Abstract
This work was undertaken to study the effects of testosterone on the coronary, mesenteric, renal and iliac circulations and to determine the mechanisms of action involved. In prepubertal pigs of both sexes anaesthetized with sodium pentobarbitone, changes in left circumflex or anterior descending coronary, superior mesenteric, left renal and left external iliac blood flow caused by intra-arterial infusion of testosterone were assessed using electromagnetic flowmeters. Changes in heart rate and arterial blood pressure were prevented by atrial pacing and by connecting the arterial system to a pressurized reservoir containing Ringer solution. In 12 pigs, intra-arterial infusion of testosterone for 5 min to achieve a stable intra-arterial concentration of 1 μg l−1 increased coronary, mesenteric, renal and iliac blood flow without affecting the maximum rate of change of left ventricular systolic pressure (left ventricular dP/dtmax) and filling pressures of the heart. In a further five pigs, a concentration-response curve was obtained by graded increases in the intra-arterial concentration of the hormone between 0.125 and 8 μg l−1. The mechanisms of these responses were studied in the 12 pigs by repeating the experiment after haemodynamic variables had returned to the control values before infusions. In six pigs, blockade of muscarinic cholinoceptors and adrenoceptors with atropine, propranolol and phentolamine did not affect the responses caused by intra-arterial infusion of testosterone performed to achieve a stable intra-arterial concentration of 1 μg l−1. In the same pigs and in the remaining six pigs, the increases in coronary, mesenteric, renal and iliac blood flow caused by intra-arterial infusion of testosterone performed to achieve a stable intra-arterial concentration of 1 μg l−1 were prevented by intra-arterial injection of Nω-nitro-l-arginine methyl ester. The present study shows that intra-arterial infusion of testosterone dilated coronary, mesenteric, renal and iliac circulations. The mechanism of this response involved the release of nitric oxide.
Although oestrogens and progesterone have been shown to elicit a widespread systemic vasodilatation (Vacca et al. 1999; Molinari et al. 2001a,b), the available information on the acute effects of testosterone on regional vascular beds and their mechanisms has been scarce and controversial. Most of the reported in vivo studies have involved the coronary circulation, where it was shown in men with coronary artery disease that testosterone causes coronary vasodilatation (Rosano et al. 1999; Shapiro et al. 1999; Webb et al. 1999), has antianginal effects (Hamm, 1942; Walker, 1942; Sigler & Tulgan, 1943; Lesser, 1946) and improves exercise-induced ST segment depression (Rosano et al. 1993). Testosterone also causes coronary vasodilatation in anaesthetized dogs, the mechanism of which was reported to involve nitric oxide release and ATP-sensitive K+ channels (Chou et al. 1996). In the isolated heart of the rat and of the guinea pig, testosterone was reported to cause coronary vasoconstriction (Schror et al. 1994; Ceballos et al. 1999). In isolated coronary artery preparations, testosterone has been reported to cause an endothelial-independent relaxation of arterial rings of the rabbit (Yue et al. 1995) and of arterial strips of the pig (Crews & Khalil, 1999), and to potentiate contractile responses or reduce coronary relaxation in arterial rings of the pig (Teoh et al. 2000a, b). Apart from the coronary arteries, testosterone has been reported to relax aortic arterial rings in rabbits and rats (Yue et al. 1995; Honda et al. 1999).
The present study was therefore designed to quantify the primary in vivo effects of acute administration of testosterone on coronary, mesenteric, renal and iliac blood flow in prepubertal pigs of both sexes and to determine the mechanisms involved. This was achieved by performing experiments of intra-arterial infusion of the hormone whilst keeping heart rate and arterial blood pressure constant to avoid secondary interference by haemodynamic and reflex effects.
Methods
The experiments were carried out in 17 domestic pigs of both sexes, weighing 68–76 kg. The animals, which were fasted overnight without witholding water, were anaesthetized with intramuscular ketamine (20 mg kg−1; Parke-Davis, Milan, Italy) followed after about 15 min by intravenous sodium pentobarbitone (15 mg kg−1; Siegfried, Zofingen, Switzerland), and artificially ventilated with oxygen-enriched air with a respiratory pump (Harvard 613; Harvard Apparatus, South Natick, MA, USA). Anaesthesia was maintained throughout the experiments by a continuous intravenous infusion of sodium pentobarbitone (7 mg kg−1 h−1) and assessed as previously reported (Linden & Mary, 1983) from responses of the animals to somatic stimuli. The experiments were carried out in accordance with national guidelines (DLGS 27/01/1992, no. 116).
Pressures in the ascending aorta and in the right atrium were recorded via catheters connected to pressure transducers (Statham P23 XL; Gould, Valley View, OH, USA) inserted into the right femoral artery and the right external jugular vein, respectively. The chest was opened in the left fourth intercostal space, the pericardium was cut and an electromagnetic flowmeter probe (model BL 613; Biotronex Laboratory Inc., Chester, MD, USA) was positioned around the proximal part of the left circumflex or the anterior descending coronary artery; this was determined by their accessibility to avoid undue dissection. The abdomen was opened with a mid-line incision and three flowmeter probes were placed near the origin of the superior mesenteric, left renal and left external iliac arteries. Distal to the probe, a plastic snare was placed around each artery for the assessment of zero blood flow. Calibration of each probe was checked in vitro at the end of each experiment. Testosterone (Sigma, Milan, Italy) was administered in coronary, mesenteric, renal and iliac arteries with an infusion pump (model 22; Harvard Apparatus) using a catheter connected to a butterfly needle inserted into the arteries distal to the flowmeter probe.
Left ventricular pressure was measured by means of a catheter connected to a pressure transducer (Statham P23 XL; Gould) inserted through the left atrium. The frequency response of the catheter-manometer system was found to be flat (± 5 %) up to 40 Hz. To pace the heart, electrodes were sewn on the left atrial appendage and connected to a stimulator (model S8800; Grass Instruments, Quincy, MA, USA) which delivered pulses of 3–5 V with 2 ms duration at the required frequency. Arterial blood samples were used to measure pH, arterial partial pressures of oxygen and carbon dioxide (PO2 and PCO2) (with a gas analyser; IL 1304; IL Instrumentation Laboratory, Lexington, MA, USA), and the haematocrit. Normal values of pH, PO2 and PCO2 of 7.42 ± 0.02, 84.3 ± 3.9 mmHg and 39.8 ± 0.7 mmHg, respectively, have been reported in prepubertal pigs (Houpt, 1986). In the present study, the animals were artificially ventilated with oxygen-enriched air and values of pH and PCO2 were maintained within normal reported limits during the experiments by the infusion of a solution of 2.8 % sodium bicarbonate and by adjusting the respiratory stroke volume, when necessary (Linden & Mary, 1983).
To prevent changes in arterial blood pressure during the experiments, a cannula was introduced into the left internal mammary artery and connected to a reservoir containing Ringer solution (SIFRA-Società Italiana Farmaceutici Ravizza, Verona, Italy) kept at 38 °C. The reservoir was pressurized using compressed air, which was controlled with a Starling resistance, and pressure within the reservoir was measured by a mercury manometer. This method has been shown in anaesthetized pigs to allow the aortic blood pressure to be maintained at steady levels without significant changes in filling pressures of the heart or the haematocrit (e.g. Vacca et al. 1999; Molinari et al. 2001a,b). Coagulation of the blood was avoided by the intravenous injection of heparin (Parke-Davis; initial doses of 500 i.u. kg−1, and subsequent doses of 50 i.u. kg−1 every 30 min). The rectal temperature of the pigs was monitored and kept between 38 and 40 °C using an electric pad.
Mean and phasic aortic blood pressure, mean right atrial pressure, left ventricular pressure, and mean and phasic coronary, mesenteric, renal and iliac blood flows were monitored and recorded together with heart rate and the maximum rate of change of left ventricular systolic pressure (dP/dtmax), by using an electrostatic strip-chart recorder (Gould ES 2000; Gould). The heart rate was obtained from the electrocardiogram with a ratemeter (ECG/Biotach amplifier, model 13–461565 A; Gould). The frequency response of the differentiator used to obtain left ventricular dP/dtmax was found to be flat (± 5 %) up to 150 Hz.
To calculate coronary vascular resistance, the difference between mean aortic blood pressure and mean left ventricular pressure during diastole was considered as the coronary pressure gradient. Coronary vascular resistance was calculated as the ratio between this pressure gradient and mean diastolic coronary blood flow during the steady state. The diastolic period of measurement was defined as starting when ventricular pressure reached its minimum value after systole and ended when it increased at the end of diastole. Regional vascular resistance in the mesenteric, renal and iliac circulation was calculated as the ratio between values of mean aortic blood pressure and mean flow.
At the end of the experiment, each animal was killed by an intravenous injection of 90 mg kg−1 sodium pentobarbitone.
Experimental protocol
The experiments were begun after at least 30 min of steady-state conditions with respect to measured haemodynamic variables. In the 17 pigs, to avoid the interference of any possible changes in heart rate and arterial blood pressure during the experiments, the heart was paced to a frequency higher, by 20 beats min−1, than that observed during the steady state and the arterial system was connected to the pressurized reservoir. After at least 10 min of steady-state conditions, intra-arterial infusions of testosterone for periods of 5 min were performed. In each pig, testosterone was first infused into the coronary artery. When coronary blood flow returned to control values before infusion, usually after about 15 min, the hormone was infused into the mesenteric artery. When mesenteric blood flow returned to control values before infusion, the hormone was infused into the renal artery and then into the iliac artery. Recordings taken for 10 min during the steady state before infusion were used as controls. Measurements of haemodynamic variables were obtained during the last minute of infusion in the steady state and compared with control values.
In each pig, control values of coronary, mesenteric, renal and iliac blood flow were used to assess the infused doses of testosterone. Each dose of testosterone was dissolved in 10 ml of saline and the solution was infused at a constant rate of 2 ml min−1. In 12 of the 17 pigs, these doses were chosen to achieve a stable intra-arterial concentration of the hormone of 1 μg l−1, a value which was previously used to study the coronary effects of testosterone in anaesthetized dogs (Chou et al. 1996). This was obtained by determining the amount of testosterone to be infused at constant rate during the 5 min from the steady state rate of blood volume flow in the region to be examined. In five of the 17 pigs, a concentration-response study was carried out by gradually increasing the intra-arterial concentration of the hormone in 13 steps (14 for renal blood flow) from a minimum value of 0.125 μg l−1 to a maximum value of 8 μg l−1.
The mechanisms of the responses of measured blood flows to the intra-arterial infusion of testosterone were studied in the 12 pigs by repeating the experiments of intra-arterial infusion. In six of these animals, infusion of testosterone in each of the four arteries was performed after blockade of muscarinic cholinoceptors and adrenoceptors with intravenous administration of atropine (0.5 mg kg−1; Sigma), propranolol (0.5 mg kg−1; Sigma) and phentolamine (1 mg kg−1; Ciba Geigy, Varese, Italy). The blocking agents were injected together. In the same six pigs, further sets of intra-arterial infusion of testosterone were completed after blockade of nitric oxide synthase with the intra-arterial administration Nω-nitro-l-arginine methyl ester (l-NAME; Sigma). In the remaining six pigs, the experiments of infusion of testosterone were performed again only after the intra-arterial administration of l-NAME. The dose of l-NAME injected in each of the four arteries was 2 mg for 1 ml min−1 of measured blood flow during the control period. An intracoronary dose of l-NAME of 100 mg has been shown in anaesthetized pigs to significantly reduce by about 50–60 % the vasodilator effect of the intracoronary administration of acetylcholine at a dose of 1 μg (Vacca et al. 1996c, 1999). This reduction in the acetylcholine-induced increase in coronary blood flow was considered a reliable marker of the inhibition of the release of nitric oxide (Parent et al. 1992). Doses of l-NAME similar to those used in the present study were previously shown in anaesthetized pigs to abolish the coronary, mesenteric, renal and iliac vasodilatation caused by the intravenous infusion of 17β-oestradiol or progesterone (Vacca et al. 1999; Molinari et al. 2001a,b). All the drugs were administered in the absence of pacing of the heart and without controlling arterial blood pressure. In all subsequent experiments, the heart was paced to the same frequency as beforehand and changes in arterial blood pressure prevented. In each of the 12 pigs, testosterone was first infused in each of the four arteries after injecting l-NAME into the coronary artery. The infusions of the hormone were then repeated after injecting l-NAME into the mesenteric artery, after injecting l-NAME into the renal artery and, finally, after injecting l-NAME into the iliac artery.
Student's paired t test was used to examine changes in measured haemodynamic variables caused by intra-arterial infusion of testosterone. The nonparametric Mann-Whitney U test for unpaired data was used to compare percentage responses (calculated relative to control) to the intra-arterial infusions of testosterone. A value of P < 0.05 was considered statistically significant. Group data are presented as means ± s.d. (range).
Results
In the 17 pigs, recordings commenced approximately 5 h after the induction of anaesthesia. The mean pH, PO2 and PCO2 of arterial blood were 7.40 ± 0.02 (7.37-7.44), 117 ± 9.1 (102-131) mmHg and 39.3 ± 1.1 (38-41) mmHg, respectively, and the haematocrit was 38.5 ± 1.6 (36-42) %. Spontaneous values of heart rate, mean aortic blood pressure, left ventricular dP/dtmax and mean coronary, mesenteric, renal and iliac blood flow were 98.9 ± 8.4 (86-115) beats min−1, 92.4 ± 6.4 (80-104) mmHg, 2450 ± 229 (1995-2810) mmHg s−1, 51 ± 5.8 (40.2-61.3) ml min−1, 960 ± 93 (810-1115) ml min−1, 410 ± 49 (310-504) ml min−1 and 100 ± 10 (81-121) ml min−1, respectively.
Effects of intra-arterial infusion of testosterone
In the 12 pigs, intra-arterial infusions of the vehicle (10 ml of saline) did not cause any changes in the control values of measured haemodynamic variables. Control values of heart rate, mean aortic blood pressure, mean right atrial pressure, left ventricular end-diastolic pressure and left ventricular dP/dtmax were 116.8 ± 8.5 (106-135) beats min−1, 95.6 ± 7.1 (84-110) mmHg, 3.3 ± 0.5 (2.7-4.1) mmHg, 5.9 ± 1.1 (4.5-8.1) mmHg and 2486 ± 234 (2088-2951) mmHg s−1, respectively. Changes in these variables during the experiments of testosterone infusion were small and insignificant (at least P > 0.05). Individual changes in coronary, mesenteric, renal and iliac blood flow caused by intra-arterial infusion of testosterone are shown in Fig. 1. These changes started within about 2 min after the start of infusions, reached a steady state in about 3 min and were completely over within 15 min after the end of infusions.
Figure 1. Responses of coronary blood flow (CBF), mesenteric blood flow (MBF), renal blood flow (RBF) and iliac blood flow (IBF) to the intra-arterial infusion of testosterone in 12 pigs.
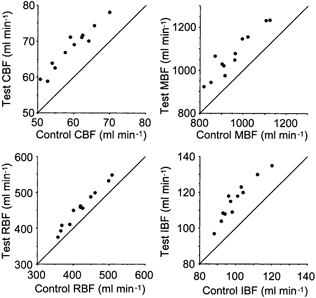
The values of blood flow obtained during the test period of measurement are plotted against the corresponding control values before the infusions. The continuous line is the line of equality.
Infusion into the coronary artery
In the 12 pigs, infusion of testosterone into the coronary artery caused an increase in coronary blood flow of 8.7 ± 1.6 (6-12.2) ml min−1 (P < 0.0005) from control values of 59.4 ± 5.7 (50.7-69.9) ml min−1 in the absence of changes in the other measured blood flows (at least P > 0.30). This increase amounted to 14.7 ± 3.1 (9.4-20.7) % of the control values and corresponded to a decrease in coronary vascular resistance of 12.2 ± 2.9 (7.1-18.3) % from control values of 1.15 ± 0.1 (0.98-1.31) mmHg ml−1 min. An example of the above response caused by testosterone in one pig is shown in Fig. 2A.
Figure 2. Example of experimental recordings showing the effects of the intra-arterial infusion of testosterone on regional blood flows in an anaesthetized pig.
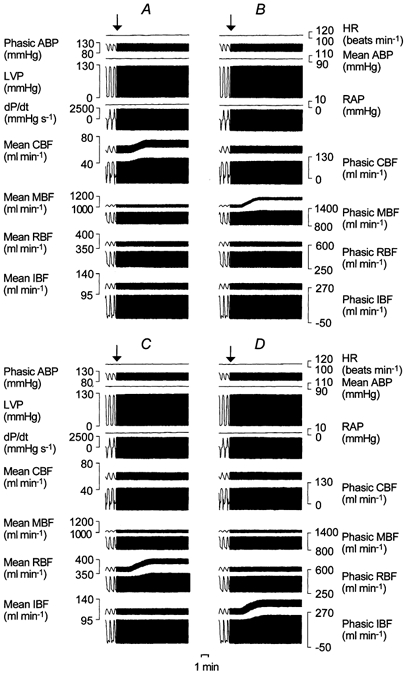
A, infusion into the coronary artery; B, infusion into the mesenteric artery; C, infusion into the renal artery; and D, infusion into the iliac artery. From top to bottom: heart rate (HR), phasic and mean aortic blood pressure (ABP), left ventricular pressure (LVP), mean right atrial pressure (RAP), left ventricular dP/dtmax, mean and phasic coronary blood flow (CBF), mean and phasic mesenteric blood flow (MBF), mean and phasic renal blood flow (RBF), mean and phasic iliac blood flow (IBF). The arrow indicates the beginning of the infusion.
Infusion into the mesenteric artery
In the 12 pigs, infusion of testosterone into the mesenteric artery caused an increase in mesenteric blood flow of 119 ± 34 (61-197) ml min−1 (P < 0.0005) from control values of 953 ± 95 (818- 1123) ml min−1 in the absence of changes in the other measured blood flows (at least P > 0.25). This increase amounted to 12.6 ± 3.9 (6.7-22.6) % of the control values and corresponded to a decrease in mesenteric vascular resistance of 11 ± 3.3 (6.5-18.4) % from control values of 0.101 ± 0.012 (0.080-0.126) mmHg ml−1 min. An example of the above response caused by testosterone in the same pig as Fig. 2A is shown in Fig. 2B.
Infusion into the renal artery
In the 12 pigs, infusion of testosterone into the renal artery caused an increase in renal blood flow of 35 ± 9 (18-50) ml min−1 (P < 0.0005) from control values of 422 ± 50 (357-507) ml min−1 in the absence of changes in the other measured blood flows (at least P > 0.10). This increase amounted to 8.3 ± 2.2 (5- 12.5) % of the control values and corresponded to a decrease in renal vascular resistance of 7.4 ± 1.8 (4.9-10) % from control values of 0.23 ± 0.03 (0.19-0.31) mmHg ml−1 min. An example of the above response caused by testosterone in the same pig as Fig. 2A and B is shown in Fig. 2C.
Infusion into the iliac artery
In the 12 pigs, infusion of testosterone into the iliac artery caused an increase in iliac blood flow of 16 ± 4 (9-22) ml min−1 (P < 0.0005) from control values of 100 ± 9 (88-120) ml min−1 in the absence of changes in the other measured blood flows (at least P > 0.15). This increase amounted to 15.5 ± 3.6 (10.2-22.9) % of the control values and corresponded to a decrease in iliac vascular resistance of 13.1 ± 2.8 (9-18.2) % from control values of 0.96 ± 0.08 (0.87-1.11) mmHg ml−1 min. An example of the above response caused by testosterone in the same pig as Fig. 2A, B and C is shown in Fig. 2D.
Comparison between responses
Comparison between the percentage increases in measured blood flows caused by intra-arterial infusions of testosterone showed that the response of renal blood flow was significantly lower than the responses of coronary, mesenteric and iliac blood flow (P < 0.001, P < 0.004 and P < 0.001, respectively). A significant difference was also found between the responses of mesenteric and iliac blood flow (P < 0.05), while the increase in coronary blood flow was not significantly different from the increases in mesenteric and iliac blood flow (P > 0.07 and P > 0.60, respectively).
Concentration-response study
In the five pigs in which a concentration-response study was performed, control values of coronary, mesenteric, renal and iliac blood flow were 55 ± 8.8 (45.3-64.9), 999 ± 83 (893-1110), 395 ± 50 (309-426) and 103 ± 15 (86-122) ml min−1, respectively. The results obtained by gradually increasing the intra-arterial concentration of testosterone from 0.125 to 8 μg l−1 are shown in Fig. 3. The threshold dose of the hormone was found to be between 0.125 and 0.25 μg l−1 for coronary, mesenteric and iliac blood flow and between 0.25 and 0.375 μg l−1 for renal blood flow. Maximal effects were observed at intra-arterial concentrations of testosterone between 3.5 and 5 μg l−1.
Figure 3. Responses of coronary blood flow, mesenteric blood flow, renal blood flow and iliac blood flow to graded increases in intra-arterial concentration of testosterone between 0.125 and 8 μg l−1 in five pigs.
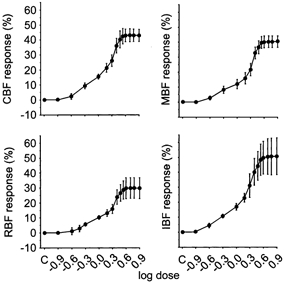
The means of percentage changes in blood flow obtained during the test periods of measurement are plotted against the logarithm of the doses. C, control value before infusions. Abbreviations as in Fig. 1. The bars indicate s.d.
Experiments after blockade of muscarinic cholinoceptors and adrenoceptors
In the six pigs examined, the administration of the blocking agents caused decreases in heart rate and mean arterial blood pressure of −13.7 ± 7.7 (-20 to +1) beats min−1 (P < 0.005) and −20.5 ± 7.1 (-31 to −9) mmHg (P < 0.0005), respectively, from control values of 100.7 ± 9.3 (88- 115) beats min−1 and 92.3 ± 7.1 (84-104) mmHg. These changes were accompanied by decreases in left ventricular dP/dtmax and mean coronary, mesenteric, renal and iliac blood flow which were −274 ± 77 (-388 to −171) mmHg s−1 (P < 0.0005) and −7.7 ± 2.1 (-10.4 to −4.6) (P < 0.0005), −156 ± 66 (-256 to −81) (P < 0.0025), −62 ± 29 (-104 to −19) (P < 0.0025) and −15 ± 8 (-23 to −5) ml min−1 (P < 0.005), respectively, from control values of 2356 ± 221 (2001-2612) mmHg s−1 and 51.2 ± 5.1 (44.6-58.1), 968 ± 93 (845-1110), 412 ± 53 (360-495) and 104 ± 9 (95-119) ml min−1, respectively. After the administration of the blocking agents, intra-arterial infusions of testosterone were performed with doses chosen to achieve a stable intra-arterial concentration of the hormone of 1 μg l−1.
Blockade of muscarinic cholinoceptors and adrenoceptors did not affect the responses of measured blood flows to intra-arterial infusion of testosterone. Group increases in coronary, mesenteric, renal and iliac blood flow were 8.9 ± 2 (5.8-9.3) (P < 0.0005), 114 ± 26 (90-150) (P < 0.0005), 33 ± 10 (20-48) (P < 0.0005) and 16 ± 4 (13-23) ml min−1 (P < 0.0005), respectively, from control values of 53.2 ± 6.9 (46.3-66.4), 819 ± 51 (751-888), 354 ± 58 (280-440) and 90 ± 10 (76-100) ml min−1, respectively. During these experiments, changes in the other measured haemodynamic variables were small and insignificant (at least P > 0.10). A comparison between increases in coronary, mesenteric, renal and iliac blood flow before and after blockade of muscarinic cholinoceptors and adrenoceptors is shown in Fig. 4.
Figure 4. The effect of blockade of muscarinic cholinoceptors and adrenoceptors on responses of coronary blood flow, mesenteric blood flow, renal blood flow and iliac blood flow to intra-arterial infusion of testosterone.
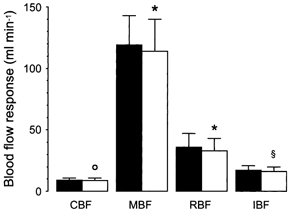
Testosterone was infused to achieve an intra-arterial concentration of 1 μg l−1 before (▪) and after blockade of cholinoceptors and adrenoceptors (□) in 6 pigs. Each column represents the mean of absolute changes from the control and the bars their s.d. Abbreviations as in Fig. 1. With respect to before blockade: * P > 0.05, ° P > 0.15, § P > 0.35.
Cardiovascular effects of l-NAME administration
In the 12 pigs, spontaneous control values of heart rate, mean aortic blood pressure and left ventricular dP/dtmax before the administrations of l-NAME were 89.9 ± 7.7 (76-102) beats min−1, 81.7 ± 12.5 (62-100) mmHg and 2242 ± 271 (1956-2800) mmHg s−1, respectively. Intra-arterial injections of l-NAME caused increases in these variables of 6.2 ± 4.8 (-3 to +12) beats min−1 (P < 0.0005), 17.4 ± 7.8 (9-33) mmHg (P < 0.0005) and 116 ± 71 (48-202) mmHg s−1 (P < 0.0005). Injection of the blocking agent into the coronary artery caused a change in mean coronary blood flow of −1.2 ± 2.4 (-5.1 to +3.2) ml min−1 (P < 0.05) from a control value of 48.2 ± 7.2 (36.2-60.9) ml min−1. Injection of l-NAME into the mesenteric artery caused a group decrease in mesenteric blood flow of −24 ± 62 (-85 to +103) ml min−1 (P > 0.10) from a control value of 867 ± 96 (746-1102) ml min−1. Injection of l-NAME into the renal artery caused a decrease in renal blood flow of 53 ± 30 (5-91) ml min−1 (P < 0.0005) from a control value of 389 ± 60 (300-498) ml min−1. Injection of l-NAME into the iliac artery caused a group decrease in iliac blood flow of −5 ± 4 (-12 to +1) ml min−1 (P < 0.0025) from a control value of 91 ± 6 (80-102) ml min−1.
Effects of l -NAME on responses to testosterone
After the administration of the blocking agent, intra-arterial infusions of testosterone were performed with doses chosen to achieve a stable intra-arterial concentration of the hormone of 1 μg l−1.
l-NAME injection into the coronary artery
In the 12 pigs, blockade of nitric oxide synthase abolished the response of coronary blood flow to the subsequent infusion of testosterone (Fig. 5) without affecting the responses of mesenteric, renal and iliac blood flow. Changes in coronary blood flow caused by testosterone were −0.1 ± 0.4 (-0.9 to +0.6) ml min−1 (P > 0.20) from control values of 53.4 ± 8 (42.6-65.7) ml min−1. The differences between the responses of mesenteric, renal and iliac blood flow before and after injection of l-NAME into the coronary artery were not significant (P > 0.20, P > 0.25 and P > 0.20, respectively).
Figure 5. The effect of intra-arterial injection of l-NAME on responses of coronary blood flow, mesenteric blood flow, renal blood flow and iliac blood flow to intra-arterial infusion of testosterone.
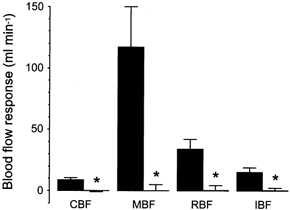
Testosterone was infused to achieve an intra-arterial concentration of 1 μg l−1 before (▪) and after the intra-arterial injection of l-NAME (□) in 12 pigs. Each column represents the mean of absolute changes from the control and the bars their s.d. Abbreviations as in Fig. 1. With respect to before injection of l-NAME: * P < 0.0005.
l-NAME injection into the mesenteric artery
In the 12 pigs, blockade of nitric oxide synthase abolished the response of mesenteric blood flow to the subsequent infusion of testosterone (Fig. 5) without affecting the responses of renal and iliac blood flow. Changes in mesenteric blood flow caused by testosterone were 0.1 ± 5 (-8 to +10) ml min−1 (P > 0.45) from control values of 848 ± 93 (735-1021) ml min−1. The differences between the responses of renal and iliac blood flow before and after injection of l-NAME into the mesenteric artery were not significant (P > 0.15 and P > 0.20, respectively).
l-NAME injection into the renal artery
In the 12 pigs, blockade of nitric oxide synthase abolished the response of renal blood flow to the subsequent infusion of testosterone (Fig. 5) without affecting the responses of iliac blood flow. Changes in renal blood flow caused by testosterone were 0.4 ± 4 (-8 to +7) ml min−1 (P > 0.35) from control values of 340 ± 40 (271-410) ml min−1. The difference between the response of iliac blood flow before and after injection of l-NAME into the renal artery was not significant (P > 0.15).
l-NAME injection into the iliac artery
In the 12 pigs, blockade of nitric oxide synthase also abolished the response of iliac blood flow to the subsequent infusion of testosterone (Fig. 5). Changes in this flow caused by testosterone were 0.4 ± 2 (-2 to +4) ml min−1 (P > 0.25) from control values of 87 ± 5 (81-94) ml min−1.
Discussion
The present study has shown in prepubertal pigs of both sexes that intra-arterial infusion of testosterone caused an increase in coronary, mesenteric, renal and iliac blood flow which was obtained in the absence of significant changes in arterial blood pressure, indicating that the hormone elicited a vasodilatation in these vascular beds. This effect of testosterone, which was not related to muscarinic cholinoceptors and adrenoceptors, involved the formation of nitric oxide.
The increase in regional blood flow observed in response to the intra-arterial infusion of testosterone can be attributed to a primary effect of the hormone and not to a secondary interference by changes in haemodynamic variables. Prevention of changes in heart rate and arterial blood pressure and absence of changes in filling pressures of the heart and left ventricular dP/dtmax excluded any interference from reflex and local metabolic and physical effects. In addition, it was possible to increase the magnitude of the response of blood flow in the four vascular regions examined by increasing the intra-arterial concentration of testosterone. Finally, infusion of saline alone at the same rate as that of testosterone did not reproduce any of the hormonal effects. Although a vasodilator effect of testosterone on the coronary circulation in vivo has been reported previously (Chou et al. 1996), the present results on the effect of the hormone on the mesenteric, renal and iliac vascular beds are new and indicate that testosterone causes widespread vasodilatation.
Blockade of muscarinic cholinoceptors and adrenoceptors did not affect the responses of vasodilatation elicited by testosterone. The dose of atropine used in this study has previously been used to block coronary muscarinic cholinoceptors in anaesthetized pigs (e.g. Vacca et al. 1999). The dose of propranolol used has also been used previously in anaesthetized pigs to block β-adrenergic receptors in the coronary (e.g. Molinari et al. 2001a) and in the mesenteric, renal and iliac vascular beds (e.g. Molinari et al. 2001b). The dose of 1 mg kg−1 of phentolamine has been shown in anaesthetized pigs to abolish the reflex coronary vasoconstriction caused by distension of the gallbladder and the uterus (Vacca et al. 1996a, 1997a) and the reflex mesenteric, renal and iliac vasoconstriction caused by distension of the stomach and the uterus (Vacca et al. 1996b, 1997b) and has previously been used to block α-adrenergic receptors (Vacca et al. 1999; Molinari et al. 2001a,b). Similar doses of the blocking agents have been used in anaesthetized pigs by other authors to obtain autonomic blockade (Gregory & Wotton, 1981). The present findings therefore excluded the involvement of muscarinic cholinoceptors and adrenoceptors in the responses of regional vasodilatation caused by testosterone, or of any possible mechanism acting through efferent vagal and sympathetic effects. Autonomic blockade resulted in a decrease of spontaneous heart rate, an effect which has previously been observed in anaesthetized pigs (Vacca et al. 1996c, 1999), and caused a small decrease in iliac vascular resistance similar to that already shown in anaesthetized pigs (Vacca et al. 1999).
The present study showed that the regional vasodilatation elicited by testosterone involved the release of nitric oxide. The regional responses to the hormone were blocked by the local administration of l-NAME. The blocking effect of l-NAME was local to the region examined, since any overflow would have been diluted and because it was possible to reproduce the effect of vasodilatation in response to intra-arterial administration of testosterone in the vascular beds which did not receive the blocking agent. The technique used in the present study does not unequivocally determine the origin of nitric oxide. However, it indicates that the response of regional vasodilatation involved nitric oxide release, since l-NAME is known to inhibit its formation generally (Henderson, 1991).
The results of this investigation indicate that there are regional differences in the increase in blood flow caused by testosterone. The percentage increase in blood flow was greater in the coronary, mesenteric and iliac circulations than in the renal circulation. A similar difference has been observed in anaesthetized pigs with respect to the vasodilatory effect of oestrogen and progesterone, where the smallest effect was found in the renal circulation (Vacca et al. 1999; Molinari et al. 2001b). All the three hormones caused substantial coronary vasodilatation and, as such, their effects were consistent with previously reported findings in men with coronary artery disease that testosterone has antianginal effects (Hamm, 1942; Walker, 1942; Sigler & Tulgan, 1943; Lesser, 1946), improves exercise-induced ST segment depression (Rosano et al. 1993) and causes coronary vasodilatation (Rosano et al. 1999; Shapiro et al. 1999; Webb et al. 1999).
The present investigation was planned to examine the acute effects of testosterone on the peripheral circulation in prepubertal pigs. The results obtained showed an effect of widespread regional vasodilatation which occurred in both sexes. It is possible that the hormone may have different effects in male or female adult pigs and also when chronically administered. However, the present findings indicate that testosterone can cause a nongenomic systemic vasodilatation by increasing the formation of nitric oxide. The potential physiological relevance of this vasodilator effect of testosterone includes maintenance of adequate blood flow to tissues serving the purpose of supplying nutrients for the anabolic role of the hormone. In addition, the vasodilator effect of testosterone and the involvement of nitric oxide release may help in vascular protection in the presence of intact endothelium.
In conclusion, the present investigation has shown that acute administration of testosterone causes regional vasodilatation in the coronary, mesenteric, renal and iliac circulation which involves the release of nitric oxide.
Acknowledgments
This research has received generous sponsorship by Università del Piemonte Orientale ‘A. Avogadro'. We thank the Azienda Ospedaliera Maggiore della Carità di Novara and Azienda Agricola Panza Maria Bianca in Podere Obiarello, Nibbia (Novara) for their help.
References
- Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, MartineZ A, YaneZ R, PereZ J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. Journal of Cardiovascular Pharmacology. 1999;33:691–697. doi: 10.1097/00005344-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arteriosclerosis Thrombosis and Vascular Biology. 1999;19:1034–1340. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- Gregory NG, Wotton SB. Autonomic and non-autonomic control of cardiovascular function in stress-sensitive pigs. Journal of Veterinary Pharmacology and Therapeutics. 1981;4:183–191. doi: 10.1111/j.1365-2885.1981.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Hamm L. Testosterone propionate in the treatment of angina pectoris. Journal of Clinical Endocrinology. 1942;2:325–328. [Google Scholar]
- Henderson AH. Endothelium in control. British Heart Journal. 1991;65:116–125. doi: 10.1136/hrt.65.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Unemoto T, Kogo H. Different mechanisms for testosterone-induced relaxation of aorta between normotensive and spontaneously hypertensive rats. Hypertension. 1999;34:1232–1236. doi: 10.1161/01.hyp.34.6.1232. [DOI] [PubMed] [Google Scholar]
- Houpt TR. The handling of swine in research. In: Stanton HC, Mersmann HJ, editors. Swine in Cardiovascular Research. II. Boca Raton, FL, USA: CRC Press; 1986. pp. 49–71. [Google Scholar]
- Lesser MA. Testosterone propionate therapy in one hundred cases of angina pectoris. Journal of Clinical Endocrinology. 1946;6:549–557. doi: 10.1210/jcem-6-8-549. [DOI] [PubMed] [Google Scholar]
- Linden RJ, Mary DASG. The preparation and maintenance of anaesthetized animals for the study of cardiovascular function. In: Linden RJ, editor. Life Sciences, Techniques in Cardiovascular Physiology. P3/1. Ireland: Elsevier Science Publishers; 1983. pp. 1–22. [Google Scholar]
- Molinari C, Battaglia A, Grossini E, Mary DASG, Stoker JB, Surico N, Vacca G. The effect of progesterone on coronary blood flow in anaesthetized pigs. Experimental Physiology. 2001a;86:101–108. doi: 10.1113/eph8602076. [DOI] [PubMed] [Google Scholar]
- Molinari C, Battaglia A, Grossini E, Mary DASG, Surico N, Vacca G. Effect of progesterone on peripheral blood flow in pre-pubertal female anesthetized pigs. Journal of Vascular Research. 2001b;38:569–577. doi: 10.1159/000051093. [DOI] [PubMed] [Google Scholar]
- Parent R, Paré R, Lavallée M. Contribution of nitric oxide to dilation of resistance coronary vessels in conscious dogs. American Journal of Physiology. 1992;262:H10–16. doi: 10.1152/ajpheart.1992.262.1.H10. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Sarrel PM, Poole WP, Collins P. Beneficial effect of oestrogen on exercise-induced myocardial ischaemia in women with coronary artery disease. Lancet. 1993;342:133–136. doi: 10.1016/0140-6736(93)91343-k. [DOI] [PubMed] [Google Scholar]
- Rosano GMC, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, Lilla della Monica P, Bonfigli B, Volpe M, Chierchia SL. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- Schror K, Morinelli TA, Masuda A, Matsuda K, Mathur RS, Halushka PV. Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pigs. European Journal of Clinical Investigation. 1994;24:50–52. doi: 10.1111/j.1365-2362.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Christiana J, Frishman WH. Testosterone and other anabolic steroids as cardiovascular drugs. American Journal of Therapy. 1999;6:167–174. doi: 10.1097/00045391-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Sigler LH, Tulgan J. Treatment of angina pectoris by testosterone propionate. New York State Journal of Medicine. 1943;43:1424–1428. [Google Scholar]
- Teoh H, Quan A, Leung SW, Man RY. Differential effects of 17β-estradiol and testosterone on the contractile responses of porcine coronary arteries. British Journal of Pharmacology. 2000a;129:1301–1308. doi: 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh H, Quan A, Man RY. Acute impairment of relaxation by low levels of testosterone in porcine coronary arteries. Cardiovascular Research. 2000b;45:1010–1018. doi: 10.1016/s0008-6363(99)00398-3. [DOI] [PubMed] [Google Scholar]
- Vacca G, Battaglia A, Grossini E, Mary DASG, Molinari C. Reflex coronary vasoconstriction caused by gallbladder distension in anesthetized pigs. Circulation. 1996a;94:2201–2209. doi: 10.1161/01.cir.94.9.2201. [DOI] [PubMed] [Google Scholar]
- Vacca G, Battaglia A, Grossini E, Mary DASG, Molinari C, Surico N. Reflex haemodynamic responses caused by distension of the uterus in anaesthetized pigs. Journal of the Autonomic Nervous System. 1997a;63:1–11. doi: 10.1016/s0165-1838(96)00124-5. [DOI] [PubMed] [Google Scholar]
- Vacca G, Battaglia A, Grossini E, Mary DASG, Molinari C, Surico N. Changes in regional blood flow in response to distension of the uterus in anaesthetised pigs. Journal of the Autonomic Nervous System. 1997b;66:7–14. doi: 10.1016/s0165-1838(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Vacca G, Battaglia A, Grossini E, Mary DASG, Molinari C, Surico N. The effect of 17β-oestradiol on regional blood flow in anaesthetized pigs. Journal of Physiology. 1999;514:875–884. doi: 10.1111/j.1469-7793.1999.875ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca G, Mary DASG, Battaglia A, Grossini E, Molinari C. The effect of distension of the stomach on peripheral blood flow in anaesthetized pigs. Experimental Physiology. 1996b;81:385–396. doi: 10.1113/expphysiol.1996.sp003943. [DOI] [PubMed] [Google Scholar]
- Vacca G, Papillo B, Battaglia A, Grossini E, Mary DASG, Pelosi G. The effects of hypertonic saline solution on coronary blood flow in anaesthetized pigs. Journal of Physiology. 1996c;491:843–851. doi: 10.1113/jphysiol.1996.sp021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TC. The use of testosterone propionate and estrogenic substance in the treatment of essential hypertension, angina pectoris and peripheral vascular disease. Journal of Clinical Endocrinology. 1942;2:560–568. [Google Scholar]
- Webb CM, McNeill JG, Hayward CS, De Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes coronary arteries and aorta. Circulation. 1995;91:1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]


