Abstract
Activation of cardiac sympathetic afferents during myocardial ischaemia causes angina and induces important cardiovascular reflex responses. Reactive oxygen species (ROS) are important chemical stimuli of cardiac afferents during and after ischaemia. Iron-catalysed Fenton chemistry constitutes one mechanism of production of hydroxyl radicals. Another potential source of these species is xanthine oxidase-catalysed oxidation of purines. Polymorphonuclear leukocytes (PMNs) also contribute to the production of ROS in some conditions. The present study tested the hypothesis that both xanthine oxidase-catalysed oxidation of purines and neutrophils provide a source of ROS sufficient to activate cardiac afferents during ischaemia. We recorded single-unit activity of cardiac afferents innervating the ventricles recorded from the left thoracic sympathetic chain (T1-5) of anaesthetized cats to identify the afferents' responses to ischaemia. The role of xanthine oxidase in activation of these afferents was determined by infusion of oxypurinol (10 mg kg−1, i.v.), an inhibitor of xanthine oxidase. The importance of neutrophils as a potential source of ROS in the activation of cardiac afferents during ischaemia was assessed by the infusion of a polyclonal antibody (3 mg ml−1 kg−1, i.v.) raised in rabbits immunized with cat PMNs. This antibody decreased the number of circulating PMNs and, to a smaller extent, platelets. Since previous data suggest that platelets release serotonin (5-HT), which activates cardiac afferents through a serotonin receptor (subtype 3,5-HT3 receptor) mechanism, before treatment with the antibody in another group, we blocked 5-HT3 receptors on sensory nerve endings with tropisetron (300 μg kg−1, i.v.). We observed that oxypurinol significantly decreased the activity of cardiac afferents during myocardial ischaemia from 1.5 ± 0.4 to 0.8 ± 0.4 impulses s−1. Similarly, the polyclonal antibody significantly reduced the discharge frequency of ischaemically sensitive cardiac afferents from 2.5 ± 0.7 to 1.1 ± 0.4 impulses s−1. However, pre-blockade of 5-HT3 receptors eliminated the influence of the antibody on discharge activity of the afferents during ischaemia. This study demonstrates that ROS generated from the oxidation of purines contribute to the stimulation of ischaemically sensitive cardiac sympathetic afferents, whereas PMNs do not play a major role in this process.
Myocardial ischaemia and reperfusion are associated with cardiovascular reflex responses as well as with chest pain. During ischaemia, activation of cardiac vagal afferents elicits reflex inhibitory cardiovascular reflexes consisting of decreases in arterial blood pressure, heart rate, and systemic vascular resistance (Oberg & Thoren, 1973). In contrast, activation of cardiac sympathetic (spinal) afferents evokes reflex excitatory cardiovascular responses (Peterson & Brown, 1971; Malliani et al. 1972; Huang et al. 1995b; Tjen-A-Looi et al. 1998; Fu & Longhurst, 2001). Clinical evidence suggests that angina pectoris can be relieved by stellate ganglionectomy or dorsal rhizotomy, but not by cervical vagotomy, indicating that cardiac nociception is transmitted by cardiac sympathetic afferents through spinal cord pathways (Birkitt et al. 1965; Palumbo & Lulu, 1965; Meller & Gebhart, 1992). Thus, dual neural innervation of sympathetic and vagal afferents relays information from the heart to the brain.
Myocardial ischaemia and reperfusion produce a number of metabolites, including lactic acid, bradykinin (BK), prostaglandins, adenosine, and reactive oxygen species (ROS), that can stimulate cardiac afferent nerve endings (Kimura et al. 1977; Berger et al. 1977; Hirsh et al. 1981; Meller & Gebhart, 1992; Grill et al. 1992). Exogenous application of these endogenous substances sensitizes and/or activates vagal and cardiac sympathetic afferents (Brown, 1967; Staszewska-Barczak et al. 1976; Baker et al. 1980; Pagani et al. 1985; Pal et al. 1989; Nganele & Hintze, 1990) For instance, we have shown that ischaemically sensitive cardiac sympathetic afferents are activated by endogenously produced BK (Huang et al. 1995a; Tjen-A-Looi et al. 1998), through the kinin B2-receptor (Tjen-A-Looi et al. 1998). Studies from other laboratories suggest that cyclooxygenase products enhance BK-induced cardiac-cardiovascular reflexes (Staszewska-Barczak et al. 1976). However, BK does not fully depend on prostaglandins to activate cardiac sympathetic afferents during myocardial ischaemia (Tjen-A-Looi et al. 1998). In contrast to BK, adenosine produced during myocardial ischaemia does not activate cardiac sympathetic afferents in cats (Pan & Longhurst, 1995). Recently, we have demonstrated that ROS are produced during brief ischaemia and reperfusion in the cat heart (O'Neill et al. 1996) and activate ischaemically sensitive cardiac sympathetic afferents to reflexly increase heart rate, arterial blood pressure, and myocardial contractility (Huang et al. 1995a,b). However, the sources that contribute to the generation of ROS and hence activation of cardiac sympathetic afferents during myocardial ischaemia are uncertain.
Ischaemia and reperfusion of the heart lead to the generation of several ROS, including hydrogen peroxide (H2O2), superoxide radicals (O2•−; Grill et al. 1987), and hydroxyl radicals (•OH); the latter species is formed by the Haber-Weiss reaction in the presence of iron (Halliwell & Gutteridge, 1990). Huang et al. (1995a) have shown that dimethyl thiourea (a chelator of several ROS species, including O2•−, H2O2, •OH and HOCl) and deferoxamine (an iron chelator and a O2•− and H2O2 scavenger) attenuate the responses of ischaemically sensitive cardiac sympathetic afferents. By contrast, iron-loaded deferoxamine, which scavenges O2•− and H2O2, does not attenuate the responses of these afferents during myocardial ischaemia. These data suggest that a number of ROS may play a role, although •OH appears to be particularly important in activating cardiac afferents during ischaemia and reperfusion.
Even though ROS scavengers reduce the responses of cardiac afferents during ischaemia, the mechanisms principally involved in the production of ROS are largely unknown. In addition to the Haber-Weiss reaction, purine metabolism is one potential source of ROS. During ischaemia the purine metabolites, hypoxanthine and xanthine, accumulate from the breakdown of ATP (Jennings et al. 1981). Xanthine oxidase converts hypoxanthine to xanthine and can be inhibited by oxypurinol. Oxypurinol thus can decrease the synthesis of ROS like O2•− and •OH during asphyxia/reventilation and anoxia/reoxygenation, respectively (Pourcyrous et al. 1993; Zweier et al. 1994). We therefore hypothesized that the inhibition of xanthine oxidase would reduce the activity of cardiac sympathetic afferents during myocardial ischaemia.
Neutrophils (polymorphonuclear leukocytes (PMNs)) constitute another potential source of ROS during myocardial ischaemia. Mounting evidence indicates that PMNs mediate irreversible injury of myocytes after prolonged myocardial ischaemia (Mullane et al. 1985; Romson et al. 1983). PMNs contain membrane-bound reduced forms of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that produce O2•−, which has antimicrobial action (Ferrari, 1994). O2•− released by activated PMNs amplifies the inflammatory response by the activation of a chemotactic factor, which allows activated PMNs to attach to endothelium, leading to injury of tissue by releasing additional oxidative enzymes such as myeloperoxidase and hydrolytic enzymes like elastase (Ferrari, 1994). Inhibition of PMN activity limits myocardial infarct size in pigs (Amsterdam et al. 1993). If PMNs produce sufficient ROS to induce tissue injury, they also could supply sufficient ROS to stimulate cardiac afferent endings during ischaemia and reperfusion. Therefore, we investigated the hypothesis that PMNs constitute an important source of ROS that stimulate cardiac sympathetic afferents during ischaemia.
To determine the role of PMNs in the activation of cardiac afferents, we used a polyclonal antibody to decrease the number of circulating PMNs. Our preliminary data indicated that the antiserum directed against PMNs to a smaller extent also decreased the number of circulating platelets. Since other data from our laboratory suggest that platelets likely play a role in the activation of cardiac sympathetic afferents during myocardial ischaemia through a serotonin subtype 3 receptor (5-HT3) mechanism (Fu & Longhurst, 2000), we performed an additional experiment to eliminate the influence of platelets; this procedure allowed us to determine if, in the absence of a 5-HT-related influence from platelets, PMNs play a major role in the activation of cardiac sympathetic afferents. These data have been presented in preliminary form (Longhurst & Tjen-A-Looi, 1998).
Methods
Surgical preparation
All experiments were performed in cats of either sex (2.3-5.9 kg). Surgical and experimental protocols used in this study were approved by the Animal Use and Care Committees at the University of California, Davis and Irvine. Anaesthesia was induced with ketamine (20-30 mg kg−1, i.m.) and maintained with α-chloralose (40-60 mg kg−1, i.v.). The trachea was intubated and respiration was maintained artificially (model 661, Harvard Apparatus, Ealing, South Natick, MA, USA). A femoral artery and vein were cannulated for measurement of blood pressure and administration of fluids and drugs, respectively. Arterial blood pressure was measured with a pressure transducer (Statham P23 ID, Gould, Cleveland, OH, USA) connected to the arterial catheter.
At the end of the experiments, animals were killed with saturated KCl injected into the circulation under deep anaesthesia. Deep anaesthesia was assured by giving an additional dose of α-chloralose (50 mg kg−1) 5 min before administering the KCl solution.
A midline sternotomy was performed, and the first to seventh left ribs and the left lung were removed. An occlusion cuff was placed around the descending thoracic aorta. Fascia overlying the left paravertebral sympathetic chain from T2 to T6 was removed. The sympathetic chain was isolated, draped over a mirror platform and covered with warm mineral oil. Small nerve filaments were teased gently from the chain between T2 and T5 with the use of an operating microscope (Zeiss, Germany) and the rostral ends were placed individually across a recording electrode. One pole of the recording electrode was grounded with a cotton thread to the animal. The recording electrode was attached to a high-impedance probe (Grass Instruments, Quincy, MA, USA). The signal was amplified (model P511 preamplifier, Grass Instruments) and processed through an audio amplifier (model AM8B audio monitor, Grass Instruments) and fed into an oscilloscope (model 549, Tektronix, Beavertown, OR, USA) for observation. The signal was recorded on a physiograph (model TA 4000, Gould, Valley View, OH, USA) and analysed offline with a computer using an EGAA program (RC Electronics, Santa Barbara, CA, USA). The location and conduction latency of each afferent nerve ending was confirmed by electrical stimulation through an electrode placed on the afferent's receptive field in the wall of the ventricle (Pan & Longhurst, 1995). Conduction distance was estimated from the receptive field along the course of the inferior cardiac nerve through the left stellate ganglion to the recording electrode on the sympathetic chain. C- and Aδ-fibre afferents were classified as those with conduction velocities of < 2.5 and 2.5-30 m s−1, respectively (Huang et al. 1995a; Pan & Longhurst, 1995)
Myocardial ischaemia was induced by constricting the coronary artery supplying the receptive field of cardiac ventricular afferents with a thread placed around the vessel (Huang et al. 1995a; Pan & Longhurst, 1995; Tjen-A-Looi et al. 1998). Regional decreases in blood flow were confirmed by observing a regional change in colour of the myocardium. Our laboratory has shown previously that this observation is associated with lactic acid production as denoted by a local reduction in tissue pH (Pan et al. 1999). Afferents were considered to be ischaemically sensitive if their discharge frequency during 5 min of myocardial ischaemia was increased and sustained at least twofold above baseline activity. All sensory endings of ischaemically sensitive afferents included in this study were located either in the left or right ventricle.
Arterial blood gases were analysed every hour during the search for cardiac afferents as well as before and after each period of myocardial ischaemia with a Radiometer blood gas analyser (model ABL 3) and were maintained within physiological limits (PO2 > 100 mmHg, PCO2 > 28–35 mmHg, pH 7.35-7.45). When necessary, arterial PO2 was increased by enriching the inspired O2 supply and pH was corrected by administering NaHCO3 (1 m, i.v.) and/or by adjusting ventilation. Body temperature was maintained at 36–38 °C with a circulating-water heating pad and a heat lamp.
Experimental protocols
In each experimental protocol we recorded the activity of one afferent per cat.
Inhibition of xanthine oxidase
Oxypurinol (Sigma) was dissolved in 10 ml of saline and 20 μl of 5 m NaOH. Oxypurinol (10 mg kg−1) was infused (at a rate of 2 ml min−1i.v.) 10 min before the second period of ischaemia (n = 9). In a different group of controls (n = 8), myocardial ischaemia was repeated at an interval of 30 min to document that the afferent response was reproducible.
Production of anti-PMN polyclonal antibody
Feline PMNs were isolated from whole blood using a modified Percoll density gradient method from Weyrich et al. 1993. Centrifugation (Beckman GPR Centrifuge, CA, USA) was done at 4 °C. Cat blood was collected with vaccutainer tubes containing 1.5 ml of acid citrate dextrose solution. Anticoagulated blood was mixed (1:1) with Dulbecco's phosphate-buffered saline (PBS, Sigma). After addition of 6 % medical grade dextran (1:5, Sigma), erythrocytes were allowed to settle. The upper plasma fraction was pipetted from the erythrocytes and centrifuged at 400 g for 20 min to obtain platelet-rich plasma (PRP). PRP was decanted from the leukocyte pellet and centrifuged at 2500 g for 10 min to obtain platelet-poor plasma (PPP). The leukocyte pellet was washed with modified Hank's balanced salt solution (mHBSS, JRH Biosciences) and centrifuged at 400 g for 10 min. Contaminating erythrocytes were depleted from the leukocyte pellet with hypotonic lysis and the suspension was recentrifuged. Isotonic Percoll (Sigma) was made by mixing Percoll with 1.5 m NaCl (9:1). The isotonic Percoll was mixed with PPP to produce three solutions with 80 %, 62 % and 50 % Percoll. Starting with the most dense solution, 4 ml of each solution was layered in a 15 ml polypropylene conical centrifuge tube. Leukocytes were resuspended in PBS, then layered on top of the gradient and centrifuged at 1500 g for 40 min. PMNs were collected at the 62–80 % interface and washed with PBS. The PMN pellet was resuspended in PBS and checked for the predominance of PMNs with haematoxylin and eosin stain. Finally, the cells were mixed with either Freund's complete or incomplete adjuvant (1:1, Pierce Chemical; Rockford, IL, USA) prior to injection to induce the production of the polyclonal antibody.
The rabbits used for production of the antibody were immunized by subcutaneous injection of PMNs with Freund's complete adjuvant. The rabbits were boosted every 2 weeks with three subsequent injections of the antigen plus incomplete Freund's adjuvant. Two weeks after completion of the immunization series, under 5 mg kg−1i.m. oxymorphine and 0.5 mg kg−1 acepromazine anaesthesia, 10 ml kg−1 of blood was collected from the rabbit through arterial puncture of the ear and the serum was stored at −70 °C until further processing. Immunoglobin was precipitated with 80 % saturated ammonium sulfate (SAS; Sigma). As described (Drenckhahn et al. 1993), antiserum was centrifuged at 3000 g for 30 min at 4 °C. An equal volume of PBS was added to the supernatant. Under constant stirring, an equal volume of 80 % SAS was added slowly. The immunoglobin was precipitated overnight at 4 °C then centrifuged at 3000 g for 30 min at 4 °C. The supernatant was discarded and the immunoglobin pellet was resuspended with PBS at half the original volume of serum. The immunoglobin then was dialysed overnight against three changes of 1 l of PBS. Thereafter, the immune serum was stored in aliquots at −70 °C. The rabbit was killed with an overdose of anaesthetic followed by exsanguination.
Effect of PMNs on cardiac afferent activity
The polyclonal antibody raised in rabbits immunized with feline PMNs (1 ml kg−1) was diluted in 20 ml of saline. Before and during the second period of myocardial ischaemia, which occurred 45 min later, the antibody was infused i.v. at a rate of 0.15-0.4 ml min−1 (n = 7). In another group of animals (n = 7), control serum from rabbits was infused at a similar rate before and during the second ischaemia. The protein concentration (spectrophotometer, Perkin-Elmer, Lambda 3B, Ridgefield, CT, USA; UV at 280 nm) of the control serum (rabbit serum, 3 mg ml−1) was matched with the serum containing the polyclonal antibody (3 mg ml−1).
Effects of PMN polyclonal antibody after 5-HT3 receptor blockade
Tropisetron (300 μg kg−1, i.v.) was administered after locating an afferent ending in the ventricle prior to ischaemia. Then the polyclonal antibody (1 ml kg−1) was diluted in 20 ml of saline and infused i.v. at a rate of 0.15-0.4 ml min−1 (n = 5); after 45 min of infusion, a second period of myocardial ischaemia was induced.
Measurement of PMN depletion
To assess the concentration of PMNs before the infusion of antibody, 1 ml of venous blood was collected. During infusion three to four blood samples were collected every 15 min to determine the effectiveness of the antibody in depleting PMNs and its influence on other blood cellular elements. Blood samples were centrifuged (Clay Adams QBC) in capillary tubes (QBC V venous tube) for 5 min. A veterinary haematology analyser QBC reader (Becton Dickonson, NJ, USA) set for the assessment of blood elements in cats was used to measure the content of the granulocytes and other blood cellular elements. The second period of myocardial ischaemia was initiated only after the granulocyte content was reduced to < 1 × 109 cells l−1.
Measurement of myeloperoxidase activity
Tissue from the ventricular region at risk and the normal ventricular tissue were stored at −70 °C until assay. Myeloperoxidase (MPO) activity was determined as reported previously (Longhurst et al. 1992). Briefly, ice-cold myocardial tissue was minced and homogenized twice (10 % w/v) with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY, USA) in 0.05 m KH2PO4 (pH 6) containing 0.5 % hexadecyltrimethyl-ammonium bromide (HTAB) for 15 s. Supernatant (100 μl) containing the MPO enzyme was added to 1.9 ml of 0.05 m KH2PO4 (pH 6) containing H2O2 (0.0005 %) and o-dianisidine (0.167 mg ml−1). The activity of the MPO supernatant was quantified kinetically on a temperature-controlled (25 °C) spectrophotometer (Perkin-Elmer, lambda 3B). Absorbency at 460 nm was recorded for several minutes and the change of absorbency over 1 min was measured during the linear portion (0.5-1.5 min). Plasma from another group of animals (n = 6) was collected to determine the relationship between MPO and the number of PMNs. Using the Percoll density gradient centrifugation method (as noted above), neutrophils were isolated from plasma collected from six animals. After determining the initial PMN concentration with a haemocytometer (Fisher Scientific), serial dilutions were used to achieve PMN concentrations of 5 × 106 and 5 × 105, which yielded 58 ± 3 and 0.6 ± 0.044 μmol H2O2 min−1. These values were close to those achieved in the control and antibody treatment conditions.
Data analysis
The discharge frequency of each afferent was averaged (impulses s−1) during 5 min of the pre-ischaemia control period, 5 min of cardiac ischaemia, and the initial 5 min of reperfusion. Data were presented as means ± s.e.m. Differences in responses of ischaemically sensitive cardiac afferents to repeated ischaemic stimuli with or without application of oxypurinol or the polyclonal antibody directed against PMNs were analysed by means of repeated measures analysis of variance on ranks, followed, when necessary, by a post hoc Student-Newman-Keuls test. Differences were considered to be significant when P < 0.05. Differences in MPO activity were analysed using Student's paired t test. Linear regression was used to determine the relationship between MPO activity and the number of PMNs. Statistical calculations were performed with SigmaStat software (Jandel Scientific Software, San Rafael, CA, USA).
Results
Oxypurinol protocol
The effect of oxypurinol as an inhibitor of xanthine oxidase was examined in nine afferents from nine animals. The activity of an ischaemically sensitive cardiac afferent innervating the left ventricle before and after infusion of oxypurinol is displayed in Fig. 1. Figure 1A shows the discharge rate of the afferent before treatment with oxypurinol during control, myocardial ischaemia and reperfusion. Oxypurinol reduced the discharge frequency of the ischaemically sensitive cardiac afferent (Fig. 1B). Overall, the responses of the group (n = 9) were reduced by an average of 44 % (Fig. 2B).
Figure 1.
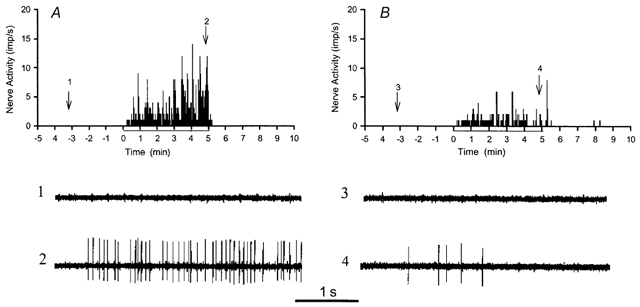
A and B, time stimulus histograms showing the frequency of action potentials (impulses s−1) in a left ventricular afferent during 5 min control, 5 min ischaemia and 5 min reperfusion before and after the administration of oxypurinol, respectively. Neurograms 1 and 2, taken at the times indicated by arrows in A, display the activity before oxypurinol, during control and ischaemia, respectively. Neurograms 3 and 4 display the activity after oxypurinol, during control and ischaemia, respectively, at the times indicated in B.
Figure 2.
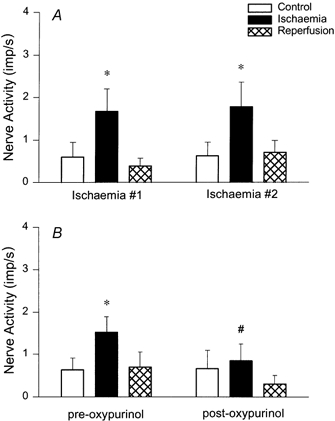
A, histogram showing group data of eight cardiac afferents yielding consistent responses during repeated ischaemia. B, group data of nine cardiac afferents responsive to ischaemia before and after administration of oxypurinol (10 mg kg−1, i.v.). * Responses during control and ischaemia were significantly different (P < 0.05). # Responses during ischaemia before and after oxypurinol likewise were significantly different (P < 0.05).
Repeated myocardial ischaemia, separated by a 30 min period of recovery without treatment, consistently stimulated the cardiac afferents in eight animals (Fig. 2A). We have observed similar reproducible cardiac afferent responses to repeated myocardial ischaemia in previous studies (Pan & Longhurst, 1995; Tjen-A-Looi et al. 1998).
Anti-PMN antibody protocol
The responses of seven ischaemically sensitive afferents were examined before and after administration of the polyclonal antibody (Fig. 3). Figure 3A shows afferent activity before depletion of PMNs during control, myocardial ischaemia and reperfusion. The response of the afferent to ischaemia was decreased by the antibody (Fig. 3B). Responses of the entire group of afferents in response to the antibody were decreased by 66 % (Fig. 4A).
Figure 3.
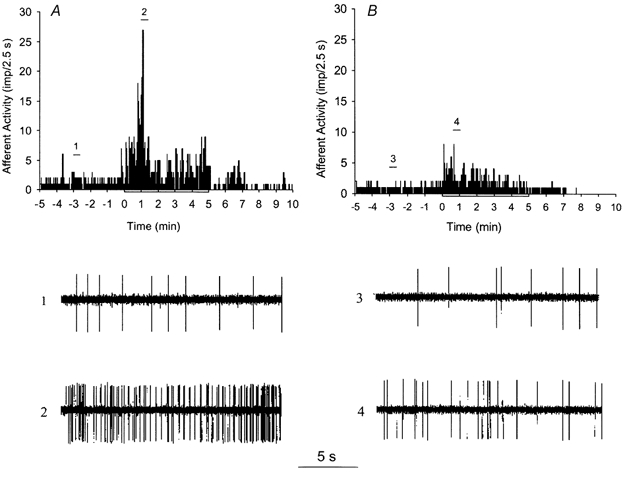
A and B, histograms showing the frequency of action potentials in a representative left ventricular afferent during control, ischaemia, and reperfusion, before and after the administration of antibody, respectively. Neurograms 1 and 2, taken at times indicated by bars in A, display the activity of the afferent before administration of antibody during control and ischaemia, respectively. Neurograms 3 and 4 display the activity of the afferent after antibody during control and ischaemia, respectively, at times shown in B.
Figure 4.
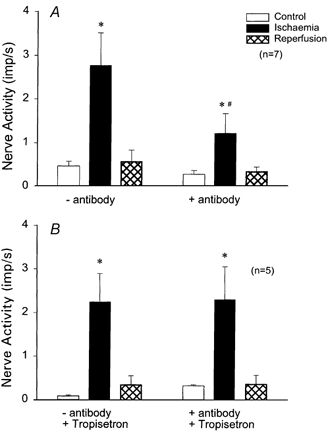
A, histograms representing group data of seven cardiac afferents responsive to ischaemia before and after administration of antibody (3 mg ml−1 kg−1, i.v.). B, group data of five cardiac afferents responsive to ischaemia, pretreated with tropisetron, a 5-HT3 antagonist, before and after administration of antibody. * Responses occurring during control and ischaemia were significantly different (P < 0.05). # Effect of ischaemia before and after antibody was significantly different (P < 0.05).
Effect of PMN antibody on cell counts
The number of circulating PMNs was decreased by 94 % during infusion of the polyclonal antibody (Table 1). Since the concentration of lymphocytes did not change, the decreased numbers of white blood cells (WBCs) reflected a decrease in granulocytes. We also observed a smaller (61 %) decrease in the concentration of platelets. Similar changes in PMNs were observed with the infusion of this antibody after pretreatment with tropisetron (Table 1). Changes in the PMNs and platelets in response to the antibody lasted for more than 1 h (P < 0.05). Cell counts did not change in the seven animals infused with control serum (Table 1).
Table 1.
Influence of rabbit serum, antiserum and PMN antibody on total white blood count, granulocytes, lymphocytes and platelets
| WBC | Granulocytes (PMNs) | Lymphocytes | Platelets | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Test | Change(%) | Control | Test | Change(%) | Control | Test | Change(%) | Control | Test | Change(%) | |
| Control serum | 15 ± 2.5 | 16 ± 2.7 | 3 | 16 ± 3 | 16 ± 3.2 | 4 | 2.9 ± 0.1 | 2.7 ± 0.2 | 7 | 249 ± 15.5 | 269 ± 15.8 | 8 |
| PMN-Ab | 12 ± 3.6 | 2.5 ± 0.5* | 79 | 10 ± 3.5 | 0.6 ± 0.1* | 94 | 1.7 ± 0.2 | 2.2 ± 0.5 | 29 | 223 ± 17.5 | 88 ± 10.6* | 61 |
| PMN-Ab + tropisetron | 18 ± 1.5 | 2.9 ± 0.3* | 84 | 15 ± 1.8 | 0.7 ± 0.09* | 95 | 2.7 ± 0.4 | 2.2 ± 0.2 | 19 | 272 ± 20.5 | 158 ± 21.4* | 42 |
Values represent mean ± s.e.m. (109 cells l−1). Control and Test represent the first and second periods of ischaemia, respectively.
Treatment significantly (P < 0.05) reduced the cell counts compared to before treatment. WBC, white blood cells; PMNs, polymorphonuclear leukocytes.
Tropisetron and anti-PMN antibody protocol
To investigate the significance of a decreased platelet count after infusion of the polyclonal antibody, we pharmacologically neutralized the potential influence of this cellular component. Thus, in five cats the responses of five ischaemically sensitive cardiac afferents to treatment with the antibody were examined after pretreatment with tropisetron, a selective 5-HT3 receptor antagonist. In contrast to the influence of the antibody without tropisetron, the response of these afferents to myocardial ischaemia did not change in response to depletion of PMNs and reduction of platelets by the antibody (Fig. 4B), following treatment with tropisetron.
Seven other cardiac afferents in seven cats demonstrated consistent responses before and after the infusion of control serum during myocardial ischaemia (Fig. 5).
Figure 5.
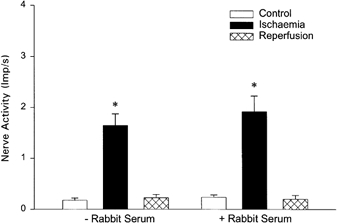
Responses of cardiac afferents during ischaemia before and after administration of control serum (n = 7). * Responses during ischaemia were significantly different compared to control (P < 0.05) but did not differ between the first and second periods of ischaemia.
Myocardial MPO activity
Compared to six animals treated with control serum the polyclonal antibody reduced myocardial tissue MPO activity (60 ± 10 vs. 10 ± 1 μmol H2O2 min−1). Isolation of neutrophils from plasma in six other animals followed by serial dilution with saline established that these MPO values corresponded to approximately 5 × 106 and 8.9 × 105 PMNs, control vs. antibody treatment, respectively. Thus, the antibody substantially reduced the amount of neutrophils in the myocardium.
Receptive fields of cardiac afferents
Most ischaemically sensitive cardiac afferents were located in the left ventricle (Fig. 6). Many were identified to be near the left circumflex and the left anterior descending coronary vessels. Conduction velocities ranged from 0.33 to 2.4 m s−1.
Figure 6.
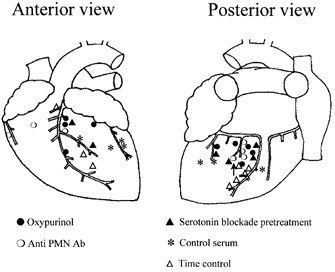
Location of receptive fields of ischaemically sensitive cardiac afferents in the left ventricle. ▵, repeated ischaemia (n = 8); •, oxypurinol (n = 9); ○, antibody (n = 7); ▴, 5-HT3 antagonist + antibody (n = 5); *, rabbit control serum (n = 7).
Discussion
This is the first study to demonstrate that prevention of purine breakdown with an inhibitor of xanthine oxidase significantly influences the responses of cardiac sympathetic afferents during ischaemia. These data suggest that ROS derived from purine degradation are important stimuli of these nerve endings during ischaemia. We also observed a significant reduction in the response of cardiac sympathetic afferents to ischaemia following depletion of circulating and myocardial PMNs with a polyclonal antibody directed against PMNs. To a smaller extent, this antibody also reduced circulatory platelets. Since platelets, through the release of serotonin, can activate cardiac afferents during ischaemia through a 5-HT3 receptor mechanism (Fu & Longhurst, 2000), we pretreated a group of animals with tropisetron, a 5-HT3 receptor antagonist. We found that pre-blockade of 5-HT3 receptors with tropisetron prevented the influence of the anti-PMN antibody on the cardiac afferent response to ischaemia. Thus, with respect to potential sources of ROS, purines, but not PMNs, appear to be important in stimulating cardiac sympathetic afferents.
Xanthine oxidase, localized in vascular endothelium, is an important enzyme in health and disease. For example, it promotes iron absorption in the small intestine (Parks & Granger, 1986) and is involved in the pathogenesis of various pathological conditions, including myocardial injury resulting from ischaemia and reperfusion (Simpson & Lucchesi, 1987). Xanthine oxidase plays a major role in the generation of ROS (Kuppusamy & Zweier, 1989; Thompson-Gorman & Zweier, 1990). In reperfused tissue, in the presence of its substrates, hypoxanthine or xanthine, xanthine oxidase reduces molecular oxygen to O2•− and H2O2, which can react further to form the very reactive species, •OH (Thompson-Gorman & Zweier, 1990; Kuppusamy & Zweier, 1989). Molecular •OH and O2•− free radicals, in turn, oxidize cellular proteins and membranes leading to cellular injury (Xia et al. 1996). Previous studies have demonstrated that oxypurinol inhibits xanthine oxidase activity (Pourcyrous et al. 1993; Zweier et al. 1994). In addition, oxypurinol reacts with PMN-generated myeloperoxidase-derived hypochlorous acid at physiological pH, which inactivates human α1-antiprotease. Oxypurinol protects human alpha α1-antiprotease against inactivation by reacting with hypochlorous acid (Grootveld et al. 1987) and thereby potentially reduces tissue injury. In ischaemic myocardial tissue, adenosine and its metabolites, inosine and hypoxanthine, are increased in interstitial fluid during brief (5 min) ischaemia (Van Wylen, 1994). In the present study we were able to demonstrate that ROS generated from the breakdown of adenosine contribute to stimulation of cardiac afferents during myocardial ischaemia.
Our study demonstrated that the inhibition of xanthine, and therefore ROS production, reduced the activation of cardiac sympathetic afferents during myocardial ischaemia. Thus, metabolites of adenosine, which can lead to the formation of ROS, play a role in the activation of cardiac sympathetic afferents during myocardial ischaemia. Previous studies by M. H. Huang et al. (1995, 1996) reported that adenosine stimulates cardiac sympathetic afferents in dogs (M. H. Huang et al. 1995, 1996). Dibner-Dunlap et al. (1993) and Thames et al. (1993) also suggested that adenosine may activate cardiac sympathetic afferents resulting in reflex sympathoexcitation in dogs (Dibner-Dunlap et al. 1993; Thames et al. 1993). In contrast, past studies found, through the direct recording of cardiac afferents in cats, that even high concentrations of adenosine and CPA, an adenosine A1 agonist, failed to stimulate ischaemically sensitive cardiac sympathetic afferents in cats during exogenous administration (Pan & Longhurst, 1995). We also found that blockade of adenosine receptors with aminophylline did not reduce the response of the cardiac afferents during ischaemia. Similar to our results, Gnecchi-Ruscone et al. (1995) have shown that aminophylline, an adenosine antagonist, does not alter the response of cardiac afferents during myocardial ischaemia in cats (Gnecchi-Ruscone et al. 1995). Differences in the experimental design (i.e. reflex vs. afferent recording study) and species studied (dog vs. cat) may lead to the differential results with respect to the role of adenosine on cardiac afferent nerve endings. Thus, current opinion is divided on the role of adenosine on cardiac afferent endings during ischaemia although there appears to be little evidence in cats that adenosine is an important mediator. Although adenosine is increased during myocardial ischaemia, the present study was not designed to study the role of adenosine in the activation of ischaemically sensitive cardiac fibres. Conversely, it appears from the current data that one of adenosine's metabolites, xanthine, can serve as a source for ROS, which are known to stimulate cardiac sympathetic afferents during ischaemia.
With respect to the influence of PMNs on sensory nerves, Bennett et al. (1998) have suggested that thermal hyperalgesia involving activation of somatic afferents, is dependent on the presence of PMNs. Thus, we speculated that these WBCs may serve as a source of ROS, like •OH (O'Neill et al. 1996), that have the capability of stimulating cardiac afferent nerve endings during myocardial ischaemia and reperfusion.
Myocardial ischaemia activates components of the complement system, which act as chemoattractants to PMNs leading to their accumulation in the myocardium (Hill & Ward, 1971; McManus et al. 1983; Rossen et al. 1985; Fletcher et al. 1993). Granulocytes are associated with reperfusion injury in ischaemic myocardium. Thus, there is a direct relationship between myocardial infarct size following reperfusion and the extent of infiltration by PMNs (Romson et al. 1983; Jolly et al. 1986), since PMNs infiltrate the myocardium early after the onset of ischaemic injury (Mallory et al. 1939; Fishbein et al. 1978; Mullane et al. 1985). Inhibition of the function of neutrophils reduces ischaemic damage in the myocardium (Bednar et al. 1985). In the present study, using a MPO assay we demonstrated an increase in the number of granulocytes after only 5 min of ischaemia, i.e. before the time required to produce infarction (Jennings & Ganote, 1974). Furthermore, we showed that depletion of PMNs with a polyclonal antibody, as measured by MPO assay, is associated with a reduced discharge frequency of cardiac sympathetic afferents during regional myocardial ischaemia.
Extrapolation of our data suggests that the antibody decreased myocardial PMNs by 82 % and circulating PMNs by 94 %. However, the antibody also decreased the number of circulating platelets, presumably because the rabbits in which the antibodies were produced were immunized with platelet antigens. Although the 61 % decrease in circulating platelets was less than the decreases in circulating and myocardial PMNs, infusion of anti-PMN polyclonal antibody may have influenced platelet activity. In this context, our recent data suggest that platelet activation during brief myocardial ischaemia may serve as a stimulus of cardiac sympathetic afferents through a 5-HT3 receptor mechanism (Fu & Longhurst, 2000). Thus, to reduce the confounding influence of platelet activation, we pretreated animals with tropisetron, a 5-HT3 receptor antagonist, a manoeuvre that eliminated the antibody-related reduction of afferent discharge during ischaemia. These data suggest, therefore, that platelets rather than PMNs, are important cellular constituents in the ischaemia-related activation of cardiac sympathetic afferents.
In summary, in addition to the Haber-Weiss reaction, purine metabolism through the xanthine oxidase pathway appears to be an important mechanism in the increased activity of cardiac sympathetic afferents during ischaemia. Conversely, PMNs do not appear to be required for this response. Once activated, these sensory nerves provide signals that ultimately lead to angina pectoris and important sympathoexcitatory cardiovascular reflexes. Such reflexes are deleterious since they can cause cardiac tachyarrythmias and peripheral vasoconstriction leading to further imbalances between myocardial oxygen supply and demand and, hence, worsen the ischaemic condition. These results amplify our past studies demonstrating that brief ischaemia of the cat heart leads to the production of ROS, which play an important role in stimulating ischaemically sensitive cardiac sensory nerves.
Acknowledgments
The authors thank Roberta Holt for her technical assistance. This work was supported by grants from the National Institute of Health, Bethesda, MD, USA, HL52156, HL66217 and the American Heart Association beginning grant-in-aid 9960007Y.
References
- Amsterdam EA, Pan H-L, Rendig S, Symons D, Fletcher MP, Longhurst JC. Limitation of myocardial infarct size in pigs with a dual lipoxygenase-cyclooxygenase blocking agent by inhibition of neutrophil activity without reduction of neutrophil migration. Journal of the American College of Cardiology. 1993;22:1738–1744. doi: 10.1016/0735-1097(93)90605-z. [DOI] [PubMed] [Google Scholar]
- Baker DG, Coleridge HM, Coleridge JCG, Nerdrum T. Search for a cardiac nociceptor: stimulation by bradykinin of sympathetic afferent nerve endings in the heart of the cat. Journal of Physiology. 1980;306:519–536. doi: 10.1113/jphysiol.1980.sp013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar M, Smith B, Pinto A, Mullane KM. Nafazatrom-induced salvage of ischemic myocardium in anesthesized dogs is mediated through inhibition of neutrophil function. Criculation Research. 1985;57:131–141. doi: 10.1161/01.res.57.1.131. [DOI] [PubMed] [Google Scholar]
- Bennett G, Al-Rashed S, Hoult JRS, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998;77:315–322. doi: 10.1016/S0304-3959(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Berger HJ, Zaret BL, Speroff L, Cohen LS, Wolfson S. Cardiac prostaglandin release during myocardial ischemia induced by atrial pacing in patients with coronary heart disease. American Journal of Cardiology. 1977;39:481–486. doi: 10.1016/s0002-9149(77)80154-9. [DOI] [PubMed] [Google Scholar]
- Birkitt DA, Apthorp GH, Chamberlain DA, Hayward GW, Tuckwell EG. Bilateral upper thoracic sympathectomy in angina pectoris: results in 52 cases. British Medical Journal. 1965;2:187–190. doi: 10.1136/bmj.2.5455.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM. Excitation of afferent cardiac sympathetic nerve fibres during myocardial ischaemia. Journal of Physiology. 1967;190:35–53. doi: 10.1113/jphysiol.1967.sp008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner-Dunlap ME, Kinugawa T, Thames MD. Activation of cardiac sympathetic afferents: effects of exogenous adenosine and adenosine analogues. American Journal of Physiology. 1993;265:H395–400. doi: 10.1152/ajpheart.1993.265.1.H395. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, JÖns T, Schmitz F. Production of polyclonal antibodies against proteins and peptides. In: Asai DJ, editor. Methods in Cell Biology: Antibodies in Cell Biology. Vol. 37. San Diego: Academic Press; 1993. pp. 7–56. [DOI] [PubMed] [Google Scholar]
- Ferrari R. Oxygen-free radicals at myocardial level: effects of ischemia and reperfusion. In: Armstrong D, editor. Free radicals in Diagnostic Medicine. New York: Plenum Press; 1994. pp. 99–111. [DOI] [PubMed] [Google Scholar]
- Fishbein MC, Maclean D, Maroko PR. Histopathologic evolution of myocardial infarction. Chest. 1978;73:843–849. doi: 10.1378/chest.73.6.843. [DOI] [PubMed] [Google Scholar]
- Fletcher MP, Stahl GL, Longhurst JC. C5a-induced myocardial ischaemia: role for CD 18-dependent PMN localization and PMN-platelet interactions. American Journal of Physiology. 1993;34:H1750–1761. doi: 10.1152/ajpheart.1993.265.5.H1750. [DOI] [PubMed] [Google Scholar]
- Fu L-W, Longhurst JC. Activation of ischemically sensitive cardiac afferents by serotonin through 5-HT3 receptors. Society for Neuroscience Abstracts. 1998;24:1622. [Google Scholar]
- Fu L-W, Longhurst JC. Activated platelets stimulate cardiac afferents through a 5-HT receptor mechanism. FASEB Journal. 2000;14:A377. [Google Scholar]
- Gnecchi-Ruscone T, Montano N, Contini M, GuaIZZ M, Lombardi F, Malliani A. Adenosine activates cardiac sympathetic afferent fibers and potentiates the excitation induced by coronary occlusion. Journal of the Autonomic Nervous system. 1995;53:175–184. doi: 10.1016/0165-1838(94)00169-k. [DOI] [PubMed] [Google Scholar]
- Grill HP, Zweier ML, Kuppusamy ML, Weisfeldt ML, Flaherty JT. Direct measurement of myocardial free radical generation in an in vivo model: effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. Journal of the American College of Cardiology. 1992;20:1604–1611. doi: 10.1016/0735-1097(92)90457-x. [DOI] [PubMed] [Google Scholar]
- Grill HP, Zweier P, Kuppusamy ML, Weisfeldt ML, Flaherty JT. Direct measurement of myocardial free radical generation in an in vivo model: effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. Journal of the American College of Cardiology. 1992;20:1604–1611. doi: 10.1016/0735-1097(92)90457-x. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Halliwell B, Moorhouse CP. Action of uric acid, allopurinol and oxypurinol on the myeloperoxidase-derived oxidant hypochlorous acid. Free Radical Research Communications. 1987;4:69–76. doi: 10.3109/10715768709088090. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease. An overview. Methods in Enzymology. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Hirose M, Furukawa S, Hayakawa H, Kimura E. Changes in hemodynamics and bradykinin concentration in coronary sinus blood in experimental coronary artery occlusion. Japanese Heart Journal. 1977;18:679–689. doi: 10.1536/ihj.18.679. [DOI] [PubMed] [Google Scholar]
- Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts in rats. Journal of Experimental Medicine. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh PD, Hillis LD, Cambel WB, Firth BG, Willerson JT. Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. New England Journal of Medicine. 1981;304:685–691. doi: 10.1056/NEJM198103193041201. [DOI] [PubMed] [Google Scholar]
- Huang H-S, Pan H-L, Stahl GL, Longhurst JC. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. American Journal of Physiology. 1995a;269:H888–901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Huang H-S, Stahl GL, Longhurst JC. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. American Journal of Physiology. 1995b;268:H2114–2124. doi: 10.1152/ajpheart.1995.268.5.H2114. [DOI] [PubMed] [Google Scholar]
- Huang MH, Horackova M, Negoescu RM, Wolf S, Armour JA. Polysensory response characteristics of dorsal root ganglion neurones that may serve sensory functions during myocardial ischaemia. Cardiovascular Research. 1996;32:503–515. [PubMed] [Google Scholar]
- Huang MH, Sylvén C, Horackova M, Armour A. Ventricular sensory neurons in canine dorsal root ganglia: effects of adenosine and substance P. American Journal of Physiology. 1995;269:R318–324. doi: 10.1152/ajpregu.1995.269.2.R318. [DOI] [PubMed] [Google Scholar]
- Jennings RB, Ganote CE. Structure changes in myocardium during acute ischemia. Circulation Research. 1974;34/35:156–172. [PubMed] [Google Scholar]
- Jennings RB, Reimer KA, Hill ML, Mayer SE. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circulation Research. 1981;49:892–900. doi: 10.1161/01.res.49.4.892. [DOI] [PubMed] [Google Scholar]
- Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL, Lucchesi BR. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. American Heart Journal. 1986;112:682–690. doi: 10.1016/0002-8703(86)90461-8. [DOI] [PubMed] [Google Scholar]
- Kimura E, Hashimoto K, Furukawa S, Hayakawa H. Changes in bradykinin level in coronary sinus blood after the experimental occlusion of a coronary artery. American Heart Journal. 1973;85:635–647. doi: 10.1016/0002-8703(73)90169-5. [DOI] [PubMed] [Google Scholar]
- Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. Journal of Biological Chemistry. 1989;264:9880–9884. [PubMed] [Google Scholar]
- Longhurst JC, Benham RA, Rendig SV. Increased concentration of leukotriene B4 but not thromboxane B2 in intestinal lymph of cats during brief ischemia. American Journal of Physiology. 1992;31:H1482–1485. doi: 10.1152/ajpheart.1992.262.5.H1482. [DOI] [PubMed] [Google Scholar]
- Longhurst JC, Tjen-A-Looi S. Both neutrophils (PMNs) and xanthine oxidase (XO) contribute to activation of cardiac sympathetic afferents in cats. Journal of the Autonomic Nervous System. 1998;8:283. [Google Scholar]
- McManus LM, Kolb WP, Crawford MH, O'Rourke RA, Grover FL, Pinckard RN. Complement localization in ischemic baboon myocardium. Laboratory Investigation. 1983;48:436–477. [PubMed] [Google Scholar]
- Malliani A, Peterson DF, Bishop VS, Brown AM. Spinal sympathetic cardio-cardiac reflexes. Circulation Research. 1972;30:158–166. doi: 10.1161/01.res.30.2.158. [DOI] [PubMed] [Google Scholar]
- Mallory G, White P, Salcedo-Salgar J. The speed of healing myocardial infarction: a study of the pathologic anatomy in seventy-two cases. American Heart Journal. 1939;18:647–671. [Google Scholar]
- Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501–524. doi: 10.1016/0306-4522(92)90398-l. [DOI] [PubMed] [Google Scholar]
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. Journal of Pharmacological Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Nganele DM, Hintze TH. Cardiac chemical reflex control of preload in conscious dogs. American Journal of Physiology. 1990;258:H1055–1063. doi: 10.1152/ajpheart.1990.258.4.H1055. [DOI] [PubMed] [Google Scholar]
- Oberg B, Thoren PN. Circulatory responses to stimulation of medullated and non-medullated afferents in the cardiac nerve in the cat. Acta Physiologica Scandinavica. 1973;87:121–132. doi: 10.1111/j.1748-1716.1973.tb05373.x. [DOI] [PubMed] [Google Scholar]
- O'Neill CA, Fu L-W, Halliwell B, Longhurst JC. Hydroxyl radical production during myocardial ischemia and reperfusion in cats. American Journal of Physiology. 1996;271:H660–667. doi: 10.1152/ajpheart.1996.271.2.H660. [DOI] [PubMed] [Google Scholar]
- Pagani M, PiINELLIZZ P, Furlan R, GuETTIZZ S, Rimoldi O, Sandrone G, Malliani A. Analysis of the pressor sympathetic reflex produced by intracoronary injections of bradykinin in conscious dogs. Circulation Research. 1985;56:175–183. doi: 10.1161/01.res.56.2.175. [DOI] [PubMed] [Google Scholar]
- Pal P, Koley J, Bhattacharyya S, Gupta JS, Koley B. Cardiac nociceptors and ischemia: role of sympathetic afferents in cat. Japanese Journal of Physiology. 1989;39:131–144. doi: 10.2170/jjphysiol.39.131. [DOI] [PubMed] [Google Scholar]
- Palumbo LT, Lulu DJ. Anterior transthoracic upper dorsal sympathectomy. Archives in Surgery. 1965;92:247–257. doi: 10.1001/archsurg.1966.01320200087014. [DOI] [PubMed] [Google Scholar]
- Pan H-L, Longhurst JC, Eisenach JC, Chen S-R. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischaemia in cats. Journal of Physiology. 1999;518:857–866. doi: 10.1111/j.1469-7793.1999.0857p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H-L, Longhurst JC. Lack of a role of adenosine in activation of ischemically sensitive cardiac sympathetic afferents. American Journal of Physiology. 1995;269:H106–113. doi: 10.1152/ajpheart.1995.269.1.H106. [DOI] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiologica Scandinavica. 1986;548:87–99. [PubMed] [Google Scholar]
- Peterson DF, Brown AM. Pressor reflexes produced by stimulation of afferent fibers in the cardiac sympathetic nerves of the cat. Circulation Research. 1971;28:605–610. doi: 10.1161/01.res.28.6.605. [DOI] [PubMed] [Google Scholar]
- Pourcyrous M, Leffler CW, Bada HS, Korones SB, Busija DW. Brain superoxide anion generation in asphyxiated piglets and the effect of indomethacin at therapeutic dose. Pediatric Research. 1993;34:366–369. doi: 10.1203/00006450-199309000-00025. [DOI] [PubMed] [Google Scholar]
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rossen RD, Swain JL, Michael LH, Weakley S, Giannini E, Entman ML. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle: a possible initiator of an extra myocardial mechanism of ischemic injury. Circulation Research. 1985;57:119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- Simpson PJ, Lucchesi BR. Free radicals and myocardial ischemia and reperfusion injury. Journal of Laboratory Clinical Medicine. 1987;110:113–130. [PubMed] [Google Scholar]
- Staszewska-Barczak J, Ferreira SH, Vane JR. An excitatory nociceptive cardiac reflex elicited by bradykinin and potentiated by prostaglandins and myocardial ischemia. Cardiovascular Research. 1976;10:314–327. doi: 10.1093/cvr/10.3.314. [DOI] [PubMed] [Google Scholar]
- Thames MD, Kinugawa T, Dibner-Dunlap ME. Reflex sympathoexcitation by cardiac sympathetic afferents during myocardial ischemia. Role of adenosine. Circulation. 1993;87:1698–1704. doi: 10.1161/01.cir.87.5.1698. [DOI] [PubMed] [Google Scholar]
- Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. Journal of Biological Chemistry. 1990;265:6656–6663. [PubMed] [Google Scholar]
- Tjen-A-Looi S, Bonham A, Longhurst JC. Interactions between cardiac sympathetic and cardiac vagal afferents in the nucleus tractus solitarius. American Journal of Physiology. 1997;272:H2843–2851. doi: 10.1152/ajpheart.1997.272.6.H2843. [DOI] [PubMed] [Google Scholar]
- Tjen-A-Looi S, Pan H-L, Longhurst J. Endogenous bradykinin activates ischemically sensitive cardiac visceral afferents through kinin B2-receptors. Journal of Physiology. 1998;510:633–641. doi: 10.1111/j.1469-7793.1998.633bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wylen DG. Effect of ischemic preconditioning on interstitial purine metabolite and lactate accumulation during myocardial ischemia. Circulation. 1994;5:2283–2289. doi: 10.1161/01.cir.89.5.2283. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Ma X-L, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. Journal of Clinical Investigation. 1993;91:2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Khatchikian G, Zweier JL. Adenosine deaminase inhibition prevents free radical-mediated injury in the postischemic heart. Journal of Biological Chemistry. 1996;271:10096–10102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Thompson-Gorman S, Klunk D, Lutty GA. Measurement and characterization of free radical generation in reoxygenated human endothelial cells. American Journal of Physiology. 1994;266:700–708. doi: 10.1152/ajpcell.1994.266.3.C700. [DOI] [PubMed] [Google Scholar]


