Abstract
It has been postulated that the mammary kinin system may play a role in modulating mammary blood flow. Until the present study, the local release of bradykinin (BK) or other kinin system constituents into the mammary vasculature had not been reported and there were also conflicting findings on the action of BK on udder vasculature. Udders were removed from healthy lactating cows at slaughter. Pairs of ipsilateral quarters were perfused with Tyrode solution through the external pudendalis artery and drained via the cranial superficial epigastric vein. Mammary secretion was collected through teat cannulae. The perfusion pressure was linearly related to perfusate flux between 60 and 210 ml min−1 and the flow rate was adjusted (110-150 ml min−1) to give a basal pressure of 85 mmHg. PO2, PCO2 and pH in the venous effluent perfusate stabilised at 157 ± 10 mmHg, 50.1 ± 2.4 mmHg and 7.1 ± 0.03, respectively. The venous effluent contained immunoreactive BK and BK precursor, tissue kallikrein activity, and bradykinin-destroying enzyme. The concentration of BK stabilised at 378 ± 48 pg (ml perfusate)−1, that of trypsin-activated BK precursor was 679 ± 59 pg BK equivalents ml−1 and that of tissue kallikrein, measured as cleavage of d-Val.Leu.Arg-p-nitroanilide (d-Val.Leu.Arg-pNA), was 5.5 ± 1.7 nmol p-NA h−1 ml−1. Arterial infusion of phenylephrine (0.49-490 μM) produced increases in perfusion pressure (vasoconstriction). Acetylcholine (ACh) (0.55-55 μM) and BK (0.1-10 μM) produced only vasodilatation. BK (EC50 = 1.00±0.04 μM) was a more potent vasodilator than ACh (EC50 = 9.57±0.49 μM). The basal BK concentration was 250 times below the threshold for vasoactivity. The udder produced a milk-like secretion, which was dependent on perfusate flow and contained a concentration of BK which remained unchanged from 60 to 180 min of perfusion (231 ± 31 pg ml−1) unlike that in the venous effluent which doubled between 60 and 120 min. Thus, in addition to its secretion into milk, BK, together with its precursor and tissue kallikrein, is continuously released into the vasculature of the isolated, perfused, lactating bovine udder.
The formation of kinin peptides in milk was first reported by Guth (1959), who found that bovine colostrum contained a precursor that could be activated by the peptidase, salivary kallikrein, to release kinin-like activity. This was confirmed by Moriya et al. (1966). Guth called this kinin colostrokinin. Colostrokinin was found to be a peptide with a Mr between 1000 and 2000 (Yamazaki & Moriya, 1969; Beretta et al. 1972). Even after calving, normal bovine milk was found to contain kinin precursor (kininogen) (Werle & Trautschold, 1960; Leach et al. 1967). The kininogen contained in bovine milk was purified (Posati et al. 1972; Wilson et al. 1989) and found to consist of two molecules, one with a Mr > 68 000, the other with a Mr between 16 000 and 17 000 (Wilson et al. 1989). Both kininogens released mainly the nonapeptide, bradykinin (BK, Mr 1060.2), when incubated with the intestinal enzymes, pancreatic kallikrein or trypsin. Free BK in milk was first detected by Leach et al. (1967) in bulk herd milk and formally characterised as such by Wilson et al. (1989) who extracted it from pasteurised skimmed bovine milk.
The first measurements of free BK concentrations in untreated bovine milk from individual animals were made by Eshraghi et al. (1999), who reported a mean concentration of 120.7 ± 21.0 pg ml−1 in milk from 13 healthy animals. This compared with a mean level of 145.6 ± 11.6 pg ml−1 in venous blood sampled from the jugular veins of healthy animals. They further showed that the concentrations of BK in milk greatly increased in the presence of mastitic infections. It remains to be determined whether BK has any functional importance in the mammary gland. It has been proposed that the mammary vasculature is regulated by locally produced vasoactive agents (Prosser et al. 1996) and that BK may have an important role in this respect. Until the present study, the release of BK or other kinin system constituents into the mammary vasculature had not been demonstrated.
In addition to a possible role in the control of udder blood flow, other putative roles in mammary gland function have also been ascribed to the kinin system. Kinins liberated from milk have been proposed as the agents that increase neonatal gut permeability to aid absorption of milk proteins (Guth, 1959) or antibodies (Yamazaki & Moriya, 1969). BK has been proposed as a physiological milk ejection stimulant (Houvenaghel et al. 1968; Oguro et al. 1982). A role in the modulation or induction of healthy breast tissue growth has been postulated for tissue kallikrein present in human milk (Magklara et al. 1999). It has also been suggested that mammary tissue-kallikrein may contribute to regulation of fluid and salt secretion (Hermann et al. 1995). The detection of tissue kallikrein in malignant human breast tissue led Hermann et al. (1995) to suggest a pathophysiological role for the enzyme in mammary carcinogenesis via the activation of metalloproteinases, or possibly via the action of released kinins in enhancing vascularisation or mitogenicity. The ability of the udder to form BK and the other kinin system components may also be implicated in the pathogenesis of infectious mastitis. Levels of both BK (Eshraghi et al. 1999) and active kallikrein (Zeitlin et al. 1999) in bovine milk are greatly raised in mastitic udders, particularly in the presence of bacterial infection (Eshraghi et al. 1999). Initial studies indicate that these increases are brought about by a direct action of pathogenic bacteria on the kinin system constituents secreted by the udder and are not part of the normal inflammatory mechanisms (Zeitlin et al. 2000).
The isolated perfused udder has to date had little use as a physiological model. A literature database search for the 37 years from 1964 through 2001 produced less than 40 publications, largely from three research groups, reporting the use of this model for any purpose. The use of the isolated perfused bovine udder was first reported by Peeters & Massart in 1947. The perfusion medium was initially bovine blood. Peeters and colleagues subsequently, in a series of studies, used blood-perfused goat udders to study mammary metabolism (e.g. Massart-Leen et al. 1986). The isolated perfused goat udder was also used for a series of mammary metabolic studies using erythrocytes suspended in albumin-containing Krebs solution as the perfusion medium (Hardwick & Linzell, 1960; Linzell et al. 1972). More recently, the isolated bovine udder perfused with Tyrode solution has been used in a series of studies by Kietzmann and his colleagues (e.g. Kietzmann et al. 1993; Bäumer & Kietzmann, 2001) as an elegant model to study the cutaneous absorption of topical drugs. The model of Kietzmann and his colleagues was used as a starting point for the model described in the present paper.
In the present study, organs obtained from the local abattoir and set up within 40 min of slaughter, have been used to evaluate the isolated perfused bovine udder as a model for studying the local release of BK and its vasomotor action in the bovine mammary vascular bed. The model has been used to determine whether components of the kinin-forming system can be detected in the udder vascular perfusate in the absence of plasma. Preliminary results from this study have been communicated to the British Pharmacological Society (Eshraghi & Zeitlin, 1997).
Methods
The isolated perfused bovine udder
Udders from healthy lactating cows of various breeds, weights and stages of lactation, were obtained from the local abattoir. The udders were dissected immediately after slaughter of cows, which were California Mastitis Test (CMT) negative and also showed no clinical signs of mastitis. The udder weight range was 12.0-15.5 kg.
The skin and the superficial layer of the mammary gland suspensory apparatus, which is attached to skin, were cut laterally towards the pelvic symphysis. The concave base of the mammary gland, together with some parts of the gracilis muscles and two intact large mammary lymph nodes were cut. The external pudendalis artery was freed and immediately flushed with 700 ml gassed (95 % O2-5 % CO2), heparinised (1000 IU l−1) Tyrode solution. The organ was wrapped in plastic sheet, packed in ice and immediately transferred to the laboratory for perfusion within 40 min of slaughter and dissection. Udders found to have damaged mammary parenchyma were not used.
Udder perfusion
The udders were suspended on a metal tubular framework that allowed the teats to hang freely. The external pudendalis artery was cannulated and half of the organ (two ipsilateral quarters) was perfused with Tyrode solution (NaCl, 8 g l−1; KCl, 200 mg l−1; CaCl2.2H2O, 265 mg ml−1; MgCl2.6H2O, 213 mg l−1; NaHCO3, 1 g l−1; NaH2PO4 65 mg ml−1; glucose, 1.1 g l−1; pH 7.4) at 38 °C, gassed with 95 % O2-5 % CO2. Venous drainage took place via the cannulated cranial superficial epigastric vein (milk vein). The isolated udder was warmed using a thermostatically controlled electric blanket at 37 °C. During an initial equilibration period (30 min), the perfusion rate was set at 110–150 ml min−1 and, except in the early study examining the relationship between pressure and flow, adjusted to give a basal perfusion pressure of 85 mmHg, the mean arterial blood pressure of adult cattle (Olsen & Booth, 1971). The perfused quarters were cannulated via the teat canals using teat cannulae (Ravanshir Ltd, Iran). Both the vascular perfusate and the mammary secretion were only in contact with plastic or siliconised surfaces.
The perfusion pressure (mmHg) was continuously monitored using a pressure transducer and recorder (Gould TA240S, Gould Electronic Ltd), via a T-tube attached to the arterial cannula. Skin and perfusate temperatures were recorded using a commercial thermometer. A blood gas machine (Rocket, Terumo Ltd, Japan) was used to monitor PO2, PCO2 and pH. All experiments were terminated at 180 min or earlier. Udders were routinely weighed at the start and end of each experiment. They showed a weight increase in the range of 4–10 % (see Discussion). A weight increase over 11 % was arbitrarily set as a routine criterion for exclusion of the organ from the experiment.
A vasodilator response to ACh was used to confirm the functional integrity of the vascular endothelium. At the end of each experiment, trypan blue solution (1 %) was infused via the arterial cannula to determine the extent of the glandular perfusion indicated by blueing of the tissues. Organs were excluded from the study if they did not show even blueing of the mammary parenchyma and the teat cistern of the perfused half of the udder, accompanied by blueing of the ‘pseudo-milk’ secretion.
Dose-response curves
After an initial equilibration period of 30 min, the perfusion flow rate was adjusted to give a basal perfusion pressure of 85 mmHg. This did not change significantly throughout the experiments. ACh, phenylephrine and BK were each dissolved at various concentrations in 1 ml fresh distilled water. The drugs or vehicle alone were infused through a three-way tap into the fast flowing arterial perfusate (110-150 ml min−1) at approximately 5 % of the perfusion flow rate, at a rate suitable to give the indicated final perfusate concentrations.
Radioimmunoassay of bradykinin
Perfusate or ‘pseudo-milk’ samples (10 ml) were collected in chilled (4 °C) polypropylene tubes containing EDTA (1 mg ml−1 final concentration) and frozen (-15 °C) for storage. Samples were thawed and extracted on C18 SepPak Vac cartridges eluted with 60 % acetonitrile, evaporated until dry at 40 °C under a nitrogen stream and radioimmunoassayed for BK (Eshraghi et al. 1999). The mean recovery of synthetic BK using this method was 92.3 ± 1.3 % (mean ± s.e.m., n = 6).
Bradykinin precursor assay
BK precursor was assayed as total releasable immunoreactive BK following activation with trypsin. Perfusate (0.5 ml) was pre-incubated (37 °C) in polypropylene tubes, with 0.5 ml Tris buffer (0.1 m, pH 8.5) for 10 min and TPCK-trypsin (chymotrypsin-free; Sigma Ltd) was added (0.1 ml, 100 μg ml−1). The tubes were closed and incubated for 2 h. The reaction was terminated by heating on a boiling water bath for 10 min. Enzyme blank incubates contained pre-boiled trypsin. BK was extracted and radioimmunoassay was performed as described above. The results were recorded as pg (releasable BK equivalents) per ml.
Assay of BK destroying activity
Perfusate was diluted (1:1) with Michaelis barbital buffer (barbital sodium, 0.058 m; HCl, 0.042 m; pH 7.4) and incubated with synthetic BK (10 μg ml−1) for 180 min, in a water bath at 37 °C. The incubation was terminated by boiling for 10 min. Control incubates contained pre-boiled perfusate. BK concentrations in the incubates were determined using reverse phase HPLC (RP-HPLC) on a C18 ODS column (Phenomenex Ltd; S5, ODS2; length, 250 mm; diameter, 3.2 mm; particle size, 5 μm). BK was eluted from the column at 0.5 ml min−1 using an acetonitrile gradient (25-70 %) in water containing 0.09 % trifluoroacetic acid and detected in a UV detector (Beckman System Gold, Model 166) at 210 nm. The retention time for the BK peak was 26.03 min, while Lys-BK and des-Arg-BK eluted at 25.07 min and 29.79 min, respectively. Percentage destruction was calculated by comparing the difference between BK concentrations from incubates containing boiled (control) or unboiled (test) perfusate.
Assay of tissue kallikrein-like amidase activity
Perfusate (1 ml) was pre-incubated with Tris buffer (0.8 ml, 0.1 m, pH 8.5) for 10 min. The incubation was started by the addition of the tissue kallikrein substrate, d-Val.Leu.Arg-pNA, 0.1 ml, 1.5 mm; Sigma Ltd) and continued for 4 h. The incubation was terminated by the addition of 0.1 ml trichloroacetic acid (TCA, 12 % solution). In control incubates, the TCA was added before the substrate, to pre-inactivate the enzymic activity. Change in absorbance at 405 nm, due to pNA cleavage, was monitored using a spectrophotometer. For the purpose of this study, a unit of tissue kallikrein activity (KU) was taken as the release of 1 nmol of pNA in 1 h.
Statistics
All values are given as means ± s.e.m. Except where stated otherwise, two- or one-way ANOVA as appropriate, with Newman-Keuls multiple comparisons post hoc tests have been used for statistical analysis of the data. Significance is taken at P ≤ 0.05. The two-tailed P value is always quoted.
Results
Udder perfusion
The udders were set up and perfusion was commenced within 40 min of slaughter. In an initial study, the relationship between flow rate and perfusion pressure was examined. After an initial 30 min equilibration period, the flow rate was varied between 30 and 210 ml min−1 (Fig. 1). It can be seen from Fig. 1 that over the range tested, the graph of perfusion pressure versus flow rate is highly linear, flattening a little below a flow rate of 60 ml min−1. For all subsequent studies, the perfusate flow was adjusted to give a basal perfusion pressure of 85 mmHg. This usually required a flow rate between 110 and 150 ml min−1.
Figure 1. The relationship between perfusion pressure and flow in isolated perfused bovine udders.
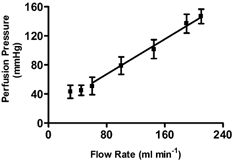
Udders were perfused with Tyrode solution (38 ° C) via the cannulated external pudendalis artery and drained via the cannulated milk vein. The organs were covered with an electric blanket maintained thermostatically at 37 ° C. The inflow pressure was recorded using a pressure transducer. Values are given as mean ± s.e.m. (n = 5). The relationship was highly linear down to a flow rate of 60 ml min−1 (r2 = 0.9921, P < 0.0001, two-tailed).
The initial perfusion conditions were based on those used by Kietzmann et al. (1993), who allowed the preparation to equilibrate for 15 min before commencing experimental studies. In the experiments to establish basal parameters, the perfusion was run for a 30 min equilibration period before measurements were commenced. Perfusate PO2, PCO2, and pH, and perfusate and skin temperatures were then recorded for a further 145 min (Fig. 2). Although examination of the graph in Fig. 2A indicates slightly greater variation in mean PO2 during the first 90 min, this did not reach significance (P > 0.05) and settled at between 157 ± 10 and 163 ± 8 mmHg during the final 60 min. However, the mean PCO2, fell significantly between 35 and 180 min (P = 0.0002), with the main fall, from 64.7 ± 2.7 to 50.1 ± 2.4 mmHg (P < 0.01) occurring between 35 and 90 min. There was no significant change in PCO2 between 90 and 180 min. Predictably, the fall in PCO2 was accompanied by a small but statistically significant increase in pH between 35 and 180 min (P = 0.0009; Fig. 2B). As with the PCO2, the main change in pH (from 6.8 ± 0.04 to 7.1 ± 0.03, P < 0.01) occurred between 35 and 90 min. There was no significant change in pH between 90 min (pH 7.1 ± 0.03) and 180 min (pH 7.1 ± 0.06). The temperature of the venous effluent perfusate reached equilibrium rapidly, and by 30 min had stabilised, remaining between 36.1 ± 0.08 and 36.6 ± 0.06 °C throughout the experiment (Fig. 2C). The skin temperature likewise quickly stabilised. The mean value at 35 min was 34.9 ± 0.06 °C and at 60 min was 35.4 ± 0.20 °C, although this was not a significant change. Thereafter, the skin temperature remained between 0.8 and 1.0 °C below that of the venous effluent throughout the experiment.
Figure 2. Changes in physicochemical parameters with time in isolated bovine udder during 180 min perfusion.
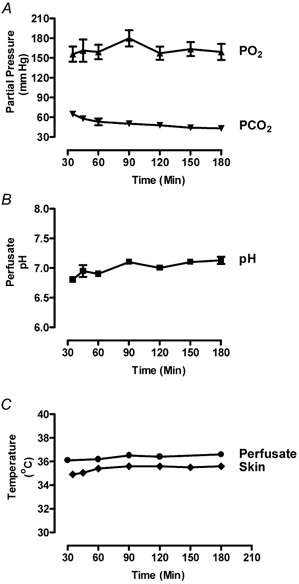
The perfusion flow rate was adjusted (110-150 ml min−1) to give a basal perfusion pressure of 85 mmHg, the conditions were otherwise as for Fig. 1 (all graphs show means ± s.e.m.). The data were analysed using one-way ANOVA with the Newmann-Keuls post hoc test. A, partial pressures of O2 and CO2 in the venous effluent perfusate. PO2 showed no significant change (n = 5). PCO2 fell significantly only between 35 and 90 min (P < 0.01, n = 5). B, pH of venous effluent, which showed significant change (P < 0.01, n = 5) between 35 and 90 min but not thereafter. C, temperatures of skin (n = 5) and venous effluent (n = 3) perfusate which did not change significantly.
Assay of kinin system components
Further groups of udders were perfused as before for 180 min, to determine basal levels for the spontaneous release of BK, BK precursor, tissue kallikrein and kininase activity. The venous effluent was collected for assay at timed intervals between 35 and 180 min after onset of perfusion. A radioimmunoassay for BK was used to measure the concentrations of free BK and total BK precursor (determined as total trypsin-releasable BK). Active tissue kallikrein was monitored by measuring the hydrolysis of a specific tissue kallikrein substrate (d-Val.Leu.Arg-pNA) and total BK-destroying activity was determined by incubating venous effluent samples with BK (10 μg ml−1) and measuring the percentage loss using RP-HPLC.
It can be seen from Fig. 3A that between 35 min and 180 min after the start of perfusion, the concentration of BK fell steeply from an initial value of 512 ± 113 pg ml−1 to 206 ± 46 pg ml−1 at 60 min (P = 0.046) and then rose steadily to stabilise at 400 ± 72 pg ml−1 at 150 min. This biphasic V-shaped change was not mirrored by the other kinin system components. The total concentration of activateable BK precursor fell consistently from 35 min to 180 min (Fig. 3B). The reduction (27.8 %, P = 0.0171) was more rapid between 35 and 60 min than during the remaining 2 h (26.6 %, P = 0.0179). The BK precursor concentration changed little after 120 min and had stabilised in the region of 679 ± 59 pg BK equivalents ml−1 by the end of the experiment. Although the graph of the time course of tissue kallikrein activity (Fig. 3C) appears to show a steady fall from the initial mean value of 11.8 ± 2.7 KU ml−1 until 90 min and stabilisation at 5.5 ± 1.7 KU ml−1 to the end of the experiment at 180 min, the changes did not quite reach statistical significance. The BK-destroying activity (Fig. 3D) of the venous effluent fell relatively rapidly (P = 0.044) between 35 min (12.9 ± 0.6 % h−1 ml−1) and 60 min (10.0 ± 1.0 % h−1 ml−1) and then remained stable until the end of the experiment.
Figure 3. Changes in concentrations of kinin system components with time in isolated bovine udder during 180 min perfusion.
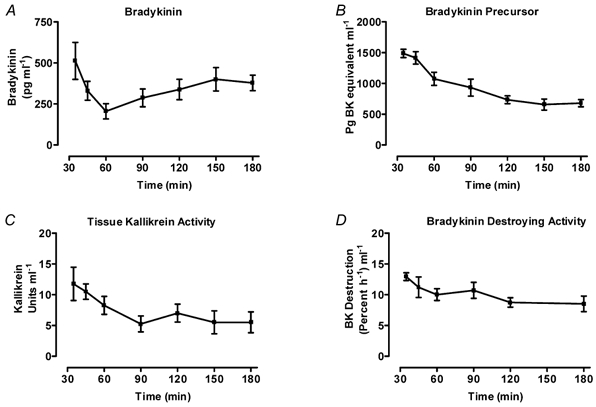
The conditions were as for Fig. 2. Owing to the differing requirements for sample collection, the four studies were carried out on four different groups of udders. Assays were carried out on venous effluent perfusate sampled at timed intervals between 35 min and 180 min. (All graphs show mean ± s.e.m.) A, concentrations of immunoreactive BK measured by radioimmunoassay. The values fell significantly between 35 and 60 min (P = 0.046) and rose to plateau at 150 min, reaching significance at 180 min (P = 0.040, n = 5). B, concentrations of total BK precursor measured as immunoreactive BK released after incubation (0.1 m Tris buffer, pH 8.5, 37 °C, 2 h) with chymotrypsin-free trypsin (9 μg ml−1). The most rapid fall occurred between 35 and 60 min (P = 0.0171) but continued more slowly between 60 and 180 min (P = 0.0179). The concentration changed little (P > 0.05) after 120 min (n = 4). C, concentrations of tissue kallikrein amidase activity measured as rate of p-nitroaniline release from a specific tissue kallikrein tripeptide substrate (d-Val.Leu.Arg-pNA). Perfusates (1 ml) were incubated (37 °C, 4 h) with substrate (150 μM) in Tris buffer (0.8 ml, 0.1 m, pH 8.5). The release of pNA was measured on a spectrophotometer as change in absorbance at 405 nm. A unit of tissue kallikrein activity (KU) is taken as the release of 1 nmol of pNA in 1 h (n = 5). D, concentrations of bradykinin-destroying activity determined by incubating perfusate (3 h, 37 °C, pH 7.4) with synthetic BK (10 μg ml−1). The residual BK was assayed using RP-HPLC. The enzyme concentration fell (P = 0.044) between 35–60 min and then remained unchanged (P > 0.05) until 180 min (n = 4).
The effluent perfusate thus contained all of the main kinin system components, free BK, BK precursor, tissue (i.e. glandular) kallikrein and kinin-destroying activity. Their levels clearly had not reached basal values after 35 min perfusion and up to 60 min appeared still to be falling, possibly as the result of a washout effect of previously accumulated material. However, from 60 min onward the BK concentration rose again to reach an equilibrium basal value, while the concentrations of the other components continued to fall until basal values were achieved. By 150 min after onset of perfusion, all of the kinin system components had settled at their basal values for this perfusion model.
Vasoactivity of bradykinin in isolated perfused udder
A study was carried out to determine the dose-response curve for the action of BK on vascular tone measured as perfusion pressure. At the outset of the study, there was no reason to expect the system to have sufficient intrinsic tone to respond appropriately to a vasodilator drug. It was therefore necessary to confirm that perfusion pressure in the bovine udder gave an apt response to a known vasoconstrictor drug and a vasodilator other than BK. Dose-response curves were therefore determined for phenylephrine, an α1-adrenoceptor agonist, and acetylcholine (ACh). ACh in particular was used because, like BK, it has vasodilator activity which is dependent on endothelial nitric oxide (NO) release, although via activation of unrelated receptors. A vasodilator response to ACh thus served as a check that there had been no ischaemic damage of the vascular endothelium between the dissection of the udders and the onset of perfusion.
ACh, at perfusate concentrations between 0.055 μM and 550 μM, produced a fall in perfusion pressure (vasodilatation) (Fig. 4A) with the main response between 0.55 and 55 μM ACh. Phenylephrine, between 0.049 and 4909 μM, produced an increase in perfusion pressure, with the main response lying between 0.49 and 490 μM (Fig. 4B). BK, between 0.01 and 50 μM produced vasodilatation, with the main response occurring between 0.1 μM and 10 μM (Fig. 4C). Although the dose-response curve to BK was not parallel to that of ACh (as might be expected since they act on unrelated receptor systems), a comparison of EC50 values indicates that BK (EC50 = 1.00 ± 0.04 μM) is much more potent than ACh (EC50 = 9.57 ± 0.49 μM) in its vasodilator action.
Figure 4. Effect of arterial infusion of acetylcholine, phenylephrine and bradykinin on vascular tone measured as perfusion pressure in isolated perfused bovine udders.
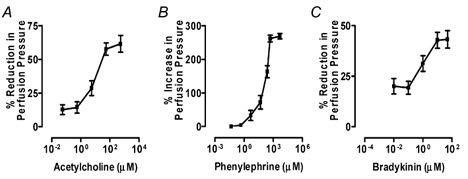
Perfusion conditions were as for Fig. 1. Perfusion pressure was monitored via the arterial cannula. Perfusate flow rate was adjusted at the start of each experiment to give a basal perfusion pressure of 85 ± 2.6 mmHg. This did not change significantly between the start and end of the experiments. Perfusion flow rate was in the range 110–140 ml min−1. Graphs show means ± s.e.m. A, graph for acetylcholine (n = 4) shows a reduction in perfusion pressure indicating vasodilatation. B, graph for phenylephrine (n = 3) shows an increase in perfusion pressure indicating vasoconstriction. C, graph for BK (n = 5) shows a reduction in perfusion pressure indicating vasodilatation.
'Pseudo-milk’ secretion
The fact that the perfused isolated bovine mammary gland produces a milk-like secretion has been noted by earlier workers (Peeters & Massart, 1947; Hardwick & Linzell, 1960; Kietzmann et al. 1993). The first two groups used their udder models primarily to study the production of this secretion. In the present study we have termed this material ‘pseudo-milk', because it was formed in the absence of blood and is unlikely to have a normal constitution. However, because we had already shown the presence of free BK in normal bovine milk (Eshraghi et al. 1999), we tested whether the pseudo-milk also contained detectable free BK. To exclude the possibility that the appearance of the pseudo-milk was merely the result of passive let-down of previously accumulated secretion, we tested the effect of halting the intravascular perfusion on its production. Figure 5A shows that by 3 min after switching off the perfusion pump, the rate of pseudo-milk production had fallen by 50 % from the initial value of 8.1 ± 0.5 g min−1 (P < 0.05) and continued to fall to 3.6 ± 0.2 g min−1 by 5 min. After 10 min the perfusion flow was re-started, and the rate of pseudo-milk production immediately rose again nearly to the initial value. The production of pseudo-milk was thus clearly dependent on perfusion flow and was not merely the result of non-specific drainage of pre-formed milk. In the udders used to study BK in the venous effluent (see Fig. 3A), the BK concentration in the pseudo-milk was also measured. When the perfused udders were allowed to equilibrate for 30 min and the pseudo-milk was collected at timed intervals as above (Fig. 5B), the BK concentration showed an initial fluctuation and then did not change significantly from 60 to 180 min. The mean concentration of BK settled at a basal value lying between 225 ± 34 and 243 ± 22 pg ml−1. Initially, immediately after 30 min equilibration, the BK level in milk was lower than that in perfusate. From 45 to 90 min, the milk concentration was greater than that in perfusate and from 120–180 min, the relationship stabilised with the BK concentration in pseudo-milk at 61 % of that in perfusate.
Figure 5. The release and bradykinin content of the mammary secretion of isolated perfused bovine udders ('pseudo-milk').
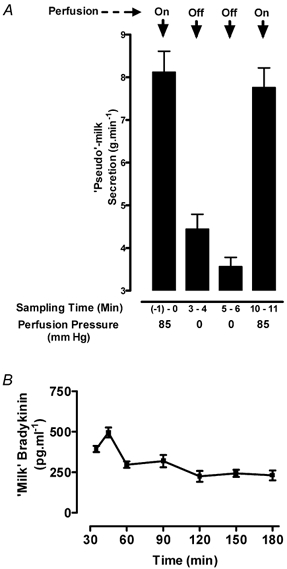
A, dependence of pseudo-milk production on perfusion flow. The passive let-down of ‘pseudo-milk’ from the cannulated teat was collected and weighed for 1 min intervals at the time points indicated. The perfusion flow was stopped at 0 min and re-instated at 6 min. The final sample was taken at 10 min. Both the reduction in milk release on halting perfusion and the increase after 4 min re-perfusion were highly significant (P < 0.001). B, the bradykinin concentration in pseudo-milk monitored between 35 and 180 min in the same udders for which perfusate BK was monitored in Fig. 3A. The values at the first two time points were significantly greater than all subsequent values (P < 0.01). Thereafter the concentration showed no significant change. Values are expressed as means ± s.e.m., n = 4.
Discussion
Bradykinin is a potent vasodilator nonapeptide. It is cleaved from precursor kininogen proteins found in blood and other body fluids, by specific kallikrein proteinases. Plasma kallikrein (Mr range, 85 000–88 000) occurs mainly in blood and lymph, and tissue (or ‘glandular') kallikreins (Mr range, 24 000–45 000) are found in most tissues, but particularly in glands and secretory tissues (Bhoola et al. 1992). Kallikrein activity has been isolated from bovine mammary tissue (Peeters et al. 1976). Milk from healthy cows contains at least three enzymes acting on kallikrein substrates, including a tissue kallikrein (Magklara et al. 1999; Zeitlin et al. 1999) and plasma kallikrein (Zeitlin et al. 1999) while quarter milk from healthy cows contains free BK, with a mean concentration of 120.7 ± 21.0 pg ml−1 (Eshraghi et al. 1999). The mean basal BK concentration in milk from healthy animals showed little fluctuation when monitored over a period of 17 days. Whether BK has a functional role in the mammary gland is as yet unclear.
There has been relatively little research on the local control of mammary vasculature and the first, and so far only, published review of the research literature is that of Prosser and colleagues in 1996, who concluded that the manner in which mammary blood flow is controlled was largely unknown. While it is clear that the mammary sympathetic nerve supply is important in maintaining mammary vascular tone, Prosser et al. (1996) suggested that local release of vasoactive agents also has a major regulatory role in the mammary vasculature. Amongst the vasodilator agents that Prosser et al. considered, parathyroid hormone-related protein, insulin-like growth factor-I, prostacyclin, nitric oxide and endothelin have been shown to be produced by the mammary gland. Prosser et al. (1996) also thought that since mammary tissue contained tissue kallikrein (Peeters et al. 1976), the kinins should also be considered as potentially important mammary vasodilators.However, no previous studies have determined whether there is local release of BK or other kinin system constituents from the bovine (or any other) mammary organ into the circulation. In addition, there have been conflicting findings concerning the effect of BK on udder vasculature. In the present study, the isolated perfused lactating bovine udder has been used to examine these questions and to establish basal values for this model.
The perfusion conditions have been adapted from those described by Kietzmann et al. (1993). Although this group particularly studied percutaneous drug absorption and cutaneous pathophysiology, Thompson (1980) has demonstrated that 98 % of lactating udder blood flow passes through the secretory tissue. The isolated perfused lactating udder is thus a potentially useful model for the study of the udder glandular vasculature. Kietzmann et al. (1993) perfused bovine udders with Tyrode solution gassed with 95 % O2-5 % CO2. They perfused the organs at a rate of 60–100 ml min−1, which they pointed out was lower than the physiological blood supply of the udder in vivo, but which provided adequate dermal perfusion. In the present study, the perfusion pressure was found to be linearly related to flow rate between 30 and 210 ml min−1, the resultant pressure ranging between 43 and 146 mmHg. A similarly linear relationship between arterial pressure and flow rate has been demonstrated in a single goat's udder (Linzell et al. 1972). The arterial perfusion rate was adjusted (usually 110–150 ml min−1) to give a basal perfusion pressure of 85 mmHg, the mean arterial pressure in adult cattle (Olsen & Booth, 1971). The mean basal pressure did not change significantly during the course of the experiments.
One drawback to the use of crystalloid perfusion fluids such as Tyrode solution for the perfusion as described by Kietzmann et al. (1993) is that there is inevitably some degree of oedema. In the present study, the inclusion of dextran or albumin to reduce oedema was considered. However, one aspect of using bovine udders as isolated preparations is that they tend to be very large organs. The organ weight range in the present study was 12.0-15.5 kg. As a result, they consumed perfusate materials in almost ‘industrial’ quantities, making the routine inclusion of such colloids in the perfusion fluid impractical. Kietzmann et al. (1993) found that during a 6 h perfusion with Tyrode solution, although the udders showed a 14 % mean weight increase, skin fold thickness measured as a sign of oedema showed no significant increase. To minimise any problems that might be associated with the onset of oedema, the length of the present experiments was limited to 3 h (or less as required), while a weight increase above 11 %, was arbitrarily set as a criterion for exclusion from the study. The organ weight increase in the present study ranged from 4–10 %.
It is common experience with isolated perfused tissues that following the change from in vivo perfusion with blood to in vitro perfusion with medium, there is a significant equilibration period during which physical and biochemical parameters may be quite variable. This equilibration period is often surprisingly long. Neither Hardwick & Linzell (1960) nor Peeters & Massart (1947) studying isolated goat and cow udders, respectively, mentioned using an initial equilibration period prior to measuring experimental parameters. Kietzmann et al. (1993) used a 15 min initial period of equilibration. In the present study, because equilibrium basal values were being established, the udders were given a longer initial equilibration period of 30 min before measuring parameters for a further 150 min (Fig. 2). Although during this period, PO2 did not change significantly, PCO2 fell to 43.0 ± 2.1 mmHg accompanied by a small rise in mean pH from 6.8 to 7.1. The temperatures of the effluent perfusate and of the skin had presumably adjusted rapidly during the initial 30 min equilibration period since they did not show any further significant change. Where such physicochemical changes were detected, they were all completed by 90 min after commencement of the perfusion.
The isolated perfused bovine udder is a relatively little used experimental model and some comment should be made about it before considering the main conclusions from this study. It proved to be a robust and reproducible model. At the abattoir, the udder is normally removed as waste material immediately following slaughter; its laboratory use is therefore not accompanied by the ethical and other requirements normally associated with animal experimentation. One of the main limitations of using the bovine udder is its large size, necessitating the use of large quantities of materials. Had species not been relevant to the present study, the udders of smaller animals such as sheep (Verbeke et al. 1972) or goats (Hardwick & Linzell, 1960) might have been used. A second limitation, common to the experimental use of all viable tissue obtained from the abattoir, is the necessity for minimising the delay between slaughter and perfusion in the laboratory. This was of particular concern in the present vascular studies, since prolonged ischaemia appeared to cause inappropriate alteration in the vascular responses. Steps were therefore taken to minimise this (immediate flushing with well-gassed Tyrode solution in the abattoir; minimal delay in setting up) and vascular endothelial function was also confirmed in the laboratory. The isolated, perfused udder preparation has been used to study mammary metabolism (Wood et al. 1965; Hardwick, 1966), the mechanisms of milk production (Hardwick & Linzell, 1960) and the pharmacology (Kietzmann et al. 1993) and pathophysiology (Bäumer & Kietzmann, 2001) of the skin. The present study shows that the isolated perfused udder is also a useful model for the study of local mediator release and modulation of mammary blood flow.
Evidence for a role for the kallikrein-kinin system in the local control of bovine mammary blood flow, as postulated by Prosser et al. (1996), is based solely on the observations that kallikrein is present in bovine mammary tissue (Peeters et al. 1976) and that BK causes mammary vasodilatation (Dhondt et al. 1973; Peeters et al. 1976). There has to date been no evidence presented showing the local release of one or more components of the kinin system into the mammary vascular bed. In the present study, we have detected the presence of immunoreactive BK and BK precursor, tissue kallikrein and bradykinin-destroying enzyme in the venous effluent from the bovine udder perfused with Tyrode solution. The mean concentrations of all of these kinin system components fell relatively steeply from 35 min (when measurements commenced) to 60 min (Fig. 3), probably due to wash-out of pre-formed material even after the initial 30 min equilibration period. The free BK concentration then slowly rose, to plateau at 400 pg ml−1 at 150 min after the start of perfusion. This time course differed from those of the other three kinin system components which either stabilised at 60 min (BK degrading enzyme), or continued to fall until 90 min (tissue kallikrein) or 150 min (BK precursor) after the onset of perfusion. The fact that all of the concentrations had reached level baselines by 150 min suggests that these are the basal concentrations for release or formation of these substances in this model. Although tissue kallikrein has been reported to be present in both human (Magklara et al. 1999) and bovine (Zeitlin et al. 1999) milk, it has not previously been shown to be released into the mammary circulation in either species. Since active kallikrein and BK-destroying activity were both detected in the perfusate, it is clear that the BK concentration must result from a dynamic equilibrium between formation and destruction. Indeed, the local concentration of BK at the site of formation is potentially much greater than that ultimately measured in the effluent perfusate.
At 180 min the total releasable BK stored as BK precursor was 679 ± 59 pg (ml perfusate)−1, which was some 70 % greater than the concentration of free BK. The concentration of BK precursor apparently released by the gland in the perfused organ would be boosted in vivo by that already present in the plasma supplying the udder. The implication of this is that in the isolated organ perfused with Tyrode solution and also in the functioning gland in vivo, it is the rate of release and activation of kallikrein and not the concentration of BK precursor that is likely to be the limiting factor determining the rate of formation of active BK.
The mean concentration of free BK in the venous effluent had stabilised at 378 ± 48 pg ml−1 by the end of the perfusion. This was some 260 % of the mean concentration of 145.6 ± 11.6 pg ml−1 previously measured in the jugular venous blood of six healthy lactating animals (Eshraghi et al. 1999). At this stage, the reason for the much greater value found in the perfusate is unclear. In general, circulating kinins are kept at a low level in vivo both by circulating peptidases and, more importantly, by clearance and destruction by kininases in the lung. In smaller animals such as cats, dogs and humans the half-life (T1/2) has been variously estimated at 17–30 s (Bhoola et al. 1992; Cyr et al. 2001). Circulating levels are thus normally extremely small. They are only raised in the vicinity of tissues secreting kallikrein or kinins, or when the local or systemic equilibrium between formation and destruction is tipped in favour of formation such as in the acid environment of inflamed tissue. Furthermore, although kininase activity was detected in the udder perfusate, the level of kininase in systemic blood may well be much greater.
The observation that the basal concentration of free endogenous BK in the perfusate was circa 0.4 nm, raised the possibility that in vivo the release of endogenous BK in the mammary gland might maintain a continuous vasodilator modulation on the underlying sympathetic tone. However, this was some 250 times below the threshold for vasoactivity in this model, although local concentrations within the organs may be greater. Arterial infusion of synthetic BK at concentrations above 100 nm (i.e. 0.1 ng ml−1) produced vasodilatation. This compared with reported threshold vasodilator doses in vivo in goats of 0.3 ng (Ogura et al. 1982) and in sheep of 0.5 ng (Peeters et al. 1972), following close arterial injection (neither group gave molar threshold concentrations). In contrast with these and the present study, Linzell et al. (1972) reported that in the isolated goat udder perfused with Krebs solution containing erythrocytes, BK unexpectedly produced vasoconstriction. They also found that deliberate induction of ischaemia and reperfusion in their model caused vasoconstriction, rather than the expected reactive hyperaemia. Although these authors commented that these findings were of potential physiological significance, they did not report checking for functional integrity of the vascular endothelium. They stated that some animals were slaughtered prior to utilising the udders, which in an earlier report was stated to involve a delay of 1–2 h (Hardwick & Linzell, 1960). Initial experience in the present study showed that endothelial damage caused by prolonged ischaemia could result in BK or ACh producing vasoconstriction. For this reason the organs were flushed immediately on removal with well-gassed Tyrode solution, and the delay between slaughter and perfusion (during which they were cooled on ice) was kept under 40 min. Despite the reported absence of cholinergic innervation to the mammary vascular bed, the vasodilator action of ACh in the mammary vasculature has been well established (see Prosser et al. 1996). In both the present study using isolated bovine udders and that of Oguro et al. (1982) using close arterial injection in vivo in goats, BK was a much more potent vasodilator than ACh, with a molar ratio of 1:9.6 in the present study and 1:1000 in the latter study. The large difference between these ratios could be species dependent. It could also result from an increased rate of ACh clearance in the in vivo study through the action of cholinesterases normally present in blood.
The bovine udder perfused with Tyrode solution produced a milk-like secretion ('pseudo-milk'), the production of which was dependent on perfusion flow. Normal bovine milk contains BK and the question of whether this is independent of or secondary to the availability of components of the kinin-forming system present in blood, has been considered (Eshraghi et al. 1999). In the present study, since plasma has been replaced by Tyrode solution, it was possible to examine this question further. The pseudo-milk contained free BK which, unlike that in the perfusate, showed little or no fluctuation after the first 60 min. The mean concentration at 180 min was 231 ± 31 pg ml−1, which was much less than the 378 ± 48 pg ml−1 occurring concomitantly in the perfusate. The fact that the pseudo-milk was secreted in the absence of plasma, provides support to the conclusion of Eshraghi et al. (1999), that the presence of BK in milk is independent of blood-based mechanisms. Clarification of whether the large difference between the final equilibrium concentrations of BK in pseudo-milk and perfusate results from differences in the rates of formation or of destruction, must await measurement of the other kinin system components in the pseudo-milk.
To summarise the present findings, the isolated perfused lactating bovine udder has been shown capable of releasing BK into both the vascular lumen and the ‘pseudo-milk’ secretion in the absence of blood plasma. The organ is also able to release both tissue kallikrein and bradykinin precursor into the vascular perfusate. Exogenous BK has been shown to have a consistent dose-related vasodilator action in this model, although at concentration levels several orders higher than that detected in the venous effluent. This discrepancy in itself does not exclude BK from the role suggested by Prosser et al. (1996) as a modulator of vascular tone in the udder. These are basal concentrations. Furthermore, endogenous BK is considered to act primarily as an autocrine/paracrine agent. The udder was found to release kinin-destroying activity into the vascular perfusate that would reduce the level of exogenous BK reaching the vascular receptors. Local levels at the site of BK formation are likely to be much higher.
Further studies using the isolated perfused udder with receptor antagonists, enzyme inhibitors and stimulants of mammary function will help determine whether the kinin system or other local factors, if any, are involved in the modulation of udder mammary vascular tone and blood flow.
Acknowledgments
The project was supported in part by University of Strathclyde Research and Development Project Grant, no. 823. H.R.E is grateful to the Iranian Ministry of Higher Education for a Scholarship. The authors thank the staff of the Glasgow Abattoir for their kind cooperation.
References
- BÄUMER W, Kietzmann M. Effects of steroidal and non-steroidal antiphlogistic drugs on eicosanoid synthesis in irritated skin: studies with the isolated perfused bovine udder. Journal of Pharmacy and Pharmacology. 2001;53:743–747. doi: 10.1211/0022357011775875. [DOI] [PubMed] [Google Scholar]
- Beretta C, Gallina G, Ormas P, Faustini R. Effects of a crude preparation of colostrokinin compared with those of bradykinin on isolated rat duodenal segments. Pharmacological Research Communications. 1972;4:87–97. [Google Scholar]
- Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacological Reviews. 1992;44:1–80. [PubMed] [Google Scholar]
- Cyr M, Lepage Y, Blais C, Gervais N, Cugno M, Rouleau J-L, Adam A. Bradykinin and des-Arg9-bradykinin metabolic pathways and kinetics of activation of human plasma. American Journal of Physiology – Heart and Circulatory Physiology. 2001;281:H275–283. doi: 10.1152/ajpheart.2001.281.1.H275. [DOI] [PubMed] [Google Scholar]
- Dhondt G, Houvenaghel A, Peeters G, Verschooten F. Influence of vasoactive hormones on blood flow through the mammary artery in lactating cows. Archives Internationales de Pharmacodynamie et de Therapie. 1973;204:89–104. [PubMed] [Google Scholar]
- Eshraghi HR, Zeitlin IJ. Bradykinin release in the isolated perfused bovine mammary gland. British Journal of Pharmacology. 1997;122:147P. [Google Scholar]
- Eshraghi HR, Zeitlin IJ, Fitzpatrick JL, Ternent H, Logue D. The release of bradykinin in bovine mastitis. Life Sciences. 1999;64:1675–1687. doi: 10.1016/s0024-3205(99)00105-8. [DOI] [PubMed] [Google Scholar]
- Guth PS. Kinins produced from bovine colostrum by kallikrein and saliva. British Journal of Pharmacology. 1959;14:549–552. doi: 10.1111/j.1476-5381.1959.tb00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick DC. The fate of acetyl groups derived from glucose in the isolated perfused goat udder. Biochemical Journal. 1966;99:228–231. doi: 10.1042/bj0990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick DC, Linzell JL. Some factors affecting milk secretion by the isolated perfused mammary gland. Journal of Physiology. 1960;154:547–571. doi: 10.1113/jphysiol.1960.sp006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Buchinger P, Rehbock J. Visualization of tissue kallikrein in human breast carcinoma by two-dimensional Western blotting and immunohistochemistry. Biological Chemistry Hoppe-Seyler. 1995;376:365–370. doi: 10.1515/bchm3.1995.376.6.365. [DOI] [PubMed] [Google Scholar]
- Houvenaghel A, Peeters G, Djordjevic N. Influence des kinins du plasma et des kallikréins sur l'éjection du lait chez la brebis. Archives Internationales de Pharmacodynamie et de Therapie. 1968;171:231–232. [PubMed] [Google Scholar]
- Houvenaghel A, Peeters G, Vandaele G, Djordjevic N. Influence des kinins du plasma et des kallikréins sur l'ejection du lait chez les ruminants. Archives Internationales de Pharmacodynamie et de Therapie. 1968;76:658–679. doi: 10.3109/13813456809058733. [DOI] [PubMed] [Google Scholar]
- Kietzmann M, Loscher W, Arens D, Maass P, Lubach D. The isolated perfused bovine udder as an in vitro model of percutaneous drug absorption. Skin viability and percutaneous absorption of dexamethasone, benzoyl peroxide, and etofenamate. Journal of Pharmacological and Toxicological Methods. 1993;30:75–84. doi: 10.1016/1056-8719(93)90010-c. [DOI] [PubMed] [Google Scholar]
- Leach BE, Blalock CR, Pallansch MJ. Kinin-like activity in bovine milk. Journal of Dairy Science. 1967;50:763–764. doi: 10.3168/jds.S0022-0302(67)87509-X. [DOI] [PubMed] [Google Scholar]
- Linzell JL, Fleet IR, Mepham TB, Peaker M. Perfusion of the isolated mammary gland of the goat. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1972;57:139–161. doi: 10.1113/expphysiol.1972.sp002145. [DOI] [PubMed] [Google Scholar]
- Magklara A, Scorilas A, LopeZ-Otin C, Vizoso F, Ruibal A, Diamandis EP. Human glandular kallikrein in breast milk, amniotic fluid, and breast cyst fluid. Clinical Chemistry. 1999;45:1774–1780. [PubMed] [Google Scholar]
- Massart-Leen AM, Peeters G, Vandeputte-van Messom G, Roets E, Burvenich C. Effects of valerate and isobutyrate on fatty acid secretion by the isolated perfused mammary gland of the lactating goat. Reproduction, Nutrition, Development. 1986;26:801–814. doi: 10.1051/rnd:19860505. [DOI] [PubMed] [Google Scholar]
- Moriya H, Moriwaki C, Yamazaki K, Akimoto S, Fukushima H. Human salivary kallikrein and liberation of colostrokinin. In: Erdös EG, Back N, Sicuteri F, editors. Hypotensive Peptides. Berlin: Springer; 1966. pp. 161–173. [Google Scholar]
- Ogura K, Hashimoto H, Nakashima M. Pharmacological effects of several drugs on the myoepithelium and the vascular smooth muscle of the lactating mammary gland in goats. Archives Internationales de Pharmacodynamie et de Therapie. 1982;256:108–122. [PubMed] [Google Scholar]
- Olsen JD, Booth GD. Normal values for aortic blood pressures and heart rates of cattle. The Cornell Veterinaria. 1971;62:85–100. [PubMed] [Google Scholar]
- Peeters G, Houvenaghel A, Verbeke R, Van Sichem-Reynaert R. Effects of bradykinin and kallikrein injected into the udder artery of sheep and goats. Archives Internationales de Pharmacodynamie et de Therapie. 1972;198:397–414. [PubMed] [Google Scholar]
- Peeters G, Massart L. La perfusion de la glande mammaire isolée. Archives Internationales de Pharmacodynamie et de Therapie. 1947;74:83–89. [PubMed] [Google Scholar]
- Peeters G, Verbeke R, Houvenaghel A, Reynaert R. Isolation of kallikrein from mammary gland of cows. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1976;61:1–14. doi: 10.1113/expphysiol.1976.sp002329. [DOI] [PubMed] [Google Scholar]
- Posati LP, Holsinger VH, Fox KK, Pallansch MJ. Inhibition of milk kinin activity by phenolic antioxidants. Journal of Dairy Science. 1972;55:1557–1560. doi: 10.3168/jds.S0022-0302(72)85719-9. [DOI] [PubMed] [Google Scholar]
- Prosser CG, Davis SR, Farr VC, Lacasse P. Regulation of blood flow in the mammary microvasculature. Journal of Dairy Science. 1996;79:1184–1197. doi: 10.3168/jds.S0022-0302(96)76472-X. [DOI] [PubMed] [Google Scholar]
- Thompson GE. The distribution of blood flow in the udder of the sheep and changes brought about by cold exposure and lactation. The Journal of Physiology. 1980;302:379–386. doi: 10.1113/jphysiol.1980.sp013249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke R, Roets E, Massart-Leen AM, Peeters G. Metabolism of (U- 14 C)-l-threonine and (U-14C)-l-phenylalanine by the isolated perfused udder. The Journal of Dairy Research. 1972;39:239–250. doi: 10.1017/s0022029900014072. [DOI] [PubMed] [Google Scholar]
- Werle E, Trautschold I. Colostrokinin and its formation by kallikrein. Zeitschrift fur Biologie. 1960;112:169–180. [PubMed] [Google Scholar]
- Wilson WE, Lazarus LH, Tomer KB. Bradykinin and kininogens in milk. Journal of Biological Chemistry. 1989;264:17777–17783. [PubMed] [Google Scholar]
- Yamazaki K, Moriya H. Isolation and purification of colostrokinin from bovine colostrum. Biochemical Pharmacology. 1969;18:2303–2311. doi: 10.1016/0006-2952(69)90344-x. [DOI] [PubMed] [Google Scholar]
- Wood HG, Peeters GJ, Verbeke R, Lauryssens M, Jacobson B. Estimation of the pentose cycle in the perfused cow's udder. Biochemical Journal. 1965;96:607–615. doi: 10.1042/bj0960607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin IJ, Allan L, Eshraghi HR, Logue D. Kallikreins in bovine milk. The Journal of Physiology. 1999;521.P:74P. [Google Scholar]
- Zeitlin IJ, Ng RSK, Eshraghi HR, Logue D. Activation by bacterial pathogens of bradykinin (BK) formation in bovine milk. The Journal of Physiology. 2000;527.P:144P. [Google Scholar]


