG cells. * P < 0.05, ** P < 0.005, *** P < 0.0005 compared to the value before the application.
Immunocytochemical staining of NPY receptors
To confirm the expression of NPY receptors in cultured DRG cells, an immunocytochemical study using an antiserum against NPY Y1 receptor protein was performed. A Nomarski image of DRG neurones cultured for 48 h and their immunoreactivity for Y1 receptors are shown in Fig. 7. Of a total 149 neurones tested, 128 neurones (85.9 %) were positive for Y1 receptor immunoreactivity, while the remaining 21 neurones (14.1 %) were negative. The percentage of positive cells obtained in this study was higher than that reported by others on DRG neurones in situ (≈20 %; Mantyh et al. 1994; Zhang et al. 1994a, 1994b). Therefore, to investigate the influence of culture on the number of Y1 receptor immunoreactive cells, we performed additional immunostaining of DRG cells before (0 h), 24 h, 48 h, and 7 days after the start of culture. The percentages of Y1 receptor-positive cells were similar throughout the culture period, 78.2 % (241/308) at 0 h, 85.1 % (302/355) at 24 h, 85.4 % (311/364) at 48 h, and 76.7 % (260/339) at 7 days, indicating no influence of the culture period on the expression of Y1 receptors. Since specific antibodies for NPY Y2 receptors are not available, expression of Y2 receptors in cultured DRG cells could not be examined.
Figure 7. Immunofluorescence of NPY Y1 receptors in cultured DRG cells.
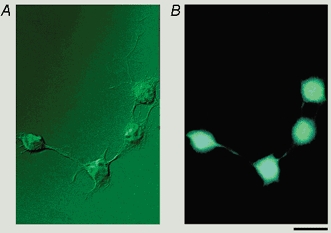
A, Nomarski image of cultured DRG cells. B, immunofluorescent image of the same cells using antibody for NPY Y1 receptor visualised by FITC. Scale bar, 20 μm.
Studies on signal transduction mechanisms for the NPY effect
Receptors for NPY are coupled to G proteins, which inhibit adenylate cyclase activity and thereby intracellular cAMP synthesis (Kassis et al. 1987; Westlind-Danielsson et al. 1987; Aakerlund et al. 1990; Michel et al. 1990; Michel 1991; Herzog et al. 1992; Larhammar et al. 1992; Shigeri & Fujimoto, 1992; Wan & Lau, 1995; Larhammar, 1996; Larhammar et al. 1998). It has been confirmed that Y1 and Y2 receptors are also coupled to these signal transduction pathway (Aakerlund et al. 1990; Wan & Lau, 1995; Larhammar, 1996; Larhammar et al. 1998). Previous studies on axonal transport have shown that elevation of intracellular cAMP and subsequent activation of protein kinase A activity potentiates axonal transport (Takenaka et al. 1994; Takenaka & Kawakami, 1996; Hiruma et al. 2000a). Thus, we hypothesised that the inhibitory response of axonal transport to NPY is mediated by activation of Gi proteins and by subsequent inhibition of the adenylate cyclase/protein kinase A system. We tested this hypothesis and obtained the following results.
Pre-treatment for 4 h with pertussis toxin (200 ng ml−1), an inhibitor of Gi and Go proteins, completely blocked the inhibitory effect of NPY (1 μM) on anterograde and retrograde axonal transport in each of five DRG neurones tested (Fig. 8), suggesting that the inhibitory effect of NPY is mediated by pertussis toxin-sensitive Gi and/or Go proteins.
Figure 8. Effects of pre-treatment with pertussis toxin on the response to NPY.
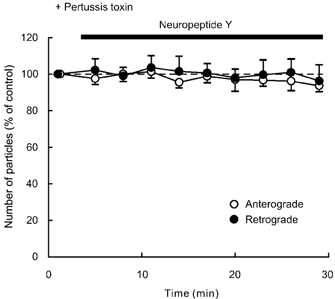
Data represent percentage changes in the number of transported particles. Neuropeptide Y (NPY, 1 μM) was applied after pre-treatment (for 4 h) with pertussis toxin (200 ng ml−1), a G protein inhibitor. Note that pre-treatment with pertussis toxin blocked the inhibitory response to NPY.
Application of SQ-22536 (100 μM), an adenylate cyclase inhibitor, decreased the number of particles in anterograde and retrograde directions in each of five neurones tested (Fig. 9A). This decrease reached approximately 50–60 % of control at 11–17 min after the start of the application, and this low level was sustained throughout the application. Treatment with H-89 (1 μM), a protein kinase A inhibitor, gave similar results to SQ-22536 (n = 5; Fig. 10A). Prior exposure of a cell to 100 μM SQ-22536 (n = 5) or 1 μM H-89 (n = 5) occluded the effect of 1 μM NPY (Fig. 9B and Fig. 10B). Each application of 1 mm dbcAMP (n = 5), a membrane permeable cAMP analogue, and 1 μM forskolin (n = 5), an adenylate cyclase activator, resulted in a transient increase in the number of particles transported in both directions (Fig. 11A and Fig. 12A). The number of particles peaked at 5–8 min after the start of each application of these drugs, and thereafter gradually declined. The peak values were approximately 140–150 % of control in both dbcAMP- and forskolin-treated cells. The application of 1 mm dbcAMP (n = 5) or 1 μM forskolin (n = 5) in combination with NPY negated the decreasing effect of NPY alone (Fig. 11B and Fig. 12B). Thus, NPY probably inhibited endogenous adenylate cyclase activity, resulting in a decrease in endogenous cAMP levels. Then, the decrease in endogenous cAMP levels was counteracted by exogenous cAMP, dbcAMP. These results suggest that the inhibitory response to NPY is presumably mediated by suppression of adenylate cyclase and subsequent reduction in intracellular cAMP levels and protein kinase A activity. Furthermore, these results suggest that the involved G proteins are Gi proteins that inhibit adenylate cyclase activity.
Figure 9. Effects of SQ-22536 (A) or NPY after SQ-22536 treatment (B).
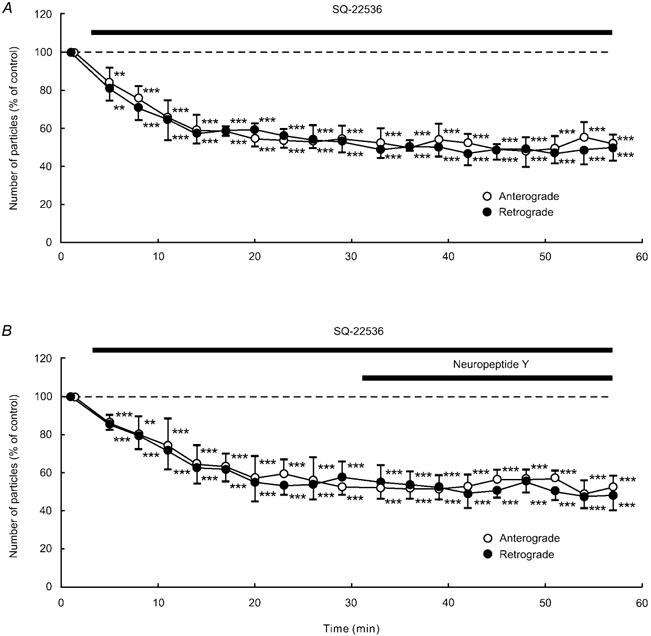
Data represent percentage changes in the number of transported particles. Cells were treated with the adenylate cyclase inhibitor SQ-22536 (100 μM) alone (A) or NPY (1 μM) after SQ-22536 (100 μM) treatment (B). Note that SQ-22536 inhibited anterograde and retrograde axonal transport, but the subsequently applied NPY failed to produce a further decrease in axonal transport. Each data point indicates the mean (± s.d.) of the values obtained from five DRG cells. * P < 0.05, ** P < 0.005, *** P < 0.0005 compared to the value before the application of SQ-22536.
Figure 10. Effects of H-89 (A) or NPY after H-89 treatment (B).
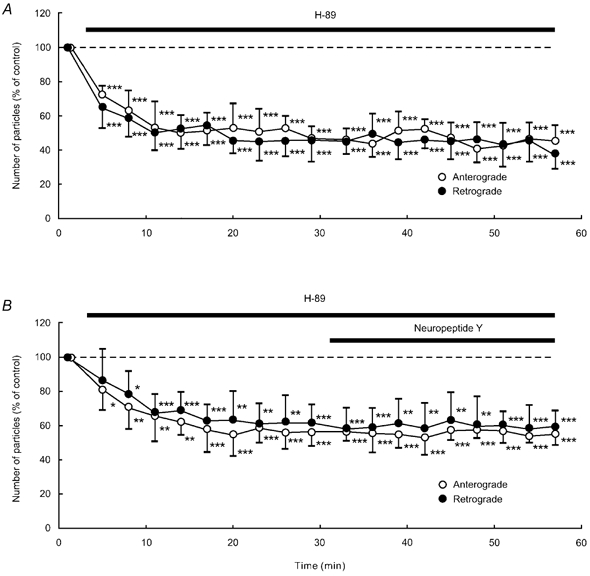
Data represent percentage changes in the number of transported particles. Cells were treated with the protein kinase A inhibitor H-89 (1 μM) alone (A), or NPY (1 μM) after treatment with H-89 (1 μM; B). Note that H-89 inhibited anterograde and retrograde axonal transport, but the subsequently applied NPY failed to produce a further decrease in axonal transport. Each data point indicates the mean (± s.d.) of the values obtained from five DRG cells. * P < 0.05, ** P < 0.005, *** P < 0.0005 compared to the value before the application of H-89.
Figure 11. Effects of dbcAMP (A) and a combination of dbcAMP and NPY (B).
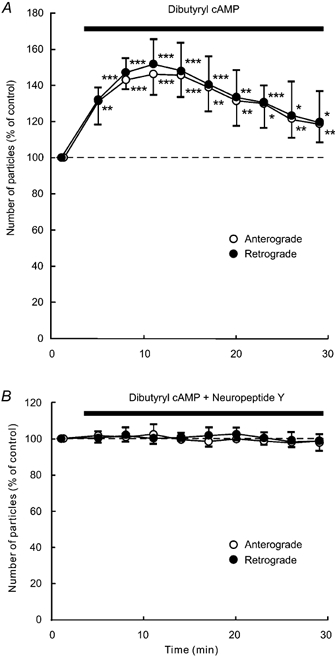
Data represent percentage changes in the number of transported particles. Cells were treated with membrane-permeable cAMP analogue dbcAMP (1 mm) alone (A), or a combination of dbcAMP (1 mm) and NPY (1 μM; B). Note that NPY counteracted the effect of dbcAMP. Each data point indicates the mean (± s.d.) of the values obtained from five DRG cells. * P < 0.05, ** P < 0.005, *** P < 0.0005 compared to the value before the application.
Figure 12. Effects of forskolin (A) and a combination of forskolin and NPY (B).
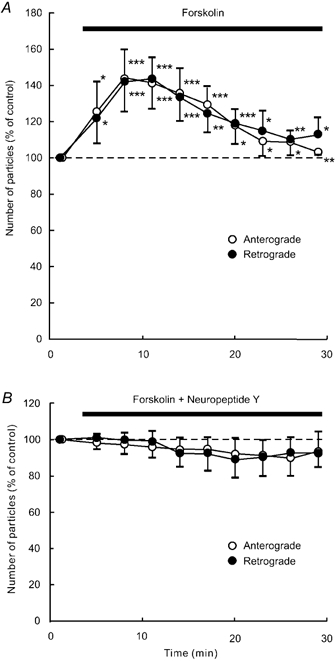
Data represent percentage changes in the number of transported particles. Cells were treated with the adenylate cyclase activator forskolin (1 μM) alone (A), or a combination of forskolin (1 μM) and NPY (1 μM; B). Note that NPY counteracted the effect of forskolin. Each data point indicates the mean (± s.d.) of the values obtained from five DRG cells. * P < 0.05, ** P < 0.005, *** P < 0.0005 compared to the value before the application.
Discussion
Inhibitory effect of NPY on axonal transport
The present results provide direct evidence that NPY inhibits both anterograde and retrograde axonal transport in cultured adult mouse DRG neurones. This response was rapid and reversible, which could be detected by video-enhanced microscopy. The present study also revealed that the inhibitory response to NPY was concentration dependent. The median inhibitory concentrations (IC50) for anterograde and retrograde axonal transport were similar, 7.5 × 10−9m in an anterograde direction and 9 × 10−9m in a retrograde direction. The instantaneous velocities in anterograde and retrograde directions were also reduced by NPY. Taking into account these results, NPY seems to act on mechanisms common to both anterograde and retrograde axonal transport. In this respect, the molecular target for the action of NPY on axonal transport is unknown. Since different types of motor proteins are responsible for each anterograde and retrograde axonal transport (Hirokawa 1998; Hirokawa et al. 1998), NPY is not likely to act on individual motor proteins themselves. Probably, NPY acts on mechanisms interacting with every motor protein, such as energy metabolism or microtubule assembly and disassembly. Further studies are necessary to clarify these mechanisms.
Physiologically, DRG neurones express NPY receptors (Mantyh et al. 1994; Zhang et al. 1994a, 1994b, 1995; Hökfelt et al. 1998; Marchand et al. 1999; Zhang et al. 1999). Under physiological conditions, NPY is released from sympathetic and parasympathetic neurones (Lundberg et al. 1990; Schalling et al. 1991), the adrenal medulla (Schalling et al. 1991), and intrinsic spinal neurones (Mark et al. 1997). It is suggested that NPY released from these tissues can reach the DRG neurones through the circulation (Zhang et al. 1994a; Hökfelt et al. 1998). Thus, the inhibitory action of NPY on axonal transport may be operational under physiological conditions. Alternatively, once peripheral sensory nerves have been damaged by injury or inflammation, the synthesis and release of NPY is markedly changed. Immunoreactivity for NPY is observed in injured-side DRG neurones but not in normal DRG neurones (Wakisaka et al. 1991, 1992; Landry et al. 2000). In the spinal cord where primary afferent fibres terminate, the expression of NPY is also increased after peripheral nerve injury (Wakisaka et al. 1991, 1992). Neuropeptide Y is released from central terminals of DRG neurones after nerve injury (Mark et al., 1998), while such release does not occur in a normal state (Mark et al. 1997). Thus, NPY may exert its inhibitory effect on axonal transport not only in a normal state but also in an injury state.
It has been suggested that NPY released in response to nerve injury possesses antinociceptive effects (Hua et al. 1991; Broqua et al. 1996; Naveilhan et al. 2001). Application of NPY into the dorsal horn of the spinal cord inhibits the release of substance P from sensory neurones (Duggan et al. 1991). Substance P is one of the major transmitters conveying nociceptive signals to the spinal cord (De Koninck & Henry, 1991; Salter & Henry, 1991). Therefore, NPY is implicated to exert an antinociceptive effect by blocking directly the release of transmitters from DRG neurones (Duggan et al. 1991). It has been suggested that axonal transport is closely related to transmitter release (Wooten et al. 1975; Giachetti & Said, 1979; Lundberg et al. 1989; Keast & Stephensen, 2000). In particular, neuropeptides, synthesised within the cell body, are axonally transported toward axon terminals (Giachetti & Said, 1979; Steiner et al. 1984; Lundberg et al. 1989; Sossin & Scheller, 1991). In fact, blockade of axonal transport by colchicine reduces the release of sensory neuropeptides (Lecci et al. 1996). Thus, the present results are in agreement with the inhibitory action of NPY on peptide release described by others (Duggan et al. 1991).
Cellular mechanisms of the NPY effect
The present study has also clarified the cellular mechanisms for the inhibitory effect of axonal transport induced by NPY. The present immunocytochemical study revealed that a large portion (85.9 %) of DRG cells cultured for 48 h were immunoreactive for the NPY Y1 receptor. The NPY Y1 agonist [Leu31,Pro34]-NPY and the NPY Y2 agonist NPY13-36 both inhibited anterograde and retrograde axonal transport. Thus, both Y1 and Y2 receptors are likely to participate in mediating the inhibitory effect of NPY on axonal transport.
We further investigated the signal transduction mechanisms involved in the NPY-induced inhibition of axonal transport. Pre-treatment with pertussis toxin, a G protein inhibitor, blocked the inhibitory effect of NPY on axonal transport. Each application of the adenylate cyclase inhibitor SQ-22536 and the protein kinase A inhibitor H-89 mimicked and occluded the effect of NPY. The membrane-permeable cAMP, dbcAMP, and the adenylate cyclase activator, forskolin, each produced a transient increase in axonal transport. The application of dbcAMP or forskolin in combination with NPY negated the decreasing effect of NPY alone. Thus, it is assumed that NPY may reduce adenylate cyclase activity, causing a decrease in endogenous cAMP. The decrease in endogenous cAMP can be counteracted by the action of exogenous cAMP, dbcAMP. These results suggest that the NYP receptor-mediated inhibition of axonal transport results from the activation of Gi proteins and subsequent inhibition of adenylate cyclase and protein kinase A activity. This is consistent with previous studies showing that all NPY receptors including Y1 and Y2 receptors are coupled to G proteins and their activation leads to inhibition of cAMP synthesis (Kassis et al. 1987; Westlind-Danielsson et al. 1987; Aakerlund et al. 1990; Michel et al. 1990; Wahlestedt et al. 1990; Michel, 1991; Larhammar et al. 1992; Herzog et al. 1992; Shigeri & Fujimoto, 1992; Wan & Lau, 1995; Larhammar, 1996; Larhammar et al. 1998). The present results are also supported by previous studies on axonal transport. It has been shown that membrane-permeable cAMP increases both anterograde and retrograde axonal transport in certain types of cultured neurones (Takenaka et al. 1994; Hashimoto et al. 1997) including mouse DRG neurones (Hiruma et al. 2000a). Furthermore, the adenylate cyclase/protein kinase A cascade has been proposed as an important pathway that regulates both anterograde and retrograde axonal transport (Takenaka et al. 1994; Takenaka & Kawakami, 1996; Hiruma et al. 2000a). The present study as well as previous studies noted that the increasing effect of dbcAMP and forskolin was transient, while the inhibitory effect of application of protein kinase A inhibitors such as H-89 and KT5720 was sustained during the application. The reason for this is not yet clear. Perhaps, because the intracellular energy supply should be limited, the excessive increase in axonal transport may be suppressed by mechanisms for effective use of energy.
We have previously shown that some neuropeptides and mediators regulate axonal transport of DRG neurones. Substance P (Hiruma et al. 2000b) and histamine (Amano et al. 2001) inhibit axonal transport, whereas calcitonin gene-related peptide (Hiruma et al. 2000b) and prostaglandin E2 (Hiruma et al. 2000a) enhance axonal transport. Alternatively, we have clarified that various intracellular mechanisms are responsible for the regulation of axonal transport. For example, axonal transport is inhibited by increases in concentrations of intracellular Ca2+ (Kanai et al. 2001) or Cl− (Hiruma et al. 1999b), by suppression of protein kinase C (Hiruma et al. 1999a), or by activation of Ca2+/calmodulin protein kinase II (Kanai et al. 2001). As described above, the activation of cAMP-dependent protein kinase A, which is related to prostaglandin E2 (Hiruma et al. 2000a), enhances axonal transport. Although it has not yet been studied, various neuropeptides, neurotransmitters, and chemical mediators are each possible links to such intracellular signallings for the regulation of axonal transport. The present study firstly shows the role of NPY on axonal transport and its signal transduction mechanisms, i.e. the inhibition of the cAMP/adenylate cyclase/protein kinase A pathway.
As described above, the present immunocytochemical study using an antiserum against NPY Y1 receptor proteins revealed that the majority (85.9 %) of cultured mouse DRG cells were positive for NPY Y1 receptors. In the present experiment on axonal transport, NPY and its agonists were effective on each neurone tested. In agreement with the present data, electrophysiological studies reported by Walker et al. (1988) indicated that NPY inhibited Ca2+ current in every cultured DRG cell tested. However, previous ligand binding studies have shown that NPY receptors are expressed in only 5–20 % of DRG neurones in situ in normal rats, rabbits, and monkeys (Mantyh et al. 1994). In situ hybridisation study has also shown that NPY Y1 receptor mRNA is expressed in 20 % of normal DRG neurones of the rat (Zhang et al. 1994a). Similarly, immunohistochemical studies using antibody to the C-terminal portion of the Y1 receptor have shown that approximately 20–25 % of the total neurones of rat dorsal root ganglia in situ are Y1 receptor immunoreactive in rats (Zhang et al. 1994a). One possible explanation for such differences in the ratio of NPY receptor expression is that it is due to the preparations where the cell size is differently distributed. It has been described that Y1 receptors are present mainly in small-sized (Zhang et al. 1994a, 1994b, 1995, 1999) and small- and medium-sized (Marchand et al. 1999) DRG neurones in a normal state. In isolated and cultured DRG cells of the adult rat, large DRG cells with diameter > 50 μm or with cell area > 1000 μm2 are rare (Gold et al. 1996; Hu et al. 1997; Segond von Banchet et al. 1999), whereas more than 20 % of the adult rat DRG neurones are such large-sized cells in situ (Battaglia & Rustioni, 1988; McCarthy & Lawson, 1990; Mantyh et al. 1994; Zhang et al. 1997). In our preparations, approximately 3 % of cultured DRG cells were large cells with diameter > 50 μm. Since large cells are lost in the process of cell isolation as described by Gold et al. (1996), the ratio of Y1-positive cells detected in this study may be higher than that obtained by in situ study. Our results indicated that the proportion of Y1-positive cells was not affected by the period of culture. Therefore, culture conditions do not seem to influence the expression of the Y1 receptor.
In conclusion, we have demonstrated that NPY, acting at NPY Y1 and Y2 receptors, inhibits anterograde and retrograde axonal transport. The effect seems to be mediated by pertussis toxin-sensitive G proteins and the adenylate cyclase/protein kinase A pathway. These results suggest that NPY is one of the modulatory factors for axonal transport under physiological and pathological conditions.
Acknowledgments
This work was partly supported by the Academic Frontier Project from the Ministry of Education, Science, Sports and Culture, Japan to T.K., and a Grand-in-Aid for Scientific Research (no. 11670638) from the Ministry of Education, Science, Sports and Culture, Japan to H.H.
References
- Aakerlund L, Gether U, Fuhlendorff J, SchwartZ TW, Thastrup O. Y1 receptors for neuropeptide Y are coupled to mobilization of intracellular calcium and inhibition of adenylate cyclase. FEBS Letters. 1990;260:73–78. doi: 10.1016/0014-5793(90)80069-u. [DOI] [PubMed] [Google Scholar]
- Amano R, Hiruma H, Nishida S, Kawakami T, Shimizu K. Inhibitory effect of histamine on axonal transport in cultured mouse dorsal root ganglion neurons. Neuroscience Research. 2001;41:201–206. doi: 10.1016/s0168-0102(01)00275-9. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. Journal of Comparative Neurology. 1988;277:302–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- Brimijoin S, Lundberg JM, Brodin E, HÖKFELT T, Nilsson G. Axonal transport of substance P in the vagus and sciatic nerves of the guinea pig. Brain Research. 1980;191:443–457. doi: 10.1016/0006-8993(80)91293-7. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-martin B, Riviere PJM, Junien J-L, Dahl SG. Antinociceptive effects of neuropeptide Y and related peptides in mice. Brain Research. 1996;724:25–32. doi: 10.1016/0006-8993(96)00262-4. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Henry JL. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proceedings of the National Academy of Sciences of the USA. 1991;88:11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan AW, Hope PJ, Lang CW. Microinjection of neuropeptide Y into the superficial dorsal horn reduces stimulus-evoked release of immunoreactive substance P in the anaesthetized cat. Neuroscience. 1991;44:733–740. doi: 10.1016/0306-4522(91)90092-3. [DOI] [PubMed] [Google Scholar]
- FernandeZ HL, Hodges-Savola CA. Axoplasmic transport of calcitonin gene-related peptide in rat peripheral nerve as a function of age. Neurochemical Research. 1994;19:1369–1377. doi: 10.1007/BF00972465. [DOI] [PubMed] [Google Scholar]
- Forman DS, Lynch KJ, Smith RS. Organelle dynamics in lobster axons: anterograde, retrograde and stationary mitochondria. Brain Research. 1987;412:96–106. doi: 10.1016/0006-8993(87)91443-0. [DOI] [PubMed] [Google Scholar]
- Giachetti A, Said SI. Axonal transport of vasoactive intestinal peptide in sciatic nerve. Nature. 1979;281:574–575. doi: 10.1038/281574a0. [DOI] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Harmar A, Keen P. Synthesis, and central and peripheral axonal transport of substance P in a dorsal root ganglion-nerve preparation in vitro. Brain Research. 1982;231:379–385. doi: 10.1016/0006-8993(82)90374-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Hori H, Kawakami T, Kusakabe T, Takenaka T. Effect of dibutyryl cyclic AMP on axoplasmic transport in the hippocampus. Brain Research. 1997;755:343–346. doi: 10.1016/s0006-8993(97)00230-8. [DOI] [PubMed] [Google Scholar]
- Herzog H, Hort YJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proceedings of the National Academy of Sciences of the USA. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Okada Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Current Opinion in Cell Biology. 1998;10:60–73. doi: 10.1016/s0955-0674(98)80087-2. [DOI] [PubMed] [Google Scholar]
- Hiruma H, Ichikawa T, Kobayashi H, Hoka S, Takenaka T, Kawakami T. Prostaglandin E2 enhances axonal transport and neuritogenesis in cultured mouse dorsal root ganglion neurons. Neuroscience. 2000a;100:885–891. doi: 10.1016/s0306-4522(00)00347-x. [DOI] [PubMed] [Google Scholar]
- Hiruma H, Maruyama H, Katakura T, Simada ZB, Nishida S, Hoka S, Takenaka T, Kawakami T. Axonal transport is inhibited by a protein kinase C inhibitor in cultured isolated mouse dorsal root ganglion cells. Brain Research. 1999a;826:135–138. doi: 10.1016/s0006-8993(99)01249-4. [DOI] [PubMed] [Google Scholar]
- Hiruma H, Nishida S, Katakura T, Kusakabe T, Takenaka T, Kawakami T. Extracellular potassium rapidly inhibits axonal transport of particles in cultured mouse dorsal root ganglion neurites. Journal of Neurobiology. 1999b;38:225–233. doi: 10.1002/(sici)1097-4695(19990205)38:2<225::aid-neu5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Hiruma H, Saito A, Ichikawa T, Kiriyama Y, Hoka S, Kusakabe T, Kobayashi H, Kawakami T. Effects of substance P and calcitonin gene-related peptide on axonal transport in isolated and cultured adult mouse dorsal root ganglion neurons. Brain Research. 2000b;883:184–191. doi: 10.1016/s0006-8993(00)02892-4. [DOI] [PubMed] [Google Scholar]
- HÖkfelt T, Broberger C, Zhang X, DieZ M, Kopp J, Xu Z-Q, Landry M, Bao L, Schalling M, Koistinaho J, Dearmond SJ, Prusiner S, Gong J, Walsh JH. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Research Reviews. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Hu H-Z, Li Z-W, Si J-Q. Evidence for the existence of substance P autoreceptor in the membrane of rat dorsal root ganglion neurons. Neuroscience. 1997;77:535–541. doi: 10.1016/s0306-4522(96)00451-4. [DOI] [PubMed] [Google Scholar]
- Hua X-Y, Boublik JH, Spicer MA, Rivier JE, Brown MR, Yaksh TL. The antinociceptive effects of spinally administered neuropeptide Y in the rat: systematic studies on structure-activity relationship. Journal of Pharmacology and Experimental Therapeutics. 1991;258:243–248. [PubMed] [Google Scholar]
- Kanai A, Hiruma H, Katakura T, Sase S, Kawakami T, Hoka S. Low-concentration lidocaine rapidly inhibits axonal transport in cultured mouse dorsal root ganglion neurons. Anesthesiology. 2001;95:675–680. doi: 10.1097/00000542-200109000-00021. [DOI] [PubMed] [Google Scholar]
- Kassis S, Olasmaa M, Terenius L, Fishman PH. Neuropeptide Y inhibits cardiac adenylate cyclase through a pertussis toxin-sensitive G protein. Journal of Biological Chemistry. 1987;262:3429–3431. [PubMed] [Google Scholar]
- Kashihara Y, Sakaguchi M, Kuno M. Axonal transport and distribution of endogenous calcitonin gene-related peptide in rat peripheral nerve. Journal of Neuroscience. 1989;9:3796–3802. doi: 10.1523/JNEUROSCI.09-11-03796.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. Journal of Comparative Neurology. 2000;424:577–587. [PubMed] [Google Scholar]
- Keen P, Harmar AJ, Spears F, Winter E. Biosynthesis, axonal transport and turnover of neuronal substance P. Ciba Foundation Symposium. 1982;91:145–164. doi: 10.1002/9780470720738.ch9. [DOI] [PubMed] [Google Scholar]
- Landry M, Holmberg K, Zhang X, HÖKFELT T. Effect of axotomy on expression of NPY, galanin, and NPY Y1 and Y2 receptors in dorsal root ganglia and the superior cervical ganglion studied with double-labeling in situ hybridization and immunohistochemistry. Experimental Neurology. 2000;162:361–384. doi: 10.1006/exnr.1999.7329. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regulatory Peptides. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Blomqvist AG, Yee F, Jazin E, Yoo H, Wahlested C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. Journal of Biological Chemistry. 1992;267:10935–10938. [PubMed] [Google Scholar]
- Larhammar D, SÖDERBERG C, Lundell I. Evolution of the neuropeptide Y family and its receptors. Annals of the New York Academy of Sciences. 1998;839:35–40. doi: 10.1111/j.1749-6632.1998.tb10729.x. [DOI] [PubMed] [Google Scholar]
- Lecci A, Patacchini R, De Giorgio R, Corinaldesi R, Theodorsson E, Giuliani S, Santicioli P, Maggi CA. Functional, biochemical and anatomical changes in the rat urinary bladder induced by perigangliar injection of colchicine. Neuroscience. 1996;71:285–296. doi: 10.1016/0306-4522(95)00422-x. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Lacroix J-S, Pernow J. Neuropeptide Y and sympathetic neurotransmission. Annals of the New York Academy of Sciences. 1990;611:166–174. doi: 10.1111/j.1749-6632.1990.tb48930.x. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Rudehill A, Sollevi A, Fried G, Wallin G. Co-release of neuropeptide Y and noradrenaline from pig spleen in vivo : importance of subcellular storage, nerve impulse frequency and pattern, feedback regulation and resupply by axonal transport. Neuroscience. 1989;28:475–486. doi: 10.1016/0306-4522(89)90193-0. [DOI] [PubMed] [Google Scholar]
- McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34:623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Rogers S, Demaster E, Ghilardi JR, Mosconi T, Kruger L, Mannon PJ, Taylor IL, Vigna SR. Some sensory neurons express neuropeptide Y receptors: potential paracrine inhibition of primary afferent nociceptors following peripheral nerve injury. Journal of Neuroscience. 1994;14:3958–3968. doi: 10.1523/JNEUROSCI.14-06-03958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand JE, Cepeda MS, Carr DB, Wurm WH, Kream RM. Alterations in neuropeptide Y, tyrosine hydroxylase, and Y-receptor subtype distribution following spinal nerve injury to rats. Pain. 1999;79:187–200. doi: 10.1016/s0304-3959(98)00165-1. [DOI] [PubMed] [Google Scholar]
- Mark MA, Colvin LA, Duggan AW. Spontaneous release of immunoreactive neuropeptide Y from the central terminals of large diameter primary afferents of rats with peripheral nerve injury. Neuroscience. 1998;83:581–589. doi: 10.1016/s0306-4522(97)00402-8. [DOI] [PubMed] [Google Scholar]
- Mark MA, Jarrott B, Colvin LA, Macmillan SJA, Duggan AW. The release of immunoreactive neuropeptide Y in the spinal cord of the anaesthetized rat and cat. Brain Research. 1997;754:195–203. doi: 10.1016/s0006-8993(97)00061-9. [DOI] [PubMed] [Google Scholar]
- Michel MC. Receptors for neuropeptide Y: multiple subtypes and multiple second messengers. Trends in Pharmacological Sciences. 1991;12:389–394. doi: 10.1016/0165-6147(91)90610-5. [DOI] [PubMed] [Google Scholar]
- Michel MC, Schlicker E, Fink K, Boublik JH, GÖTHERT M, Willette RN, Daly RN, Hieble JP, Rivier JE, Motulsky HJ. Distinction of NPY receptors in vitro and in vivo. I. NPY-(18–36). discriminates NPY receptor subtypes in vitro. American Journal of Physiology. 1990;259:E131–139. doi: 10.1152/ajpendo.1990.259.1.E131. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao J-X, Xu X-J, Wiesenfeld-Hallin Z, Thorén P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Arenas E, Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Okada Y, Sato-Yoshitake R, Hirokawa N. The activation of protein kinase A pathway selectively inhibits anterograde axonal transport of vesicles but not mitochondria transport or retrograde transport in vivo. Journal of Neuroscience. 1995;15:3053–3064. doi: 10.1523/JNEUROSCI.15-04-03053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Henry JL. Responses of functionally identified neurones in the dorsal horn of the cat spinal cord to substance P, neurokinin A and physalaemin. Neuroscience. 1991;43:601–610. doi: 10.1016/0306-4522(91)90319-j. [DOI] [PubMed] [Google Scholar]
- Schalling M, Franco-Cereceda A, Hemsén A, Dagerlind Å, Seroogy K, Persson H, HÖKFELT T, Lundberg JM. Neuropeptide Y and catecholamine synthesizing enzymes and their mRNAs in rat sympathetic neurons and adrenal glands: studies on expression, synthesis and axonal transport after pharmacological and experimental manipulations using hybridization techniques and radioimmunoassay. Neuroscience. 1991;41:753–766. doi: 10.1016/0306-4522(91)90365-u. [DOI] [PubMed] [Google Scholar]
- Segond von Banchet G, Petersen M, Schaible H-G. Expression of neurokinin-1 receptors on cultured dorsal root ganglion neurons from the adult rat. Neuroscience. 1999;90:677–684. doi: 10.1016/s0306-4522(98)00408-4. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Fujimoto M. Two different signal transductions of neuropeptide Y1 receptor in SK-N-MC cells. Biochemical and Biophysical Research Communications. 1992;187:1565–1571. doi: 10.1016/0006-291x(92)90481-y. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Scheller RH. Biosynthesis and sorting of neuropeptides. Current Opinion in Neurobiology. 1991;1:79–83. doi: 10.1016/0959-4388(91)90013-w. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Docherty K, Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. Journal of Cellular Biochemistry. 1984;24:121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Kawakami T. Signal transduction mechanism responsible for changes in axoplasmic transport caused by neurotransmitters. Neurochemical Research. 1996;21:553–556. doi: 10.1007/BF02527752. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Kawakami T, Hikawa N, Gotoh H. Axoplasmic transport of mitochondria in cultured dorsal root ganglion cells. Brain Research. 1990;528:285–290. doi: 10.1016/0006-8993(90)91669-8. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Kawakami T, Hori H, Bandou Y. Effect of neurotransmitters on axoplasmic transport: how adrenaline affects superior cervical ganglion cells. Brain Research. 1994;643:81–85. doi: 10.1016/0006-8993(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Grundemar L, HÅKANSON R, Heilig M, Shen GH, Zukowska-Grojec Z, Reis DJ. Neuropeptide Y receptor subtypes, Y1 and Y2. Annals of the New York Academy of Sciences. 1990;611:7–26. doi: 10.1111/j.1749-6632.1990.tb48918.x. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neuroscience Letters. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain Research. 1992;598:349–352. doi: 10.1016/0006-8993(92)90206-o. [DOI] [PubMed] [Google Scholar]
- Walker MW, Ewald DA, Perney TM, Miller RJ. Neuropeptide Y modulates neurotransmitter release and Ca2+ currents in rat sensory neurons. Journal of Neuroscience. 1988;8:2438–2446. doi: 10.1523/JNEUROSCI.08-07-02438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CP, Lau BHS. Neuropeptide Y receptor subtypes. Life Sciences. 1995;56:1055–1064. doi: 10.1016/0024-3205(95)00041-4. [DOI] [PubMed] [Google Scholar]
- Westlind-Danielsson A, Undén A, Abens J, Andell S, Bartfai T. Neuropeptide Y receptors and the inhibition of adenylate cyclase in the human frontal and temporal cortex. Neuroscience Letters. 1987;74:237–242. doi: 10.1016/0304-3940(87)90156-x. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Kopin IJ, Axelrod J. Effects of colchicine and vinblastine on axonal transport and transmitter release in sympathetic nerves. Annals of the New York Academy of Sciences. 1975;253:528–534. doi: 10.1111/j.1749-6632.1975.tb19226.x. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Wamsley JK, Kuhar MJ. Anterograde transport of opioid receptors in rat vagus nerves and dorsal roots of spinal nerves: pharmacology and sensitivity to sodium and guanine nucleotides. Experimental Brain Research. 1990;81:267–278. doi: 10.1007/BF00228115. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Xu Z-Q, Kopp J, Arvidsson U, Elde R, HÖKFELT T. Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proceedings of the National Academy of Sciences of the USA. 1994a;91:11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ji R-R, Nilsson S, Villar M, Ubink R, Ju G, Wiesenfeld-Hallin Z, HÖKFELT T. Neuropeptide Y and galanin binding sites in rat and monkey lumbar dorsal root ganglia and spinal cord and effect of peripheral axotomy. European Journal of Neuroscience. 1995;7:367–380. doi: 10.1111/j.1460-9568.1995.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shi T, Holmberg K, Landry M, Huang W, Xiao H, Ju G, HÓkfelt T. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proceedings of the National Academy of Sciences of the USA. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tong Y-G, Bao L, HÖKFELT T. The neuropeptide Y Y1 receptor is a somatic receptor on dorsal root ganglion neurons and a postsynaptic receptor on somatostatin dorsal horn neurons. European Journal of Neuroscience. 1999;11:2211–2225. doi: 10.1046/j.1460-9568.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wiesenfeld-Hallin Z, HÖKFELT T. Effect of peripheral axotomy on expression of neuropeptide Y receptor mRNA in rat lumbar dorsal root ganglia. European Journal of Neuroscience. 1994b;6:43–57. doi: 10.1111/j.1460-9568.1994.tb00246.x. [DOI] [PubMed] [Google Scholar]


