Abstract
We have shown previously that thromboxane A2 (TXA2), which may be released by the anti-tumour drug irinotecan and by platelet-activating factor (PAF), causes Cl− secretion in the isolated rat colon. In the present study, the involvement of TXA2 in nitric oxide-induced Cl− secretion in isolated rat colon was investigated. In colonic mucosa set between Ussing chambers, the NO donor sodium nitroprusside (SNP; 100 μM) caused Cl− secretion, an effect that was almost completely inhibited by the NO scavenger carboxy-PTIO at 200 μM. The SNP-induced Cl− secretion was inhibited in a concentration-dependent manner by the TXA2 receptor antagonist ONO-3708 (IC50 = 2 μM) and the TX synthase inhibitor Y-20811 (IC50 = 0.4 μM). SNP significantly increased the release of TXA2 (measured as TXB2 release) from the mucosa. The SNP-induced increases in Cl− secretion and TXA2 release were blocked by a NO-sensitive guanylate cyclase inhibitor (ODQ). Dibutyryl cGMP (500 μM) also induced Cl− secretion, which was sensitive to ONO-3708 (10 μM) and Y-20811 (1 μM), and increased the release of TXA2 from the mucosa. PAF-induced (10 μM) Cl− secretion was inhibited by carboxy-PTIO (200 μM) and ODQ (10 μM), whereas irinotecan-induced (500 μM) Cl− secretion was not significantly inhibited by these drugs. A stable TXA2 analogue (STA2) but not SNP (100 μM) changed the membrane potential of epithelial cells in isolated colonic crypts under the whole-cell current-clamp condition. These results indicate that PAF elicits the NO-cGMP pathway and then stimulates the release of TXA2, which is a stimulant of colonic Cl− secretion. In contrast, the NO-cGMP pathway is not involved in the TXA2-mediated Cl− secretion induced by irinotecan.
Thromboxane A2 (TXA2) is a well-known potent inducer of platelet aggregation and vasoconstriction (Coleman et al. 1994). We have found recently that in isolated rat colon, the epithelial Cl− secretion induced by the anti-tumour drug irinotecan and by platelet-activating factor (PAF) is mediated by the release of TXA2 from the subepithelial layer of the mucosa (Sakai et al. 1997; Suzuki et al. 2000b). Eicosanoids including TXA2, in the colonic mucosa are mostly produced in this subepithelial layer (Craven & DeRubertis, 1983). However, it is not clear how irinotecan and PAF stimulate the production and release of TXA2 from the subepithelium. We have found that endogenous TXA2 and exogenous application of the stable TXA2 analogue 9,11-epithio-11,12-methano-thromboxane A2 (STA2) act directly on epithelial crypt cells, resulting in the stimulation of Cl− secretion in the colon (Sakai et al. 1997; Ikari et al. 1999; Suzuki et al. 2000a). However, expression of the TXA2 receptor in this cell type has not previously been reported.
Sodium nitroprusside (SNP), a nitric oxide (NO) donor, increases Cl− secretion in the rat (Tamai & Gaginella, 1993; Wilson et al. 1993; Stoner et al. 2000), guinea-pig (MacNaughton, 1993) and human colon (Stack et al. 1996). This NO-induced Cl− secretion is mediated by the production of cyclo-oxygenase metabolites (MacNaughton, 1993; Wilson et al. 1993; Stack et al. 1996). Cyclo-oxygenase produces a number of biologically active eicosanoids such as prostaglandin (PG) E2, PGD2, PGF2α, PGI2 and TXA2, but it has not been specified which eicosanoid contributes to the NO-induced Cl− secretion in the colon. Moreover, the endogenous activator of NO production and release in the colon remains unclear.
In the present study, we used isolated rat colonic mucosa and colonic crypts to examine the involvement of NO in TXA2-, PAF- and irinotecan-induced Cl− secretion, and demonstrate for the first time that the NO-cGMP-TXA2 pathway mediates PAF-induced Cl− secretion. Some of these results have been presented to The Physiological Society (Sakai et al. 2002).
Methods
Chemicals
Irinotecan (7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxycamptothecin; Daiichi Pharmaceutical, Tokyo, Japan and Yakult Honsha, Tokyo, Japan), STA2 (ONO Pharmaceutical, Osaka, Japan), PGE2 (Toray Industries, Tokyo, Japan), 7-[2α,4α-(dimethylmethano)-6β-(2-cyclopentyl-2β-hydroxyacetamido)-1α-cyclohexyl]5(Z)-heptenoic acid (ONO-3708; ONO Pharmaceutical Co.) and sodium 4-[α-hydroxy-5- (1-imidazolyl)- 2-methybenzyl]-3,5-dimethylbenzoate dehydrate (Y-20811; Yoshitomi Pharmaceutical Industries, Fukuoka, Japan) were generous gifts of their respective manufacturers. Sodium nitroprusside dehydrate (SNP), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, sodium salt (carboxy-PTIO) and furosemide (frusemide) were obtained from Wako Pure Chemical Industries (Osaka, Japan). 5-Nitro-2-(3-phenylpropylamino)-benzoate (NPPB) was from Research Biochemicals International (Natick, MA, USA). N2,O2'-Dibutyryl guanosine 3′,5′-cyclic monophosphate sodium salt (DBcGMP) was from Yamasa (Choushi, Japan). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxaline-1-one (ODQ) was from Sigma (St Louis, MO, USA). PAF (1-alkyl-2-acetoyl-sn-glycero-3-phosphocholine) was from Avanti Polar Lipids (Alabaster, AL, USA). Irinotecan, NPPB and ODQ were dissolved in dimethyl sulphoxide, and PGE2, furosemide and PAF were dissolved in ethanol. The concentrations of dimethyl sulphoxide and ethanol in the final aqueous solutions never exceeded 0.5 %.
Solutions
The Parsons solution for tissue preparation and Ussing chamber experiments contained (mm): 107 NaCl, 4.5 KCl, 25 NaHCO3, 1.8 Na2HPO4, 0.2 NaH2PO4, 1.25 CaCl2, 1 MgSO4 and 12 glucose. The solution was gassed with carbogen (5 % CO2 in 95 % O2) at a pH of 7.4. Where indicated, a low-chloride solution (7 mm Cl−) was used in which NaCl was replaced by sodium gluconate and supplemented with 4.5 mm CaSO4 in order to compensate for the calcium-buffering properties of gluconate. The calcium-free EDTA solution for the isolation of crypts from distal colon contained (mm): 127 NaCl, 5 KCl, 1 MgCl2, 5 EDTA, 10 Hepes, 5 glucose, 5 sodium pyruvate and 10 mg ml−1 bovine serum albumin (BSA). The pH of the solution was adjusted to 7.4 with NaOH. The high-potassium Tyrode solution for the storage of the crypts contained (mm): 100 potassium gluconate, 30 KCl, 20 NaCl, 1.25 CaCl2, 1 MgCl2, 10 Hepes, 12 glucose, 5 sodium pyruvate and 1 mg ml−1 BSA. The pH was adjusted to 7.4 with KOH. Whole-cell patch-clamp experiments were carried out with the following solutions. The extracellular bathing solution contained (mm): 140 NaCl, 5.4 KCl, 1.25 CaCl2, 1 MgCl2 and 10 Hepes; the pH was adjusted to 7.4 with NaOH. The intracellular pipette solution contained (mm): 100 potassium gluconate, 30 KCl, 10 NaCl, 2 MgCl2, 0.1 EGTA, 10 Hepes and 2 ATP; the pH was adjusted to 7.2 with KOH.
Tissue preparation
The following procedures were performed in accordance with the guidelines presented by the Animal Care and Use Committee of Toyama Medical and Pharmaceutical University. The mucosa-submucosa preparation (hereafter, simply described as the mucosa) was obtained from female Wistar rats (Japan SLC, Shizuoka, Japan) weighing 140–200 g. The animals had free access to water and food until the day of the experiment. Animals were killed rapidly by stunning and cervical dislocation. The serosa and muscularis propria were stripped away by hand to obtain the mucosa preparation of the distal part of the colon descendens.
Ussing chamber experiments
The tissue was set between modified Ussing chambers and bathed with 4 ml of Parsons solution incubated at 37 °C on either side of the mucosa. The exposed surface of the tissue measured 0.3 cm2. Short-circuit current (Isc) was measured continuously at zero voltage difference with an amplifier (CEZ-9100, Nihon Kohden, Tokyo, Japan). The fluid resistance was compensated. The direction of Isc from the mucosal-to-serosal side is expressed as positive: that is, an increase in Cl− movement from the serosal-to-mucosal side (Cl− secretion) corresponded to an increase in Isc. The transepithelial potential difference (Pd) under open-circuit conditions was measured in the current-clamp mode of the amplifier, and the reference was taken on the serosal side. Tissue conductance (Gt) was determined from the deviation of Isc in response to a command voltage pulse of 0.5 mV (its duration was 100 ms).
Assay of TXB2
The mucosa was set between the Ussing chambers. Since TXA2 quickly transforms into TXB2 in aqueous solutions, we measured the concentration of TXB2 in the bathing solution using an enzyme immunoassay kit (Amersham Pharmacia Biotech, Buckinghamshire, UK). After several changes of bathing solutions, the mucosa was incubated for 35 min and the control solution was collected from the serosal or mucosal side. Fresh solutions were introduced successively and incubated for 15 min. Then, SNP or DBcGMP at the required concentration was added to the serosal solution, followed by a 20 min incubation period, and the test solution was collected. To assess the effects of inhibitor on the SNP-induced release of TXB2, the resulting solutions after the 35 min incubation period in the presence of SNP plus the inhibitor were collected. Control and test solutions (1 ml of each) were immediately frozen, freeze-dried and dissolved with 0.25 ml of the assay kit buffer to determine the concentration of TXB2. Data are expressed as the amount of TXB2 in the serosal solution (4 ml) of the chamber.
Preparation of isolated colonic crypts
Crypts were isolated from the distal colon as described previously (Ecke et al. 1996). Briefly, the distal colon was resected and turned inside-out. The inverted sac was filled with 3–5 ml of the calcium-free EDTA solution, and incubated in the calcium-free solution for 10 min at 35 °C. Crypts isolated during this incubation were collected and resuspended in high-potassium Tyrode solution.
Patch-clamp recordings
The crypts were fixed to a glass poly-l-lysine-coated coverslip, which was mounted in a 680 μl chamber. The chamber solution was continuously exchanged throughout an experiment at a perfusion rate of 800 μl min−1. Whole-cell current-clamp experiments of the crypt cells were performed using an EPC-9 patch-clamp system (HEKA Elektronik, Lambrecht, Germany), as described previously (Sakai & Takeguchi, 1993; Sakai et al. 1997). The liquid junction potential between the pipette and the bathing solutions was corrected. The reference for describing the patch-potential was taken on the extracellular side of the membrane. Experiments were performed at 35–37 °C.
RNA isolation and RT-PCR
Total RNA was prepared from isolated colonic crypts by the acidic phenol method (Chomczynski & Sacchi, 1987). For RT-PCR, 2 μg of the RNA was random primed (Pd(N)6; 200 ng) in a final volume of 20 μl in the presence of 200 units of Superscript II reverse transcriptase (Gibco BRL, Life Technologies, Rockville, MD, USA). The RT reaction was carried out as described previously (Sakai et al. 1999, 2001). One-twentieth of the RT sample was incubated with Taq DNA polymerase (Promega) and 15 pmol of specific sense and antisense primers based on the rat TXA2 receptor sequence. The nucleotide positions of sense and antisense primers were 301–320 (5-TGCCGCCTTTGCCACTTCAT-3′) and 758–778 (5′-CCAGCAAGGGCATCCAA-CACA-3′), respectively. Forty cycles of PCR (95 °C for 30 s, 58 °C for 30 s, 68 °C for 60 s) were performed. One-tenth of the PCR product was loaded onto a 2 % agarose gel and electrophoresed.
Statistics
Results are presented as the mean ± s.e.m. Differences between groups were analysed by one-way analysis of variance (ANOVA), and correction for multiple comparisons was made using Dunnett's multiple comparison test. If necessary, Tukey's multiple comparison test was used. Comparison between the two groups was made by using Student's t test. Statistically significant differences were assumed at P < 0.05.
Results
NO-elicited Cl− secretion in isolated rat colon
SNP increased Isc across the rat colonic mucosa in a concentration-dependent manner (EC50 = 20 μM) with a plateau phase that was stable for at least 30 min (Fig. 1A and B). If the mucosa was pre-treated with the NO scavenger carboxy-PTIO (200 μM at the serosal side), the effect of SNP (100 μM) was almost completely abolished (Fig. 1C and D), indicating that the SNP-induced increase in Isc is mediated by the release of NO. Carboxy-PTIO (200 μM) had no effect on the baseline Isc (in the absence of SNP): the values before and after the addition of carboxy-PTIO were 34.2 ± 3.1 and 32.4 ± 3.7 μA cm−2, respectively (n = 6, P > 0.05). The SNP-induced increase in Isc was completely inhibited by furosemide (100 μM at the serosal side; Fig. 1E), an inhibitor of the basolateral Na+-K+-2Cl− cotransporter (Chipperfield, 1986), and also by 100 μM NPPB (at the mucosal side; Fig. 1F), a blocker of the apical Cl− channel of rat colon (Diener & Rummel, 1989; Sakai et al. 1995, 1997). In the low-chloride solution containing 7 mm Cl−, SNP (100 μM) had almost no effect on Isc (Fig. 1G).
Figure 1. Sodium nitroprusside (SNP)-induced Cl− current in isolated rat colonic mucosa.

A, 100 μM SNP was added to the serosal side. A typical trace of the SNP-induced current (short-circuit current, Isc) is shown. B, concentration-dependent effects of SNP. The Isc values at the SNP-elicited plateau phase were read and the results are expressed as the net increases from the control Isc measured immediately before the addition of SNP (ΔIsc). n = 6-10. C, 200 μM carboxy-PTIO was added to the serosal side 10 min before the addition of SNP (100 μM). After 20 min, the solutions at both the serosal and mucosal sides were replaced three times with fresh Parsons solutions. Then 100 μM SNP was added. A typical trace of Isc is shown. D, the experimental protocol was the same as that in C. The net increases in Isc 15 min after the addition of SNP in the presence (left column) or absence (right column) of carboxy-PTIO were recorded. n = 5. ** P < 0.01. E, 100 μM furosemide was added to the serosal side after the SNP (100 μM)-elicited plateau phase was observed. A typical trace of Isc from five experiments is shown. F, 100 μM NPPB was added to the mucosal side after the SNP (100 μM)-elicited plateau phase was observed. A typical trace of Isc from five experiments is shown. G, low-chloride solutions were used at both the serosal and mucosal sides. 100 μM SNP was added to the serosal side. A typical trace of Isc from four experiments is shown.
NO-elicited Cl− secretion is mediated by the cGMP pathway
The SNP-induced increase in Isc was abolished by 10 μM ODQ (at the serosal side), an inhibitor of soluble guanylate cyclase (Fig. 2A and B). ODQ (10 μM) did not affect the baseline Isc (in the absence of SNP): the values before and after the addition of ODQ were 28.7 ± 1.2 and 27.0 ± 1.1 μA cm−2, respectively (n = 5, P > 0.05). DBcGMP (500 μM at the serosal side) increased Isc, an effect that was inhibited by furosemide (100 μM at the serosal side; Fig. 2C) and NPPB (100 μM at the mucosal side; Fig. 2D), indicating that SNP-elicited Cl− secretion is mediated by the cGMP pathway. In the low-chloride solution containing 7 mm Cl−, DBcGMP (500 μM) had almost no effect on Isc (Fig. 2E).
Figure 2. DBcGMP-induced Cl− current in the colonic mucosa.
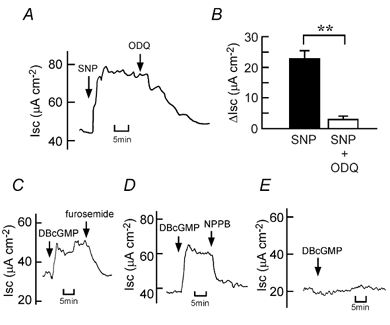
A, 10 μM ODQ was added to the serosal side after the SNP (100 μM)-elicited plateau phase was observed. A typical trace of Isc is shown. B, 100 μM SNP-induced net increases in Isc were recorded just before addition of 10 μM ODQ (left column). When the effect of ODQ reached a steady state, the Isc values were read, and they are expressed as ΔIsc (right column). n = 8. ** P < 0.01. C, 100 μM furosemide was added to the serosal side after the DBcGMP (500 μM)-elicited plateau phase was observed. A typical trace of Isc from four experiments is shown. D, 100 μM NPPB was added to the mucosal side after the DBcGMP (500 μM)-elicited plateau phase was observed. A typical trace of Isc from four experiments is shown. E, low-chloride solutions were employed at both the serosal and mucosal sides. DBcGMP (500 μM) was added to the serosal side. A typical trace of Isc from four experiments is shown.
NO- and cGMP-elicited Cl− secretion is mediated by the production of TXA2
Here, we used a specific TXA2 receptor antagonist, ONO-3708 (Suga et al. 1987), and a specific TX synthase inhibitor, Y-20811 (Mikashima et al. 1986). Previous reports showed that ONO-3708 (10 μM) did not antagonize various prostanoid receptors for PGE2, PGF2α and PGI2, and did not inhibit cyclo-oxygenase (Kondo et al. 1989), and that Y-20811 (100 μM) had no effect on cyclo-oxygenase and PGI2 synthase (Mikashima et al. 1986). In addition, we have confirmed previously that ONO-3708 (10 μM) and Y-20811 (1 μM) did not affect baseline Isc (in the absence of secretagogues) in the rat colon (Sakai et al. 1997).
The SNP (100 μM)-elicited Cl− current was inhibited in a concentration-dependent manner by ONO-3708 (Fig. 3A and B) and Y-20811 (Fig. 3C and D). The IC50 values for ONO-3708 and Y-20811 were 2 and 0.4 μM, respectively. SNP-elicited increases in Pd and Gt were also significantly inhibited by ONO-3708 (10 μM; Fig. 3E and F) and Y-20811 (1 μM; Fig. 3G and H). Similar to the case for SNP (Fig. 3), the DBcGMP (500 μM)-induced increases in Isc, Pd and Gt were inhibited by ONO-3708 (10 μM; Fig. 4A, B, E and F) and Y-20811 (1 μM; Fig. 4C, D, G and H). ONO-3708 (0.3-10 μM) and Y-20811 (0.1-3 μM) did not significantly affect the PGE2 (0.5 μM)-induced increase in Isc (Fig. 5).
Figure 3. Inhibitory effects of a TXA2 receptor antagonist (ONO-3708) and a thromboxane synthase inhibitor (Y-20811) on the SNP-induced Cl− current.
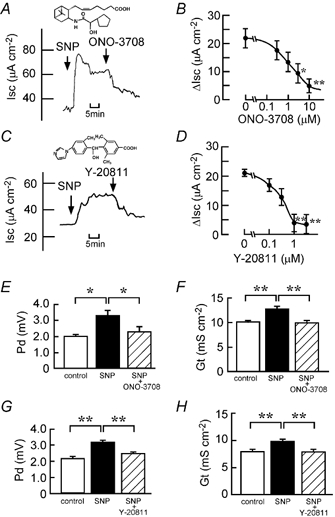
SNP (100 μM) was added to the serosal side. A and C, ONO-3708 (10 μM at the serosal side; A) or Y-20811 (1 μM at both the serosal and mucosal sides; C) was added after the SNP-elicited plateau phase was observed. Typical traces of Isc are shown. Insets, chemical structures of ONO-3708 (A) and Y-20811 (C). B and D, concentration-dependent inhibitory effects of ONO-3708 (B) and Y-20811 (D). ONO-3708 or Y-20811 was added cumulatively when the plateau phase of Isc was observed after the addition of SNP. SNP-induced net increases in Isc were recorded when the effect of the inhibitor reached a steady state. Smaller values of ΔIsc mean a greater degree of inhibition (n = 6 (B) and n = 5 (D)). *,** Significantly different from the value in the absence of the inhibitor (P < 0.05 and 0.01, respectively). E-H, the potential difference (Pd; E and G) and tissue conductance (Gt; F and H) values were measured at three different times: (1) just before the addition of SNP (open columns), (2) when values of Isc reached a steady state after the addition of SNP (filled columns) and (3) after the additional application of ONO-3708 (E and F) or Y-20811 (G and H; hatched columns). n = 5. *,** P < 0.05 and 0.01, respectively.
Figure 4. Inhibitory effects of ONO-3708 and Y-20811 on the DBcGMP-induced Cl− current.
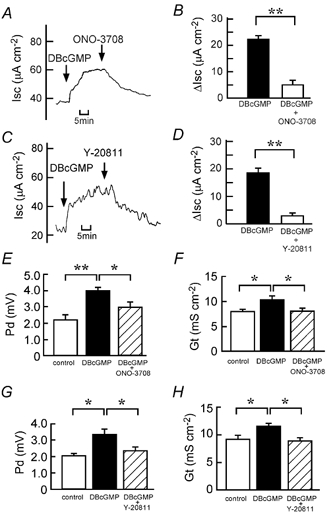
DBcGMP (500 μM) was added to the serosal side. A and C, ONO-3708 (10 μM at the serosal side; A) or Y-20811 (1 μM at both the serosal and mucosal sides; C) was added after the DBcGMP-elicited plateau phase was observed. Typical traces of Isc are shown. B and D, the 500 μM DBcGMP-induced net increases in Isc were recorded just before the addition of 10 μM ONO-3708 (B) or 1 μM Y-20811 (D; filled columns). When the effect of the inhibitor reached a steady state, the Isc values were read, and they are expressed as ΔIsc (open columns). n = 4. ** P < 0.01. E-H, the Pd (E and G) and Gt (F and H) values were measured at three different times: (1) just before the addition of DBcGMP (open columns), (2) when values of Isc reached a steady state after the addition of DBcGMP (filled columns) and (3) after the additional application of ONO-3708 (E and F) or Y-20811 (G and H; hatched columns). n = 4. *,** P < 0.05 and 0.01, respectively.
Figure 5. Effects of ONO-3708 and Y-20811 on the PGE2-elicited current.
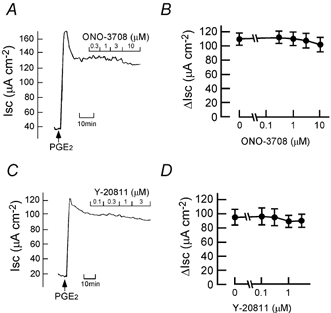
A and C, in the plateau phase observed after the addition of PGE2 (0.5 μM at the serosal side), 0.3-10 μM ONO-3708 (A) or 0.1-3 μM Y-20811 (C) was added cumulatively as indicated. Typical traces are shown. B and D, the experimental protocols were the same as those in A and C. Data are expressed as net increases from the Isc recorded just before the addition of PGE2 (ΔIsc). n = 4.
Stimulated release of TXA2 by the NO-cGMP pathway in the colon
In accordance with above results, SNP increased the release of TXA2 (measured as TXB2) in a concentration-dependent manner, from the distal colon into the bathing solution (Fig. 6A). The effects were significant at 10–100 μM, and this concentration range was effective for the SNP-induced increase in Isc (Fig. 1B). DBcGMP (500 μM) also stimulated the release of TXB2 from the colon (Fig. 6B). The SNP-increased release of TXB2 was significantly inhibited by carboxy-PTIO (200 μM) and ODQ (10 μM; Fig. 7).
Figure 6. SNP- and DBcGMP-induced release of TXB2 into the bathing solution from mucosa set between Ussing chambers.
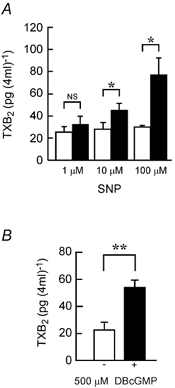
The mucosa was incubated for 35 min in the absence of SNP and DBcGMP, then the TXB2 concentration in the serosal solution was determined (open columns). Fresh Parsons solution was introduced and the mucosa was incubated successively for 15 min in the absence of SNP and DBcGMP, and for 20 min in the presence of 1–100 μM SNP (A) or 500 μM DBcGMP (B). Then the TXB2 concentration in the serosal solution was determined (filled columns). n = 4. *,** P < 0.05 and 0.01, respectively. NS, P > 0.05.
Figure 7. Inhibitory effects of the NO scavenger carboxy-PTIO and the soluble guanylate cyclase inhibitor (ODQ) on the SNP-induced release of TXB2.
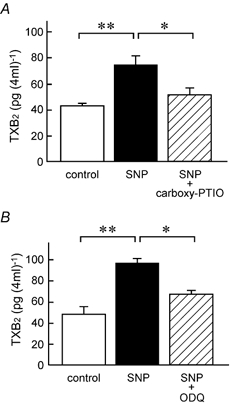
A, TXB2 concentrations in the serosal solution incubated without SNP and carboxy-PTIO (open columns), with 100 μM SNP (filled columns), and with 100 μM SNP plus 200 μM carboxy-PTIO (hatched column) were determined in each mucosa as described in Methods. B, TXB2 concentrations in the mucosal solution incubated without SNP and ODQ (open columns), with 100 μM SNP (filled columns), and with 100 μM SNP plus 10 μM ODQ (hatched column) were determined. All chemicals were added to the serosal side. n = 4 (A) and 3 (B). *,** P < 0.05 and 0.01, respectively.
Involvement of the NO-cGMP pathway in the TXA2-mediated Cl− secretion induced by PAF
We have found recently that PAF and irinotecan dose-dependently induce Cl− secretion, an action that is mediated mainly by the release of TXA2 in isolated rat colon (Sakai et al. 1997; Suzuki et al. 2000b). Figure 8 shows that increases in Isc caused by PAF and irinotecan depend upon the presence of Cl− ions in the solutions. Next, we examined whether the NO-cGMP pathway is involved in PAF- and irinotecan-induced Cl− secretion. Interestingly, the PAF-elicited increases in Isc, Gt and Pd were almost completely inhibited by carboxy-PTIO (Fig. 9A, B, E and F) and ODQ (Fig. 9C, D, G and H), indicating that PAF-induced Cl− secretion is mediated by the NO-cGMP-TXA2 pathway. In contrast, the irinotecan-elicited increases in Isc, Gt and Pd were not significantly inhibited by either carboxy-PTIO (Fig. 10A, B, E and F) or ODQ (Fig. 10C, D, G and H).
Figure 8. Platelet-activating factor (PAF)- and irinotecan-induced Cl− current in isolated rat colonic mucosa.
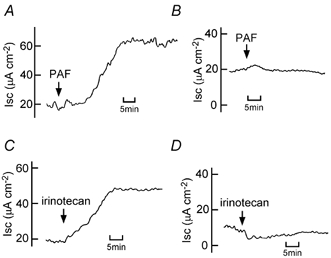
A and B, 10 μM PAF was added to the serosal side. Isc was recorded in the presence of Parsons solution containing 114 mm Cl− (A) or the low-chloride solution containing 7 mm Cl− (B). Typical examples from four experiments are shown. C and D, 500 μM irinotecan was added to both the serosal and mucosal sides. Isc was recorded in the presence of Parsons solution containing 114 mm Cl− (C) or the low-chloride solution containing 7 mm Cl− (D). Typical examples from four experiments are shown.
Figure 9. Inhibitory effects of a NO scavenger carboxy-PTIO and a soluble guanylate cyclase inhibitor (ODQ) on the PAF-induced Cl− current.
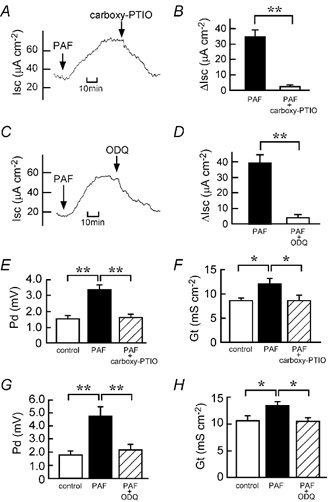
PAF (10 μM) was added to the serosal side. A and C, 200 μM carboxy-PTIO (A) or 10 μM ODQ (C) was added to the serosal side after the PAF-elicited plateau phase was observed. Typical traces of Isc are shown. B and D, the 10 μM PAF-induced net increases in Isc were recorded just before the addition of 200 μM carboxy-PTIO (B) or 10 μM ODQ (D) (filled columns). When the effect of the inhibitor reached a steady state, the Isc values were read, and they are expressed as ΔIsc (open columns). n = 5. ** P < 0.01. E-H, the Pd (E and G) and Gt (F and H) values were measured at three different times: (1) just before the addition of PAF (open columns), (2) when values of Isc had reached a steady state after the addition of PAF (filled columns) and (3) after the additional application of carboxy-PTIO (E and F) or ODQ (G and H) (hatched columns). n = 5. *,** P < 0.05 and 0.01, respectively.
Figure 10. Effects of carboxy-PTIO and ODQ on the irinotecan-induced Cl− current.
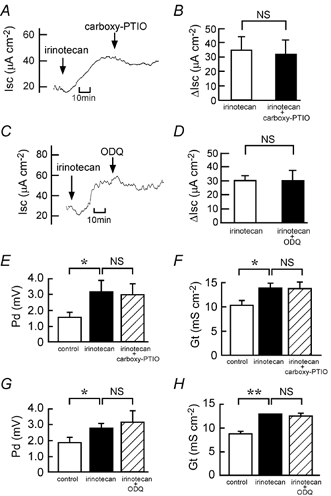
Irinotecan (500 μM) was added to both the serosal and mucosal sides. A and C, 200 μM carboxy-PTIO (A) or 10 μM ODQ (C) was added to the serosal side after the irinotecan-elicited plateau phase was observed. Typical traces of Isc are shown. B and D, the 500 μM irinotecan-induced net increases in Isc were recorded just before (open columns) and 30 min after (filled columns) the addition of 200 μM carboxy-PTIO (B) or 10 μM ODQ (D). n = 4. NS, P > 0.05. E-H, the Pd (E and G) and Gt (F and H) values were measured at three different times: (1) just before the addition of irinotecan (open columns), (2) when values of Isc had reached a steady state after the addition of irinotecan (filled columns) and (3) after the additional application of carboxy-PTIO (E and F) or ODQ (G and H; hatched columns). n = 4 (E and F) and 3 (G and H). *,** P < 0.05 and 0.01, respectively. NS, P > 0.05.
Effect of NO on the membrane potential of the cells in isolated colonic crypts
The Ussing chamber experiments indicated an indirect action of NO on epithelial Cl− secretion. In the whole-cell current-clamp mode, SNP (100 μM) had no effect on the membrane potential of cells in isolated crypts (Fig. 11A and C). In contrast, STA2 (0.1 μM), a stable analogue of TXA2, induced a significant depolarization of the cells (Fig. 11B and C).
Figure 11. Effects of SNP and STA2 on the membrane potential of single cells in isolated colonic crypts.
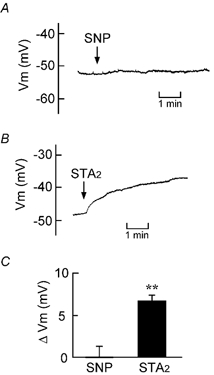
A and B, typical traces of the membrane potential (Vm) of the cells located at the middle of crypts. Vm was recorded at zero-current-clamp mode. The bathing solution containing 100 μM SNP (A) or 0.1 μM STA2 (B) was perfused from the indicated time. C, the Vm values were read just before and 5 min after the addition of SNP (left column) or STA2 (right column), and they are expressed as net changes from the control Vm (ΔVm) recorded immediately before the addition of SNP or STA2. ** Significantly different from zero (P < 0.01). n = 5 (left) and 3 (right).
Expression of TXA2 receptor mRNA in colonic crypt cells
In RT-PCR experiments, a set of specific primers that can amplify a partial cDNA fragment (478 bp) of the rat TXA2 receptor was used. Figure 12 shows that RT-PCR from total RNA of isolated rat colonic crypts gives a 478 bp product. Sequencing revealed that this product had 100 % homology with the rat TXA2 receptor (Kitanaka et al. 1995; data not shown).
Figure 12. Expression of TXA2 receptor mRNA in colonic crypt cells.
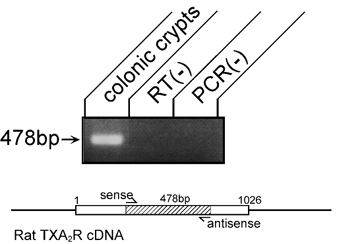
Gel analysis of the RT-PCR product from isolated colonic crypts. A single band of 478 bp was detected by ethidium bromide staining (colonic crypts). No band was detected in negative control experiments of RT-PCR without reverse transcriptase (RT(-)) or Taq DNA polymerase (PCR(-)). Inset, a scheme of this PCR experiment is shown. TXA2R, TXA2 receptor; sense, a sense primer; antisense, an antisense primer.
Discussion
Figure 13 summarizes the model for PAF- and irinotecan-induced Cl− secretion, the compounds used in this study and their proposed sites of action.
Figure 13. A scheme of the PAF- and irinotecan-induced Cl− secretion in rat colonic mucosa, and proposed sites of action of the compounds used in this study.
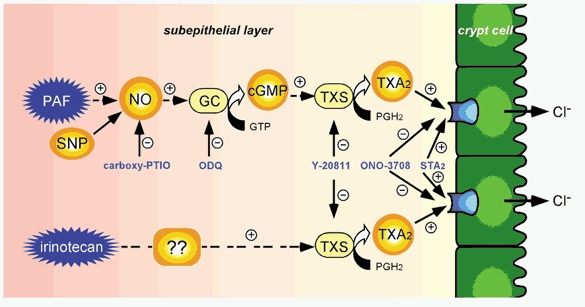
GC, soluble guanylate cyclase; TXS, thromboxane synthase; +, activation; -, inhibition.
NO-induced Cl− secretion in the colon is mediated by the production of cyclo-oxygenase metabolites (MacNaughton, 1993; Wilson et al. 1993; Stack et al. 1996). The Cl− secretion induced by cGMP is also mediated by the production of cyclo-oxygenase metabolites (Nobles et al. 1991). However, it has not been clarified which eicosanoid is involved in NO- and cGMP-induced Cl− secretion in the colon. Wilson et al. (1996) reported that SNP significantly increased the release of PGE2 without affecting the release of TXB2 in a solution containing diced pieces of rat colonic mucosa, but this diced mucosa experiment did not show whether the SNP-induced PGE2 release actually stimulated colonic Cl− secretion.
In the study presented here, we have shown from Ussing chamber experiments that SNP- and DBcGMP-elicited Cl− secretion was blocked by the TXA2 receptor antagonist, ONO-3708, and the TX synthase inhibitor, Y-20811 (Fig. 3 and Fig. 4), and that SNP and DBcGMP stimulated the release of TXB2 from the colonic mucosa (Fig. 6). A soluble guanylate cyclase inhibitor, ODQ, blocked the Cl− secretion (Fig. 2). The SNP-induced increases in Cl− secretion and TXB2 release were blocked by the NO scavenger, carboxy-PTIO (Fig. 1 and Fig. 7). Furthermore, STA2, a stable analogue of TXA2, stimulated Cl− secretion (Sakai et al. 1997; Suzuki et al. 2000a). NO-induced endogenous cGMP is not directly effective in epithelial colonic crypt cells, because STA2 but not SNP is able to activate the Cl− channels in the epithelial cells of isolated rat colonic crypts (Fig. 11). We therefore suggest that NO- and cGMP-induced Cl− secretion in the colon is mediated mainly by the production of TXA2.
We have recently reported that both PAF and irinotecan cause TXA2-mediated Cl− secretion in the rat colon (Sakai et al. 1997; Suzuki et al. 2000a,b). PAF and irinotecan stimulate the production of TXA2 in the subepithelium, and the released TXA2 acts on epithelial colonic crypt cells (Sakai et al. 1997; Suzuki et al. 2000a,b). The expression of TXA2 receptor mRNA in the colonic crypt cells was confirmed (Fig. 12). In the present study, we have demonstrated for the first time that PAF-induced Cl− secretion but not irinotecan-induced Cl− secretion is mediated by the production of NO (Fig. 9 and Fig. 10). NO may be released from macrophages, mast cells, phagocytes and/or neutrophils in the colonic mucosa (Wilson et al. 1993; Stack et al. 1996). To date, the mechanism for the NO-independent TXA2 release elicited by irinotecan remains unknown. Irinotecan is a DNA topoisomerase I inhibitor and has been used clinically as an anti-tumour drug. One of the major dose-limiting toxicities of irinotecan is severe diarrhoea, and the irinotecan-induced Cl− secretion in the colon may be one of the factors responsible for this symptom (Sakai et al. 1995, 1997; Suzuki et al. 2000a).
In our present study, SNP increased colonic Cl− secretion in a concentration-dependent manner (Fig. 1B). The results were obtained from in vitro experiments on rat distal colonic mucosa. Several other studies have also shown that NO stimulates colonic Cl− secretion (MacNaughton, 1993; Tamai & Ganginella, 1993; Wilson et al. 1993; Stack et al. 1996; Stoner et al. 2000). Contrary to these reports of NO-stimulated Cl− secretion, several research groups have reported that endogenous NO has a proabsorptive effect in the jejunum and ileum (Barry et al. 1994; Mailman, 1994; Maher et al. 1995; Schirgi-Degen & Beubler, 1995). For example, intravenous infusion of Nω-nitro-l-arginine methyl ester (l-NAME), a NO synthase inhibitor, into a ligated rat jejunal loop, reversed the net fluid absorption to produce net secretion. The PGE2-induced secretion was increased by treatment with l-NAME and blocked by SNP, although mucosal cGMP and cAMP levels after l-NAME treatment were not different from control values (Schirgi-Degen & Beubler, 1995). Furthermore, a murine model of colitis induced by trinitrobenzene sulphonic acid resulted in prolonged impairment of colonic epithelial secretion, and the decrease in Cl− secretion is suggested to be due to the inhibiting actions of NO produced by inducible NO synthase (MacNaughton et al. 1998; Asfaha et al. 2001). At present, it is difficult to explain these intestinal secretory and absorptive effects of NO. One possible explanation is that NO has dual actions, depending on the state of the colon (i.e. normal and colitis). Another possibility is that the effects of NO would be different in the jejunum, ileum and colon according to their different physiological functions.
In summary, in the present study we have found that in the rat colon, NO-induced Cl− secretion is mediated by an increase in the production of cGMP and subsequent stimulation of TXA2 release from subepithelial cells, and that this NO-cGMP-TXA2 pathway is stimulated by PAF but not by irinotecan. Further studies are necessary to clarify the pathophysiological roles of the Cl− secretion induced by the PAF-NO-cGMP-TXA2 pathway in the colon and to examine the signalling mechanisms involved in the irinotecan-stimulated release of TXA2.
Acknowledgments
We thank Dr Andrew F. James (University of Bristol, Bristol, UK) for his careful reading of this manuscript. This work was supported in part by Grants-in-Aid from Japan Society for the Promotion of Science (to H.S. and N.T.) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.S. and N.T.), and by grants from the Uehara Memorial Foundation, Takeda Science Foundation, Suzuken Memorial Foundation, The Research Foundation for Pharmaceutical Sciences and Tamura Foundation for Promotion of Science and Technology (to H.S.). T. Suzuki is a research fellow of Japan Society for the Promotion of Science.
References
- Asfaha S, MacNaughton WK, Appleyard CB, Chadee K, Wallace JL. Persistent epithelial dysfunction and bacterial translocation after resolution of intestinal inflammation. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2001;281:G635–644. doi: 10.1152/ajpgi.2001.281.3.G635. [DOI] [PubMed] [Google Scholar]
- Barry MK, Aloisi JD, Pickering SP, Yeo CJ. Nitric oxide modulates water and electrolyte transport in the ileum. Annals of Surgery. 1994;219:382–388. doi: 10.1097/00000658-199404000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield AR. The (Na+-K+-Cl−) co-transport system. Clinical Science. 1986;71:465–476. doi: 10.1042/cs0710465. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. Classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacological Reviews. 1994;46:205–229. [PubMed] [Google Scholar]
- Craven PA, Derubertis FR. Patterns of prostaglandin synthesis and degradation in isolated superficial and proliferative colonic epithelial cells compared to residual colon. Prostaglandins. 1983;26:583–604. doi: 10.1016/0090-6980(83)90196-x. [DOI] [PubMed] [Google Scholar]
- Diener M, Rummel W. Actions of the Cl− channel blocker NPPB on absorptive and secretory transport processes of Na+ and Cl− in rat descending colon. Acta Physiologica Scandinavica. 1989;137:215–222. doi: 10.1111/j.1748-1716.1989.tb08741.x. [DOI] [PubMed] [Google Scholar]
- Ecke D, Bleich M, Greger R. Crypt base cells show forskolin-induced Cl− secretion but no cation inward conductance. Pflügers Archiv. 1996;431:427–434. doi: 10.1007/BF02207282. [DOI] [PubMed] [Google Scholar]
- Ikari A, Sakai H, Sato T, Takeguchi N. Thromboxane A2 receptor linked with the Ca2+ pathway in rat colonic crypt cells. Biochemical and Biophysical Research Communications. 1999;258:708–712. doi: 10.1006/bbrc.1999.0674. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Hashimoto H, Sugimoto Y, Sawada M, Negishi M, Suzumura A, Marunouchi T, Ichikawa A, Baba A. cDNA cloning of a thromboxane A2 receptor from rat astrocytes. Biochimica et Biophysica Acta. 1995;1265:220–223. doi: 10.1016/0167-4889(94)00225-4. [DOI] [PubMed] [Google Scholar]
- Kondo K, Seo R, Naka M, Kitagawa T, Wakitani K, Sakata M, Kira H, Okegawa T, Kawasaki A. Effects of ONO-3708, an antagonist of the thromboxane A2/prostaglandin endoperoxide receptor, on platelet aggregation and thrombosis. European Journal of Pharmacology. 1989;163:253–261. doi: 10.1016/0014-2999(89)90194-5. [DOI] [PubMed] [Google Scholar]
- MacNaughton WK. Nitric oxide-donating compounds stimulate electrolyte transport in the guinea pig intestine in vitro. Life Sciences. 1993;53:585–593. doi: 10.1016/0024-3205(93)90716-g. [DOI] [PubMed] [Google Scholar]
- MacNaughton WK, Lowe SS, Cushing K. Role of nitric oxide in inflammation-induced suppression of secretion in a mouse model of acute colitis. American Journal of Physiology. 1998;275:G1353–1360. doi: 10.1152/ajpgi.1998.275.6.G1353. [DOI] [PubMed] [Google Scholar]
- Maher MM, Gontarek JD, JimeneZ RE, Cahill PA, Yeo CJ. Endogenous nitric oxide promotes ileal absorption. Journal of Surgical Research. 1995;58:687–692. doi: 10.1006/jsre.1995.1108. [DOI] [PubMed] [Google Scholar]
- Mailman D. Differential effects of lumenal l-arginine and NG-nitro-l-arginine on blood flow and water fluxes in rat ileum. British Journal of Pharmacology. 1994;112:304–310. doi: 10.1111/j.1476-5381.1994.tb13069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikashima H, Ochi H, Muramoto Y, Yasuda H, Tsuruta M, Maruyama Y. Effects of Y-20811, a long-lasting thromboxane synthetase inhibitor, on thromboxane production and platelet function. Thrombosis Research. 1986;43:455–468. doi: 10.1016/0049-3848(86)90090-3. [DOI] [PubMed] [Google Scholar]
- Nobles M, Diener M, Mestres P, Rummel W. Segmental heterogeneity of the rat colon in the response to activators of secretion on the cAMP-, the cGMP- and the Ca2+-pathway. Acta Physiologica Scandinavica. 1991;142:375–386. doi: 10.1111/j.1748-1716.1991.tb09171.x. [DOI] [PubMed] [Google Scholar]
- Sakai H, Diener M, Gartmann V, Takeguchi N. Eicosanoid-mediated Cl− secretion induced by the antitumor drug, irinotecan (CPT-11), in the rat colon. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:309–314. doi: 10.1007/BF00233252. [DOI] [PubMed] [Google Scholar]
- Sakai H, Lingueglia E, Champigny G, Mattei M -G, Lazdunski M. Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. Journal of Physiology. 1999;519:323–333. doi: 10.1111/j.1469-7793.1999.0323m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Sato T, Hamada N, Yasue M, Ikari A, Kakinoki B, Takeguchi N. Thromboxane A2, released by the anti-tumor drug irinotecan, is a novel stimulator of Cl− secretion in isolated rat colon. Journal of Physiology. 1997;505:133–144. doi: 10.1111/j.1469-7793.1997.133bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Suzuki T, Murota M, Takeguchi N. Nitric oxide/cyclic GMP-induced Cl− secretion in isolated rat colon is mediated by production of thromboxane A2. (Abstract) Journal of Physiology. 2002;539.P:3P–4P. doi: 10.1113/jphysiol.2002.021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Takeguchi N. Small-conductance Cl− channels in rabbit parietal cells activated by prostaglandin E2 and inhibited by GTPγS. Journal of Physiology. 1993;461:201–212. doi: 10.1113/jphysiol.1993.sp019509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Ukai M, Ikari A, Asano S, Takeguchi N. Is rabbit CLCA1 related to the basolateral Ca2+-dependent Cl− channel of gastric parietal cells? Japanese Journal of Physiology. 2001;51:121–125. doi: 10.2170/jjphysiol.51.121. [DOI] [PubMed] [Google Scholar]
- Stack WA, FilipowicZ B, Hawkey CJ. Nitric oxide donating compounds stimulate human colonic ion transport in vitro. Gut. 1996;39:93–99. doi: 10.1136/gut.39.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner MC, Scherr AM, Lee JA, Wolfe LG, Kellum JM. Nitric oxide is a neurotransmitter in the chloride secretory response to serotonin in rat colon. Surgery. 2000;128:240–245. doi: 10.1067/msy.2000.107608. [DOI] [PubMed] [Google Scholar]
- Suga H, Hamanaka N, Kondo K, Miyake H, Ohuchida S, Arai Y, Kawasaki A. ONO-3708: an effective thromboxane A2 antagonist possessing 3-acylaminopinane skeleton. Advances in Prostaglandin, Thromboxane, and Leukotriene Research. 1987;17:799–802. [PubMed] [Google Scholar]
- Suzuki T, Sakai H, Ikari A, Takeguchi N. Inhibition of thromboxane A2-induced Cl− secretion by antidiarrhea drug loperamide in isolated rat colon. Journal of Pharmacology and Experimental Therapeutics. 2000a;295:233–238. [PubMed] [Google Scholar]
- Suzuki T, Sakai H, Takeguchi N. Thromboxane A2-mediated Cl− secretion induced by platelet-activating factor in isolated rat colon. European Journal of Pharmacology. 2000b;400:297–303. doi: 10.1016/s0014-2999(00)00405-2. [DOI] [PubMed] [Google Scholar]
- Tamai H, Gaginella TS. Direct evidence for nitric oxide stimulation of electrolyte secretion in the rat colon. Free Radical Research Communications. 1993;19:229–239. doi: 10.3109/10715769309056511. [DOI] [PubMed] [Google Scholar]
- Wilson KT, Vaandrager AB, De Vente J, Musch MW, De Jonge HR, Chang EB. Production and localization of cGMP and PGE2 in nitroprusside-stimulated rat colonic ion transport. American Journal of Physiology. 1996;270:C832–840. doi: 10.1152/ajpcell.1996.270.3.C832. [DOI] [PubMed] [Google Scholar]
- Wilson KT, Xie Y, Musch MW, Chang EB. Sodium nitroprusside stimulates anion secretion and inhibits sodium chloride absorption in rat colon. Journal of Pharmacology and Experimental Therapeutics. 1993;266:224–230. [PubMed] [Google Scholar]


