Abstract
The phasic modulation of wrist flexor corticomotor disinhibition has previously been demonstrated during the flexion phase of rhythmical passive flexion-extension of the human wrist. Here we ask if rhythmical bimanual flexion-extension movements of the wrists of neurologically intact subjects, modulate inhibitory activity in the motor cortex. In the first experiment intracortical inhibition was assessed when one wrist was passively flexed and extended on its own, with the addition of the opposite limb voluntarily moving synchronously in a mirror symmetric pattern, and also in a near-symmetric asynchronous pattern. Two subsequent experiments investigated firstly the modulation of spinal reflex pathway activity during the same three movement conditions, and secondly the effect of contralateral wrist movement alone on the excitability of corticomotoneuronal pathways to a static test limb. When the wrist flexors of both upper limbs were shortening simultaneously (i.e. synchronously), intracortical inhibition associated with flexor representations was suppressed to a greater extent than when the two muscles were shortening asynchronously. The results of the three experiments indicate that modulation of inhibitory activity was taking place at the cortical level. These findings may have further application in the study of rehabilitation procedures where the effects of simultaneous activation of affected and unaffected upper limbs in hemiparetic patients are to be investigated.
In a recent study, Lewis and colleagues demonstrated that the excitability of corticomotoneuronal (CM) pathways to flexor carpi radialis was modulated phasically while the limb was driven passively through wrist flexion and extension (Lewis et al. 2001). Importantly, marked potentiation (disinhibition) was observed during the flexion phases of movement even though muscle quiescence was maintained throughout the entire movement cycle. This pattern of modulation was attributed to movement-elicited afference.
Following injury, the release of intracortical inhibition is thought to be a substrate of cortical reorganization (Jacobs & Donoghue, 1991; Ziemann et al. 2001). In primary motor cortex (M1) of rats, Jacobs and colleagues induced disinhibition by administering a γ-aminobutyric acid (GABA) receptor antagonist (bicuculline methobromide) directly into the forelimb representation in M1 (Jacobs & Donoghue, 1991). Administering the GABA antagonist resulted in forelimb movements not only being elicited when that area was electrically stimulated, but also when the neighbouring vibrissa representation was stimulated. The expansion of the cortical area from which movements could be elicited suggests that latent intracortical connections were unmasked with this procedure. In human subjects, Ziemann and colleagues examined practice-dependent cortical plasticity by inducing GABA-related cortical disinhibition using an ischaemic nerve block applied to the forearm. They also increased cortical inhibition by the administration of the GABA receptor agonist lorazepam (Ziemann et al. 2001), while paired-pulse transcranial magnetic stimulation (TMS) was used to assess the activity of cortical inhibitory interneurons within M1 (e.g. Kujirai et al. 1993). The results of the experiment by Ziemann et al. suggested that the down-regulation of cortical inhibition facilitated practice-dependent plasticity during practice of upper limb ballistic movements. Plasticity was evident from an increase in the excitability of muscle representations in M1, and an increase in peak acceleration of the ballistic movement. Liepert et al. (2000) examined cortical excitability of the unaffected and affected hemispheres of recovering post-stroke hemiparetic subjects. They found a down-regulation of cortical inhibition in M1 of the affected hemisphere relative to the unaffected hemisphere, and relative to M1 of neurologically intact control subjects. The above studies in neurologically intact and neurologically impaired subjects provide evidence of an association between down-regulated intracortical inhibition and cortical reorganization.
The aim of the present experiments was to examine the regulation of inhibitory mechanisms in human M1 during different patterns of rhythmical bimanual movements performed in active and passive contexts. It is well known that mirror symmetric kinematic patterns of upper limb movement are performed more reliably than all other patterns (Carson, 1995). Conversely, the acquisition of novel patterns of bimanual rhythmical co-ordinated movement has a time course of several days (Fontaine et al. 1997), which is consistent with skill acquisition, where there is evidence of cortical plasticity associated with motor learning (Pascual-Leone et al. 1994). Cortical plasticity associated with motor recovery usually has a time course of many weeks (Cicinelli et al. 1997), and there is some limited evidence that synchronous activation of upper limb muscles assists motor recovery of the affected limb in post-stroke hemiparetic patients (Mudie & Matyas, 2000; Whittal et al. 2000). The authors of the latter studies suggested that a facilitation effect from the non-paretic to the paretic limb might have accounted for the observed motor recovery.
In the present experiments, flexor carpi radialis (FCR) and extensor carpi radialis (ECR) CM pathway or spinal pathway excitability was examined during synchronous and asynchronous bimanual wrist flexion-extension. A tracking paradigm was employed where one wrist was being passively and rhythmically flexed and extended by a computer-controlled servomotor, while the neurologically intact participants performed active voluntary flexion and extension movements of the opposite wrist at the same frequency. The dynamics of wrist flexion-extension in a tracking paradigm, where an active limb tracks the spatiotemporal pattern of a driven limb, have been shown to share many similarities with bilaterally active paradigms (Stinear & Byblow, 2001). Based on the evidence outlined above, we designed the present experiments to contrast the regulation of intracortical inhibition between synchronous and asynchronous patterns of bimanual co-ordinated movement.
Methods
Electromyography (EMG)
EMG signals were collected from 10 mm diameter Hydrospot Ag-AgCl electrodes (Physiometrix Inc., USA), fixed with tape 1 cm apart (i.e. at 2 cm centres) midway between the musculotendinous junctions of the subdominant FCR and ECR muscles following standard skin preparation. EMG signals were amplified (Grass P511AC EMG amplifiers, Grass Instrument Division, RI, USA), band-pass filtered at 30 Hz-1 kHz (-6 dB cut-off points), sampled at a rate of 2000 Hz by a MacLab acquisition system and displayed using PowerLab Scope v3.6.4 software (ADInstruments, Castle Hill, NSW), and stored to disk for off-line analysis. For each stimulus, 120 ms of EMG data were collected, of which 20 ms were pre-stimulus.
Transcranial magnetic stimulation
Transcranial magnetic stimulation was delivered to the M1 contralateral to the test limb from a Magstim Model 200 unit, or for paired-pulse cortical stimulation, two Model 200 units via a BiStim unit (Magstim Company, Dyfed, UK). A figure-of-eight coil (70 mm coil diameter) held tangentially to the scalp was used, with the handle held posterior and orthogonal to the assumed plane of the central sulcus. Subjects wore a tight fitting cotton cap with pre-marked co-ordinates in a 1 cm grid pattern. Six MEPs (motor evoked potentials) were collected and examined on-line simultaneously from FCR and ECR for each co-ordinate of the 1 cm grid pattern surrounding a position 3 cm lateral to the vertex. The ‘hot-spot’ was taken to be the co-ordinate where MEP amplitudes were greater than amplitudes of adjacent co-ordinates for a given stimulus intensity. All subsequent stimuli were delivered to the hot-spot. The rest threshold for FCR was defined as the highest stimulus intensity that produced no more than four of eight consecutive MEPs with an amplitude of ≈50 μV while the FCR was at rest. The active threshold was taken as the lowest stimulus intensity that produced at least four of eight consecutive MEPs that were discernible (≈100 μV) from background EMG during weak isometric contraction (approximately 5 % maximum voluntary contraction, MVC). In Experiments 1 and 3, the conditioning stimulus intensity was set at 90 % of active threshold, and this level was checked to ensure no MEPs were evident in the FCR of the muscle at rest. If so, the intensity level was reduced until MEPs were no longer elicited. The test stimulus intensity was initially set at 120 % of rest threshold and adjusted up as required to ensure that the amplitude of test MEPs elicited at rest and suppressed by conditioning stimuli delivered at an inter-stimulus interval of 2 ms (Kujirai et al. 1993) were greater than 100 μV. Although this parameter setting procedure was prioritized to FCR, subjects' ECR parameters were satisfactorily set at the same time, as evidenced by the effectiveness of conditioning.
H-reflex testing
In Experiment 2, H-reflex responses were recorded from the FCR of the driven limb by stimulating the median nerve using a Grass S48 stimulator together with isolation (Grass SIU7) and constant current (Grass CCU1C) units, and a monopolar configuration was used to enhance current distribution around the cathode (Jayakar, 1993). To minimize the variability in the intensity of the induced current over the median nerve at the elbow that can result from task-related movement of the stimulating electrodes, the stimulation site was chosen where the cathode was attached to the skin over the nerve on the medial surface of the upper limb where the nerve courses superficially along the border of the adjacent biceps and triceps brachii. The anode was placed over the ipsilateral acromion process.
Passive movement apparatus
The purpose-built apparatus comprised two steel-framed tables, 61 cm wide × 44 cm deep × 73 cm high, with aluminium top plates. Both units were fitted with a forearm rest incorporating two adjustable stabilizing posts mounted either side of the forearm. Each unit had a hand plate with two posts on the dorsal side to locate and stabilize the hands in a posture where the palmar surfaces of the hands would face each other when the two units were placed side by side in front of the subject. Each hand plate was mounted on a vertical spindle so the wrist joint could rotate freely around the axis of the spindle. Each spindle was connected to a potentiometer to provide angular displacement signals to a PC LabVIEW programme, via a National Instruments 16 bit A-D converter (PCI-MIO-16XE-50). Holes in each top plate accurately specified the angle of the hand plate to the forearm midline in five-degree increments from −90 to +90 with zero being the angle when the ventral surface of the forearm and the palmar surface of the hand plate were at zero degrees to each other. Steel pins were placed in the holes forming physical limits for calibration of the equipment, and to prevent over-rotation of the hand plate. A Brushless AC Servomotor (Baldor, Fort Smith, AR, USA) mounted underneath one unit was driven by a Baldor D3S Motor Drive and a PMAC motor control card (Delta Tau Data, Northridge, CA, USA) from a second PC. The hand in this unit was designated as ‘driven'. Two safety switches were included in the driven unit design: a foot switch for the subject and a hand switch for the experimenter. The contralateral unit was identical to the driven unit, excluding the motor and safety devices, and was designated as ‘active'. Subjects were seated in front of the apparatus, with seat height, forearm-locating posts and hand-fixing posts individually adjusted for stability and comfort. The subdominant limb was always designated as the test limb, and in Experiments 1 and 2 was always the driven limb.
Protocols and analysis
All subjects provided their written informed consent to participate in the study. The University of Auckland Human Subjects Ethics Committee approved the study in accordance with the Declaration of Helsinki.
Experiment 1
Data from eight right-handed subjects (six male, two female), who were able to perform the bimanual patterns without activating the muscles of the driven limb, were included in the analysis. Subjects' average handedness score was 63 % (range, 48–91 %; Oldfield, 1971), and their mean age was 28 (range, 21–48 years).
In order to investigate intracortical inhibitory mechanisms, the level of background EMG activity must be maintained as low as possible, because even low-level voluntary muscle activation has been shown to down-regulate intracortical inhibition (Ridding et al. 1995). Therefore, CM pathway excitability was assessed in the subdominant passively driven limb, using the purpose-built apparatus.
There were three movement conditions, all involving 100 deg of wrist flexion-extension about a neutral wrist angle of 0 deg, at a cycle rate of 1 Hz. In all conditions, the limb from which EMG data were collected always performed the same task. Firstly, a unimanual condition where the driven limb was passively moved while the contralateral limb was at rest; secondly, an inphase pattern where the active limb tracked the spatiotemporal characteristics of the driven limb such that the two limbs reached peak flexion and peak extension simultaneously; and thirdly, a novel pattern where subjects tracked the movement of the driven limb with their active limb at a phase angle of 60 deg (active hand lag). Voltages generated from potentiometers mounted on the manipulanda produced a Lissajous figure on an oscilloscope placed in front of the subject, so that they could continuously monitor the phase angle of their limbs throughout the novel pattern trials. The Lissajous figure produced by the voltage difference between the hand displacement potentiometers in the novel pattern was a broad ellipse with the long axis at an angle of 45 deg to the horizontal. A transparency with a pair of concentric ellipses of appropriate size, shape and orientation was placed over the oscilloscope screen. Subjects were required to maintain the oscilloscope trace (Lissajous figure) between the concentric lines. During a series of 12 trials each lasting 50 s, EMG responses to 16 non-conditioned and 16 conditioned stimuli were collected for each movement condition at a point midway between peak flexion and extension. Four MEPs during flexion and four MEPs during extension were collected in each trial. Non-conditioned and conditioned trials were alternated for each of the three movement conditions to form a block of six trials. Blocks of trials were repeated four times to control for order effects. Sixteen non-conditioned and 16 conditioned responses were also collected while the target muscles were at rest. Throughout data collection, background EMG was monitored by the experimenter and subjects were constantly reminded to maintain their driven limb passive. A sample of EMG during maximum voluntary contraction was also collected. Limb displacement data were saved to disk and analysed off-line. For the novel pattern, responses to TMS were accepted for analysis if they occurred when the between-limb phase angle was within the range 40 deg and 90 deg (i.e. within a one-quarter movement cycle). MEP peak-to-peak amplitudes were calculated using custom routines and averaged for each subject, muscle, movement and stimulus condition. For each subject, MEPs from FCR with high amplitude background EMG were discarded so that responses were only included in subsequent analysis if the group means of background EMG expressed as a percentage of MVC were equivalent across the three movement conditions (unimanual, 3.86 %; inphase, 3.87 %; novel, 4.28 %; repeated measures ANOVA, P = 0.07). A similar selection process for ECR was used although levels remained lower for the novel pattern (1.6 % MVC) than inphase (1.87 % MVC) and unimanual conditions (1.83 % MVC; repeated measures ANOVA, P = 0.004). Background motor unit firing, amplifier noise and cross-talk as assessed in static trials with the muscles at rest contributed 0.6 % MVC for FCR and 0.4 % MVC for ECR. To test for an effect of phasic modulation, where conditioned MEP amplitudes were expected to be smaller than non-conditioned amplitudes, means (normalized to the maximum MEP amplitude for each subject and muscle) were analysed using Student's one-tailed t tests. To examine the modulation of cortical inhibition by movement condition, differences between means of conditioned responses expressed as a percentage of non-conditioned responses were tested for significance using Student's one-tailed t tests. A significance level of 0.05 was adopted for the present analysis (and the analysis of the following two experiments).
Experiment 2
We were able to obtain reliable H-reflexes in FCR muscle from five of eight subjects initially examined. Two male and three female neurologically intact subjects with a mean age of 23 (range, 22–25 years) participated in the experiment. Three subjects were right handed with an average handedness score of 66 % (range, 33–96 %), while two were left handed with handedness scores of −65 % and −100 % respectively.
H-reflex responses were recorded from the subdominant limb at rest (static condition), and when this limb was passively driven through wrist flexion-extension during three movement conditions: (i) while the two hands were cycling in a mirror symmetric pattern (inphase condition), or (ii) the active hand was cycling with a phase lag of 60 deg (the novel pattern condition), or (iii) when the driven hand only was cycling (unimanual condition). For all movement conditions the amplitude of wrist flexion-extension was 100 deg. Cycling frequency was 0.8 Hz, and during bimanual trials an auditory metronome was used to pace the active hand. The cycling frequency was lowered to 0.8 Hz from the 1.0 Hz used in Experiment 1 to assist subjects in the maintenance of temporal accuracy and EMG quiescence. This 20 % reduction in cycling frequency was not expected to change the pattern of phasic modulation, as Lewis et al. (2001) found similar patterns of modulation at frequencies of 0.6 Hz and 1.0 Hz. In bimanual trials, subjects were instructed to coincide their active dominant wrist peak flexion in time with the auditory cue. The amplitude of the active hand manipulandum was constrained by foam-covered stops positioned 5 deg beyond the 50 deg peak flexion and extension. Subjects were instructed to flex and extend as close as possible to, but not collide heavily with, the stops. Low-tension springs attached to the active hand manipulandum provided resistance only when the manipulandum was close to peak flexion and peak extension. This latter device helped subjects to define the movement amplitude and to cycle rhythmically. A box was placed over the driven hand to prevent vision of driven hand movement potentially distracting subjects from the precise timing of active hand movement. Furthermore, vision of the driven hand in the present experiment was prevented to remove potential influences from visual feedback-related descending inputs to spinal-level circuitry. Descending inputs to spinal circuitry are thought to enhance the selectivity of muscle activation patterns through spinal-level presynaptic inhibitory mechanisms (Meunier & Pierrot-Deseilligny, 1998). The driven limb median nerve was stimulated with rectangular wave pulses of 1 ms duration during all experimental conditions. Data collection began by noting the maximum M response with the FCR at rest (Mmax). The stimulus intensity for subsequent trials was set at a level producing a response of ≈10 % Mmax. This level produced H-reflex responses that were considerably depressed compared with responses at rest, ensuring adequate scope for movement-task-related facilitation. Furthermore, H-reflex responses at ≈10 % Mmax during movement were never less than 0.25 mV, and were typically 0.5 mV, ensuring there was adequate scope for amplitude depression. Two blocks of three movement trials provided 10 responses for each movement condition and cycle phase combination. Stimuli were alternately delivered at mid-flexion and mid-extension (zero-degree crossings). Three static trials provided 30 responses at rest. Static trials preceded and followed the first block of movement trials, and a static trial followed the second block of movement trials. To ensure repetitive stimulation did not depress the response over the duration of data collection, up to a minute was allowed to elapse between trials, and the next trial was not commenced until an H response at rest was obtained that was indistinguishable from H responses obtained from prior static trials. Displacement data were collected for subsequent off-line inspection to ensure that H responses were included in analyses only when the required phase relations were met. The mean amplitudes of M responses for each condition and cycle phase were calculated and expressed as a percentage of Mmax.
The mean amplitudes of H responses for each condition and both cycle phases were calculated and normalized to static values. The mean r.m.s. amplitudes of background EMG expressed as a percentage of MVC were calculated for static responses and for each movement condition and cycle phase. To test for an effect of cycle phase, mean H responses from potentiated and inhibited cycle phases were tested for significance with Student's one-tailed t tests. To test for an effect of movement condition, mean H responses during flexion were tested using Student's two-tailed t tests. Background EMG means for each condition were tested for significance with Student's two-tailed t tests.
Experiment 3
Two female and five male neurologically intact subjects with a mean age of 27 (range, 22–36 years) participated in the experiment. Six subjects were right handed with an average handedness score of 81 % (range, 33–100 %), and one was left handed with a score of −65 %.
MEPs in response to TMS were recorded from FCR and ECR of the subdominant test limb at rest, located in the passive movement unit with the hand piece fixed at 0 deg, and the contralateral limb was voluntarily flexed and extended 100 deg in the active unit. Subjects were instructed to coincide their active hand peak flexion in time with an auditory cue at 0.8 Hz, as in Experiment 2. Stimulator intensities were determined for each subject as in Experiment 1, and vision of the driven hand was prevented for the reasons described above. Trials of non-conditioned and conditioned stimuli were alternated, with a stimulus being delivered in each of six temporally equal cycle phases during each trial. These coincided with the following displacement angles (cycle phases 1–6 respectively): +44 deg, 0 deg, −44 deg, −44 deg, 0 deg and +44 deg, where +50 deg was peak flexion and −50 deg was peak extension. To control for order effects, stimuli were delivered in a pseudo-random order of cycle phases. The temporal spacing of cycle phases was chosen so that stimuli would be delivered during two cycle phases for each muscle (e.g. 4 and 5 for FCR) where the limbs would be in a spatial relationship analogous to the patterns investigated in Experiment 1. Specifically, stimuli delivered at 0 deg (cycle phases 2 and 5) probed the static limb CM pathways when the limbs were in a spatial relationship analogous to bimanual inphase movement. Stimuli delivered at −44 deg (cycle phase 4) during the flexion phase probed the static limb FCR pathway when the limbs were in a spatial relationship analogous to bimanual movement where the active hand lagged the driven hand by 60 deg (i.e. the novel pattern). Similarly, stimuli delivered at +44 deg (cycle phase 1) during the extension phase probed the static limb ECR pathway when the limbs were in a spatial relationship analogous to 60 deg of active hand phase lag. Displacement data were collected for off-line inspection so that the cycle phases in which MEPs occurred could be identified. Mean non-conditioned and conditioned MEP amplitudes for each muscle, movement condition and cycle phase were calculated and normalized to static values.
From group data, MEP amplitudes normalized to static values for each muscle were inspected, and means from the most potentiated cycle phase and the most inhibited cycle phase were examined with Student's one-tailed t tests for an effect of cycle phase. Conditioned MEP responses obtained from FCR in cycle phases 4 and 5 (analogous to novel and inphase respectively) were expressed as a percentage of non-conditioned responses, and examined using Student's two-tailed t tests for effects of cycle phase. A similar analysis was made of ECR responses comparing cycle phases 1 and 2 (analogous to novel and inphase respectively).
Results
Experiment 1
Figure 1 illustrates the modulation of MEP amplitude in one representative subject's responses. Two traces for each condition have been overlaid to demonstrate low intra-individual variability in MEP amplitude. Phasic modulation and the effect of conditioning were evident.
Figure 1. EMG traces from FCR of one representative subject illustrating modulation of MEP amplitude.
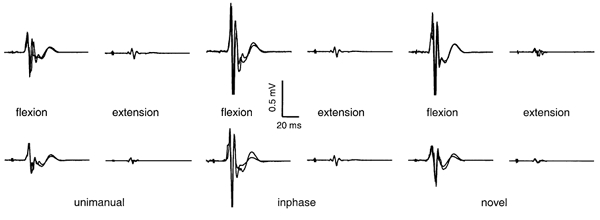
Top row, non-conditioned responses; bottom row, conditioned responses. Each panel contains an overlay of two responses.
The analysis of MEP amplitude from group data is presented in Fig. 2. Significant phasic modulation was evident in both conditioned and non-conditioned responses in FCR, where the mean MEP amplitude was greater during flexion than during extension, for the three movement conditions. The mean MEP amplitudes from ECR demonstrated a similar effect of cycle phase where the mean MEP amplitude was greater during extension than during flexion, except in the unimanual condition where conditioned responses did not differ significantly between phases.
Figure 2. Modulation of MEP amplitude by cycle phase from Experiment 1.
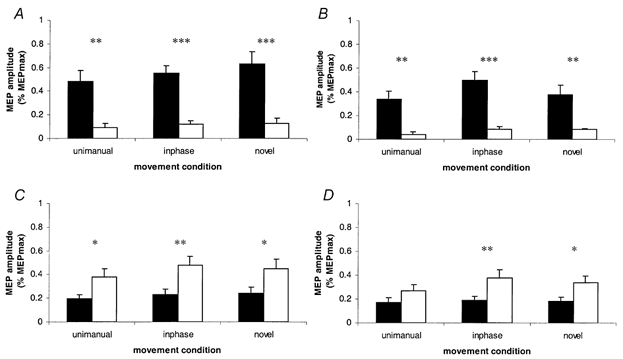
Means of MEP amplitudes normalized to the maximum MEP amplitude for each subject. Black columns represent mid-flexion responses; open columns represent mid-extension responses. A and B, FCR non-conditioned and conditioned responses respectively; C and D, ECR non-conditioned and conditioned responses respectively. Error bars represent +1 s.e.m. *P = <0.05; **P = <0.01; ***P = <0.001. Levels of significance are from Student's one-tailed t tests, d.f. 1, 7.
The analysis of intracortical inhibition in the cortical representation of both muscles is presented in Fig. 3. When mean conditioned MEP amplitudes from FCR were expressed as a percentage of mean non-conditioned responses, there was less cortical inhibition (i.e. more disinhibition) during the flexion cycle phase for inphase and unimanual movement than for static responses.
Figure 3. Cortical disinhibition in the representation of FCR (A) and ECR (B) from Experiment 1.
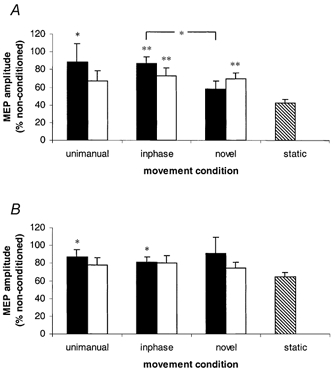
Mean MEP amplitudes of conditioned responses expressed as a percentage of non-conditioned responses. Black columns represent mid-flexion responses; open columns represent mid-extension responses; the hatched column represents static responses. Error bars represent +1 s.e.m. *P = <0.05; **P = <0.01, from Student's one-tailed t tests, d.f. 1, 7. The bracketed asterisk indicates there is a difference between inphase and novel means during flexion. The remaining levels of significance are from movement condition means compared with static means.
During the flexion cycle phase, mean inhibition for the novel pattern of movement did not reliably differ from static values (P = 0.09), and less inhibition (i.e. a higher percentage value of non-conditioned MEP amplitude) was evident for the inphase pattern (mean, 87 %) than the novel pattern (mean, 58 %) of movement (P = 0.021). While the unimanual and inphase means were similar in size, the difference between the unimanual and static means did not reach statistical significance (Fig. 2A). The statistically significant difference in means between inphase and novel patterns resulted from conditioned responses (normalized to the maximum MEP for each subject) being lower for the novel (mean, 0.38) than the inphase (mean, 0.50) pattern (P = 0.02), while the mean non-conditioned responses (novel, 0.63; inphase mean, 0.55) did not reliably differ (P = 0.10). During the extension cycle phase, less inhibition was evident for the two bimanual conditions than static values. Analysis of intracortical inhibition in the representation of ECR revealed that during the extension cycle phase, no effect of movement condition was demonstrated, while in the flexion cycle phase, less inhibition was evident during inphase and unimanual conditions than the static condition.
The main finding from the present experiment was that a novel pattern of bimanual co-ordinated movement did not down-regulate intracortical inhibition in the M1 contralateral to the passively driven limb. Disinhibition was demonstrated most strongly during the inphase pattern, as well as when the target limb alone was being driven. These findings suggest cortical inhibition assists in the maintenance of asynchronous patterns of homologous FCR muscle activation. A similar effect of movement condition was not revealed for ECR. This latter finding may be an indication that the cortex maintains greater individual control of wrist flexors than wrist extensors. Such an interpretation is consistent with previously reported anatomical and physiological differences between wrist flexors and extensors. For example, forearm and intrinsic hand flexors functioning as manipulators are innervated by neurons that are topographically discrete from those that innervate wrist and finger extensors functioning as stabilizers (Ghez, 1991; Rothwell, 1994). There is also evidence that flexor motor units of the hand are more efficient than extensor motor units. A unit change in the firing rate of corticomotoneurons facilitating flexor motor units results in a greater change in torque than an equivalent change in the firing rate of corticomotoneurons facilitating extensor motor units (Cheney et al. 1991).
The down-regulation of cortical inhibition during FCR shortening in the present study has been demonstrated previously (Lewis et al. 2001). Lewis and colleagues demonstrated modulation of intracortical inhibition in the M1 of a passively driven limb measured across a range of wrist angles during flexion and extension. After accounting for the effects of static wrist position, the marked potentiation of responses during FCR shortening and inhibition during lengthening (i.e. phasic modulation) suggested a mechanism of movement-elicited cortical disinhibition. In the present study, responses were recorded when the hand was passing through a wrist angle of 0 deg, a position that coincided with the same 1/8th movement cycle where responses were maximally potentiated in the former study. In the present study, an effect of bimanual movement pattern was only evident for FCR during the potentiated (flexion) cycle phase. It is unlikely that unwanted muscle activation can explain the modulation of cortical inhibition by the pattern of bimanual movement in FCR. Although the levels of background EMG were similar to those reported by Ridding and colleagues (Ridding et al. 1995), where cortical disinhibition was found to be down-regulated by a low level of voluntary contraction, the levels of background EMG in the present experiment were equivalent across the three movement conditions. A likely explanation for the down-regulation of cortical inhibition during muscle shortening is that antagonist muscle spindle outputs lead to a suppression of intracortical inhibitory activity. Support for this interpretation has been demonstrated in an experiment involving weak isometric contraction of forearm muscles and sub-threshold electrical stimulation of peripheral mixed nerves evoking antagonist muscle afference (Aimonetti & Nielsen, 2001). These authors reported decreased intracortical inhibition and increased intracortical facilitation. They suggested the facilitation or disinhibition was being evoked at the cortical level.
It is unlikely that the significantly different levels of inhibition for the two bimanual patterns in the present experiment were mediated at the spinal level. If the effect of pattern had been mediated at the spinal level, the amplitudes of both non-conditioned and conditioned responses should have been equivalent. Since an effect of pattern was only evident in conditioned responses, this effect is probably mediated cortically. However, the data from the present experiment cannot rule out an effect of pattern at the spinal level. The second experiment was therefore designed to determine if a similar effect of pattern could be demonstrated from H-reflex data. The same three movement conditions employed in the first experiment were investigated. Modulation by movement condition in H-reflex data would have indicated that spinal circuitry had been at least partly responsible for the modulation of FCR CM pathway excitability, as demonstrated in the first experiment. Conversely, a lack of modulation in H-reflex responses by movement task, coupled with the lack of modulation revealed in the non-conditioned responses in the first experiment, would suggest the modulation was cortical in origin.
Experiment 2
Figure 4 illustrates the mean amplitudes of H responses normalized to static values. Responses were larger in the flexion phase than the extension phase during unimanual and inphase movement, while flexion and extension means during the novel pattern of movement failed to reach the adopted level of significance. Mean amplitudes of H responses normalized to static values during the flexion cycle phase failed to reveal an effect of movement condition.
Figure 4. Modulation of H-reflexes from Experiment 2.
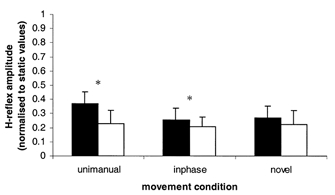
Mean amplitude of H-reflex responses normalized to static values during mid-flexion responses (black columns) and mid-extension responses (open columns) for the three movement conditions. Error bars represent +1 s.e.m. *P = <0.05, indicating significance between flexion and extension responses. Levels of significance are from Student's one-tailed t tests, d.f. 1, 4.
The main finding from the second experiment was the absence of an effect of pattern in the H-reflex data recorded from the passively driven limb during flexion. Phasic modulation was evident in the H-reflex data, where higher amplitude responses were obtained during flexion than extension phases of the movement cycle during the unimanual and inphase movement conditions. A similar pattern of phasic modulation of H-reflexes has been previously observed in the upper limb (Carson et al. 1999), and also the lower limb (Cheng et al. 1995), during passive movement. The Carson et al. (1999) study demonstrated that H-reflexes in FCR at rest were modulated in a similar manner when the contralateral wrist only was actively flexing and extending. Specifically, higher amplitude H-reflexes were obtained during the flexion phase of the contralateral limb than the extension phase. During this same condition, all H-reflex responses were suppressed compared with responses recorded when both limbs were at rest. The results of the present experiment support those of Carson et al. (1999) with respect to phasic modulation and the suppression of responses during movement relative to static values. The results support our conclusion that the effects of pattern on conditioned MEPs elicited from TMS in Experiment 1 were unlikely to be of spinal origin. To define with precision where the modulation was taking place would require an investigation utilizing anodal transcranial electrical stimulation (TES) that stimulates pyramidal cells directly (Day et al. 1987), bypassing the intracortical circuitry capable of modulating M1 outputs in response to sensorimotor inputs. Eliciting responses to TES in forearm muscles at rest is a difficult and uncomfortable procedure, but may become the subject of a future experiment. A question of immediate concern to us was to what extent the active movement of the contralateral limb in isolation may have contributed to the pattern of CM excitability (and disinhibition) observed in the test (passive) limb. To explore this issue we examined CM excitability of a static limb during active flexion and extension of the contralateral limb.
Experiment 3
From Fig. 5, it is evident that rhythmical flexion-extension of one wrist phasically modulated the excitability of CM pathways to FCR and ECR of the contralateral limb maintained at rest. This modulation was evident for both non-conditioned and conditioned responses. The most potentiated and the most inhibited cycle phases were identified from an inspection of mean MEP amplitudes for each cycle phase and muscle. Differences between these selected means were significant when tested using one tailed t tests. For responses from FCR, the most potentiated and most inhibited cycle phases were 4 and 1 for non-conditioned, and 4 and 2 for conditioned responses, respectively. For responses from ECR, the most potentiated and most inhibited cycle phases were 1 and 4 for non-conditioned, and 1 and 5 for conditioned responses, respectively. For both muscles the most potentiated cycle phases always fell during the period when the muscle of the contralateral active limb was voluntarily contracting. Conversely, the most inhibited cycle phases always fell during the period when the muscle of the contralateral active limb was being stretched.
Figure 5. Phasic modulation of MEP amplitudes from Experiment 3.
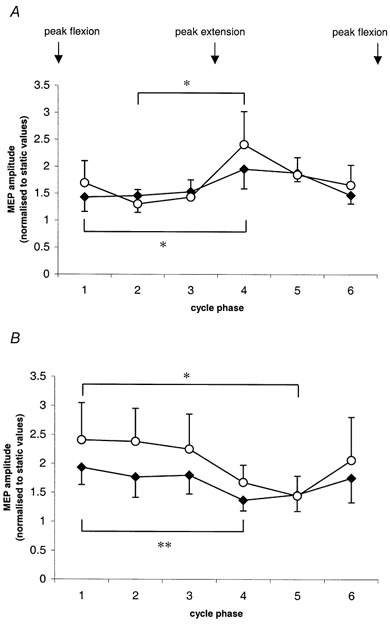
A, from FCR. B, from ECR. Cycle phases 1–6 relate to contralateral hand movement. ○, conditioned responses; ♦, non-conditioned responses. Error bars represent 1 s.e.m. Brackets and asterisks above conditioned data points and below non-conditioned data points indicate significance between most potentiated and most inhibited cycle phases using Student's one-tailed t tests; d.f. 1, 6; *P < 0.05; **P < 0.01.
Mean conditioned responses from FCR at cycle phase 4 were 92 % of the mean non-conditioned responses, and at cycle phase 5, 73 % of non-conditioned responses. The difference in these two means failed to reach the adopted level of significance using two-tailed t tests. Mean conditioned responses from ECR at cycle phase 1 were 116 % of the mean non-conditioned responses, and at cycle phase 2, 132 % of non-conditioned responses. The difference in these two means also failed to reach the adopted level of significance using two-tailed t tests.
There were two main findings from the third experiment. Firstly, the excitability of the CM pathways to wrist flexors and extensors of the static limb at rest was phasically modulated. Specifically, these pathways were potentiated when the contralateral homologous muscles were contracting during voluntary rhythmical flexion- extension. This pattern of modulation has also been observed in FCR pathways when the limb was passively driven (Experiment 1; Carson et al. 2000; Lewis et al. 2001). What makes this finding interesting was that the excitability of CM pathways to muscles at rest was up-regulated by voluntary contraction of contralateral homologous muscles. It is known that contraction of a muscle in the human hand increases CM pathway excitability to the contralateral homologous muscle at rest (Stedman et al. 1998; Stinear et al. 2001). This effect is thought to occur as a result of transcallosal inhibitory interneurons suppressing the action of intracortical inhibitory interneurons (Schnitzler et al. 1996). Similarly, in the present experiment, modulation of excitability of CM pathways to wrist effectors at rest may be the result of a down-regulation of inhibitory intracortical interneurons. A possible functional relevance of these high levels of pathway excitability to muscles at rest may be to ensure homologous muscles are maintained in readiness to contract simultaneously during tasks such as grasping an object with both upper limbs.
The second finding from the third experiment was that the greater disinhibition demonstrated in the first experiment during the inphase pattern compared with the novel pattern was not demonstrated at cycle phases in the present experiment analogous to those two patterns of movement. For FCR the limbs were in a spatial relationship analogous to 60 deg of active hand phase lag (i.e. the novel pattern) during cycle phase 4, and in a spatial relationship analogous to the inphase pattern during cycle phase 5. Conditioned MEP amplitude means (as a percentage of non-conditioned responses) appeared to be higher in cycle phase 4 than 5, although there was no statistical difference. The importance of this finding is that it supports the interpretation of data from Experiment 1, that the greater disinhibition for the inphase pattern of movement was the result of the simultaneous shortening of homologous FCR muscles. In contrast, in Experiment 3, inhibition during the two cycle phases analogous to the inphase and novel patterns was equivalent. Therefore, the greater disinhibition for the inphase pattern in Experiment 1 was unlikely to be the sole result of voluntary contraction of the contralateral FCR. Tracking the active hand movement in Experiment 1 may have contributed to the observed modulation of intracortical inhibition. The visual tracking component of the required task in Experiment 1 was not included in the second and third experiments for reasons described in the Methods section, Experiment 2. The role of visual tracking in the modulation of cortical inhibitory circuits during the performance of synchronous and asynchronous patterns of bimanual movement has yet to be investigated.
Discussion
Together, the results of all three present experiments indicate the following. Movement-elicited afference derived from a passively moving limb modulates inhibitory processes associated with the passively moving limb. This modulation is occurring within the GABA-mediated inhibitory cortical neurons within M1. Intracortical inhibition is maintained when both upper limbs are moving in asynchrony, but is down-regulated when both upper limbs are moving in synchrony. From a functional perspective, a down-regulation of inhibition during asynchronous bimanual movement may be counter-productive to the maintenance of independent muscle activation.
Motor cortex disinhibition is thought to be a neural mechanism underlying post-stroke functional recovery. For example, Liepert et al. (2000) demonstrated disinhibition of the affected cortex in acute stroke subjects relative to the unaffected cortex and to neurologically intact controls. Responses were recorded from the first dorsal interosseous muscle at rest. These authors argued that the disinhibition was not pathological, but a compensatory mechanism, because in their subjects M1 and presumably the intracortical inhibitory interneurons had been spared. Furthermore, there was a tendency for subjects with less pronounced disability to have greater levels of disinhibition.
Synchronized voluntary bimanual movements have been utilized as novel upper limb rehabilitation protocols with some success (Mudie & Matyas, 2000; Whittal et al. 2000). These findings, and those of Liepert et al. (2000), suggest that functional recovery of the upper limb and the suppression of cortical inhibition may be associated with cortical plasticity. In future experiments we intend to employ this passive-active movement paradigm to examine CM excitability in recovering hemiparetic patients.
Acknowledgments
The authors extend their thanks to Richard Carson and Jeffrey Summers for their helpful suggestions on this work. J.W.S. is supported by a fellowship from the Foundation for Research Science and Technology (NZ). Funding for this project was provided by a University of Auckland staff grant to W.B.
References
- Aimonetti J-M, Nielsen JB. Changes in intracortical excitability induced by stimulation of wrist afferents in man. Journal of Physiology. 2001;534:891–902. doi: 10.1111/j.1469-7793.2001.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R, Riek S, Smethurst C, Parraga J, Byblow W. Neuromuscular-skeletal constraints upon the dynamics of unimanual and bimanual coordination. Experimental Brain Research. 2000;131:196–214. doi: 10.1007/s002219900272. [DOI] [PubMed] [Google Scholar]
- Carson RG. The dynamics of isometric bimanual coordination. Experimental Brain Research. 1995;105:465–476. doi: 10.1007/BF00233046. [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S, Bawa P. Electromyographic activity, H-reflex modulation and corticospinal input to forearm during active and passive rhythmic movements. Human Movement Science. 1999;18:307–343. [Google Scholar]
- Cheney P, FetZ E, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Progress in Brain Research. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Brooke JD, Misiaszek JE, Staines WR. The relationship between the kinematics of passive movement, the stretch of extensor muscles of the leg and the change induced in the gain of the soleus H reflex in humans. Brain Research. 1995;672:89–96. doi: 10.1016/0006-8993(94)01321-8. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke organization of brain-motor output to the hand: a 2–4 month follow-up with focal magnetic brain stimulation. Electroencephalography and Clinical Neurophysiology. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Day B, Rothwell JC, Thompson P, Dick J, Cowan J, Berardelli A, Marsden CD. Motor cortex stimulation in intact man II: multiple descending volleys. Brain. 1987;110:1191–1209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- Fontaine RJ, Lee TD, Swinnen SP. Learning a new bimanual coordination pattern: reciprocal influences of intrinsic and to-be-learned patterns. Canadian Journal of Experimental Psychology. 1997;51:1–9. doi: 10.1037/1196-1961.51.1.1. [DOI] [PubMed] [Google Scholar]
- Ghez C. The control of movement. In: Kandel E, SchwartZ J, Jessel T, editors. Principles of Neuroscience. 3. Prentice-Hall; 1991. pp. 539–540. chap. 35. [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jayakar P. Physiological principles of electrical stimulation. In: Devinsky O, Beric A, Dogali M, editors. Electrical and Magnetic Stimulation of the Brain and Spinal Cord. New York: Raven Press; 1993. pp. 17–28. [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD, Carson RG. Phasic modulation of corticomotor excitability during passive movement of the upper limb: effects of movement frequency and muscle specificity. Brain Research. 2001;900:282–294. doi: 10.1016/s0006-8993(01)02369-1. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clinical Neurophysiology. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of I a afferents in humans. Experimental Brain Research. 1998;119:415–426. doi: 10.1007/s002210050357. [DOI] [PubMed] [Google Scholar]
- Mudie HM, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disability and Rehabilitation. 2000;22:23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallet M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor J, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. Journal of Physiology. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J. Control of Voluntary Movement. London: Chapman & Hall; 1994. [Google Scholar]
- Schnitzler A, Kessler KR, Beneke R. Transcallosally mediated inhibition of interneurons within human primary motor cortex. Experimental Brain Research. 1996;112:381–391. doi: 10.1007/BF00227944. [DOI] [PubMed] [Google Scholar]
- Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle and Nerve. 1998;21:1033–1039. doi: 10.1002/(sici)1097-4598(199808)21:8<1033::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Walker KS, Byblow WD. Symmetric facilitation between motor cortices during contraction of ipsilateral hand muscles. Experimental Brain Research. 2001;139:101. doi: 10.1007/s002210100758. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Phase transitions and postural deviations during bimanual kinesthetic tracking. Experimental Brain Research. 2001;137:467–477. doi: 10.1007/s002210000665. [DOI] [PubMed] [Google Scholar]
- Whittal J, Waller S, Silver KHC, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]


