Abstract
In small segments of circular smooth muscle bundle isolated from the guinea-pig gastric antrum, depolarization of the tissue with intracellular current stimuli evoked regenerative slow potentials after a refractory period of 5–10 s. The refractory period changed inversely with the amplitude and duration of the stimulating depolarization. Thapsigargin (an inhibitor of calcium-ATPase at internal stores), 2-aminoethoxydiphenyl borate (2-APB, an inhibitor of inositol 1,4,5-trisphosphate (IP3)-receptor-mediated Ca2+ release), and carbonyl cyanide m-chlorophenyl-hydrazone (a mitochondrial protonophore) reduced the amplitude of slow potentials, with no significant alteration of the refractory period. Bisindolylmaleimide I or chelerythrine (inhibitors of protein kinase C, PKC) increased the refractory period and inhibited the amplitude of slow potentials. These results indicate that the refractory period and amplitude of slow potentials are related to the activation of PKC and the amount of Ca2+ released from the internal stores through activation of IP3 receptors, respectively. Acetylcholine (ACh) reduced the refractory period and increased the amplitude of slow potentials: the former was antagonized by chelerythrine and the latter by 2-APB. The results suggest that ACh has dual actions; stimulation of the metabolism of inositol phosphate and activation of PKC. Phorbol-12-myristate-13-acetate, a selective stimulant of PKC, at low concentrations (< 10 nm) mimicked the actions of ACh and at high concentrations reduced the frequency of slow potentials and increased the refractory period. The possible involvement of the concentration-dependent differences in the actions of phorbol ester on the translocation of PKC was considered.
Gastrointestinal smooth muscles are spontaneously active and generate slow waves and action potentials (Tomita, 1981). Interstitial cells of Cajal (ICC) distributed in the gastric wall are thought to be involved in the generation of these spontaneous activities (Sanders, 1996; Huizinga et al. 1997). In the gastric wall of laboratory animals such as the guinea-pig and mouse, many types of interstitial cells (IC) may be identified, such as those distributed at the myenteric layers (IC-MY) and those found within circular muscle bundles (IC-IM; Komuro et al. 1996; Burns et al. 1997). The pacemaker cells for gastric activities may be IC-MY, which generate driving potentials with initial fast spike and subsequent plateau components of 10–15 s duration. The potentials are propagated to circular and longitudinal smooth muscles in an electrotonic manner through gap junctions (Dickens et al. 1999). Slow waves generated in gastric smooth muscle of the guinea-pig consist of voltage-sensitive and voltage-insensitive components (Ohba et al. 1975; Tomita, 1981), and only the voltage-sensitive component is easily inhibited by caffeine (Dickens et al. 1999). A driving potential generated in IC-MY is propagated to circular muscles to form the voltage-insensitive component of a slow wave, and this potential triggers the voltage-sensitive component (Dickens et al. 1999). In the gastric muscles of W/WV mutant mice lacking IC-IM, the caffeine-sensitive component of the slow waves is absent, suggesting that this component is elicited by electrotonic spread of potentials generated in IC-IM (Dickens et al. 2001).
In isolated circular muscles of the guinea-pig antrum with no attached IC-MY, however, regenerative slow potentials that are sensitive to caffeine appear periodically (Suzuki & Hirst, 1999; Nose et al. 2000; Fukuta et al. 2002; Hirst et al. 2002). These slow potentials are also evoked by depolarization of the membrane, with a minimum latency of about 1 s. This long latency is thought to be required for the production of unidentified messengers, possibly including inositol 1,4,5-trisphosphate (IP3), in response to depolarization of the membrane (Suzuki & Hirst, 1999; Suzuki, 2000). The possible involvement of increased production of IP3 during the generation of spontaneous activity has also been considered in the pyloric circular muscle of the guinea-pig stomach (Van Helden et al. 2000) and the murine small intestine (Malysz et al. 2001). When two depolarizing stimuli are applied to a segment of circular muscle from the guinea-pig gastric antrum, a refractory period of about 5 s is seen prior to the generation of a slow potential, and this refractory period may be one of the important factors for the determination of the maximum frequency of slow waves (Nose et al. 2000).
The present experiments were carried out to investigate the factors affecting the refractory period for generation of slow potentials in an isolated segment of circular smooth muscle of the guinea-pig gastric antrum. A small segment of smooth muscle, the size being about one-tenth of the length constant of the tissue (equal to 2.2 mm, Osa & Kuriyama, 1970), was impaled by two intracellular electrodes, and electrical responses were recorded simultaneously from two different cells, as reported previously (Suzuki & Hirst, 1999). Cells were stimulated by current injection via one electrode to evoke slow potentials, and the effects of chemicals known to inhibit IP3 receptors, protein kinase C (PKC) or the Ca2+ pump at the internal membrane were tested. Some of these chemicals increased concentrations of intracellular Ca2+ ([Ca2+]i), with depolarization of the membrane (Fukuta et al. 2002). The membrane potential is one of the factors that modulates the frequency and amplitude of slow potentials (Nose et al. 2000; Fukuta et al. 2002). Thus, the effects of these chemicals on slow potentials were tested using low concentrations that did not modulate membrane potential, so as to facilitate the evaluation of the effects. The results indicate that the slow potential is an active response that requires a refractory period for its generation, and the amplitude of slow potentials may be determined by the amount of Ca2+ released from an internal store, possibly through IP3-receptor stimulation, whereas the refractory period is causally related to the activation of PKC. Some of these experimental results have been reported in brief at the 43rd Annual Meeting of the Japanese Smooth Muscle Society (Suzuki et al. 2001).
Methods
Male albino guinea-pigs, weighing 250–300 g, were anaesthetized with fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether (sevoflurane; Maruishi Pharmaceutical, Osaka, Japan) and decapitated. All animals were treated ethically according to the guiding principles for the care and use of animals in the field of physiological sciences, approved by The Physiological Society of Japan. The stomach was excised and then opened by cutting along the small curvature in Krebs solution. The mucosal layers were removed by cutting with fine scissors, and smooth muscle tissues were isolated from the antral region. The circular tissue preparation (single bundle 80–100 μm wide and 200–250 μm long) was prepared by mechanical removal of the longitudinal muscle layer with fine forceps. The preparation was pinned out on a Sylgard plate (silicone elastomer, Dow Corning, Midland, MI, USA) at the bottom of the recording chamber (volume, approximately 0.5 ml), and superfused with warmed (35 °C) Krebs solution at constant flow rates (about 2 ml min−1). The recording chamber was mounted onto the stage of an inverted microscope (Nikon IX-70, Tokyo, Japan). These methods are essentially the same as those reported by Suzuki & Hirst (1999).
Electrical responses of smooth muscle cells were recorded using conventional microelectrode methods. Glass capillary microelectrodes filled with 0.5 m KCl had the tip resistances ranged between 150 and 250 MΩ. Two microelectrodes were inserted into the same tissue, and electrical responses were recorded simultaneously from two cells. Experiments were carried out when signals recorded from the two electrodes were synchronized. Current pulses with 0.5-10 nA intensity (duration, 1–2 s) were applied to one electrode, and electrotonic potentials produced were recorded by the second electrode. Membrane potential changes, recorded using a high-input-impedance amplifier (Axoclamp-2B, Axon Instruments, Foster City CA, USA), were displayed on a cathode-ray oscilloscope (SS-7602, Iwatsu, Osaka, Japan) and stored on a personal computer for later analysis. Ionic composition of the Krebs solution was as follows (mm): Na+ 134; K+ 5.9, Mg2+ 1.2, Ca2+ 2.5, HCO3− 15.5, H2PO4− 1.2, Cl− 134, glucose 15.5. The solution was aerated with O2 containing 5 % CO2, and the pH of the solution was 7.1-7.2.
Drugs used were acetylcholine chloride (ACh), caffeine, carbonyl cyanide m-chloroprene hydrazones (CCCP), nifedipine and thapsigargin, (purchased from Sigma Chemical, USA), and 2-aminoethoxydiphenyl borate (2-APB), bisindolylmaleimide I (BIM), chelerythrine chloride, phorbol-12-myristate-13-acetate (PMA), and ryanodine (purchased from Calbiochem, San Diego, CA, USA). 2-APB, BIM, CCCP, chelerythrine, PMA and nifedipine were dissolved in dimethyl sulphoxide to make stock solutions, and they were added to Krebs solution to make the desired concentrations. Other chemicals were dissolved in distilled water as a stock solution, and diluted further with Krebs solution to desired concentrations (the volume ratios of the dilution were over 1:1000). The dilution procedures did not alter the pH of the Krebs solution.
Values measured are expressed as the mean ± s.e.m. or s.d. Differences between values were tested using an unpaired Student's t test, and probabilities less than 5 % (P < 0.05) were considered significant.
Results
Depolarization-evoked slow potentials
In most segments of circular smooth muscle bundles isolated from the guinea-pig gastric antrum, electrical responses recorded simultaneously from two cells were synchronized, indicating that they were electrically coupled. The cell pairs with no synchronized activity (less than 5 % of cell pairs examined) were not used in the present experiments. Most cells examined demonstrated periodic generation of regenerative slow potentials. A burst of spike potentials was often generated on top of each slow potential. The membrane potential between slow potentials was not stable, and random generation of transient small fluctuations (unitary potentials, Edwards et al. 1999) was observed. Nifedipine (1 μM) abolished the spike potentials, with no interruption to the generation of slow potentials or to the unitary potentials, while caffeine (1 mm) abolished all activity. In the presence of nifedipine, slow potentials with amplitudes ranging between 22 and 45 mV (mean, 33.9 ± 0.9 mV, n = 52) and durations measured at the foot ranging between 5 and 12 s (mean, 7.0 ± 0.2 s, n = 52) were generated at frequencies ranging between 0.2 and 3.1 min−1 (mean, 1.28 ± 0.12 min−1, n = 52). These properties of electrical responses were similar to those reported previously (Suzuki & Hirst, 1999; Edwards et al. 1999). All experiments were carried out in the presence of 1 μM nifedipine, to exclude the possible involvement of voltage-gated Ca2+ channels in the evoked responses.
Application of a current pulse (1-2 s duration) via one electrode produced electrotonic potentials in other cells, which could be recorded by the second electrode. The electrotonic potentials thus produced could be recorded in all cell pairs with synchronized activity. Current injection via one electrode produced a depolarizing potential in the second electrode, and the amplitude of the electrotonic depolarization increased with the amplitude of the injected current. When the amplitude of depolarization exceeded a certain level, muscles produced regenerative slow potentials. The evoked slow potentials had a latency of 1–3 s after onset of the depolarization, as reported previously (Suzuki & Hirst, 1999). Experiments were carried out to stimulate muscles for 1 s with depolarizing pulses of supramaximal intensity, at various times after cessation of a spontaneously generated slow potential. When pulses were applied within 1–2 s after the cessation of a spontaneous slow potential, only electrotonic potentials were produced (Fig. 1A). Pulses applied 3–7 s after the cessation of a spontaneous slow potential evoked electrotonic potentials and subsequent small incomplete slow potentials, which looked like clusters of unitary potentials (Fig. 1B). Stimulation of muscles 7–10 s after spontaneous slow potentials elicited either electrical responses similar to those seen in Fig. 1B or slow potentials with amplitudes similar to those generated spontaneously, occurring approximately 1 s after the electrotonic potentials (Fig. 1C). Increasing the time between the cessation of a slow potential and the application of a depolarizing pulse to more than 10 s evoked a slow potential with an amplitude similar to those evoked spontaneously. The relationship between the time of application of pulses after cessation of spontaneous slow potentials and the peak amplitude of the evoked potentials (Fig. 1D) indicated that there was a period of 1–3 s during which no response was evoked by depolarizing pulses. For the period 3–7 s after cessation of slow potentials, depolarizing responses evoked by stimulating pulses increased roughly linearly. Stimulation of muscles after an interval exceeding 8 s produced complete regenerative slow potentials. As a consequence, the relationship between the interval between cessation of a slow potential and delivery of the stimulus and the amplitude of the evoked response had a discontinuity around 3–5 s after cessation of the slow potentials. Inconsistent responses were evoked when stimulation was applied during the discontinuous period. Application of pulses at intervals longer than 10 s produced reproducible amplitudes of slow potentials. These results indicate that there is a refractory period following a slow potential in the gastric smooth muscles, and during this period, generation of a succeeding slow potential is inhibited.
Figure 1. Generation of slow potentials by depolarizing pulses.
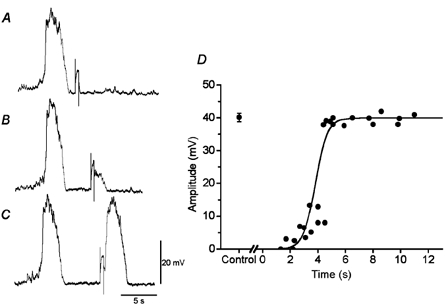
During simultaneous recordings of membrane potential from two cells in a segment of circular smooth muscle tissue from the guinea-pig gastric antrum, current pulses of 3 nA intensity and 1 s duration were applied to one electrode, and the evoked responses were recorded at the second electrode. Current pulses were applied about 2 s (A), 7 s (B) and 12 s (C) after the cessation of a spontaneous slow potential. D, summary of the relationship between the time of application of current pulses after cessation of slow potentials and the amplitude of potentials evoked by the depolarization. The line in the figure was drawn as a sigmoidal fitting of all data. Control, amplitude of slow potentials generated spontaneously before application of current pulses (mean ± s.d., n = 25).
In some preparations in which the frequency of spontaneous slow potentials was extremely low (below 0.2 min−1), attempts were made to evoke slow potentials by imposing depolarizing pulses, and the refractory period for generation of a second slow potential was also measured. The conditioning pulses were applied at intervals over 1 min, and the test pulses were applied at various times after cessation of the conditioning slow potentials. The results indicated that there was also a comparable length (5-12 s) of refractory period for the generation of slow potentials following evoked slow potentials (data not shown), indicating that the refractory properties of evoked slow potentials were similar to those of spontaneously generated slow potentials.
Figure 2A shows the amplitude of responses evoked by depolarizing pulses with a fixed duration (1 s) and at three different intensities, applied at various times after the cessation of a slow potential. When 2 nA current pulses were applied, the membrane was depolarized by 6.8 ± 0.3 mV (n = 8), and a slow potential was generated with a refractory period of about 10 s. Increasing the intensity of current to 4 nA depolarized the membrane by 12.5 ± 0.4 mV (n = 10), which resulted in a reduction of the time required for the generation of a slow potential to about 5 s. Depolarization of the membrane by 22.4 ± 0.4 mV (n = 9) using an 8 nA pulse elicited a slow potential of full size at about 2.7 s delay. These results indicate that the refractory period for generation of a slow potential is a function of the amplitude of depolarization. When the latency for generation of slow potentials after cessation of spontaneous slow potentials was plotted against the amplitude of depolarization produced by 1 s pulses (Fig. 2B), the relationship was reciprocal with the threshold depolarization for the generation of slow potential at 8.7 ± 2.0 mV (n = 11). The refractory period for the generation of slow potentials was also shortened with increasing amplitude of depolarization, and the value was similar above 15–18 mV depolarization. The mean value of the refractory period for generation of slow potentials was 3.6 ± 0.8 s (n = 10) when the membrane was depolarized over 15 mV.
Figure 2. The generation of slow potentials evoked by depolarizing stimuli.
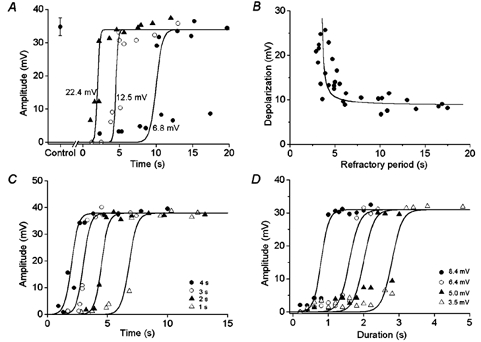
A, during simultaneous recordings of membrane potential from two cells in a segment of circular smooth muscle tissue of the guinea-pig gastric antrum, 1 s current pulses at three different intensities (2, 4 and 8 nA) were applied via one electrode, and the amplitudes of evoked electrical responses measured at the second electrode were plotted as a function of time after cessation of the preceding spontaneous slow potential. Current pulses of 2 nA, 4 nA and 8 nA depolarized the membrane by 6.8 ± 0.3 mV (n = 8, •), 12.5 ± 0.4 mV (n = 10, ○) and 22.4 ± 0.4 mV (n = 9, ▴), respectively. Control, amplitude of spontaneously generated slow potentials (mean ± s.d., n = 36). B, the relationship between amplitude of depolarization produced by 1 s pulses and the refractory period for generation of slow potentials. The curve in the figure was given by an equation, (y + 9) = (1/x + 4), where y is the amplitude of depolarization and x is the refractory period. C, the relationship between the time for stimulation of muscles after cessation of spontaneous slow potentials and the amplitude of evoked responses produced by four different durations of pulses (▵, 1 s; ▴, 2 s; ○, 3 s; •, 4 s) with fixed amplitude (6.4 ± 0.6 mV, n = 51) using 3 nA current pulses in a segment of circular smooth muscle bundle. D, stimulation of a circular smooth muscle bundle from the gastric antrum with four different intensities of current pulses (2, 3, 4 and 5 nA) depolarized the membrane by 3.5, 5.0, 6.4 and 8 mV, respectively. The graph shows the relationship between the duration of depolarization at four different amplitudes (▵, 3.5 mV; ▴, 5.0 mV; ○, 6.4 mV; •, 8.4 mV) and the amplitudes of evoked responses.
The effectiveness of depolarizing pulses in evoking slow potentials varied with their duration and intensity. An example of the effect of changing the duration of depolarization on the latency for the generation of slow potentials is shown in Fig. 2C. When a fixed intensity of stimulation (3 nA) was applied, the time required for generation of slow potentials was shortened successively by increasing the duration of pulses from 1 s to 4 s. These results indicate that the refractory period for generation of a slow potential decreases with increasing duration of depolarization.
The effects of changes in the duration of depolarizing pulses on the responses of muscles were also investigated at various amplitudes of depolarization (Fig. 2D). When the membrane was depolarized by 3.5 mV, the duration of depolarization required to evoke slow potentials was about 3.2 s. Increasing the amplitude of depolarization to 5.0, 6.4 and 8.4 mV reduced the required duration of depolarization to about 2.4, 1.8 and 1 s, respectively. These results indicate that the relationship between the amplitude and duration of depolarization required for the generation of a slow potential was again reciprocal, and smaller amplitude depolarizations required a longer duration for the generation of a slow potential.
Modulation of slow potentials by inhibitors of the internal Ca2+ stores or intracellular signalling
Experiments were carried out to investigate the factors determining the refractory period for the generation of slow potentials, using several types of chemicals known to modulate cellular functions through the functional inhibition of internal Ca2+ stores or intracellular signalling. Chemicals tested were thapsigargin (an inhibitor of the uptake of Ca2+ into the internal Ca2+ stores through Ca-ATPase inhibition; Thastrup et al. 1990), 2-APB (an inhibitor of the IP3-receptor-mediated Ca2+ release; Maruyama et al. 1997), ryanodine (an inhibitor of the calcium-induced release of Ca2+ from the internal stores; Feher & Lipford, 1985), CCCP (a mitochondrial protonophore that inhibits the uptake of Ca2+ into mitochondria; Duchen, 1999), and chelerythrine and BIM (inhibitors of the translocation of PKC; Herbert et al. 1990; Toullec et al. 1991).
High concentrations of thapsigargin (> 1 μM) depolarized the membrane and abolished spontaneous and evoked slow potentials (data not shown). The concentration of thapsigargin used was 0.3 μM, which did not alter the resting membrane potential (control, −65.1 ± 2.8 mV; in thapsigargin, −64.8 ± 2.5 mV, n = 6; P > 0.05). In the presence of thapsigargin for over 20 min, the amplitude of spontaneous slow potentials was decreased (control, 40.2 ± 1.3 mV; in thapsigargin, 32.2 ± 1.4 mV, n = 6; P < 0.05), with no significant alteration of the frequency of slow potentials (control, 0.69 ± 0.18 min−1; in thapsigargin, 0.71 ± 0.25 min−1, n = 6; P > 0.05). The refractory period for the generation of slow potentials evoked by 1 s pulses remained unchanged in the presence of thapsigargin (control, 4.3 ± 0.8 s; in thapsigargin, 4.8 ± 1.2 s, n = 6; P > 0.05) (Fig. 3). The inhibitory actions of thapsigargin did not recover after up to 2 h of washing. These results suggest that the reduction of internal Ca2+ stores mainly reduces the amplitude of slow potentials, with no marked alteration in the refractory period.
Figure 3. Effects of thapsigargin on evoked slow potentials.
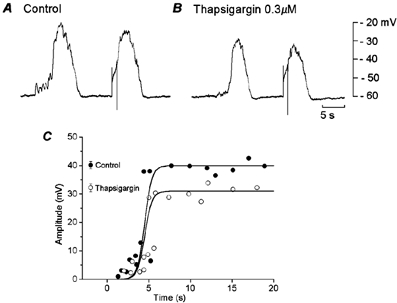
During simultaneous recordings of membrane potential from two cells in a segment of circular smooth muscle tissue of the guinea-pig gastric antrum, muscle was stimulated by current pulses of 1 s duration and 3 nA intensity, in the absence (A, control) and presence of 0.3 μM thapsigargin (B). A and B were recorded from the same pair of cells. C, the relationship between the time of application of stimulating pulses after cessation of spontaneous slow potentials and amplitude of responses evoked by the pulses, in the absence (•, control) and presence of 0.3 μM thapsigargin (○).
2-APB (> 10 μM) depolarized the membrane by 5–10 mV and abolished spontaneously generated slow potentials, as reported previously (Fukuta et al. 2002). Application of a lower concentration of 2-APB (5 μM) for over 10 min reduced the amplitude of spontaneous slow potentials (control, 34.0 ± 2.1 mV; in 2-APB, 29.5 ± 3.2 mV, n = 7; P < 0.05), with no significant alteration to the resting membrane potential (control, −63.8 ± 2.0 mV; in 2-APB, −62.8 ± 2.1 mV, n = 7; P > 0.05). The refractory period for the generation of slow potentials was not significantly altered by 2-APB (control, 5.7 ± 1.5 s; in 2-APB, 5.9 ± 1.6 s, n = 7; P > 0.05) (Fig. 4A, B, E). The inhibitory actions of 2-APB were reversible, and 20–30 min washing was required for recovery.
Figure 4. Effects of 2-aminoethoxydiphenyl borate (2-APB) and La3+ on slow potentials.
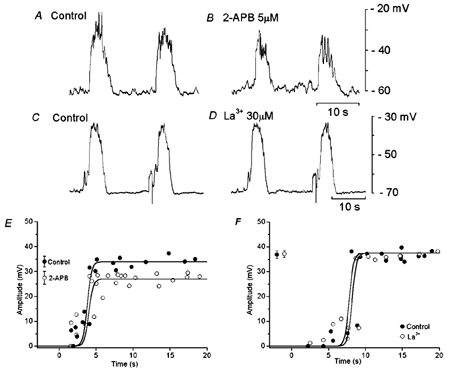
During simultaneous recordings of membrane potential from two cells in a segment of circular smooth muscle tissue from the guinea-pig gastric antrum, 1 s current pulses (A and B, 3.0 nA; C and D, 2.5 nA) were applied to one electrode, and evoked slow potentials were recorded in the absence (A, C) and presence of 5 μM 2-APB (B) or 30 μM La3+ (D). The amplitudes of responses evoked in the second electrode were plotted as a function of the interval between the cessation of a spontaneous slow potential and the initiation of a current pulse, in the absence and presence of 5 μM 2-APB (E) or 30 μM La3+ (F). Mean amplitudes (mean ± s.d.) of slow potentials generated in the absence (•) and presence of 2-APB or La3+ (○) are shown at the left hand side of each figure (E, n = 19 for 2-APB, P < 0.05; F, n = 23 for La3+, P > 0.05).
The effect of La3+ on slow potentials was also examined, since 2-APB has inhibitory actions on non-selective cation channels (Prakriya & Lewis, 2001) and La3+ inhibits these channel conductances (Hoth & Penner, 1993). Application of La3+ (30 μM) for 10–40 min did not alter the amplitude or refractory period of slow potentials (Fig. 4C, D, F). The resting membrane potential was also unchanged by La3+ (control, −63.5 ± 3.5 mV, in La3+, −64.1 ± 2.8 mV; n = 10, P > 0.05). In separate experiments, a lower concentration of La3+ (10 μM) did not reduce the refractory period or alter the amplitude of slow potentials (n = 2, data not shown).
Exposing tissues to solutions containing 10 μM ryanodine for 30–60 min did not alter the amplitude of spontaneous slow potentials (control, 35.5 ± 3.5 mV; in ryanodine, 35.1 ± 3.0 mV; n = 6; P > 0.05), or the resting membrane potential (control, −62.1 ± 1.5 mV; in ryanodine, −62.3 ± 1.8 mV, n = 6; P > 0.05). The refractory period for the generation of slow potential was 6.7 ± 1.9 s, and this value was unaltered in the presence of ryanodine (6.1 ± 1.9 s; n = 6; P > 0.05). Experiments were repeated three times to expose tissues to the ryanodine-containing solution for over 2 h, and no significant change was detected (data not shown). Increasing the concentration of ryanodine to 30 μM did not produce any significant alteration in the amplitude, frequency or the refractory period of slow potentials (n = 2, data not shown).
These results suggest that the inhibition by 2-APB of slow potentials is due mainly to the blockade of IP3 receptors, and not due to the inhibition of non-selective cation currents. Furthermore, IP3 receptors, but not ryanodine receptors, may be involved in the generation of slow potentials, as one of the determinants of the amplitude of slow potentials.
High concentrations of CCCP (> 1 μM) depolarized the membrane and abolished slow potentials in gastric smooth muscle cells (Fukuta et al. 2002). In the present experiments, the concentrations of CCCP tested on slow potentials ranged between 0.1 and 0.3 μM, which did not alter the resting membrane potential. A typical example of the effects of 0.1 μM CCCP on slow potentials is shown in Fig. 5. CCCP applied for 10–30 min reduced the amplitude of spontaneous and evoked slow potentials (control, 28.7 ± 1.7 mV, n = 15; in CCCP, 23.1 ± 1.7 mV; n = 18; P < 0.05), with no change in the membrane potential (control, −65.5 ± 3.5 mV, n = 10; in CCCP, −64.8 ± 2.8 mV, n = 8; P > 0.05) or the refractory period for slow potential generation (about 4.5 s). Similar experiments repeated in five different tissues indicated that the refractory period was not significantly altered by CCCP (control, 5.5 ± 1.5 s; in CCCP, 5.7 ± 1.7 s; P > 0.05). The inhibition by CCCP of slow potentials was not removed after superfusion of the tissue with CCCP-free solution for up to 2 h. These results suggest that mitochondrial functions are involved in determining the amplitude, but not the refractory period of slow potentials.
Figure 5. Effects of carbonyl cyanide m-chloroprene hydrazones (CCCP) on evoked slow potentials.
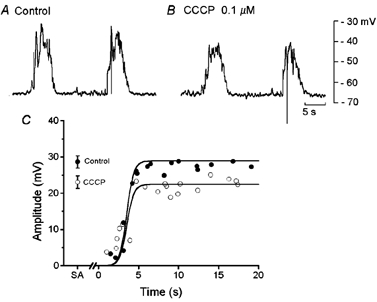
During simultaneous recordings of membrane potential from two cells in a segment of circular smooth muscle tissue from the guinea-pig gastric antrum, current pulses of 1 s duration and 2 nA intensity were applied at about 10 s after cessation of a spontaneously generated slow potential, in the absence (A, control) and presence of 0.1 μM CCCP (B). A and B were recorded from the same pair of cells. C, the relationship between the interval between the cessation of a slow potential and the initiation of a current pulse and the amplitude of the evoked response, in the absence (•, control) and presence of 0.1 μM CCCP (○). SA, amplitude of slow potentials generated spontaneously in the absence (•) and presence of CCCP (○; mean ± s.d.; n = 17 for control and n = 19 for CCCP; P < 0.05).
Chelerythrine and BIM, known inhibitors of PKC, at concentrations higher than 1 μM, depolarized the membrane potential and abolished slow potentials and unitary potentials (data not shown). Experiments were carried out to observe the effects of chelerythrine in concentrations that allowed the generation of spontaneous slow potentials with no alteration in the membrane (equal to 0.1-0.3 μM). Application of 0.3 μM chelerythrine for 10–40 min reduced the frequency (control, 1.88 ± 0.58 min−1; in chelerythrine, 1.10 ± 0.23 min−I; n = 6; P < 0.05) and amplitude of slow potentials (control, 30.3 ± 2.5 mV; in chelerythrine, 27.5 ± 2.2 mV; n = 6; P < 0.05), with no change in the resting membrane potential (control, −65.7 ± 3.8 mV; in chelerythrine, −66.5 ± 4.1 mV; n = 6; P > 0.05). In the presence of chelerythrine, the refractory period for the generation of slow potentials was increased (control, 7.6 ± 1.8 s; in chelerythrine, 10.8 ± 1.3 s; n = 6; P < 0.05), with an accompanying reduction in amplitude of the evoked slow potentials by about 20 % (Fig. 6A–D).
Figure 6. Effects of chelerythrine and bisindolylmaleimide I (BIM) on slow potentials.
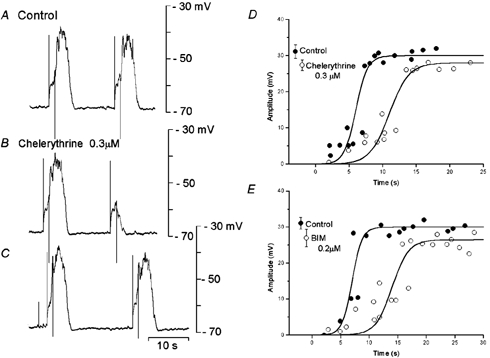
A segment of circular smooth muscle tissue was stimulated by pulses (1.2 s duration, 2.5 nA intensity) at about 10 s (A and B) and 15 s (C) after the cessation of an evoked slow potential, in the absence (A, control) and presence of 0.3 μM chelerythrine (B and C). The amplitudes of the evoked responses were measured, in the absence (•, control) and presence (○) of 0.3 μM chelerythrine (D) or 0.2 μM BIM (E). Mean amplitudes (± s.d.) of the conditioning slow potentials are shown at the left hand sides of each graph (D, n = 9 for control and chelerythrine, P < 0.05; E, n = 9 for control and n = 12 for BIM, P < 0.05). A, B and C were recorded from the same pair of cells. D and E were obtained from different tissues.
BIM exhibited effects similar to those of chelerythrine on slow potentials, and application of 0.1 μM BIM for over 10 min reduced the amplitudes of slow potentials (control, 31.1 ± 1.6 mV; in BIM, 27.7 ± 2.4 mV; n = 8; P < 0.05), with no change in the resting membrane potential (control, −66.3 ± 2.5 mV; in BIM, −65.8 ± 3.2 mV; n = 8; P > 0.05). In the presence of BIM, the refractory period for the generation of slow potentials was increased (control, 6.1 ± 2.8 s; in BIM, 12.8 ± 3.8 s; n = 8; P < 0.05) and the amplitude of evoked slow potentials was reduced by about 10 % (Fig. 6E). The inhibitory actions of chelerythrine or BIM were irreversible, for up to 2 h of washing. These results suggest that the inhibition of PKC increases the refractory period and reduces the amplitude of slow potentials.
Effects of activation of PKC by PMA on slow potentials
Experiments were carried out to test the effects of activation of PKC with PMA on evoked slow potentials. High concentrations of PMA (> 1 μM) abolished spontaneous and evoked slow potentials, with no significant change in the resting membrane potential (control, −65.5 ± 3.5 mV; in 1 μM PMA, −65.1 ± 2.8 mV; n = 7; P > 0.05). The concentrations of PMA tested, between 1 and 100 nm, did not alter the resting membrane potential. Figure 7 shows the effects of 1 and 100 nm PMA (> 10 min exposure) on slow potentials produced spontaneously and evoked by 1.5 s pulses. The resting membrane potential was not altered by PMA (control, −64.3 ± 3.0 mV; in 1 nm PMA, −64.5 ± 5.1 mV, n = 7, P > 0.05; in 100 nm PMA, −64.1 ± 2.5 mV, n = 7, P > 0.05). The frequency of spontaneously generated slow potentials (1.76 ± 0.79 min−1) was not altered in the presence of 1 nm PMA (1.66 ± 0.85 min−1; n = 7; P > 0.05) and was decreased in the presence of 100 nm PMA (0.8 ± 0.65 min−1, n = 7; P < 0.05). The refractory period of the evoked slow potentials (5.5 ± 0.8 s, n = 6) was decreased in the presence of 1 nm PMA (4.0 ± 1.0 s; n = 6; P < 0.05) and increased in the presence of 100 nm PMA (10.5 ±2.5 s; n = 6; P < 0.05). The actions of PMA persisted after removal of this chemical from the superfusate for up to 3 h. These results indicate that PMA has dual actions, low concentrations reduce and high concentrations increase the refractory period. The amplitude of spontaneous slow potentials was not altered by 1 nm PMA and tended to decrease in 100 nm PMA (Fig. 7).
Figure 7. Effects of phorbol-12-myristate-13-acetate (PMA) on slow potentials.
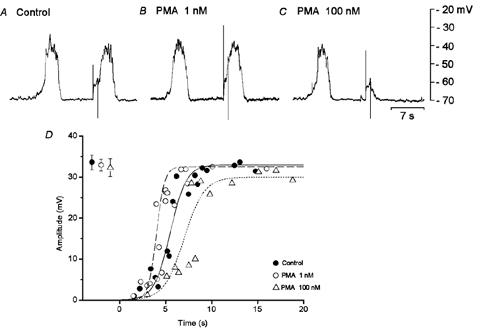
In an isolated single circular muscle bundle from the guinea-pig stomach antrum, membrane potentials were recorded from a pair of cells. Depolarizing pulses (1.5 s duration, 3 nA) were applied at about 5 s intervals following the cessation of a spontaneously generated slow potential, in the absence (A) and presence of PMA (B, 1 nm; C, 100 nm). All responses were recorded from the same cell. D, relationship indicating the amplitude of depolarizing responses evoked by stimulating pulses applied at various times after cessation of a slow potential, in the absence (•) and presence of PMA (○, 1 nm; ▵, 100 nm). Values shown at the left hand side indicate the mean amplitude of spontaneously generated slow potentials in the absence and presence of PMA (mean ± s.d., n = 17 for control, n = 17 for 1 nm PMA and n = 13 for 100 nm PMA, P > 0.05 for any given pair).
Effects of ACh on slow potentials
None of the concentrations of ACh tested (30-50 nm) altered the resting membrane potentials of gastric smooth muscle cells, as reported previously (Komori & Suzuki, 1986). ACh increased the frequency and amplitude of spontaneously generated slow potentials by 15–20 % of control values (frequency: 1.35 ± 0.35 times; n = 12; amplitude: 1.21 ± 0.31 times, n = 12). The amplitude of evoked slow potentials was also increased by ACh by 1.25 ± 0.25 times (n = 12), with an associated reduction in the refractory period by 30–60 % of control (Fig. 8A and B).
Figure 8. Effects of ACh and 2-APB on slow potentials.
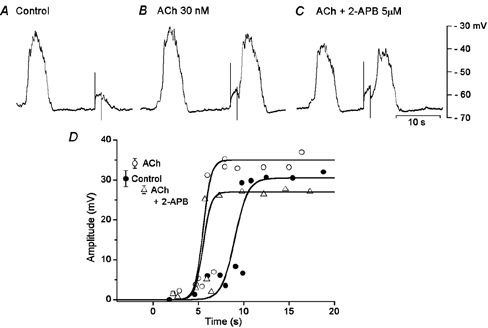
An isolated segment of circular smooth muscle of guinea-pig gastric antrum was stimulated with depolarizing pulses (1.5 s duration, 2 nA) in the absence (A, control) and presence of 30 nm ACh (B) and ACh + 5 μM 2-APB (C). All responses were recorded from the same cell (the resting membrane potential, −64 mV). D, amplitudes of responses evoked by depolarizing pulses applied at various times after the cessation of a spontaneously generated slow potential, in the absence (•) and presence of ACh (○) and ACh + 2-APB (▵). The values shown at the left hand side of the graph indicate the amplitude of spontaneous slow potentials (mean ± s.d.; P < 0.05 compared to control).
Experiments were carried out to test the effects of 2-APB, chelerythrine and BIM on these actions of ACh. As shown in Fig. 8C, 2-APB (5 μM) added in the presence of ACh reduced the amplitude of slow potentials. In five preparations, the mean amplitude of slow potentials was reduced to 82.3 ± 8.5 % of control by 2-APB. The refractory period for the generation of slow potentials, once reduced by ACh, was not further altered by addition of 2-APB (in ACh, 5.9 ± 1.1 s; in ACh + 2-APB, 5.6 ± 1.0 s; n = 5; P > 0.05; Fig. 8C and D). These results indicate that ACh decreases the refractory period and increases the amplitude of slow potentials, and 2-APB antagonizes only the latter action.
The effects of BIM (0.2 μM) on slow potentials enhanced by ACh are shown in Fig. 9. BIM increased the refractory period that had been reduced by ACh (in ACh, 5.1 ± 0.2 s; ACh + BIM, 8.5 ± 0.2 s; n = 6; P < 0.05), with no change in the membrane potential (in ACh, −63.8 ± 2.1 mV; in ACh + BIM, −63.5 ± 2.5 mV; n = 6; P > 0.05). However, the amplitude of slow potentials enhanced by ACh was not significantly reduced by BIM (in ACh, 35.3 ± 2.5 mV; in ACh + BIM, 35.8 ± 1.8 mV, n = 6; P > 0.05).
Figure 9. Effects of ACh and BIM on slow potentials.
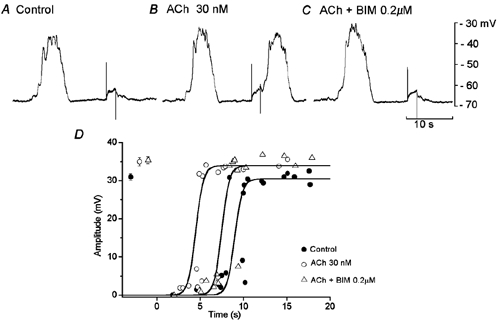
In an isolated segment of circular smooth muscle of guinea-pig gastric antrum, membrane potential responses to stimulation by depolarizing pulses (2 s duration, 2 nA) were recorded, in the absence (A, control) and presence of 30 nm ACh (B) and ACh + 0.2 μM BIM (C). All responses were recorded from the same cell. D, amplitude of responses evoked by depolarizing pulses applied at various times after the cessation of a spontaneous slow potential, in the absence (•) and presence of 30 nm ACh (○) and ACh with 0.2 μM BIM (▵). Values shown at the left hand side of the graph indicate the amplitude of spontaneous slow potentials (mean ± s.d.; P < 0.05 compared to control).
In a separate series of experiments, the effects of chelerythrine on slow potentials enhanced by ACh were also observed. In the presence of 30 nm ACh, chelerythrine (0.3 μM) again increased the refractory period (in ACh, 5.6 ± 1.3 s; in ACh + chelerythrine, 11.0 ± 2.1 s; n = 4; P < 0.05), with no change in the amplitude of slow potentials (in ACh, 33.4 ± 1.3 mV; in ACh + chelerythrine, 33.3 ± 1.4 mV; n = 10; P > 0.05). Increasing the concentration of chelerythrine to 1 μM resulted in a marked increase in the refractory period (in ACh, 4.8 ± 1.3 s; in ACh + chelerythrine, 13.9 ± 4.1 s; n = 3; P < 0.05) and a reduction in amplitude of slow potentials (in ACh, 39.8 ± 1.1 mV; in ACh + chelerythrine, 32.1 ± 1.4 mV; n = 3; P < 0.05). Thus, the inhibition of PKC antagonizes the ACh-induced reduction of the refractory period for slow potentials, with no alteration to the amplitude of slow potentials. Stronger inhibition of PKC results in an inhibition of the actions of ACh on both the refractory period and the amplitude of slow potentials.
Discussion
The present experiments indicated that in small segments of circular smooth muscle bundles isolated from the guinea-pig gastric antrum, slow potentials were evoked by depolarizing the membrane with current pulses, irrespective of the presence or absence of spontaneously generated slow potentials. The evoked slow potentials had refractory periods lasting several seconds, and the duration of these refractory periods varied inversely with the amplitude and duration of the conditioning depolarization. Thus, slow potentials are generated in an all-or-none fashion, as in the case of nerve action potentials. The refractory period for excitation of nerves is related to the time required for the recovery of voltage-gated Na+ channels from depolarization-induced inactivation. Spectral analysis of the components of slow potentials indicates that the potentials are formed by a summation of unitary potentials (Edwards et al. 1999). If this is the case, the evoked slow potentials may be the result of the instantaneous generation of unitary potentials in response to depolarization.
Experiments were carried out to test the effects of several types of chemicals that are known to modulate cellular functions on the refractory period for generation of evoked slow potentials. Most chemicals tested (i.e. thapsigargin, 2-APB, CCCP, chelerythrine and BIM) depolarized the membrane and inhibited spontaneous activities including slow potentials and unitary potentials when the concentrations were high (> 1 or 10 μM). Therefore, concentrations of chemicals tested were reduced to levels that did not alter the resting membrane potential (usually < 1 μM), and these often produced indistinguishable effects. However, experiments carried out in the absence of any change in the membrane potential facilitated the analysis and evaluation of the effects of chemicals tested.
The refractory period of slow potentials was increased by inhibiting the activity of PKC with chelerythrine or BIM, but was not altered by inhibiting the Ca2+ pump at the internal Ca2+ store with thapsigargin, by inhibiting the IP3-mediated release of Ca2+ from internal stores with 2-APB or by inhibiting Ca2+ handling in the mitochondria with CCCP. On the other hand, inhibitors of the release of Ca2+ from internal stores, such as thapsigargin and 2-APB, reduced the amplitude of slow potentials with no marked alteration of the refractory period. These results suggest that the refractory period for the generation of slow potentials is related to the activation of PKC, whereas their amplitude is determined mainly by the amount of Ca2+ released from internal stores. Thus, the mechanisms determining the refractory period of slow potentials differ from those that determine the amplitude.
In circular smooth muscle bundles isolated from the guinea-pig gastric antrum, slow potentials are generated by depolarization of the membrane, with a minimum latency of about 1 s (Suzuki & Hirst, 1999), which is comparable to the delay required for the production of IP3 in response to stimulation with agonists (Somlyo & Somlyo, 1994). These observations allowed the speculation that membrane depolarization produces unidentified second messengers that accelerate the release of Ca2+ from the internal stores (Suzuki, 2000). Slow waves are absent in the gastric smooth muscle of mutant mice lacking expression of the IP3 receptor (Suzuki et al. 2000). 2-APB, an inhibitor of the IP3-receptor-mediated release of Ca2+ from internal stores (Maruyama et al. 1997), abolishes slow waves (Hirst & Edwards, 2001) and slow potentials (Fukuta et al. 2002) in gastric smooth muscles of the guinea-pig. In murine small intestine, inhibition of spontaneous activity by xestospongine C is mediated by the blockade of the release of Ca2+ from the IP3-sensitive stores (Malysz et al. 2001). These results suggest that the production of IP3 is a key factor in the generation of slow potentials during depolarization, and that the amount of Ca2+ released would determine the amplitude of slow potentials, possibly by activation of calcium-activated Cl− channels (Hirst et al. 2002). The observation of increased production of receptor-mediated IP3 during depolarization of the membrane (Ganitkevich & Isenberg, 1993) supports this suggestion. In addition to elevated production of IP3, PKC is also activated by stimulation with several types of agonists (Nishizuka, 1986) and by depolarization of the membrane, possibly through an elevation of [Ca2+]i (Kong et al. 1991; Maasch et al. 2000). The results of the present experiments suggest that this is also the case in antral smooth muscle tissues, and PKC may be one of the essential factors for the initiation of slow potentials in response to depolarizing stimuli.
It is interesting to consider how PKC might be involved in the generation of depolarization-evoked slow potentials. PKC, being activated by Ca2+, translocates to the membrane side (Nishizuka, 1986) and activates membrane-bound functional proteins such as phospholipase C (PLC), possibly by increasing the influx of Ca2+ through activation of ion channels such as capacitative Ca2+ channels (Ma et al. 2001). The reduction of [Ca2+]o or chelating internal Ca2+ with BAPTA abolishes evoked slow potentials in gastric muscles (Suzuki & Hirst, 1999; Fukuta et al. 2002), allowing speculation that the increased influx of extracellular Ca2+ is required for the generation of slow potentials. Voltage-gated influx of Ca2+ may be negligible, since experiments have been carried out in the presence of nifedipine. 2-APB and La3+ are known inhibitors of capacitative Ca2+ conductances (Broad et al. 2001; Missiaen et al. 2001; Prakriya & Lewis, 2001), and these chemicals do not alter the refractory period for slow potential generation, suggesting that the influx of Ca2+ through these ion channels is also not significantly involved in the generation of evoked slow potentials.
The slow potentials generated spontaneously had identical refractory periods to those evoked by depolarizing pulses. The cellular mechanisms of the generation of these two types of slow potentials may also be similar, since both are abolished by low concentrations (0.3-1 mm) of caffeine (Suzuki & Hirst, 1999). These results could be extrapolated to indicate that PKC is a key requirement for the initiation of spontaneous slow potentials. In fact, higher concentrations of chelerythrine or BIM abolished spontaneous slow potentials. In the gastric muscles of IP3-receptor knock-out mice, slow waves are absent, but spike potentials are still generated spontaneously (Suzuki et al. 2000), suggesting that PKC is located upstream of IP3 in the signalling pathways for the initiation of slow potentials. The spontaneous generation of slow potentials is inhibited by preventing Ca2+ handling in mitochondria with CCCP (Fukuta et al. 2002), suggesting that rhythmic activation of PKC could be maintained by Ca2+ released from the mitochondria. However, the present experiments provided contradictory results in response to CCCP. It remains unclear how mitochondrial functions are related to the generation of evoked slow potentials in gastric muscles.
ACh reduced the refractory period of slow potentials by activation of PKC, since the actions were antagonized by inhibitors of PKC. ACh also increased the amplitude of slow potentials, and this increase was antagonized by 2-APB, but not by inhibitors of PKC. This indicates that the facilitated production of IP3 by ACh with activation of PLC increased the amplitude of slow potentials through the increased release of Ca2+ from the internal stores. These results agree with the concepts that ACh activates both PKC and PLC (Nishizuka, 1986). ACh is a major transmitter of gastric motor nerves, and enhances spontaneous electrical and mechanical responses in the guinea-pig gastric muscles (Komori & Suzuki, 1986, 1988). The results obtained in the present experiments suggest that the facilitated generation of slow waves by ACh is related to the reduction of the refractory period of slow potentials due to activation of PKC.
It is expected that both ACh and PMA reduce the refractory period and increase the amplitude of slow potentials, since ACh stimulates PLC through activation of G-proteins and PKC, possibly indirectly through elevated production of diacylglycerol, whereas PMA stimulates PKC directly (Nishizuka, 1986). However, the results obtained here indicate that only low concentrations of PMA mimicked the actions of ACh on slow potentials. In cultured A7r5 smooth muscle cells, phorbol 12,13-dibutyrate has concentration-dependent dual actions on the translocation of PKCα; low concentrations exert their effects at subplasmalemmal sites and high concentrations exert their effects at perinuclear sites (Li et al. 2001). Similar dual actions of PMA on gastric muscles are also thought possible. Alternatively, high concentrations of PMA could induce a sustained activation of PKC through which the rhythmic oscillation of [Ca2+]i is prevented in pacemaker cells.
Taken together, the results suggest an importance of PKC for the refractory period of slow potentials. As the depolarization of the membrane activates PKC for translocation (Kong et al. 1991; Maasch et al. 2000), it is speculated that the refractory period for slow potential generation is related to the recovery of PKC being available for translocation, or alternatively the refractory period is related to the time required for the supply of sufficient amount of PKC for translocation in a certain proportion of pacemaker cells. IP3 may also be involved in the generation of slow potentials, but possibly acting downstream of PKC, as a determinant of the amplitude of slow potentials through regulation of the amount of Ca2+ released from the internal stores. The unitary potentials may be propagated from IC-IM through gap junctions (Dickens et al. 2001), and slow potentials are the summed potentials of these unitary potentials (Edwards et al. 1999). These results allow speculation that the depolarization of the smooth muscle membrane is conducted to IC-IM and evokes unitary potentials, possibly through activation of PKC.
Acknowledgments
The authors are grateful to Dr Frank R. Edwards for critical reading of the manuscript. The present experiments were supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to H. S. (No. 14570044).
References
- Broad LM, Braun F-J, Lievremont J-P, Bird GS, Kurosaki T, Putney JW., Jr Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. Journal of Biological Chemistry. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GDS. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. Journal of Physiology. 2001;531:827–833. doi: 10.1111/j.1469-7793.2001.0827h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. Journal of Physiology. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Contribution of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. Journal of Physiology. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. Journal of Physiology. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher JJ, Lipford GB. Mechanism of action of ryanodine on cardiac sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1985;813:77–86. doi: 10.1016/0005-2736(85)90347-5. [DOI] [PubMed] [Google Scholar]
- Fukuta H, Kito Y, Suzuki H. Spontaneous electrical activity and associated changes in calcium concentration in the guinea-pig gastric smooth muscle. Journal of Physiology. 2002;540:249–260. doi: 10.1113/jphysiol.2001.013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary artery. Journal of Physiology. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochemical and Biophysical Research Communications. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig antrum involves a delayed increase in [Ca2+]i and Cl− channels. Journal of Physiology. 2002;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Edwards FR. Generation of slow wave in the antral region of the guinea-pig stomach – a stochastic process. Journal of Physiology. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cell. Journal of Physiology. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Vanderwinden J-M, Rumessen J. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends in Pharmacological Sciences. 1997;8:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- Komori K, Suzuki H. Distribution and properties of excitatory and inhibitory junction potentials in circular smooth muscle of the guinea-pig stomach. Journal of Physiology. 1986;370:339–355. doi: 10.1113/jphysiol.1986.sp015938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K, Suzuki H. Modulation of smooth muscle activity by excitatory and inhibitory nerves in the guinea-pig stomach. Comparative Biochemistry and Physiology. 1988;91:311–319. doi: 10.1016/0742-8413(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Komuro T, Tokui K, Zhou DS. Identification of the interstitial cells of Cajal. Histology and Histopathology. 1996;11:769–786. [PubMed] [Google Scholar]
- Kong SK, Choy YM, Fung KP, Lee CY. Membrane depolarization induces protein kinase C translocation and voltage operated calcium channel opening in PU5–1. 8 cells. Protein kinase C as a negative feedback modulator for calcium signaling. Second Messengers Phosphoproteins. 1991;13:117–130. [PubMed] [Google Scholar]
- Li C, FultZ MF, Geng W, Ohno S, Norton M, Wright GL. Concentration-dependent phorbol stimulation of PKCα localization at the nucleus or subplasmalemma in A7r5 cells. Pflügers Archiv. 2001;443:38–47. doi: 10.1007/s004240100627. [DOI] [PubMed] [Google Scholar]
- Ma R, Pluznick J, Kudlacek P, Sansom SC. Protein kinase C activates store-operated Ca2+ channels in human glomerular mesangial cells. Journal of Biological Chemistry. 2001;276:25759–25765. doi: 10.1074/jbc.M011241200. [DOI] [PubMed] [Google Scholar]
- Maasch C, Wagner S, Lindschau C, Alexander G, Buchner K, Gollasch M, Luft FC, Haller H. Protein kinase α targeting is regulated by temporal and spatial changes in intracellular free calcium concentration [Ca2+]i. FASEB Journal. 2000;14:1653–1663. doi: 10.1096/fj.14.11.1653. [DOI] [PubMed] [Google Scholar]
- Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP3-sensitive calcium release in the murine small intestine. American Journal of Physiology. 2001;280:G439–448. doi: 10.1152/ajpgi.2001.280.3.G439. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2-APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. Journal of Biochemistry. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Callewaert G, De Smedt H, Parys JB. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29:111–116. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Perspectives on the role of protein kinase C in stimulus-response coupling. Journal of National Cancer Investigation. 1986;76:363–370. [PubMed] [Google Scholar]
- Nose K, Suzuki H, Kannan H. Voltage-dependency of the frequency of slow waves in antrum smooth muscle of the guinea-pig stomach. Japanese Journal of Physiology. 2000;50:625–633. doi: 10.2170/jjphysiol.50.625. [DOI] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. Journal of Physiology. 1975;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T, Kuriyama H. The membrane properties and decremental conduction of excitation in the fundus of the guinea-pig stomach. Japanese Journal of Physiology. 1970;20:626–639. doi: 10.2170/jjphysiol.20.626. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB). occurs independently of IP3 receptors. Journal of Physiology. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Cellular mechanisms of myogenic activity in gastric smooth muscle. Japanese Journal of Physiology. 2000;50:289–301. doi: 10.2170/jjphysiol.50.289. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. Journal of Physiology. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kito Y, Fukuta H, Yamamoto Y. Possible involvement of IP3 in the generation of slow potentials in smooth muscle of the guinea-pig stomach. Journal of Smooth Muscle Research. 2001;5:J-29. in Japanese. [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptors. Journal of Physiology. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proceedings of the National Academy of Sciences of the USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow wave) in gastrointestinal smooth muscles. In: BÜlbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle. London: Edward Arnold; 1981. pp. 127–156. [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. Journal of Biological Chemistry. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Van Helden DF, ImtiaZ MS, Nurgaliyeva K, Von Der Weid P-Y, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. Journal of Physiology. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


