Abstract
Reuptake of extracellular noradrenaline (NA) into superior cervical ganglion (SCG) neurones is mediated by means of the noradrenaline transporter (NAT, uptake 1). We now demonstrate by single-cell RT-PCR that mRNA of the organic cation transporter 3 (OCT3, uptake 2) occurs in rat SCG neurones as well. Furthermore, our RT-PCR analyses reveal the presence of mRNA for novel organic cation transporters 1 and 2 (OCTN1 and OCTN2), but not for OCT1 or OCT2 in the ganglion. Making use of the NAT as a powerful, neurone-specific transporter system, we loaded[3H]-N-methyl-4-phenylpyridinium ([3H]-MPP+) into cultured rat SCG neurones. The ensuing radioactive outflow from these cultures was enhanced by desipramine and reserpine, but reduced (in the presence of desipramine) by the OCT3 inhibitors cyanine 863, oestradiol and corticosterone. In contrast, cyanine 863 enhanced the radioactive outflow from cultures preloaded with [3H]-NA. Two observations suggest that a depletion of storage vesicles by cyanine 863 accounts for the latter phenomenon: first, the primary radioactive product isolated from supernatants of cultures loaded with [3H]-NA was the metabolite [3H]-DHPG; and second, inhibition of MAO significantly reduced the radioactive outflow in response to cyanine 863. The outflow of [3H]-MPP+ was significantly enhanced by MPP+, guanidine, choline and amantadine as potential substrates for OCT-related transmembrane transporters. However, desipramine at a low concentration essentially blocked the radioactive outflow induced by all of these substances with the exception of MPP+, indicating the NAT and not an OCT as their primary site of action. The MPP+-induced release of [3H]-MPP+ was fully prevented by a combined application of desipramine and cyanine 863. No trans-stimulation of [3H]-MPP+ outflow was observed by the OCTN1 and OCTN2 substrate carnitine at 100 μM. Our observations indicate an OCT-mediated transmembrane transport of [3H]-MPP+. Amongst the three OCTs expressed in the SCG, OCT3 best fits the profile of substrates and antagonists that cause trans-stimulation and trans-inhibition, respectively, of [3H]-MPP+ release.
Reliable chemical signal transduction at synaptic sites requires that after release the neurotransmitter is rapidly removed from the synaptic cleft. In the sympathetic nervous system, this effect is mediated by the noradrenaline transporter (NAT, uptake 1) which carries noradrenaline (NA) back into presynaptic axonal varicosities by high-affinity reuptake (Graefe & Boenisch, 1988; Boenisch & Leiden, 1998). However, it has long been known that NA is also removed by a second transport system (uptake 2) that was initially characterized by its low affinity (Iversen, 1965) and by means of pharmacological tools (Trendelenburg, 1988).
The molecular identity of uptake 2 was finally unravelled by two groups who were independently able to clone the transporter (Gründemann et al. 1998b; Kekuda et al. 1998; Wu et al. 2000b). Hydropathy plots suggest 12 putative transmembrane segments, a feature shared with members of a large group of transporter molecules named ‘amphiphilic solute facilitators’ (ASF; Marger & Saier, 1993; Gründemann et al. 1998b; Kekuda et al. 1998). They mediate transmembrane transport of a wide range of substances, including endobiotic and xenobiotic organic anions and cations (Marger & Saier, 1993; Koepsell, 1998; Koepsell et al. 1999). The cloned equivalent of uptake 2 was named organic cation transporter 3 (OCT3; Kekuda et al. 1998).
OCT3 shares about 50 % structural homology with the previously described organic cation transporters 1 and 2 (OCT1, OCT2; Gründemann et al. 1998b; Kekuda et al. 1998; Wu et al. 2000b). However, structural and functional inconsistencies with OCT1 and OCT2 have also led to the suggestion of a different name (extraneuronal transporter for monoamine uptake, EMT; Gründemann et al. 1998b, 1999).
OCT3 has a fairly widespread tissue distribution, as shown by RT-PCR as well as by Northern blot techniques (Gründemann et al. 1998b; Wu et al. 1998a, 2000b). It is markedly expressed in rat placenta (Kekuda et al. 1998) and in a human kidney cell line, Caki-1 (Gründemann et al. 1998b), which served as sources for cloning the rat and human OCT3, respectively. In contrast to low levels of OCT1 and OCT2 (Gorboulev et al. 1997; Gründemann et al. 1998a; Koepsell, 1998; Wu et al. 1998a), OCT3 is also clearly expressed in the CNS (Gründemann et al. 1998b; Wu et al. 1998a).
In keeping with the traditional view of an extraneuronal localization (Trendelenburg, 1988), uptake 2 was found in astrocytes and in various human glioma cell lines (Russ et al. 1996; Streich et al. 1996). However, in situ hybridization suggests the presence of OCT3 mRNA in neural cell populations of the rat cerebellum and hippocampus (Wu et al. 1998a) and of mouse retina neurones (Rajan et al. 2000), implying a functional role of OCT3 in nerve cells as well.
We thought that the superior cervical ganglion (SCG) would be a good candidate for exploration of this hypothesis. SCG neurones synthesize and release NA and might therefore use uptake 2/OCT3 as a reserve mechanism for the removal of NA from the extracellular space (Trendelenburg, 1988). Our work, based on cell cultures of the SCG, provides evidence for the first time not only for the presence of OCT3 mRNA in neurones of the sympathetic nervous system, but also for the functional expression of an (outward-directed) transport mechanism with OCT3-like properties.
Methods
Cell culture
Superior cervical ganglia (SCG) were dissected from 2- to 5-day-old Sprague-Dawley rat pups humanely killed, as required by the guidelines of the Animal Care Committee, by decapitation and dissociated as described previously (Boehm & Huck, 1995). Ganglia were freed from adherant connective tissue and blood vessels, cut into three or four pieces, and incubated in collagenase (1.5 mg ml−1; Sigma cat. no. C-9891) and dispase (3.0 mg ml−1; Boehringer Mannheim cat. no. 165859) for 20 min at 36.5 °C. Subsequently, the ganglia were trypsinized (0.25 % trypsin in Tyrode solution; Worthington cat. no. 3703) for 15 min at 36.5 °C, dissociated by trituration, and plated onto 5 mm discs (punched out of tissue culture dishes, Nunc 153066, 30 000 cells per disc) coated with collagen (Becton-Dickinson cat. no. 354236) for release experiments. For uptake experiments and the analysis of NA metabolites, cells were plated onto collagen-coated multiwells (Nunc cat. no. 150628, 120 000 cells per dish) or 35 mm tissue culture dishes (Nunc cat. no. 153066, 120 000 cells per dish), respectively.
The culture medium consisted of Dulbecco's modified Eagle's medium (DMEM; Gibco-Invitrogen cat. no. 31885-023), supplemented to contain 2.2 g l−1 glucose, 10 mg l−1 insulin (Sigma cat. no. I-5500), 25 000 IU l−1 penicillin, 25 mg l−1 streptomycin (Gibco-Invitrogen cat. no. 15140-148), 30 μg l−1 nerve growth factor (NGF; R&D Systems cat. no. 556-NG), and 5 % heat-inactivated fetal calf serum (FCS; Gibco-Invitrogen cat. no. 10108-165).
Neurone-enriched cultures were obtained by density-centrifugation of triturated cells through 33 % Percoll (Amersham-Pharmacia Biotech cat. no. 17-089102) in Tyrode solution. Pellets were resuspended in the culture medium described above, but supplemented in the absence of FCS with ovalbumin (0.1 %, Sigma cat. no. A-5503) and a mixture consisting of (mg l−1, final concentration): insulin 5, human transferrin 100, progesterone 0.0063, putrescine 16.11 and Na+ selenite 0.0052 (N2-supplement, Gibco-Invitrogen cat. no. 17502-014). Thirty thousand cells were seeded into glass rings of 8 mm diameter in order to confine them to the centre of laminin-coated (Becton-Dickinson cat. no. 354232) 35 mm tissue dishes (Nunc cat. no. 153066) and treated with 1 μmol l−1 cytosine β-d-arabinofuranoside (ARA-C, Sigma cat. no. C-1768) after 1 day.
The cells were kept in vitro in an atmosphere of 5 % CO2-95 % air (serum-free: 7 % CO2 and 93 % air) at 36.5 °C for 3–6 days before transmitter release, uptake experiments, HPLC analysis of [3H]-labelled NA metabolites, or RT-PCR analysis. Cultures kept for more than 4 days were fed after 2 or 3 days by replacing half of the culture medium.
PC12 cells were obtained from the European Collection of Cell cultures (ECACC, Salisbury, Wilts, UK), plated onto collagen-coated culture dishes (Nunc) and kept in OPTIMEM (Gibco-Invitrogen cat. no. 51985-018), supplemented with 25 000 IU l−1 penicillin, 25 mg l−1 streptomycin, 5 % heat-inactivated FCS and 10 % horse serum (Gibco-Invitrogen cat. no. 26050-039). Once per week, cell cultures were split; the medium was changed twice per week.
RT-PCR analysis
Preparation of RNA and reverse transcription (RT)
Neurone-enriched cell cultures (20 000 cells per dish, 3 days in vitro) and tissue homogenates from kidney, SCG and dorsal root ganglia of 5-day-old rat pups were lysed using the acidic guanidine thiocyanate-phenol method. Total RNA was extracted twice with acidic phenol : chloroform : isoamylalcohol (25 : 24 : 1) and precipitated with ethanol (96 %). Any remaining chromosomal DNA was digested with RNase-free DNase I (Roche). Reverse transcription was performed using 0.2 ml thin-walled PCR tubes (Costar) according to the protocol of the supplier (BcaBest RT-PCR kit, TaKaRa Shuzo Biomedical Group, Otsu, Shiga, Japan). In brief, 10 μl RT-buffer (2 × concentrated), MgSO4 (5 mm), deoxynucleotide mix (dNTPs, 0.5 mm), random nonamers (2.5 μM), RNAse inhibitor (20 U) and BcaBest reverse transcriptase (22 U) were premixed. Half a microgram of total RNA extracted from kidney or SCG tissue, or of the RNA extracted from two neurone-enriched cell cultures were then added, and the volume was adjusted to 20 μl with RNAse-free water. The temperature profile was as follows: 2 min at 65 °C; 1 min at 30 °C; temperature ramp 30–65 °C at 1.1 °C min−1; 12 min at 65 °C; 3 min at 94 °C; and storage at 4 °C. The resulting cDNA was diluted to 100 μl (1 : 5) with sterile water and analysed for the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and the organic cation transporters rOCT1 (rat OCT1), rOCT2, rOCT3, and the ‘novel’ organic cation transporters rOCTN1 and rOCTN2 by conventional PCR.
Single-cell RT (SC-RT) for the detection of GAPDH and rOCT3
SCG cultures prepared from 5-day-old rat pups were rinsed twice with a sterile Hepes-buffered solution. Single neurones were then sucked into patch clamp electrodes prefilled with 5 μl RNAse-free water under visual control with an inverted phase contrast microscope. The electrode contents were expelled into a 0.2 ml thin-walled PCR tube, and DNA was digested by DNase I (Roche) in a total volume of 10 μl for 15 min at 37 °C. Following DNase-I inactivation by a 10 min incubation at 70 °C, RT reactions were performed according to the protocol of the Sigma Enhanced Avian RT-PCR kit. Primers were annealed by incubations of single-neurone RNAs with random nonamers (final concentration 2.5 μM) and RNase inhibitor (20 U) for 10 min at 70 °C and for 10 min at 25 °C. The cDNA was synthesised by adding 20 U reverse transcriptase, 2 μl RT-buffer (10 × concentrated), dNTPs (final conconcentration 100 μM), and RNase-free water up to a total volume of 20 μl. The reaction mixture was incubated at 42 °C for 10 min followed by a temperature ramp (1 °C min−1) up to 65 °C and an additional 15 min plateau phase at 65 °C. The reaction was terminated by heating to 85 °C for 2 min. The cDNA thus obtained from a single neurone was subjected to SC-PCR to detect the expression of GAPDH and rOCT3. In order to minimise the degradation of RNAs, sterile gloves were worn during the procedures, and tubes and their contents were kept on ice whenever possible.
PCR
The primer sequences for the PCR amplification of GAPDH, rOCT1, rOCT2, rOCT3, rOCTN1 and rOCTN2 were developed from GenBank sequences with commercially available software (VectorNTI). Oligonucleotides were custom synthesised and HPLC purified (VBC Genomics, Vienna, Austria). The primers used in these studies included, in 5′-3′ orientation (nt: nucleotides; Tann: annealing temperature):
GAPDH (accession no. X02231; nt 360-1036) Tann: 52 °C
sense ACT GGC GTC TTC ACC ACC AT
antisense TCC ACC ACC CTG TTG CTG TA
rOCT1 (accession no. X78855; nt 1300-1777) Tann: 55 °C
sense GAT CTT TAT CCC GCA TGA GC
antisense TTC TGG GAA TCC TCC AAG TG
rOCT2 (accession no. D83044; nt 1401-1863) Tann: 57 °C
sense GCT TGG GTA GAA TGG GCA TC
antisense GTG AGG TTG GTT TGT GTG GG
rOCT3 (accession no. AF055286; nt 2095-2561) Tann: 52 °C
sense GCC TTG CAG TGT GCT TCA C
antisense GGA ACC TCA GTG GCT TTG G
rOCTN1 (accession no. AF169831; nt 1652-2017) Tann: 58 °C
sense AGA GGG TTC AG ATG TGG A
antisense CAC AAG TCC AGT TTG GTG C
rOCTN2 (accession no. AF110416; nt 1877-2375) Tann: 61 °C
sense GCT CAC GGA TGG GGC ATC T
antisense GCA GAG CAG CCA CAG TCC A
Conventional PCR was performed with a thermal cycler (Biometra, T-gradient) in 0.2 ml thin-walled PCR tubes. RT products and SC-RT products were amplified in a total volume of 20 μl containing 1 mm dNTPs, PCR-buffer, 0.25 U Ex-taq DNA polymerase (TaKaRa), and 0.5 μM OCTs or 0.25 μM GAPDH sense and antisense primers. Expression of GAPDH as an indicator for cDNA integrity was monitored by the amplification of 0.25 μl RT product. One microlitre of diluted RT product was used as a template for the amplification of OCTs. For SC-PCR, OCT3 mRNA of isolated SCG neurones was identified by the amplification of 4 μl SC-RT reaction product, whereas 1 μl of SC-RT reaction product was analysed for the expression of GAPDH.
The thermal cycler program used for PCR included an initial denaturation step at 94 °C for 3 min followed by 36 cycles (or 45 cycles for single cell analysis) with 30 s at 94 °C, 25 s at the respective annealing temperatures and 45 s at 72 °C. After a final extension step at 72 °C for 4 min, PCR products were separated by electrophoresis in 1.5 % agarose gels and visualised using ethidium bromide.
In each SC-RT PCR experiment, negative controls to exclude contamination from extraneous RNA were run with 5 μl of Hepes-buffered rinsing solution taken off the cultures. For conventional PCR, DNA contamination from extraneous sources was checked by replacing the cDNA templates with water. To ensure that genomic DNA did not contribute to the PCR products, RNA preparations were processed in the normal manner, except that reverse transcriptase was omitted. All of these controls were consistently negative. Representative amplicons were sequenced (Sequencing Service, VBC Genomics) and found to match the published sequences.
Loading of cultures with [3H]-noradrenaline and [3H]-N-methyl-4-phenylpyridinium ([3H]-MPP+) for superfusion
The techniques of labelling cultures of rat sympathetic neurones with [3H]-noradrenaline (NA) and subsequent superfusion have previously been described in detail (Boehm & Huck, 1995). The cultures were incubated in 0.035 μM [3H]-NA or 0.025 μM [3H]-MPP+ in culture medium containing 1 mm ascorbic acid for 60 min. Thereafter, culture discs were transferred to small chambers and superfused with a buffer containing (mmol l−1): NaCl 120, KCl 3.0, CaCl2 2.0, MgCl2 2.0, glucose 20, Hepes 10, fumaric acid 0.5, Na-pyruvate 5.0 and ascorbic acid 0.57, adjusted to pH 7.4 with NaOH, 25 °C, at a superfusion rate of 1.1 ml min−1. After a 40 min washout period in the presence of CaCl2, the cultures were rinsed for an additional 20 min period with a CaCl2-free buffer before collections of 4 min fractions were started with no CaCl2 present in the superfusion buffer. Residual radioactivity was determined by extracting cultures with 1 % (in H2O) sodium dodecyl sulphate (SDS, Sigma cat. no. L-4509). Radioactivity in extracts and collected fractions was determined by liquid scintillation counting, with appropriate quench corrections if required due to the presence of cyanine 863.
Calculation of basal and stimulation-evoked tritium outflow
Fractional rates (expressed as %) of [3H]-outflow were obtained by dividing the radioactivity of a 4 min fraction by the total radioactivity of a culture (i.e. the sum of all fractions plus the extract of all residual radioactivity retained by the culture) at the beginning of the corresponding 4 min collection period. Quantitative comparisons of the effects of substances were made by subtracting extrapolated basal release from the measured radioactive outflow of seven fractions (nos 7–13 or nos 8-14; area under curve, AUC; shown in Fig. 2B as the sum of vertical bars). Summed AUC values were divided by the total radioactivity of the respective culture and multiplied by 100. Extrapolated basal release was obtained by fitting the first six (or 7) data points of basal release to a single-exponential function (SigmaPlot, SPSS Inc., Chicago, IL, USA; see Fig. 2B for an example). Adequacy of fits was judged by eye. Unless indicated otherwise, data are shown as means ± s.e.m. Significance of differences for unpaired observations was evaluated by the signed rank test according to Mann-Whitney.
Figure 2. Reserpine and desipramine effects in SCG cultures loaded with [3H]-MPP+.
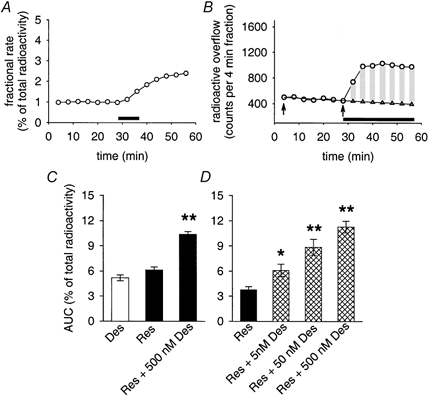
A, fractional rate of spontaneous release of [3H]-MPP+ and the release in response to 0.3 μM reserpine (application indicated by bar). Data points are means from 3 individual cultures included in the same release experiment (s.e.m. values smaller than the size of symbols). B, radioactive outflow from a single SCG culture shown as counts per 4 min fraction in the absence and presence of 0.5 μM desipramine (indicated by horizontal bar). Circles show data points; triangles indicate the points obtained by fitting the basal release between arrows to a single-exponential function and by an extrapolation to times after adding desipramine. Vertical bars indicate difference between extrapolated basal release and the release in the presence of desipramine. Summation of bars yields an area under curve (AUC) that we set in relation to the total radioactivity obtained from the culture. AUC for the experiment shown was 6.83 % (summed difference between basal and stimulated release: 3766 counts min−1; total radioactivity: 55 123 counts min−1). C, stimulated release in the presence of 0.5 μM desipramine (Des), in response to a transient (2 fraction, i.e. 8 min, see A) exposure of cultures to 0.3 μM reserpine (Res), and after a combined treatment with reserpine followed by desipramine (Res + 500 nm Des; see also top trace in Fig. 4A). Bars indicate means ± s.e.m. of AUC calculations from 23 (desipramine), 49 (reserpine) and 85 individual cultures (reserpine + desipramine). Stimulated release in response to either desipramine or reserpine was significantly different from (extrapolated) basal radioactive outflow (P < 0.01, Mann-Whitney U test). Combined treatment with reserpine followed by desipramine was significantly different from each treatment in isolation (**P < 0.01, Mann-Whitney U test). D, concentration dependency of desipramine effects after treating cultures transiently with 0.3 μM reserpine (as shown in A and top trace in Fig. 4A). Bars indicate means ± s.e.m. of AUC calculations from 9 individual cultures. All indicated concentrations of desipramine were found to enhance radioactive outflow significantly (* P < 0.05 for 5 nm desipramine, ** P < 0.01 for 50 and 500 nm desipramine, Mann-Whitney U test).
Analysis of [3H]-NA metabolites
Cells grown on collagen-coated 35 mm tissue culture dishes were loaded with [3H]-NA for 1 h as detailed above. Cultures were then rinsed seven times during a 45 min washout period with CaCl2-containing superfusion buffer, and finally three times for 15 min with CaCl2-free buffer containing inhibitors of reuptake or enzymes as indicated in the Results. After a 30 min incubation period at 36.5 °C, supernatants (700 μl) supplemented to contain 1 mmol l−1 ascorbic acid and 0.1 mol l−1 perchloric acid were collected and stored for analysis at −20 °C. The analysis was performed with combined HPLC (Waters Spherisorb 5 μm ODS2 4.6 × 250 mm HPLC column; UV detector, Bio-Rad cat. no. 1706) and liquid scintillation detection (Packard FLO-ONE Beta, 0-18.5 keV; cell-type, liquid; update time 6 s). Columns were loaded with 300 μl thawed sample supplemented to contain 0.4 mm NaHSO3 and 1 μg each of the following internal standards: DOMA (dl-3,4-dihydroxymandelic acid, Sigma cat. no. D-0135), DHPG (dl-3,4-dihydroxyphenyl glycol, Sigma cat. no. D-9753), VMA (3-methoxy-4-hydroxymandelic acid, Sigma cat. no. H-0131), MHPG (3-methoxy-4-hydroxyphenylglycol, Sigma cat. no. H-1377), NA (noradrenaline, Sigma cat. no. A-7257), adrenaline (Sigma cat. no. E-4250) and NMN (normetanephrine, Sigma cat. no. N-7127). Elution was done at a flow rate of 1.6 ml min−1 with a mobile phase containing 50 mm citric acid, 20 mm Na2HPO4, 3.5 mm 1-heptanesulphonic acid Na-salt (Fluka cat. no. 221554) and 0.125 % triethylamine (Sigma cat. no. T-0886). Scintillation detection was performed by post-column adding of 3.2 ml min−1 Ultima-Flo M (Packard, total flow rate 4.8 ml min−1).
Chemicals
Substances not specified above were from the following sources: (-)-[ring-2,5,6-3H]-norepinephrine (56.9 Ci mmol−1; NEN, cat. no. NET678); [3H]-N-methyl-4-phenylpyridinium ([3H]- MPP+, 78 Ci mmol−1, NEN cat. no. NET914; or 80 Ci mmol−1, ARC cat. no. ART-849); cyanine 863 (1-ethyl-2-(1,4-dimethyl-2-phenyl-6-pyrimidinylidene)-methyl) quinolinium chloride, ICN cat. no. 157977); clorgyline hydrochloride (RBI cat. no. M-004); MPP+-iodide (RBI cat. no. D-048); 3,5-dinitrocatechol (OR-486; RBI cat. no. D-131); amantadine HCl (Sigma cat. no. A-1260); corticosterone (Sigma cat. no. C-2630); β-oestradiol (Sigma cat. no. E-8875); d-tubocurarine chloride (d-TC, Sigma cat. no. T-2379); choline chloride (Sigma cat. no. C-7017); tetraethylammonium chloride (TEA, Sigma cat. no. T-2265); guanidine hydrochloride (Sigma cat. no. G-4505); desipramine hydrochloride (Sigma cat. no. D-3900); and reserpine (Sigma cat. no. R-0875). DMSO (dimethyl sulphoxide, Sigma cat. no. D-8779) used to dissolve corticosterone or β-oestradiol never exceeded a final concentration of 0.1 %. Other chemicals were from Merck, analytical grade. Drugs were stored as stock solutions in H2O or DMSO at −20 °C and diluted to their final concentrations in CaCl2-free superfusion buffer.
Results
OCT mRNA in SCG neurones
We searched tissue extracts from postnatal rat kidney, intact SCG and neurone-enriched SCG cell cultures for the expression of five OCTs (OCT1, OCT2, OCT3, OCTN1 and OCTN2) by RT-PCR. mRNA of OCT3, OCTN1 and OCTN2 was present in all preparations (Fig. 1A and B), whereas OCT1 and OCT2 were seen in the kidney but not in the SCG (Fig. 1A). Likewise, OCT3, OCTN1 and OCTN2 mRNA was found in NGF-naïve PC12 cells, whereas DRG neurones contained mRNA for OCTN1 and OCTN2, but not for OCT3 (Fig. 1D).
Figure 1. RT-PCR products for OCT1, OCT2, OCT3, OCTN1 and OCTN2.
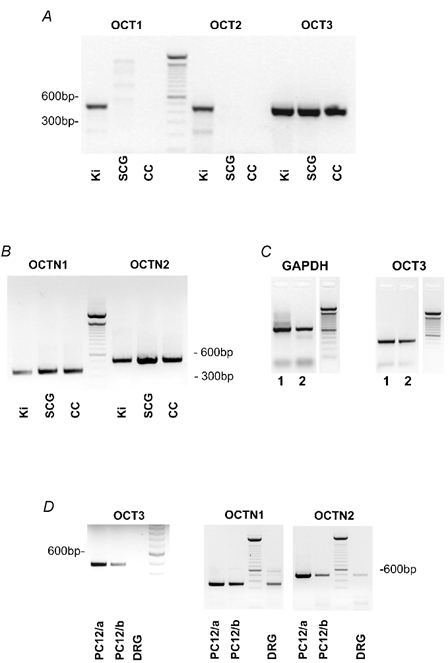
A and B, cDNA derived from kidney (Ki, 5-day-old rats), superior cervical ganglion (SCG, 5-day-old rats) and neurone-enriched cell cultures from the SCG (CC, 5-day-old rats; cultures 3 days in vitro). Note that OCT3, OCTN1 and OCTN2, but not OCT1 or OCT2 mRNA is present in the SCG. C, single-cell RT-PCR from two representative neurones (1, 2) that were positive for the housekeeping gene, GAPDH (left panel). The same neurones also proved positive for OCT3 (right panel). D, RT-PCR products for OCT3, OCTN1 and OCTN2 determined in two different NGF-naïve PC12 cell cultures (PC12/a and PC12/b), and in rat dorsal root ganglia (DRG, 5-day-old rats). OCTN1 and OCTN2, but not OCT3 mRNA was detected in the rat DRG, though all were present in PC12 cultures.
We then probed cultured SCG neurones identified by phase contrast microscopy with single-cell RT-PCR for the presence of OCT3. Out of 17 neurones that proved positive for the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 13 were also positive for OCT3 (Fig. 1C). These observations suggest that the organic cation transporters OCT3, OCTN1 and OCTN2 may be expressed in a differential manner in neurones of the peripheral nervous system.
MPP+ release in cell cultures of the rat SCG
Trans-inhibition experiments
Next we looked for a functional expression of OCTs, but rather than concentrating on uptake we focused our attention on the release of the cation MPP+, an established substrate of OCT3 (Russ et al. 1992; Wu et al. 1998a, 2000b; Gründemann et al. 1999; Rajan et al. 2000). As described below (Accumulation of MPP+ in SCG cultures), [3H]-MPP+ was first loaded into SCG sympathetic neurones by means of the NAT as a powerful, nerve cell-specific transporter system (Schroeter et al. 2000). We then studied the outward-directed transfer of MPP+, since OCT3 in this direction would stand a much better chance to compete against the NAT due to the pronounced (inward-directed) polarity of the latter transporter (Graefe & Boenisch, 1988). Our experimental protocol included the release of preloaded [3H]-MPP+ under resting conditions, the use of the storage vesicle-depleting agent reserpine, and cis- and trans-inhibition of the NAT by desipramine.
Dissociated cell cultures of the rat SCG released small amounts of preloaded [3H]-MPP+ at a fractional rate of 1.29 ± 0.05 % (mean ± s.e.m., n = 162 individual cultures). Upon loading the cells with [3H]-MPP+, a major fraction of radioactivity was accumulated in synaptic vesicles and thus not available as a substrate for outward-directed transmembrane transporters. Hence, basal release was significantly enhanced by (transient) exposure of cultures to 0.3 μM reserpine (Fig. 2A, C and D), an irreversible inhibitor of the vesicular monoamine transporter (VMAT; Henry et al. 1998). Basal fractional release of [3H]-MPP+ was also enhanced when 0.5 μM desipramine was included in the superfusion buffer (2.40 ± 0.06 %; mean ± s.e.m., n = 99; Fig. 2B and C). Desipramine at low concentration is an established and potent inhibitor of the NAT that leaves OCT3/EMT unaffected (see Discussion for a brief review of the literature). Our observation therefore indicates that in the absence of desipramine, reuptake by the NAT back into cells substantially masked the leakage of [3H]-MPP+ out of cultured sympathetic neurones. Co-applications of reserpine and desipramine had additive effects (Fig. 2C and D). We found desipramine concentrations as low as 5 nm effective in inhibiting reuptake of [3H]-MPP+ by the NAT (Fig. 2D).
Owing to the hydrophilic properties of MPP+, only limited quantities of the substance may have left the cells by simple diffusion (Russ et al. 1992), posing the question of how it was possible for the substance to pass the plasma membrane. Since our PCR analysis revealed the presence of OCT3 mRNA, we tested the hypothesis that [3H]-MPP+ outflow might occur by means of this transporter. In fact, three established inhibitors of the OCT3, namely cyanine 863, oestradiol and corticosterone (Russ et al. 1993; Wu et al. 1998a; Rajan et al. 2000), and the type II cation d-tubocurarine (d-TC) were found to reduce the release of MPP+ (Fig. 3). This phenomenon, called ‘trans-inhibition', is fairly common and has been observed in OCTs in general and in uptake 2 in particular (Raiteri et al. 1977; Trendelenburg, 1988; Russ et al. 1996; Nagel et al. 1997; Yabuuchi et al. 1999; Budiman et al. 2001). Note, however, that trans-inhibition of radioactive outflow was only seen after blockade of reuptake by the NAT, and that cyanine 863 and related substances did not significantly affect radioactive outflow in the absence of desipramine (Fig. 3D and E). Cyanine 863, oestradiol, corticosterone and d-TC not only effectively antagonized basal release (Fig. 3), but also the outflow of [3H]-MPP+ in response to treatment with reserpine (Fig. 4). These results clearly indicate that MPP+ does not leak from cultures by simple diffusion, but by some kind of transporter-mediated mechanism. As argued in the Discussion, out of the list of known organic ion transporters, OCT3 is the candidate that best fits our observations.
Figure 3. Effects of inhibitors of OCT3 on [3H]-MPP+ outflow.
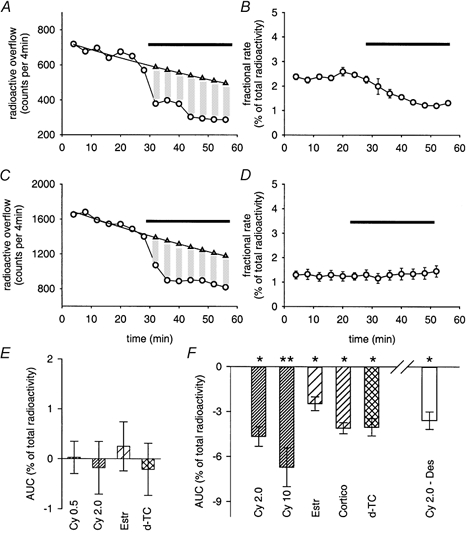
A, inhibition of [3H]-MPP+ release by 2 μM cyanine 863 (indicated by horizontal bar) in the presence of 0.5 μM desipramine. Desipramine was added to the superfusion buffer 10 min before collection of 4 min fractions was started. AUC (-4.74 %) was calculated as shown in Fig. 2B. B, same experimental approach as shown in A, but with results expressed as fractional rates. Bar indicates the presence of 2.0 μM cyanine 863. Data points are means ± s.e.m. from 3 individual cultures included in the same release experiment. C, inhibition of MPP+ release by 30 μM corticosterone (indicated by horizontal bar) in the presence of 0.5 μM desipramine. Desipramine was added to the superfusion buffer 10 min before collection of 4 min fractions was started. AUC (-4.02 %) was calculated as shown in Fig. 2B. D, fractional rates of radioactive outflow in the absence or presence of 2 μM cyanine 863 alone (indicated by bar). E, AUC values of [3H]-MPP+ outflow in the presence of cyanine 863 (0.5 and 2.0 μM), 30 μM oestradiol (Estr), or 100 μM d-tubocurarine (d-TC). Bars indicate means ± s.e.m. from 14 (0.5 μM Cy), 16 (2.0 μM Cy), 21 (30 μM Estr), and 9 individual cultures (100 μM d-TC). None of the AUC values is significantly different from zero (P > 0.05, Mann-Whitney U test). F, AUC values of MPP+ outflow in the presence of indicated substances. All cultures were treated with 0.5 μM desipramine 10 min before and during collection of 4 min fractions, except Cy 2.0 – Des, where 2.0 μM cyanine instead of desipramine was included in the superfusion buffer before and during the experiments, and where desipramine was probed for an inhibition of radioactive outflow. Abbreviations for substances are identical to E (plus Cortico: corticosterone). Bars indicate means ± s.e.m. from 8 (2.0 μM Cy), 6 (10 μM Cy), 6 (30 μM Estr), 6 (30 μM Cortico), 6 (100 μM d-TC), and 14 individual cultures (2.0 Cy – 0.5 μM Des). Significantly different from zero: * P < 0.05, ** P < 0.01 (Mann-Whitney U test).
Figure 4. Effects of inhibitors of OCT3 on reserpine-induced [3H]-MPP+ outflow.
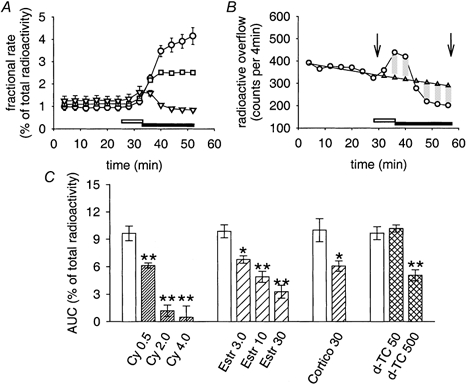
For all data shown, a radioactive outflow was induced by transient (8 min) applications of 0.3 μM reserpine (open horizontal bars in A and B), followed by 0.5 μM desipramine in the absence or presence of cyanine 863 (filled horizontal bars in A and B). A, [3H]-MPP+ outflow in response to 0.3 μM reserpine, modified by subsequent applications of: 0.5 μM desipramine (○); 0.5 μM desipramine plus 0.5 μM cyanine 863 (▪); or 0.5 μM desipramine plus 4 μM cyanine 863 (▿). Data points are mean fractional rates ± s.e.m. (n = 3). B, same experimental approach as shown in A (reserpine-induced [3H]-MPP+ release followed by co-applications of 4 μM cyanine 863 and 0.5 μM desipramine), but with data from a single culture plotted together with extrapolated basal release (▵). The AUC between arrows (-0.13 %) was calculated as shown in Fig. 2B. Note underestimation of effect of inhibitor due to its application only after reserpine. C, summary of effects of OCT3 inhibitors on reserpine-induced [3H]-MPP+ release with the experimental protocol shown in A and B. Open bars are matched controls of [3H]-MPP+ release in response to reserpine, followed by applications of 0.5 μM desipramine. Pattern-filled bars show results of inhibitors, co-applied with desipramine after the reserpine stimulus (cyanine 863 0.5, 2.0 and 4 μM, n = 8, 19 and 8, respectively, with matched controls, n = 20; oestradiol 3, 10 and 30 μM, n = 6, 13 and 9, respectively, with matched controls, n = 21; corticosterone 30 μM, n = 8, with matched controls, n = 9; d-TC 50 and 500 μM, n = 8 and 9, respectively, with matched controls, n = 18). Data are AUC values (means ± s.e.m.), calculated as shown in B and in Fig. 2B. Significantly different from control: * P < 0.05, ** P < 0.01 (Mann-Whitney U test).
Our experiments also revealed a dual effect of desipramine, since it not only enhanced (Fig. 2B and C) but could also reduce the outflow of MPP+ if applied after blockade of the OCT by 2 μM cyanine 863 (Fig. 3F). Desipramine therefore pinpoints OCT-dependent MPP+ outflow in a dual manner: it unmasks OCT-dependent release by suppressing reuptake by the NAT (cis-inhibition); and it plugs the NAT as an extra leakage pathway by means of trans-inhibition. The twofold mode of action of desipramine might explain why, in its absence, inhibitors of the OCT3 did not show consistent effects on the basal outflow of MPP+ (Fig. 3D and E). Residual radioactive outflow with both the NAT and the OCT inhibited may reflect transmembrane transport of MPP+ either by spontaneous, low rate vesicular exocytosis even in the absence of Ca2+ in the superfusion buffer, or by transporter systems yet to be identified that are not affected by desipramine or inhibitors of the OCT.
Trans-stimulation experiments
We next investigated whether small organic cations that are also substrates of OCTs might be able to enhance the outflow of [3H]-MPP+ by trans-stimulation. Trans-stimulation is equally well established as a phenomenon for OCTs as trans-inhibition (Trendelenburg, 1988; Busch et al. 1996, 1998; Nagel et al. 1997; Yabuuchi et al. 1999; Wagner et al. 2000; Ohashi et al. 2001). The organic cations tested (guanidine, choline and tetraethylammonium (TEA)) have previously been used as substrates to probe OCT3 function (Gründemann et al. 1998b, 1999; Kekuda et al. 1998; Wu et al. 1998a, 2000b). We found substantial enhancement of [3H]-MPP+ outflow in response to guanidine and choline, but the effects of the two agents were efficiently antagonized by 0.5 μM desipramine (Fig. 5A, B and E). As detailed in the Discussion, desipramine at such low concentrations leaves uptake 2/OCT3 unaffected, and the results therefore suggest that in our experiments, trans-stimulation by guanidine and choline is mediated by the NAT. Trans-stimulation of uptake 1 has been proposed previously as the mechanism that induces [3H]-NA release in response to choline (Ungell et al. 1986). Because of its purported trans-stimulation of the OCT2 (Busch et al. 1998), we also tested amantadine and found a large [3H]-MPP+ outflow in response to the antiparkinsonian drug (Fig. 5C and E). As for guanidine and choline, the [3H]-MPP+ outflow induced by 50 μM amantadine was also sensitive to desipramine and thus appeared to be mediated by the NAT. In keeping with this interpretation, we saw substantial desipramine-sensitive outflow of preloaded [3H]-MPP+ in NAT-transfected HEK293 cells (Pifl & Singer, 1999) challenged with 50 μM amantadine (data not shown). TEA, on the other hand, caused only slight enhancement of [3H]-MPP+ outflow and even acted as an inhibitor in the presence of desipramine (Fig. 5D and E). Hence, out of this list of potential substrates, none had a major effect that could be unambiguously attributed to trans-stimulation of an OCT.
Figure 5. Trans-stimulation by potential OCT substrates.
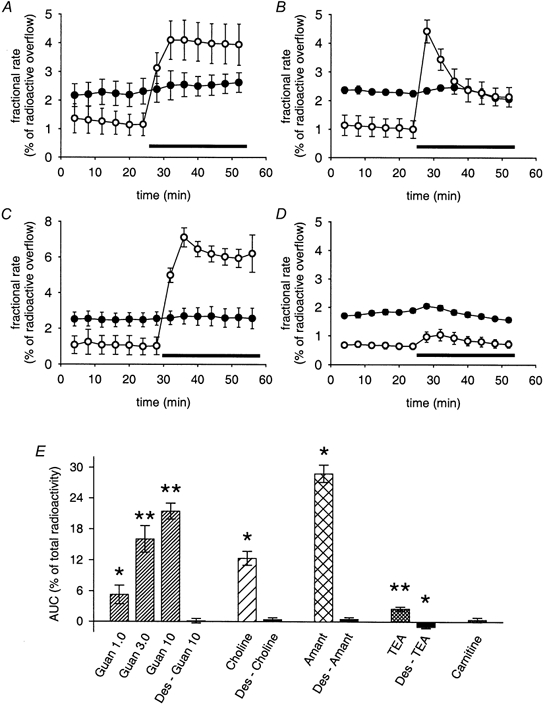
A-D, effects of potential OCT substrates on [3H]-MPP+ outflow tested in the absence (○) or presence (•) of 0.5 μM desipramine. Desipramine was added to the superfusion buffer 10 min before and during the collection of 4 min fractions. Data are plotted as fractional rates (means ± s.e.m., n = 3 for each individual data point). The following substances were used (applications indicated by bars): 10 mm guanidine (A); 10 mm choline (B); 50 μM amantadine (C); and 10 mm tetraethylammonium Cl− (TEA; D). E, summary of effects of potential OCT substrates on [3H]-MPP+ outflow with the experimental protocol shown in A-D. Pattern-filled bars show trans-stimulation by potential substrates: guanidine (Guan) 1.0, 3.0 and 10 mm, n = 6, 12 and 25 individual cultures, respectively; 10 mm choline (n = 6); 50 μM amantadine (Amant, n = 6); 10 mm TEA (n = 17); 100 μM carnitine (n = 9). Filled bars (Des-Guan 10, Des-Choline, Des-Amant and Des-TEA) demonstrate effects of desipramine (added 10 min before and during collection of fractions) on trans-stimulation (n = 6, 6, 6 and 11 individual cultures, respectively). Data are AUC values (means ± s.e.m.), calculated as shown in Fig. 2B. Significantly different from zero: * P < 0.05, ** P < 0.01 (Mann-Whitney U test).
Nevertheless, we could produce a substantial OCT-related trans-stimulation of preloaded [3H]-MPP+ by superfusion of cultures with MPP+ (Fig. 6). Hence, the release in response to 50 μM MPP+ was reduced in part by 1 μM desipramine, and in part by 2 μM cyanine 863 (Fig. 6). Full inhibition of MPP+-induced outflow of radioactivity was achieved by combined applications of 1 μM desipramine and 10 μM cyanine 863 (Fig. 6B). These observations suggest trans-stimulation of both the NAT and an OCT by MPP+ and are to be expected when probing MPP+ as an established substrate for both transporter systems (Russ et al. 1992; Pifl et al. 1996).
Figure 6. Trans-stimulation of [3H]-MPP+ outflow by MPP+.
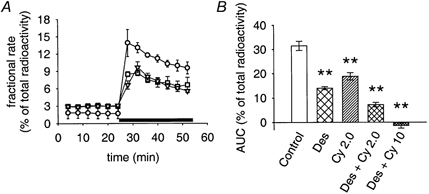
A, effects of 50 μM MPP+ (application indicated by bar) on [3H]-MPP+ outflow in the absence (○) or presence of 1 μM desipramine (▪) or 2.0 μM cyanine 863 (▿). Desipramine and cyanine 863 were added to the superfusion buffer 10 min before collection of 4 min fractions was started. Data are plotted as fractional rates (means ± s.e.m., n = 3). B, AUC values of MPP+-induced trans-stimulation in the absence of inhibitors (open bar, control), in the presence of 1 μM desipramine (Des), 2 μM cyanine 863 (Cy 2.0), or 1 μM desipramine plus cyanine 863 (Des + Cy 2.0 or Des + Cy 10 for 2 μM or 10 μM cyanine 863, respectively). Inhibitors were added to the superfusion buffer 10 min before and during collection of 4 min fractions. Bars indicate means ± s.e.m. from 24 (control), 12 (Des), 11 (Cy 2.0), 14 (Des + Cy 2.0), or 6 (Des + Cy 10) individual cultures. ** Significantly different from control (P < 0.01, Mann-Whitney U test).
Radioactive outflow in cultures loaded with [3H]-NA
Cultures loaded with [3H]-NA had fractional rates of basal radioactive outflow of 1.39 ± 0.08 % (means ± s.e.m., n = 157). The basal release increased slightly in the presence of desipramine (Fig. 7C), but considerably upon treatment of cultures with 0.3 μM reserpine (Fig. 7A and C). Cyanine 863, which had no effect by itself on [3H]-MPP+ outflow (Fig. 3D and E), and in the presence of desipramine even inhibited the release of [3H]-MPP+ (Fig. 3A, B and F), caused significant radioactive outflow in cultures preloaded with [3H]-NA (Fig. 7B and C). Likewise, d-TC and oestradiol stimulated radioactive outflow in [3H]-NA-loaded cultures (Fig. 7C), whereas neither substance had an effect on [3H]-MPP+ outflow (Fig. 3E) and again inhibited the release of [3H]-MPP+ in the presence of desipramine (Fig. 3F and Fig. 4C). Guanidine and amantadine, however, were clearly less effective in stimulating radioactive outflow from [3H]-NA-loaded cultures (Fig. 7C) than from cultures loaded with [3H]-MPP+ (Fig 5A, C and E). The somewhat unexpected stimulation of radioactive outflow from [3H]-NA-loaded cultures by oestradiol (Fig. 7C) is in line with a previous observation that corticosterone transiently increased the outflow of NA and its metabolites in the isolated perfused rat heart (Fiebig & Trendelenburg, 1978).
Figure 7. Radioactive outflow from SCG cultures loaded with [3H]-NA.
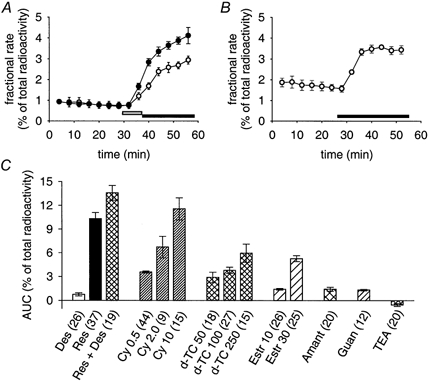
A, fractional rate of the release of radioactivity in response to 0.3 μM reserpine (○, application indicated by open bar), and in response to 0.3 μM reserpine followed by 0.5 μM desipramine (•, application indicated by filled bar). Data points are means ± s.e.m. from 3 individual cultures included in the same release experiment. B, fractional rate of radioactive outflow in response to 2.0 μM cyanine 863 (application indicated by bar). Data points are means ± s.e.m. from 3 individual cultures included in the same release experiment. C, AUC values of radioactive outflow induced by indicated test substances (number of individual cultures in parenthesis). Des: 0.5 μM desipramine; Res: 0.3 μM reserpine; Cy 0.5, 2.0, 10: cyanine 863 (μM); d-TC 50, 100, 250: d-tubocurarine (μM); Estr 10, 30: oestradiol (μM); Amant: 50 μM amantadine; Guan: 10 mm guanidine; TEA: 10 mm tetraethylammonium Cl−. Bars indicate means ± s.e.m.. All data points differ significantly from zero (P < 0.01, Mann-Whitney U test).
The conflicting results relating to the use of the radioactive tracer can be accounted for by considering the diverse properties of [3H]-NA and [3H]-MPP+. Unlike MPP+ (Sayre, 1989), free cytoplasmic NA (e.g. following the depletion of storage vesicles by reserpine) is rapidly metabolised to DHPG by the enzyme monoamine oxidase (MAO; Boenisch & Trendelenburg, 1988; Graefe & Boenisch, 1988). DHPG readily crosses the plasma membrane by simple diffusion and thus comprises the main metabolic product in perfusates from sympathetically innervated tissue (Stute & Trendelenburg, 1984; Boenisch & Trendelenburg, 1988; Graefe & Boenisch, 1988). It was therefore of interest to analyse the supernatants in our culture system for the major radioactive metabolites of [3H]-NA in response to both reserpine (as a reference substance) and cyanine 863.
Analysis of supernatants from cultures loaded with [3H]-NA
Supernatants from cultures loaded with [3H]-NA and incubated for 30 min with reserpine plus desipramine contained 78 % [3H]-DHPG as the principal radioactive product and 18 % [3H]-NA (Fig. 8A). Likewise, [3H]-DHPG (56 %) exceeded [3H]-NA contents (37 %) in cultures treated with 2 μM cyanine 863 in the presence of desipramine (Fig. 8B). The somewhat lower ratio of DHPG : NA in response to cyanine 863 (1.5 ± 0.21, n = 3) compared to reserpine (4.4 ± 0.76; mean ± s.e.m., n = 3; P > 0.05, no significant difference by Mann-Whitney U test) might indicate a moderate inhibition of MAO by cyanine 863, although this aspect was not pursued in the current project.
Figure 8. Radioactive metabolites in supernatants of cultures loaded with [3H]-NA.
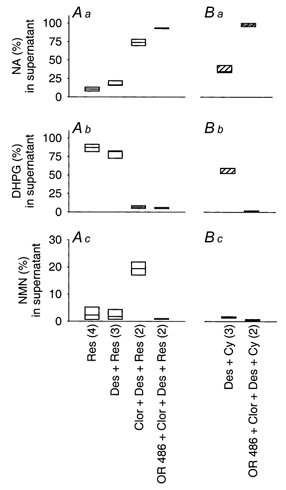
SCG cultures were loaded with [3H]-NA and rinsed for 60 min as described in Methods. Cultures were then incubated for 30 min, with either 0.3 μM reserpine (A) or 2.0 μM cyanine 863 (B) and with 0.5 μM desipramine (Des), 0.5 μM clorgyline (Clor) and 1 μM OR 486 to inhibit the NAT, MAOA and COMT, respectively. Thereafter, the radioactivity in supernatants was analysed for the presence of DOMA (dl-3,4-dihydroxymandelic acid), DHPG (dl-3, 4-dihydroxyphenyl glycol), VMA (3-methoxy-4-hydroxymandelic acid), MHPG (3-methoxy-4-hydroxyphenylglycol), NA, adrenaline and NMN (normetanephrine). Box plots indicate the relative proportion that each of the 3 major compounds ([3H]-NA, [3H]-DHPG and [3H]-NMN) contributes to the total radioactivity recovered from supernatants (expressed as %). Figures in parentheses show the number of supernatants that have been analysed for indicated experimental approaches.
Supernatants of cultures collected after a 30 min incubation and treated with the MAOA inhibitor clorgyline (Youdim et al. 1988) showed much reduced ratios of DHPG : NA in response to both reserpine (mean ratio: 0.09, n = 2) and cyanine (mean ratio: 0.01, n = 2), but appreciable amounts of NMN (19.4 and 14.5 % for reserpine and cyanine 863, respectively; shown in Fig. 8A c for reserpine), indicating that MAO is the principal enzyme to metabolise free NA, and that catechol-O-methyltransferase (COMT) partly takes over if MAO has been inhibited. Treatment of cultures with clorgyline plus the COMT inhibitor OR 486 (Nissinen et al. 1988) reduced both DHPG and NMN and resulted in the appearance of more than 95 % of the radioactivity as genuine [3H]-NA (Fig. 8A and B). Regardless of how cultures were treated, the total radioactivity in supernatants was quantitatively accounted for by the sum of [3H]-NA, [3H]-DHPG and [3H]-NMN (99.26 ± 0.41 %; mean ± s.e.m., n = 16).
Release experiments in cultures loaded with [3H]-NA, with MAO and COMT inhibited
The inhibition of MAO and COMT significantly reduced basal fractional release of radioactivity to 0.36 ± 0.04 % (mean ± s.e.m., n = 57; significantly different from the control values without enzymes inhibited: 1.28 ± 0.05 %, P < 0.01, Mann-Whitney U test). Likewise, the treatment of cultures with clorgyline, with or without OR 486, inhibited radioactive outflow in response to either cyanine 863 or reserpine (Fig. 9), indicating that radioactive outflow at rest and in response to the two substances greatly depends on the formation of more diffusible metabolites of NA. In contrast, clorgyline and OR 486 did not affect the [3H]-MPP+ outflow elicited by an exposure of cultures to reserpine plus desipramine (AUC 11.20 ± 0.60 % without enzyme inhibitors; 10.20 ± 0.57 % following treatment with clorgyline and OR 486, n = 6, P > 0.05, Mann-Whitney U test). This observation rules out the possibility that the inhibition of [3H]-NA outflow by clorgyline and OR 486 was due to a direct interference of the two substances with the transporter.
Figure 9. Effects of MAOA and COMT inhibition on the radioactive outflow in cultures loaded with [3H]-NA.
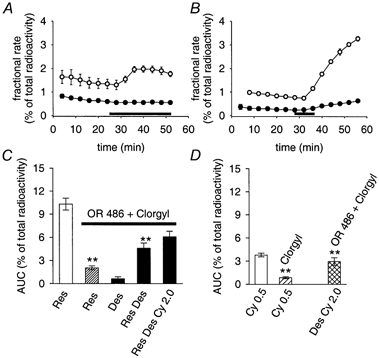
A, radioactive outflow in response to 0.5 μM cyanine 863 (application indicated by bar) from control SCG cultures (○) and from matched cultures treated with the MAOA inhibitor clorgyline (0.5 μM, •) 10 min before and during the collection of fractions. Results are shown as fractional rates (means ± s.e.m., n = 3). B, radioactive outflow in response to 0.3 μM reserpine (application indicated by bar) from control SCG cultures (○) and from matched cultures treated with clorgyline as in A (0.5 μM, •). Results are shown as mean fractional rates (s.e.m. values smaller than the size of symbols, n = 3). C, AUC values of radioactive outflow induced by indicated test substances. OR 486 + Clorgyl: cultures treated for 10 min with 1 μM of the COMT inhibitor OR 486 plus 0.5 μM clorgyline before and during the collection of fractions. Res (without enzyme inhibitors): 0.3 μM reserpine (transiently applied for 8 min as shown in B and in Fig. 7A). Data shown by the open bar are taken from Fig. 7C for a comparison, n = 37. Res (OR 486 + Clorgyl): radioactive outflow induced by 0.3 μM reserpine transiently applied in the presence of enzyme inhibitors (n = 17). Data differ significantly from results of reserpine-induced radioactive outflow without enzyme inhibitors, P < 0.01, Mann-Whitney U test. Des (OR 486 + Clorgyl): radioactive outflow induced by 0.5 μM desipramine in the presence of enzyme inhibitors (n = 26). Res Des (OR 486 + Clorgyl): radioactive outflow induced by 0.3 μM reserpine followed by 0.5 μM desipramine in the presence of enzyme inhibitors (n = 24). Data of combined applications differ significantly from results of release induced by reserpine or desipramine alone, P < 0.01, Mann-Whitney U test. Res Des Cy 2.0 (OR 486 + Clorgyl): radioactive outflow induced by 0.3 μM reserpine, followed by 0.5 μM desipramine plus 2.0 μM cyanine 863 in the presence of enzyme inhibitors (n = 24). D, Cy 0.5 (without enzyme inhibitors): radioactive outflow induced by 0.5 μM cyanine 863 (n = 9). Cy 0.5 (Clorgyl): radioactive outflow in response to 0.5 μM cyanine 863 with MAOA inhibited (n = 8). Data differ significantly from results of cyanine 863-induced release without the inhibition of MAOA, P < 0.01, Mann-Whitney U test. Des Cy 2.0 (OR 486 + Clorgyl): release induced by 2.0 μM cyanine 863 with both MAOA and COMT inhibited, and with 0.5 μM desipramine included in the superfusion buffer 10 min before and during the application of cyanine 863 (n = 9; data points significantly different from zero, P < 0.01, Mann-Whitney U test).
With MAO and COMT inhibited, desipramine caused minor radioactive outflow and enhanced the effect of reserpine (Fig. 9C), suggesting that some NAT-dependent reuptake of [3H]-NA occurs in such cultures. Whereas these effects of desipramine paralleled results from cultures loaded with [3H]-MPP+ (Fig. 2B, C and D), 2.0 μM cyanine 863 in the presence of desipramine was found to enhance (Fig. 9D) rather than to inhibit (as one might expect from observations in [3H]-MPP+-loaded cultures, see Fig. 3A, B and F) the outflow of genuine [3H]-NA (MAO and COMT inhibited). Likewise, 2.0 μM cyanine 863 did not significantly affect (and certainly did not reduce) [3H]-NA outflow in response to reserpine plus desipramine (MAO and COMT inhibited, Fig. 9C), even though cyanine 863 significantly inhibited the [3H]-MPP+ outflow ensuing with combined applications of reserpine and desipramine (Fig. 4A, B and C). In sum, cyanine 863 enhanced radioactive outflow in cultures loaded with [3H]-NA predominantly by an increased production of the readily diffusible metabolite DHPG and may thus be classified as a storage vesicle-depleting agent (Stute & Trendelenburg, 1984; Boenisch & Trendelenburg, 1988). Further implications of the differences attributed to the use of the two substrates, [3H]-NA and [3H]-MPP+, are dealt with in the Discussion.
Accumulation of MPP+ in SCG cultures
On grounds of the outward-directed transport of [3H]-MPP+ described above, we may expect at least two mechanisms for the uptake of [3H]-MPP+: an OCT (possibly OCT3), and the NAT. Cultures incubated with 64 nm [3H]-MPP+ accumulated the substrate primarily by means of the NAT, since uptake of radioactivity was reduced to about 5 % in the presence of 1 μM desipramine (Fig. 10A), which at such low concentrations would leave OCTs unaffected (detailed below in the Discussion). In keeping with this conclusion, the presence of cyanine 863 did not significantly affect the accumulation of [3H]-MPP+ over a 30 min incubation period (5.50 ± 0.10 pmol per culture in the absence of 2 μM cyanine 863; 5.43 ± 0.19 pmol per culture in its presence; means ± s.e.m., n = 6; P > 0.05 Mann-Whitney U test). These observations indicate that at low concentrations, the bulk of [3H]-MPP+ transport is handled by the NAT, and that cyanine 863-sensitive, NAT-independent mechanisms do not significantly contribute to the accumulation of radioactivity. Since the NAT is exclusively located on neurones (Schroeter et al. 2000), we may furthermore conclude that at low concentration of the substrate, MPP+ becomes selectively concentrated in neurones. Hence, the outflow ensuing on loading with [3H]-MPP+ reflects leakage from SCG neurones.
Figure 10. Accumulation of [3H]-MPP+ in rat SCG cultures by NAT-related and by NAT-unrelated mechanisms.
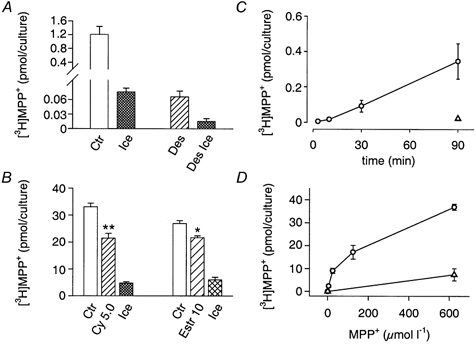
A, cultures incubated for 30 min with 64 nm [3H]-MPP+ in the absence (Ctr) or presence of 1 μM desipramine (Des). Ice and Des Ice indicate uptake measured for the two conditions on ice. Accumulation of [3H]-MPP+ is reduced to 5.4 % of the control value by the presence of 1 μM desipramine. B, effect of the OCT3 inhibitors cyanine 863 and oestradiol on the NAT-independent accumulation of 25 μM [3H]-MPP+. Cultures pretreated for 10 min with 0.3 μM reserpine and 5 μM desipramine were incubated with 5 μM desipramine and 25 μM MPP+ in the absence (Ctr) or presence of either 5 μM cyanine 863 (Cy 5.0) or 10 μM oestradiol (Estr 10). Ice indicates control uptake measured on ice. Data are shown as means ± s.e.m. (Ctr, Cy 5.0 and Estr 10, n = 6; Ice, n = 8). Cyanine (5 μM) and 10 μM oestradiol reduced accumulation to 64 and 80 %, respectively. ** P < 0.01 and * P < 0.05) denote significant differences from controls; Mann-Whitney U test). C, time-dependent accumulation of [3H]-MPP+. Cultures were incubated in the presence of 1 μM desipramine with 64 nm [3H]-MPP+ for indicated periods of time (○). ▵, same protocol, but with the accumulation of [3H]-MPP+ measured on ice. Data are shown as means ± s.e.m. (n = 5-6). D, concentration-dependent accumulation of [3H]-MPP+. Cultures were incubated for 30 min in the presence of desipramine with indicated concentrations of [3H]-MPP+ (○). ▵, same protocol, but with the accumulation of [3H]-MPP+ done on ice. Kinetic parameters deduced from the data yield a Km of 115 ± 39 μM and a Vmax of 34.1 ± 3.6 pmol per culture (means ± s.e.m., n = 4).
Nevertheless, in the presence of desipramine an additional, temperature-sensitive component of accumulation became apparent. It was linear over a 90 min incubation period (Fig. 10C), and of low affinity but high capacity for MPP+ transport (Fig. 10D). Since accumulation of [3H]-MPP+ in the presence of desipramine was also sensitive to cyanine 863 and oestradiol (Fig. 10B), it meets the requirements for an OCT3-dependent transport (Russ et al. 1992, 1996; Wu et al. 1998a; Rajan et al. 2000). Beyond these tentative experiments we did not attempt to characterize the inwardly directed transport of MPP+ further since, owing to the presence of the NAT as a potent, competing system for the uptake, our SCG cell cultures are ill suited for detailed kinetic analyses.
Discussion
The principal findings of this work are the first demonstration of mRNA for OCT3 in neurones of the rat SCG and an outward-directed, transmembrane organic cation transport from SCG neurones possibly mediated by OCT3. OCT3 (i.e. EMT; Gründemann et al. 1998b), initially named uptake 2 (Iversen, 1965), has generally been considered non-neuronal (Trendelenburg, 1988). Hence, uptake 2 has been shown in such cells as heart and blood vessel muscle cells (for review see Trendelenburg, 1988) and more recently in primary cell cultures of rat cortical astrocytes and tumour cells of glial origin (Russ et al. 1996; Streich et al. 1996). However, recent in situ hybridization data suggest that OCT3 mRNA might also be present in CNS neurones (Wu et al. 1998a) and retinal ganglion cells (Rajan et al. 2000). Our own observations based on single-cell RT-PCR and on release experiments with [3H]-MPP+ from cultured SCG nerve cells confirm and significantly extend the concept of a neuronal localization of OCTs.
For our functional studies we took advantage of the powerful neuronal transporter system NAT, which causes accumulation of substrates like NA and MPP+ almost exclusively in nerve cells. Then we were able to study neuronal release under resting conditions, upon inhibition of the NAT by desipramine, and with the storage vesicle-depleting agent reserpine. Our conclusion that OCTs play a functional role in sympathetic nerve cells rests on observations made with MPP+. MPP+ is an excellent substrate for OCT1, OCT2 and OCT3 (Russ et al. 1992; Gründemann et al. 1999; Rajan et al. 2000). It is metabolically stable (Sayre, 1989) and, as a charged molecule, shows little diffusion across the cytoplasmic membrane (Russ et al. 1992). MPP+, like NA, is stored in presynaptic vesicles due to active transport by the VMAT (Liu et al. 1992; Erickson et al. 1996).
Cultures loaded with [3H]-MPP+ showed moderate basal release that was significantly enhanced by desipramine, an established inhibitor of the NAT (Lee et al. 1982; Graefe & Boenisch, 1988). In contrast, as has been shown in a number of previous publications, desipramine at low concentrations leaves OCT3/EMT unaffected. Hence, 1 μM desipramine had no effect on [3H]-NA uptake into Caki-1 cells (Schoemig & Schönfeld, 1990). Furthermore, the presence of 0.3 μM desipramine did not affect [3H]-MPP+ uptake by the human OCT3/EMT transfected into the HEK293 cell line (Gründemann et al. 1998b). When expressed in human retinal pigment epithelial (HRPE) cells, (human) OCT3-mediated uptake of [3H]-MPP+ was inhibited by desipramine with a Ki of 14 μM (Wu et al. 2000b), even though effects at the low concentration end of the dose-response curve were not shown in this report. Likewise, the rat OCT3 appears fairly insensitive to desipramine, since upon expression in HRPE cells, uptake of [3H]-MPP+ was inhibited with an IC50 of 68 μM only. No effects were apparent in this report at 1 μM desipramine (Wu et al. 1998a). We therefore conclude that the enhancement of [3H]-MPP+ outflow seen at desipramine concentrations below 1 μM occurred by virtue of an effect on the NAT and not on OCT3.
Treatment with desipramine will not only cause cis-inhibition (Lee et al. 1982), thereby preventing inward-directed reuptake of MPP+ that had leaked from the cells, but also trans-inhibition of the NAT, thus blocking outward-directed transport (Raiteri et al. 1977). Any MPP+ that appears in the supernatant under these experimental conditions therefore must have left the cells by a transport system other than the NAT.
Outward-directed transport of [3H]-MPP+ might be mediated by OCT3
To the best of our understanding, four groups of organic ion transporters out of the ASF superfamily (Marger & Saier, 1993) might have facilitated the leakage of [3H]-MPP+ out of SCG neurones: OCTs, OCTNs, organic anion transporters (OATs) and organic anion-transporting polypeptides (OATPs). Taking into consideration the presence of mRNAs in the ganglion, the specificity of transported substrates, and our trans-stimulation and trans-inhibition experiments, we currently favour OCT3 as the transporter molecule that accounts for the phenomena we observe.
We tend to exclude both the OATs and OATPs, since to date they have not been shown to transport small (type I) organic cations like TEA or MPP+ (Noe et al. 1997; Kusuhara et al. 1999; Sekine et al. 2000). Nevertheless, OATPs are capable of transporting large (type II) organic cations (Sekine et al. 2000; Van Montfoort et al. 2001) by a type I organic cation-insensitive mechanism (Van Montfoort et al. 2001). Due to the lack of mRNA, we may also exclude OCT1 and OCT2 as transporter molecules that mediate the leak of MPP+ from SCG neurones.
The situation is less clear for OCTN1 and OCTN2, primarily because as ‘novel’ members of the OCT family we know much less about their preferences for substrates and inhibitors. Both OCTN1 and OCTN2 are inhibited by quinidine (Yabuuchi et al. 1999; Ohashi et al. 2001), but to our knowledge, the effect of neither steroids nor cyanine-related compounds (Russ et al. 1993) as potential inhibitors has been tested on this transporter subfamily. The small (type I) cation TEA appears to be a good substrate for OCTN1 and OCTN2 (Wu et al. 1998b, 1999 2000a; Seth et al. 1999; Yabuuchi et al. 1999; Tamai et al. 2000; Wagner et al. 2000). However, TEA uptake seems fairly insensitive to MPP+ (Wu et al. 1999, 2000a; Arndt et al. 2001; Ohashi et al. 2001), indicating that MPP+ is not readily accepted as a substrate by the OCTNs (Ohashi et al. 2001).
A particular feature of OCTN1 and OCTN2 is a Na+-dependent transport of carnitine (Tamai et al. 1998, 2000; Seth et al. 1999; Wu et al. 1999; Wagner et al. 2000; Ohashi et al. 2001). Furthermore, trans-stimulation phenomena for both OCTN1 and OCTN2 have been observed using TEA or carnitine as substrates (Yabuuchi et al. 1999; Wagner et al. 2000; Ohashi et al. 2001). Since neither TEA nor carnitine induced trans-stimulation of preloaded [3H]-MPP+ in our own experiments, and since MPP+ at 50 μM (a concentration possibly too low for the OCTNs) caused the phenomenon, OCTNs do not seem ideal candidates to match our observations on outward-directed transport of [3H]-MPP+. Nevertheless, we were able to detect mRNA for both transporters in the intact rat SCG, in neurone-enriched SCG cell cultures, in NGF-naïve PC12 cells and in the rat DRG. mRNA for OCTN1 and OCTN2 has been shown to occur in the central nervous system (Tamai et al. 1998; Wu et al. 1998b, 1999, 2000a).
In contrast to the OCTNs, the phenomena we observed satisfy most of the criteria required for an OCT3-dependent mechanism: OCT3 mRNA is present in neurones of the SCG; MPP+ is an excellent substrate for this transporter (Russ et al. 1992); several substances known to inhibit OCT3, such as cyanine 863, oestradiol and corticosterone (Russ et al. 1993; Rajan et al. 2000), were effectively obstructing MPP+ release by trans-inhibition; and MPP+ at 50 μM induced release by trans-stimulation in a partly cyanine 863-dependent manner. The inability of TEA and guanidine to cause MPP+ release from SCG cultures by trans-stimulation does not necessarily contradict our tentative conclusion, since there is some on-going debate concerning the capacity of the two substances to function as substrates for OCT3 (Gründemann et al. 1998b, 1999; Kekuda et al. 1998; Wu et al. 1998a, 2000b). Even if TEA and guanidine served as substrates for OCT3/uptake 2, their low rate of transport could render them either ineffective for trans-stimulation (at an intermediate Vmax) or even turn them into inhibitors (at a low Vmax; see Trendelenburg, 1988).
Is the efflux of [3H]-MPP+ of neuronal or non-neuronal origin?
The potent inhibition of [3H]-MPP+ accumulation by desipramine shown in Fig. 10 and the absence of clear-cut effects on uptake by cyanine 863 in our culture system indicate that the bulk of radioactivity (roughly 95 %) is handled by the NAT and thus concentrated in nerve cells. Hence, immediately after incubation and without washout, contents of [3H]-MPP+ in non-neuronal cells comprise 5 % of the radioactivity at the most, and a mere modulation of this small pool would be hard to detect in our release experiments. Furthermore, inhibitors of OCT3 effectively antagonized not only spontaneous release but also the [3H]-MPP+ outflow in response to reserpine, which specifically acts on the neuronal VMAT (Fig. 4). In fact, this experiment also argues against a major role of non-neuronal OCT3/uptake 2 in capturing free [3H]-MPP+. Assuming that non-neuronal cells scavenge reserpine-induced outflow of [3H]-MPP+, the inhibition of OCT3/uptake 2 in these cells by, for example, cyanine 863 should actually enhance (and not reduce, as seen in our experiments) the radioactive outflow that originates from neurones. Taken together, these observations suggest that radioactive outflow of preloaded [3H]-MPP+ originates from nerve cells, and that any stimulatory or inhibitory modulation of release is exerted by virtue of effects on neurones in our culture system.
Cyanine 863 acts as a storage vesicle-depleting agent
Results differed significantly when [3H]-NA was used instead of [3H]-MPP+ to load cultures of the SCG. Hence, cyanine 863 at concentrations that inhibited the release of [3H]-MPP+ (in the presence of desipramine) now caused substantial radioactive outflow. This outflow was largely reduced when cultures were treated with the MAOA inhibitor clorgyline, indicating that the occurrence of radioactivity in the superfusate depended on the generation of diffusible, lipophilic [3H]-NA metabolites by enzymatic degradation mediated by MAO. Our analysis of supernatants from cultures preloaded with [3H]-NA and challenged with cyanine 863 revealed that DHPG was in fact the main radioactive constituent. DHPG will rise if NA stored in presynaptic vesicles is released into the cytoplasm and thus becomes exposed to MAO. Depletion of storage vesicles by cyanine 863 might occur by dissipation of the pH gradient (weak base hypothesis; Sulzer & Rayport, 1990) and/or by an inhibition of VMAT2 in a reserpine-like manner (Schuldiner et al. 1995). A similar mechanism applies to d-TC, which only caused radioactive outflow from cultures loaded with [3H]-NA, but not with [3H]-MPP+. Depletion of storage vesicles by weak organic cations might also explain the large increase in the plasma levels of catecholamines (both adrenaline and NA) upon intravenous injections of a potent inhibitor of OCT3, disprocynium 24, in rats (Eisenhofer et al. 1996).
However, cyanine 863 enhanced the radioactive outflow even in conditions where degradation of [3H]-NA was prevented by a blockade of both MAO and COMT. Hence, metabolic breakdown with the occurrence of readily diffusible products cannot be the sole reason why radioactive outflow of [3H]-NA in response to cyanine 863 increased, whereas it decreased when [3H]-MPP+ had been used as a tracer. Two additional divergent properties of the two substrates may account for this discrepancy: non-ionic transmembrane diffusion is considerably higher for NA than for MPP+, whereas the rate constant for the OCT3-mediated, outward-directed transport of NA is ten times lower (Russ et al. 1992). Accordingly, any inhibition of transporter-related efflux will show significantly better for MPP+ than for NA.
What is the biological significance of OCT3 in SCG neurones?
Though our results indicate the presence of OCT3 in sympathetic nerve cells, its biological role remains enigmatic. Our observations are based on an artificial situation using MPP+ as a tracer. MPP+ is a permanent cation (Russ et al. 1992), it is a substrate for the NAT (at higher affinity than NA; see Pifl et al. 1996) and the VMAT (at lower affinity than NA; see Erickson et al. 1996) and it is metabolically stable (Sayre, 1989). Hence, we may expect fairly high concentrations of free cytoplasmic MPP+. Furthermore, MPP+ serves as an excellent substrate for OCT3 (Russ et al. 1992; Gründemann et al. 1998b, 1999; Wu et al. 1998a, 2000b; Rajan et al. 2000). These qualities taken together make it an ideal candidate to probe OCT3-mediated outward-directed transport. However, the carrier is driven by an electrochemical gradient (Trendelenburg, 1988; Schoemig et al. 1992; Kekuda et al. 1998; Wu et al. 1998a), suggesting the inward direction as the more physiological route for the transport of cations. Yet uptake of MPP+ by the OCT is small compared to the NAT in our culture system and, despite considerable efforts to localize the transporter, it has not been detected in axonal endings of the postganglionic sympathetic nervous system (Trendelenburg, 1988). These observations suggest that OCT3 occurs in SCG neurones either at low expression levels or in a functional configuration that differs from non-neuronal sites. Unfortunately, efforts to generate OCT3 antibodies that might help to clarify this question have been unsuccessful so far (Zwart et al. 2001).
Attempts to assess the biological significance of OCT3 have been made either by injecting inhibitors of OCT3 (such as disprocynium 24) into animals (Eisenhofer et al. 1996), or by studying mice with a functional deletion of the transporter (Zwart et al. 2001). Interestingly, acute infusions of the potent OCT3/uptake 2 antagonist, disprocynium 24, markedly affected plasma levels of catecholamines and their metabolites in rats, but had little effect on the behaviour of the animals (Eisenhofer et al. 1996). Likewise, OCT3 knockout mice are viable and fertile with no obvious physiological defect (Zwart et al. 2001), indicating that OCT3 is either not crucial for a normal function of the nervous system, or that other transporter systems compensate for the lack of OCT3. By attempting to generate OCT3-specific antibodies, and by applying our experimental protocols to the knockout model, we hope to gain insight into the biological meaning of OCT-mediated cation transport in nerve cells.
Acknowledgments
We greatly appreciate expert assistance by Gabriele Koth, Andrea Motejlek, Harald Reither and Karin Schwarz. This work was supported by the Austrian Science Foundation, Project 15084 to S. H.
References
- Arndt P, Volk C, Gorboulev V, Budiman T, Popp C, Ulzheimer-Teuber I, Akhoundova A, KoppatZ S, Bamberg E, Nagel G, Koepsell H. Interaction of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. American Journal of Physiology. 2001;281:F454–468. doi: 10.1152/ajprenal.2001.281.3.F454. [DOI] [PubMed] [Google Scholar]
- Boehm S, Huck S. α2-Adrenoreceptor-mediated inhibition of acetylcholine-induced noradrenaline release from rat sympathetic neurons: an action at voltage-gated Ca2+ channels. Neuroscience. 1995;69:221–231. doi: 10.1016/0306-4522(95)00235-b. [DOI] [PubMed] [Google Scholar]
- Boenisch H, Leiden L. Catecholamine reuptake and storage. Advances in Pharmacology. 1998;42:149–164. [PubMed] [Google Scholar]
- Boenisch H, Trendelenburg U. The mechanism of action of indirectly acting sympathomimetic agents. In: Trendelenburg U, Weiners N, editors. Handbook of Experimental Pharmacology. New York, Berlin: Springer Verlag; 1988. pp. 247–277. [Google Scholar]
- Budiman T, Bamberg E, Koepsell H, Nagel G. Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. Journal of Biological Chemistry. 2001;275:29413–29420. doi: 10.1074/jbc.M004645200. [DOI] [PubMed] [Google Scholar]
- Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, Arndt P, Ulzheimer JC, Sonders MS, Baumann C, Waldegger S, Lang F, Koepsell H. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Molecular Pharmacology. 1998;54:342–352. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. Journal of Biological Chemistry. 1996;51:32 599–32 604. doi: 10.1074/jbc.271.51.32599. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, McCarty R, Pacak K, Russ H, Schoemig E. Disprocynium24, a novel inhibitor of the extraneuronal monoamine transporter, has potent effects on the inactivation of circulating noradrenaline and adrenaline in conscious rat. Naunyn-Schmiedeberg's Archives of Pharmacology. 1996;354:287–294. doi: 10.1007/BF00171059. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Schaefer MKH, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proceedings of the National Academy of Sciences of the USA. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig ER, Trendelenburg U. The neuronal and extraneuronal uptake and metabolism of 3H-(-)noradrenaline in the perfused rat heart. Naunyn-Schmiedeberg's Archives of Pharmacology. 1978;303:21–35. doi: 10.1007/BF00496182. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA and Cell Biology. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- Graefe K-H, Boenisch H. The transport of amines across the axonal membranes of noradrenergic and dopaminergic neurones. In: Trendelenburg U, Weiners N, editors. Handbook of Experimental Pharmacology. New York, Berlin: Springer Verlag; 1988. pp. 193–245. [Google Scholar]
- Gründemann D, KÖSTER S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F, Obermüller N, Schoemig E. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. Journal of Biological Chemistry. 1998a;273:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- Gründemann D, Liebich G, Kiefer N, KÖSTER S, Schoemig E. Selective substrates for non-neuronal monoamine transporters. Molecular Pharmacology. 1999;56:1–10. doi: 10.1124/mol.56.1.1. [DOI] [PubMed] [Google Scholar]
- Gründemann D, Schechinger B, Rappold GA, Schoemig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nature Neuroscience. 1998b;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Henry J-P, Sagne C, Bedet C, Gasnier B. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochemistry International. 1998;32:227–246. doi: 10.1016/s0197-0186(97)00092-2. [DOI] [PubMed] [Google Scholar]
- Iversen LL. The uptake of catechol amines at high perfusion concentrations in the rat isolated heart: a novel catechol amine uptake process. British Journal of Pharmacology. 1965;25:18–33. doi: 10.1111/j.1476-5381.1965.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Wu X, Wang H, Fei Y-J, Leibach FH, Ganapathy V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. Journal of Biological Chemistry. 1998;273:15 971–15 979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- Koepsell H. Organic cation transporters in intestine, kidney, liver, and brain. Annual Review of Physiology. 1998;60:243–266. doi: 10.1146/annurev.physiol.60.1.243. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Gorboulev V, Arndt P. Molecular pharmacology of organic cation transporters in kidney. Journal of Membrane Biology. 1999;167:103–117. doi: 10.1007/s002329900475. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. Journal of Biological Chemistry. 1999;274:13 675–13 680. doi: 10.1074/jbc.274.19.13675. [DOI] [PubMed] [Google Scholar]
- Lee CM, Javitch JA, Snyder SH. Characterization of [3H]desipramine binding associated with neuronal norepinephrine uptake sites in rat brain membranes. Journal of Neuroscience. 1982;2:1515–1525. doi: 10.1523/JNEUROSCI.02-10-01515.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg G, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–552. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Marger MD, Saier MH. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends in Biochemical Sciences. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Nagel G, Volk C, Friedrich T, Ulzheimer JC, Bamberg E, Koepsell H. A reevaluation of substrate specificity of the rat cation transporter rOTC1. Journal of Biological Chemistry. 1997;272:31 953–31 956. doi: 10.1074/jbc.272.51.31953. [DOI] [PubMed] [Google Scholar]
- Nissinen E, Linden I-B, SchultZ E, Kaakkola S, MÄNNISTÖ PT, Pohto P. Inhibition of catechol-O-methyltransferase activity by two novel disubstituted catechols in the rat. European Journal of Pharmacology. 1988;153:263–269. doi: 10.1016/0014-2999(88)90614-0. [DOI] [PubMed] [Google Scholar]
- Noe B, Hagenbuch B, Stieger B, Meier PJ. Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proceedings of the National Academy of Sciences of the USA. 1997;94:10 346–10 350. doi: 10.1073/pnas.94.19.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi R, Tamai I, Nezu J-I, Nikaido H, Hashimoto N, Oku A, Sai Y, Shimane M, Tsuji A. Molecular and physiological evidence for multifunctionality of carnitine/organic cation transporter OCTN2. Molecular Pharmacology. 2001;59:358–366. doi: 10.1124/mol.59.2.358. [DOI] [PubMed] [Google Scholar]
- Pifl C, HornykiewicZ O, Giros B, Caron MG. Catecholamine transporters and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity: studies comparing the cloned human noradrenaline and human dopamine transporter. Journal of Pharmacology and Experimental Therapeutics. 1996;277:1437–1443. [PubMed] [Google Scholar]
- Pifl C, Singer EA. Ion dependence of carrier-mediated release in dopamine or norepinephrine transporter-transfected cells questions the hypothesis of facilitated exchange diffusion. Molecular Pharmacology. 1999;56:1047–1054. doi: 10.1124/mol.56.5.1047. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. Effect of desmethylimipramine on the release of 3H-norpinephrine induced by various agents in hypothalamic synaptosomes. Molecular Pharmacology. 1977;13:746–758. [PubMed] [Google Scholar]
- Rajan PD, Kekuda R, Chancy CD, Huang W, Ganapathy V, Smith SB. Expression of the extraneuronal monoamine transporter in RPE and neural retina. Current Eye Research. 2000;20:195–204. [PubMed] [Google Scholar]
- Russ H, Gliese M, Sonna J, Schoemig E. The extraneuronal transport mechanism for noradrenaline (uptake2) avidly transports 1-methyl-4-phenylpyridinium (MPP+) Naunyn-Schmiedeberg's Archives of Pharmacology. 1992;346:158–165. doi: 10.1007/BF00165297. [DOI] [PubMed] [Google Scholar]
- Russ H, Sonna J, Keppler K, Baunach S, Schoemig E. Cyanine-related compounds: a novel class of potent inhibitors of extraneuronal noradrenaline transport. Naunyn-Schmiedeberg's Archives of Pharmacology. 1993;348:458–465. doi: 10.1007/BF00173203. [DOI] [PubMed] [Google Scholar]
- Russ H, Staudt K, Martel F, Gliese M, Schoemig E. The extraneuronal transporter for monamine transmitters exists in cells derived from human central nervous system glia. European Journal of Neuroscience. 1996;8:1256–1264. doi: 10.1111/j.1460-9568.1996.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Sayre LM. Biochemical mechanism of action of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicology Letters. 1989;48:121–149. doi: 10.1016/0378-4274(89)90168-9. [DOI] [PubMed] [Google Scholar]
- Schoemig E, Babin-Ebell J, Russ H, Trendelenburg U. The force driving the extraneuronal transport mechanism for catecholamines (uptake 2) Naunyn-Schmiedeberg's Archives of Pharmacology. 1992;345:437–443. doi: 10.1007/BF00176622. [DOI] [PubMed] [Google Scholar]
- Schoemig E, Schönfeld C-L. Extraneuronal noradrenaline transport (uptake2) in a human cell line (Caki-1 cells) Naunyn-Schmiedeberg's Archives of Pharmacology. 1990;341:404–410. doi: 10.1007/BF00176331. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. Journal of Comparative Neurology. 2000;420:211–232. [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiological Reviews. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pflügers Archiv. 2000;440:337–350. doi: 10.1007/s004240000297. [DOI] [PubMed] [Google Scholar]
- Seth P, Wu X, Huang W, Leibach FH, Ganapathy V. Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. Journal of Biological Chemistry. 1999;274:33 388–33 392. doi: 10.1074/jbc.274.47.33388. [DOI] [PubMed] [Google Scholar]
- Streich S, Brüss M, Boenisch H. Expression of the extraneuronal monoamine transporter (uptake2) in human glioma cells. Naunyn-Schmiedeberg's Archives of Pharmacology. 1996;353:328–333. doi: 10.1007/BF00168636. [DOI] [PubMed] [Google Scholar]
- Stute N, Trendelenburg U. The outward transport of axoplasmic noradrenaline induced by a rise of the sodium concentration in the adrenergic nerve endings of the rat vas deferens. Naunyn-Schmiedeberg's Archives of Pharmacology. 1984;327:124–132. doi: 10.1007/BF00500906. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J-I, Sai Y, Kobayashi D, Oku A, Shimane M, Tsuji A. Molecular and functional characterization of organic cation/carnitine transporter family in mice. Journal of Biological Chemistry. 2000;275:40064–40072. doi: 10.1074/jbc.M005340200. [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J-I, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. Journal of Biological Chemistry. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U. The extraneuronal uptake and metabolism of catecholamines. In: Trendelenburg U, Weiners N, editors. Handbook of Experimental Pharmacology. New York, Berlin: Springer Verlag; 1988. pp. 279–319. [Google Scholar]
- Ungell A-L, Boenisch H, Graefe K-H. Choline+: a substrate of the neuronal noradrenaline carrier in the rat vas deferens. Naunyn-Schmiedeberg's Archives of Pharmacology. 1986;334:223–227. doi: 10.1007/BF00508775. [DOI] [PubMed] [Google Scholar]
- Van Montfoort JE, MÜLLER M, Groothuis GMM, Meijer DKF, Koepsell H, Meier PJ. Comparison of “type I” and “type II” organic cation transport by organic cation transporters and organic anion transporting polypeptides. Journal of Pharmacology and Experimental Therapeutics. 2001;298:110–115. [PubMed] [Google Scholar]
- Wagner CA, LÜKEWILLE U, Kaltenbach S, Moschen I, Bröer A, Risler T, Bröer S, Lang F. Functional and pharmacological characterization of human Na+-carnitine cotransporter hOCTN2. American Journal of Physiology. 2000;279:F584–591. doi: 10.1152/ajprenal.2000.279.3.F584. [DOI] [PubMed] [Google Scholar]
- Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochimica et Biophysica Acta. 2000a;1466:315–327. doi: 10.1016/s0005-2736(00)00189-9. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, Leibach FH, Ganapathy V. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. American Journal of Physiology. 2000b;279:F449–458. doi: 10.1152/ajprenal.2000.279.3.F449. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. Journal of Pharmacology and Experimental Therapeutics. 1999;290:1482–1492. [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei Y-J, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. Journal of Biological Chemistry. 1998a;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- Wu X, Prasad PD, Leibach FH, Ganapathy V. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochemical and Biophysical Research Communications. 1998b;246:589–595. doi: 10.1006/bbrc.1998.8669. [DOI] [PubMed] [Google Scholar]
- Yabuuchi H, Tamai I, Nezu J-I, Sakamoto K, Oku A, Shimane M, Sai Y, Tsuji A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. Journal of Pharmacology and Experimental Therapeutics. 1999;289:768–773. [PubMed] [Google Scholar]
- Youdim MBH, Finberg JPM, Tipton KF. Monoamine oxidase. In: Trendelenburg U, Weiners N, editors. Handbook of Experimental Pharmacology. New York, Berlin: Springer Verlag; 1988. pp. 119–192. [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Molecular and Cellular Biology. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


