Abstract
A high salt diet in some species results in elevated arterial blood pressure and alterations in vascular smooth muscle responses to agonists. Weanling male Sprague-Dawley rats were given either a high salt diet containing 8 % or a low salt diet of 0.4 % sodium chloride for a period of 4 weeks. At the end of the feeding period, tail systolic pressure was higher in the high salt than in low salt rats. The rats were then killed and the intestines removed. Vascular smooth muscle (VSM) responses were estimated from the changes in lumenal diameter of pressurised second order mesenteric resistance arteries. High salt diet resulted in enhanced VSM responses to noradrenaline. The vessels dilated in response both to acetylcholine and to sodium nitroprusside and the responses were similar in vessels from both high and low salt rats. However, vessels from high salt rats were resistant to the blocking of endothelium derived nitric oxide (EDNO) with l-NAME and the responses were instead abolished by blocking endothelium derived hyperpolarising factor (EDHF) with apamin and charybdotoxin. These results show that in Sprague-Dawley rats, a high salt diet enhances the vasoconstriction in response to noradrenaline. The vasodilatory responses to acetylcholine were not significantly changed. However, they appeared to be mediated mainly by EDHF rather than by EDNO as in the low salt animals.
There is considerable epidemiological evidence in humans linking the ingestion of large quantities of salt to the development of hypertension (Intersalt, 1988; Stamler et al. 1991). A similar association has been reported from studies of experimental animals fed high levels of salt. This is particularly marked in the genetically selected Dahl salt sensitive rats (Dahl et al. 1962), but it also occurs to a smaller extent in non-selected animals including weanling Sprague-Dawley (SD) rats (Miyajima & Bunag, 1985; Nwaigwe & Sofola, 1989; Obiefuna et al. 1991a), adult SD rats (Giardina et al. 2001), Wistar rats (Huang & Johns, 2000), dogs (Vogel, 1966) and chimpanzees (Denton et al. 1995).
The mechanisms linking high salt intake to hypertension appear to be complex and to involve alterations in both reflex function (Ferrari & Mark, 1987) and in the contractile properties of the vascular smooth muscle (Mulvany et al. 1978; Adegunloye & Sofola, 1997; Nishida et al. 1998).
It is now well established that endothelial factors are of importance in the mediation and modulation of both vasoconstriction and vasodilatation, although the effects of different levels of salt intake on these factors are much less clear. Acetylcholine induces relaxation of vascular smooth muscle mediated largely through release of endothelium-dependent relaxing factors, mainly nitric oxide (EDNO). The acetylcholine-mediated relaxation has been shown to be impaired in both spontaneously hypertensive rats (Wu et al. 1998; Izzard & Heagerty, 1999) and in Dahl salt-sensitive (DS) rats (Nishida et al. 1998). However, in unselected Sprague-Dawley rats the effects of salt loading have been inconsistent (Obiefuna et al. 1991b; Lenda et al. 2000). Part of the inconsistency may have been due to the different vessels studied: aortic rings by Obiefuna; spino-trapezius vessels by Lenda. Also in mesenteric vessels, which are known to be of major importance in blood pressure control, there is some evidence that, in addition to endothelial derived nitric oxide, endothelium-derived hyperpolarising factor (EDHF) may also be of importance (Ebeigbe et al. 1990; Adeagbo & Triggle, 1993). It is not known, however, whether the actions mediated by EDNO or by EDHF are affected by the level of salt intake.
The present study was therefore undertaken using normal Sprague-Dawley rats, to examine the effects of salt loading on the contractile and relaxation properties of mesenteric vascular smooth muscle. We particularly wished to examine the relative roles of EDNO and EDHF in any changes in relaxation responses.
Methods
Weanling Sprague-Dawley (SD) rats aged 5 weeks and weighing 160–170 g were allocated either to a diet of normal chow with 0.4 % NaCl (low) or a high salt chow with 8 % NaCl. The high salt chow was prepared by mixing 76 g of NaCl with 924 g of chow. The rats were fed on these diets for 4 weeks with water given ad libitum. Tail systolic blood pressures were determined in some of the rats before and at the end of the feeding period, by means of a cuff and pressure detecting equipment (Life Sciences Equipment, CA, USA) as described by Bunag & Teravainen (1991). All experimental procedures complied with the Animals (Scientific Procedures) Act 1986.
At the end of the feeding period, the rats were killed with carbon dioxide, the abdomen was opened and, after tying off the mesenteric vessels, the intestine was removed and placed in cold physiological salt solution (PSS) at 4 °C. The PSS consisted of (mm): NaCl 119, NaHCO3 25, KCl 4.7, CaCl2 1.6, MgSO4 7H2O 1.17, KH2PO4 1.18, sodium EDTA 0.026 and glucose 5.5. From the mesentery, second order mesenteric arteries of about 5 mm in length were dissected out and mounted between two glass cannulae in an arteriograph (Living Systems Instrumentation, Burlington, VT, USA). The vessel was perfused and superfused with PSS at 37 °C and pH 7.4 which was gassed with 5 % CO2 in oxygen. The PSS also contained 10−5m indomethacin to inhibit prostaglandin synthesis. The vessels, of diameter about 250 μm, were pressurised to 70 mmHg via a servo-controlled pressure system. The internal diameter of the vessel was determined by an inverted microscope-video tracking system (Living Systems Instrumentation, USA) and displayed on a monitor. The values of vessel diameter were also recorded using a heated stylus recorder (Devices Instruments Ltd, Hertfordshire, UK).
The vessel was allowed to equilibrate for 60–90 min, with two challenges of 10−6m noradrenaline (NA) being applied. At the end of the equilibration period, the vessel was subjected to the following procedures. (1) Cumulative doses of NA were added to the PSS to obtain concentrations of 10−8-10−5m and the concentration-response curves (CRCs) were determined. (2) Following preconstriction with NA to 50 % of the resting diameter, CRCs were determined for acetylcholine (ACh) at concentrations of 10−8-10−5m. Tests were repeated after addition of 10−4mNω-l-nitro-l-arginine methyl ester (l-NAME). (3) Following preconstriction with NA to 50 % of the resting diameter, CRCs were determined for sodium nitroprusside at concentrations of 10−8-10−5m. (4) Effects on the responses to ACh were determined after administration of l-NAME (10−4m), followed by apamin (Ap, 1 μM) and charybdotoxin (ChTX, 1 μM) to block effects of EDHF as described by Doughty et al. (1999).
All the drugs used were obtained from Sigma (Poole, Dorset, UK). Indomethacin was dissolved in 2.5 % sodium carbonate to make a stock solution of 10−2m. Fresh preparations of l-NAME and indomethacin were made on each experimental day.
Concentration-response curves were determined for NA and ACh before and after blockade of EDNO as well as after blocking EDHF. Significance tests were carried out using one-way ANOVA and unpaired Student's t test for intergroup analyses. P values less than 0.05 were taken as being significant.
Results
At the end of the experimental feeding period, the weights of the control (low salt, CR) and salt-loaded rats (SR) were 273 ± 3 g (n = 13) and 225 ± 2 g (n = 12), respectively. The tail systolic blood pressure (SBP) in the 4 weeks increased from 101 ± 4 to 121 ± 3 mmHg in control rats (n = 6) and from 99 ± 4 to 155 ± 4 mmHg in the salt rats (n = 5). The terminal SBP in the salt rats was significantly higher than in control rats (P < 0.01).
Responses to noradrenaline
The concentration-lumenal diameter response curves (CRCs) of pressurised mesenteric arteries to noradrenaline were studied in eight low salt and eight high salt rats. The contractile responses of the high salt rats were greater than those of the low salt animals (Fig. 1). The EC50 for the high salt rats was (2.45 ± 0.83) × 10−7m while that for the low salt rats was (5.77 ± 2.6) × 10−7m (P < 0.02). In addition, the dose of NA eliciting maximum constrictor response, Emax, was siginificantly lower (P < 0.03) in the high salt than in the low salt rats ((1.43 ± 0.57) × 10−6mvs. (5.43 ± 1.6) × 10−6m, P < 0.03).
Figure 1. Responses to noradrenaline.
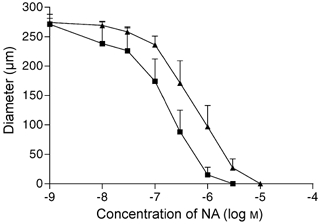
Concentration-response curves of intralumenal diameter vs. noradrenaline in low salt (CR ▴, n = 8) and high salt (SR ▪, n = 8) rats.
Responses to acetylcholine
Acetylcholine induced a dose-dependent relaxation of the preconstricted vessels in both groups of rats. Vessels were preconstricted from 267 ± 12 to 140 ± 13 μm in CR and from 251 ± 15 to 131 ± 12 μm in SR. The responses, shown in Fig. 2, were not significantly different between the groups prior to l-NAME (P > 0.05). The IC30 (concentration of acetylcholine causing a 30 % increase in diameter) values for the high and low salt rats were, respectively, 2.12 ± 0.99 and 2.54 ± 1.73 × 10−7m (P > 0.05).
Figure 2. Responses to acetylcholine.
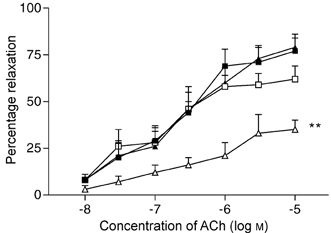
Concentration-response curves of percentage relaxation vs. acetylcholine in low salt rats (CR, n = 10), before (▴) and after (▵) l-NAME (dose 10−4m) and in high salt rats (SR, n = 10) before (▪) and after (▪) l-NAME. Relaxation curves before l-NAME were similar in both groups but the low salt group showed significantly depressed response after l-NAME (** P < 0.03).
The effects on the relaxation responses of l-NAME are shown in Fig. 2. CR vessels were preconstricted from 260 ± 10 to 126 ± 12 μm and SR vessels from 269 ± 16 to 134 +18 μm. l-NAME significantly reduced the relaxation to ACh in the low salt rats but had no significant effect in the high salt rats. For example, at the highest dose of ACh tested (10−5m) in low salt rats l-NAME reduced the relaxation from 79.2 ± 9.3 to 35.1 ± 5.2 % (P < 0.001), whereas in high salt rats the relaxations before and after l-NAME were, respectively, 77.3 ± 8.7 and 62.4 ± 7.3 % (P > 0.05).
In two experiments in each group in which the endothelium was removed by passing air bubbles through the vessels, the relaxation responses to acetylcholine were completely abolished.
Responses to sodium nitroprusside
These experiments were carried out on four rats from each group. After precontraction of the vessels with NA (from 242 ± 12 to 127 ± 20 μm in CR and from 275 ± 17 to 135 ± 14 μm in SR), and in presence of indomethacin and l-NAME, the relaxation responses to the NO donor were found to be similar in the two groups of rats (Fig. 3).
Figure 3. Responses to sodium nitroprusside.
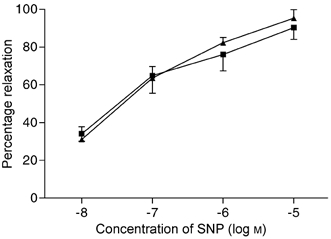
Concentration-response curves for sodium nitroprusside (SNP) in CR (▴, n = 4) and SR (▪, n = 4). The relaxation responses were similar in both groups of rats.
Responses to acetylcholine after l-NAME, apamin and charybdotoxin
The results of experiments, carried out on four rats in the low salt group and five rats in the high salt group are shown in Fig. 4. Vessels were preconstricted from 255 ± 20 to 126 ± 21 μm in CR and from 269 ± 21 to 129 ± 27 μm in SR. In low salt rats (Fig. 4A) relaxation responses to ACh were significantly inhibited by l-NAME (P < 0.03) and with the addition of apamin (Ap) and charybdotoxin (ChTx), the residual relaxation responses were abolished. In the high salt rats (Fig. 4B), the relaxation responses to ACh were little affected by l-NAME, but the relaxation responses after application of l-NAME, Ap and ChTx were also abolished. The comparisons of the effects of the inhibitors on the relaxation responses in control and salt-loaded rats to the highest dose of ACh used, i.e. 10−5m, are shown in Fig. 5.
Figure 4. Responses to acetylcholine after l-NAME, apamin and charybdotoxin.
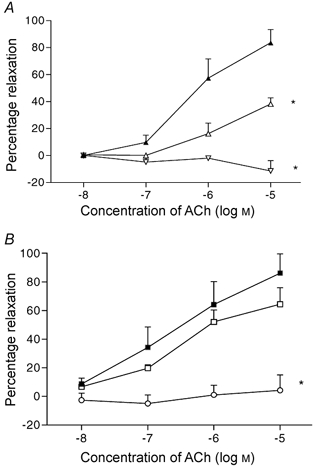
A, concentration-response relaxation curves in rats fed a low salt diet (n = 4) before blockers (▴), after l-NAME (dose 10−4m, ▵), and followed by apamin (Ap, 1 μM) and charybdotoxin (ChTx, 1 μM) (▿). B, same procedure in rats fed a high salt diet (n = 5), before blockers (▪), after l-NAME (▪), and followed by Ap and ChTx (○). * P < 0.01 vs. response curve prior to blockers.
Figure 5. Comparison of maximum relaxation responses to ACh in the presence of inhibitors.
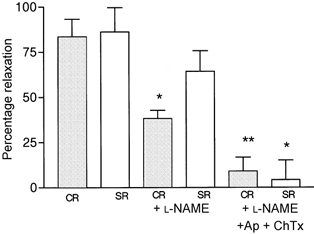
Bar diagram showing comparison of responses at the maximum dose of ACh used (10−5m), in low salt (CR) and high salt rats (SR), before inhibitors (left panels), after l-NAME (10−4m) (middle panels) and after apamin (Ap 1 μM) and charybdotoxin (ChTx 1 μM) in addition to l-NAME. * P < 0.01, ** P < 0.001 between corresponding values prior to addition of blockers.
Discussion
Dietary salt loading to various strains of rats is known to result in increases in arterial blood pressure. This effect is greatest in the genetically selected Dahl salt-sensitive animals (Dahl et al. 1962) but it also occurs in other animals including Sprague-Dawley rats (Miyajima & Bunag, 1985; Torri, 1980; Obiefuna et al. 1991a; Giardina et al. 2001) and to a lesser extent in Wistar rats (Huang & Johns, 2000; Kagota et al. 2001). The results from our study, using Sprague-Dawley rats, confirm this earlier work; systolic blood pressure was on average 34 mmHg higher in the high salt group.
Previous work has demonstrated that vessels from salt-loaded Sprague-Dawley rats constricted more powerfully in response to the application of noradrenaline (Obiefuna et al. 1991a,b; Adegunloye & Sofola, 1997). However, these investigators had used aortic ring preparations rather than the perfused small artery preparation used in this study. The perfused vessel preparations is more physiological as it is perfused along its length at a normal distending pressure (Halpern & Kelley, 1991; Buus et al. 1994; Dunn et al. 1994) Also, physiologically, it is the small arteries which are of importance in regulation of vascular resistance. Previous work has shown that perfused vessels may give responses that are not seen in ring preparations (Dunn et al. 1994; Falloon et al. 1995). Thus our work showing the enhanced responses to noradrenaline in the salt-loaded rats confirms previous findings, but using a more physiological technique. It seems quite possible that the enhanced response to this physiological vasoconstrictor would contribute to the elevated blood pressure following salt loading. This observation may also explain the enhanced vascular responsiveness in salt-sensitive essential hypertension (Bragulat et al. 2001), even under the same sympathetic tone.
Another possible factor which could contribute to elevated blood pressure following salt loading could be a change in the vasodilator function of the resistance vessels. Dilatation may be effected be endothelial release of nitric oxide and this may be stimulated in vitro by acetylcholine. In fact a normal response to acetylcholine has been used as an index of normal endothelial function (Vanhoutte et al. 1995). The effects of salt loading on responses to acetylcholine have not been consistent. Obiefuna et al. (1991b) reported no difference in the responses of aortic ring preparations, from SD rats and Kagota et al. (2001) made similar observations in aortic rings from Wistar rats. Lenda et al. (2000), however, reported that salt loading depressed acetylcholine-induced vasodilatation of pial vessels. Our study, of the responses of perfused mesenteric vessels, did not show any significant difference in the responses of the two groups of rats to acetylcholine. However, the mechanism causing this dilatation does appear to be quite different. In the low salt rats the dilatation was greatly reduced following addition of l-NAME, indicating that EDNO was the major dilating agent. In the high salt rats, however, l-NAME had no significant effect, indicating that EDNO was not a significant mechanism in dilating those vessels. The difference between the two groups was not due a difference in the responsiveness of the vessels to nitric oxide, because addition of sodium nitroprusside, a nitric oxide donor had similar effects in both groups. However, the possibility still exists that alteration in nitric oxide production can modulate sympathetic neuro-effector mechanisms as reported in normal (Thomas & Victor, 1998) or DS rats (Nishida et al. 1998) and in man (Chavoshan et al. 2002).
We found that both the residual dilatation after l-NAME in the low salt rats and the much larger dilatation in the high salt animals were completely abolished by apamin and charybdotoxin. This implies that EDHF is responsible for causing dilatation. Earlier reports have shown that combined intraluminal administration of 1 μM apamin and 1 μM charybdotoxin, inhibitors of small and intermediate potassium-activated calcium channels, respectively, effectively abolished responses to EDHF (Doughty et al. 1999). This has also been further confirmed by electrophysiological studies in which both agents when administered together cause inhibition of acetylcholine-induced hyperpolarisation and prevented EDHF-induced relaxation (Chen & Cheung, 1997; Coleman et al. 2001). Our present results suggest that, in the salt-loaded animals, EDHF is the vasodilatory mechanism of relatively greater importance and in combination with the enhanced vasoconstrictor response would result overall in an increased pressor effect.
The change in the mechanism causing vasodilatation following salt loading seems surprising, particularly in view of the observation that the overall response was not significantly affected. However, the involvement of EDHF has also previously been shown to be important in diabetic rats where the acetylcholine-induced relaxation was abolished by apamin and charybdotoxin (Wigg et al. 2001). Comparable results have been reported by Izzard & Heagerty (1999) who noted that the flow-related dilatation of pressurised mesenteric arteries of spontaneously hypertensive rats was reduced compared to normal rats and it was insensitive to inhibition with l-NAME.
It is becoming apparent that vasodilatation may be mediated through different mechanisms depending both on the vessels studied as well as on the condition. For example, Lenda et al. (2000) reported that a high salt diet resulted in reduced relaxation in vessels of spinotrapezius muscle and suggested that this was due to production of reactive oxygen radicals. Liu et al. (1999) suggested that, after l-NAME application, pial arteries of salt-loaded rats actually showed a small vasoconstriction in response to acetycholine. In Dahl salt-sensitive rats, Ruud et al. (1999) suggested the involvement of nitric oxide isoform II in the development of the hypertension.
Our observations are the first to implicate an increased role of EDHF in the relaxation occurring in response to ACh in salt-loaded animals. EDHF is known to cause significant vasodilatation in several blood vessels (Mombouli & Vanhoutte, 1997) and to be of particular importance in mesenteric resistance vessels of the rat (Ebeigbe et al. 1990; Nagao et al. 1992; Adeagbo & Triggle, 1993; Shimokawa et al. 1996). We can only speculate on the significance of our findings in relation to the development of hypertension. The overall relaxation in response to ACh was not different in our salt-loaded rats. However, ACh is not the normal physiological stimulus to the endothelium; shear stress is. It would, therefore, be of interest to examine the mechanisms of vasodilatation in response to flow in these animals.
In conclusion, we have demonstrated that a high salt diet in weanling Sprague-Dawley rats results in elevated blood pressure and this is associated with an enhanced vasoconstrictor response to noradrenaline. It does not alter vasorelaxant responses to a nitric oxide donor or to administration of acetylcholine. However, the mechanism of ACh-induced dilatation is different and appears to be associated with an enhanced production of EDHF by the vascular endothelium.
Acknowledgments
This research was supported by the British Heart Foundation. O.A.S. was a BHF Visiting Fellow. We are grateful to Mr D. Myers for technical assistance.
References
- Adeagbo AS, Triggle CR. Varying extracellular [K+]: a functional approach to separating EDHF and EDNO-related mechanisms in perfused rat mesenteric arterial bed. Journal of Cardiovascular Pharmacology. 1993;21:423–429. [PubMed] [Google Scholar]
- Adegunloye BJ, Sofola OA. Effect of dietary salt loading and high calcium diet on vascular smooth muscle responses and endothelial function in rats. Clinical and Experimental Pharmacology and Physiology. 1997;24:814–818. doi: 10.1111/j.1440-1681.1997.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–448. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- Bunag RD, Teravainen T. Tail cuff measurement of systolic hypertension in different strains of rats. Mechanisms of Ageing and Development. 1991;59:197–213. doi: 10.1016/0047-6374(91)90085-e. [DOI] [PubMed] [Google Scholar]
- Buus NH, Vanbavel E, Mulvany MJ. Differences in sensitivity of rat mesenteric arteries to agonists when studied as ring preparations or as cannulated preparations. British Journal of Pharmacology. 1994;112:579–587. doi: 10.1111/j.1476-5381.1994.tb13114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. Journal of Physiology. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cheung DW. Effect of K(+) channel blockers on ACh-induced hyperpolarisation and relaxation in mesenteric arteries. American Journal of Physiology. 1997;272:H2306–2312. doi: 10.1152/ajpheart.1997.272.5.H2306. [DOI] [PubMed] [Google Scholar]
- Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarisation factor in guinea-pig, rat and human blood vessels. Journal of Physiology. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl LK, Heine M, Tassinari L. Effects of chronic excess salt ingestion: evidence that genetic factors play an important role in susceptibility to experimental hypertension. Journal of Experimental Medicine. 1962;115:1173–1180. doi: 10.1084/jem.115.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Weisinger R, Mundy MI, Wickings EJ, Dixon A, Moisson P, Pingard AM, Shade R, Carey D, Ardaillou R. The effect of increased salt intake on blood pressure of chimpanzees. Nature Medicine. 1995;1:1009–1016. doi: 10.1038/nm1095-1009. [DOI] [PubMed] [Google Scholar]
- Doughty JM, Place F, Langton DD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. American Journal of Physiology. 1999;276:H1107–1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Wellman GC, Bevan JA. Enhanced resistance artery sensitivity to agonists under isobasic compared with isometric conditions. American Journal of Physiology. 1994;266:H417–455. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- Ebeigbe AB, Cressier F, Kunneh MK, Luu TD, Criscione L. Influence of NA-momomethyl l-arginine on endothelium-dependent relaxation in the perfused mesenteric vascular bed of the rat. Biochemical and Biophysical Research Communications. 1990;169:873–879. doi: 10.1016/0006-291x(90)91974-w. [DOI] [PubMed] [Google Scholar]
- Falloon BJ, Stephen N, Tulip JR, Heagerty AM. Comparison of small artery sensitivity and morphology in pressurised and wire-mounted preparations. American Journal of Physiology. 1995;268:H670–678. doi: 10.1152/ajpheart.1995.268.2.H670. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Mark AL. Sensitization of aortic baroreceptors by high salt diet in Dahl salt-resistant rats. Hypertension. 1987;10:55–60. doi: 10.1161/01.hyp.10.1.55. [DOI] [PubMed] [Google Scholar]
- Giardina JB, Green GM, Rinewalt AN, Granger JP, Khalil RA. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension. 2001;37:516–523. doi: 10.1161/01.hyp.37.2.516. [DOI] [PubMed] [Google Scholar]
- Halpern W, Kelley M. In vitro methodology of resistance arteries. Blood Vessels. 1991;28:245–251. doi: 10.1159/000158869. [DOI] [PubMed] [Google Scholar]
- Huang CH, Johns EJ. Impact of angiotensin II in the brain on renal sympathetic nerve activity in anaesthetised rats raised on a high salt diet. Journal of Physiology. 2000;523.P:213–214P. [Google Scholar]
- Intersalt. An international study of electrolyte excretion and blood pressure. Results of 24 h urinary sodium and potassium excretion. British Medical Journal. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard AS, Heagerty AM. Impaired flow dependent dilation in distal mesenteric arteries from the spontaneously hypertensive rat. Journal of Physiology. 1999;518:239–245. doi: 10.1111/j.1469-7793.1999.0239r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagota S, Tamashiro A, Yamaguchi Y, Suguira R, Kuno T, Nakamura K, Kunitomo M. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. British Journal of Pharmacology. 2001;134:737–744. doi: 10.1038/sj.bjp.0704300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. American Journal of Physiology. 2000;279:H7–14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension. 1999;33:686–688. doi: 10.1161/01.hyp.33.2.686. [DOI] [PubMed] [Google Scholar]
- Miyajima E, Bunag RD. Dietary salt loading produces baroreflex impairment and mild hypertension in rats. American Journal of Physiology. 1985;249:H278–284. doi: 10.1152/ajpheart.1985.249.2.H278. [DOI] [PubMed] [Google Scholar]
- Mombouli J, Vanhoutte PM. Endothelium derived hyperpolarising factor(s): updating the unknown. Trends in Pharmacological Sciences. 1997;18:252–256. [PubMed] [Google Scholar]
- Mulvany MJ, Hansen DK, Aalkjaer C. Direct evidence that the greater contractility of resistance vessels in SH rats is associated with narrowed lumen and thickened media and an increased number of smooth muscle cell layer. Circulation Research. 1978;43:854–864. doi: 10.1161/01.res.43.6.854. [DOI] [PubMed] [Google Scholar]
- Nagao T, Illiano S, Vanhoutte PM. Heterogeneous distribution of endothelium dependent relaxation resistant to N-nitro-l-arginine in rats. American Journal of Physiology. 1992;263:H1090–1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Ding J, Zhou M, Chen Q, Murakami H, Wu XZ, Kosaka H. Role of nitric oxide in vascular hyperesponsiveness to norepinephrine in hypertensive Dahl rats. Journal of Hypertension. 1998;16:1611–1618. doi: 10.1097/00004872-199816110-00007. [DOI] [PubMed] [Google Scholar]
- Nwaigwe CN, Sofola OA. Potassium but not nifedipine reduces hypertension in anaesthetised salt loaded rats. Medical Science Research. 1989;17:767–768. [Google Scholar]
- Obiefuna PCM, Ebeigbe AB, Sofola OA, Aloamaka PC. Altered responses of aortic smooth muscle from Sprague Dawley rats with salt induced hypertension. Clinical and Experimental Pharmacology and Physiology. 1991a;18:813–818. doi: 10.1111/j.1440-1681.1991.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Obiefuna PCM, Sofola OA, Ebeigbe AB. Dietary salt loading attenuates endothelium-dependent relaxation in response to histamine but not to acetylcholine in rat aortic rings. Experimental Physiology. 1991b;76:135–138. doi: 10.1113/expphysiol.1991.sp003475. [DOI] [PubMed] [Google Scholar]
- Ruud MA, Trolliet M, Hope S, Scribner AW, Daumerie G, Toolan G, Clouthier T, Loscalzo J. Salt induced hypertension in Dahl salt-resistance and salt-sensitive rats with NOS II inhibition. American Journal of Physiology. 1999;277:H732–739. doi: 10.1152/ajpheart.1999.277.2.H732. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagoo T, Ehashira K, Fujishima M, Takeshita A. The importance of hyperpolarising mechanisms increases as the vessel size decreases in endothelium-dependant relaxations in rat mesenteric artery. Journal of Cardiovascular Pharmacology. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Stamler J, Rose G, Elliot P, Dyer A, Marmount M, Kestelot H, Stamler R. Findings of the international co-operative intersalt study. Hypertension. 1991;17(suppl. I):9–15. doi: 10.1161/01.hyp.17.1_suppl.i9. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. Journal of Physiology. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri K. Salt intake and hypertension in rats. In: Kare MR, Fregly MJ, Bernard RA, editors. Biological and Behavioural Aspects of Salt Intake. New York: Academic Press; 1980. pp. 345–366. [Google Scholar]
- Vanhoutte PM, Boulanger CM, Mombouili JV. Endothelium derived relaxing factors and converting enzyme inhibition. American Journal of Cardiology. 1995;76:3–12. [PubMed] [Google Scholar]
- Vogel JA. Salt induced hypertension in the dog. American Journal of Physiology. 1966;210:186–190. doi: 10.1152/ajplegacy.1966.210.1.186. [DOI] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT Parkington & H. C. Comparison of the effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. American Journal of Physiology – Heart and Circulatory Physiology. 2001;281:H232–240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- Wu X, Totvanen J, Hutri-Kahonen N, Kahonen M, Makynen J, Korpela R, Ruskoaho H, Karjalo K, Porsti I. Comparison of the effect of supplementation with whey mineral and potassium on arterial tone in experimental hypertension. Cardiovascular Research. 1998;40:364–374. doi: 10.1016/s0008-6363(98)00180-1. [DOI] [PubMed] [Google Scholar]


