Abstract
This study explores the importance of creatine kinase (CK) in the regulation of muscle mitochondrial respiration in human subjects depending on their level of physical activity. Volunteers were classified as sedentary, active or athletic according to the total activity index as determined by the Baecke questionnaire in combination with maximal oxygen uptake values (peak V̇O2, expressed in ml min−1 kg−1). All volunteers underwent a cyclo-ergometric incremental exercise test to estimate their peak V̇O2 and V̇O2 at the ventilatory threshold (VT). Muscle biopsy samples were taken from the vastus lateralis and mitochondrial respiration was evaluated in an oxygraph cell on saponin permeabilised muscle fibres in the absence (V̇0) or in the presence (V̇max) of saturating [ADP]. While V̇0 was similar, V̇max differed among groups (sedentary, 3.7 ± 0.3, active, 5.9 ± 0.9 and athletic, 7.9 ± 0.5 μmol O2 min−1 (g dry weight)−1). V̇max was correlated with peak V̇O2 (P < 0.01, r = 0.63) and with V̇T (P < 0.01, r = 0.57). There was a significantly greater degree of coupling between oxidation and phosphorylation (V̇max/V̇0) in the athletic individuals. The mitochondrial Km for ADP was significantly higher in athletic subjects (P < 0.01). Mitochondrial CK (mi-CK) activation by addition of creatine induced a marked decrease in Km in athletic individuals only, indicative of an efficient coupling of mi-CK to ADP rephosphorylation in the athletic subjects only. It is suggested that increasing aerobic performance requires an enhancement of both muscle oxidative capacity and mechanisms of respiratory control, attesting to the importance of temporal co-ordination of energy fluxes by CK for higher efficacy.
Human exercise capacity depends on various factors, from oxygen uptake to oxygen utilisation. The relative importance of these is not yet fully understood and while O2 utilisation at the mitochondria most probably represents the ultimate step of the maximal O2 uptake (V̇O2,max), each single step in the O2 cascade contributes in an integrated way to determine O2 availability and finally V̇O2,max (Wagner, 1992). Moreover, it is well recognised that V̇O2,max sets the upper limit for energy production in endurance sport, but does not determine the final performance. As endurance capacity improves, substantial metabolic and structural adaptations take place in skeletal muscle, with a reduction in the rate of glycogen depletion, a reduced rate of lactate accumulation and enhanced fat oxidation (Gollnick, 1986; Horowitz & Klein, 2000). This occurs simultaneously with an increase in mitochondrial and capillary density (Hoppeler et al. 1985), as well as in mitochondrial enzyme changes (Holloszy & Coyle, 1984).
A simple quantitative increase in mitochondrial content, however, may not be sufficient to provide a higher endurance capacity. Indeed, each muscle type exhibits an energy metabolic profile adapted to its pattern of activity. Adaptations involve energy production components, such as glycolytic enzymes and mitochondria, as well as mechanisms of cellular energy transfer such as the creatine kinase (CK) system (Ventura-Clapier et al. 1998). The fast glycolytic muscle is adapted to a brief and intense energy utilisation based on the immediate availability of the cytosolic store of energy (mainly phosphocreatine and ATP), on the buffering effect of the CK reaction in the cytosol, and on a high glycolytic activity (Kushmerick et al. 1992; Veksler et al. 1995; Kuznetsov et al. 1996). On the other hand, because of its high oxidative capacity and an efficient coupling between energy utilisation and energy production, the slow oxidative muscle is adapted to sustain prolonged work (Ventura-Clapier et al. 1998; Kay et al. 2000; Saks et al. 2001). This is achieved by specific CK isoenzymes located in the mitochondrial intermembrane space (mi-CK) at the site of production and in close interaction with the cellular ATPases involved in excitation-contraction coupling at sites of utilisation (e.g. Na+-K+-ATPase at the sarcolemma, calcium ATPase at the sarcoplasmic reticulum, myosin ATPase at the myofilaments). Another important parameter of mitochondrial function is the sensitivity of respiration to ADP. In mitochondria, the rephosphorylation of ADP is thought to follow Michaelis-Menten kinetics, and the apparent Km for ADP (inversely proportional to the affinity of mitochondria for ADP), has been shown to be related to the metabolic profile of the muscle, being higher in muscle with higher oxidative capacity. In such oxidative muscles, addition of creatine decreases the Km for ADP, and ATP production is then coupled to phosphocreatine (PCr) resynthesis within the intermembrane space. Thus, the mechanisms for the control of mitochondrial respiration may be based on coupled multi-enzymatic systems within the intermembrane space, and on the properties of the outer mitochondrial membrane. In oxidative muscle, because mitochondrial CK (mi-CK) is coupled to the ATP/ADP translocase, newly produced ATP is channelled to the mi-CK, which phosphorylates creatine into PCr and produces ADP that is channelled back to the mitochondrial matrix (Saks et al. 1994). This system amplifies the small cytoplasmic ADP signals and controls the mitochondrial respiration rate (Saks et al. 1994). On the other hand, in glycolytic muscle the mitochondrial respiration is not controlled by the mi-CK and the accessibility of ADP to the mitochondrial matrix is high (Veksler et al. 1995).
Such a regulation of mitochondrial respiration can be demonstrated with a validated method of investigation of mitochondrial respiration in situ using saponin-skinned muscle fibres (Veksler et al. 1987; Saks et al. 1998). Respiration of skinned fibres is a unique means of assessing the function of the whole mitochondrial population maintained in its cyto-architectural environment, while controlling amounts of oxygen and substrates. This has only recently been applied to human skeletal muscle samples, and has demonstrated that the maximal tissue oxidative capacity is not affected by acute exercise (Tonkonogi et al. 1998), but increases after short-term training (Walsh et al. 2001a) or with activity level (Mettauer et al. 2001). These human studies have shown quantitative changes in oxidative capacity that were expected from earlier morphological studies (Hoppeler et al. 1985). The mitochondrial sensitivity to ADP has been suggested to be lower if the percentage of type I fibre is high and if subjects undergo a period of training (Tonkonogi et al. 1998; Walsh et al. 2001a). However, the qualitative changes in terms of coupling efficiency between energy expenditure and production, as well as the regulatory properties of the mi-CK system, have not been assessed in detail.
The aim of this study, therefore, was to investigate how mitochondrial regulation is adapted to different levels of physical activity by applying the permeabilised fibre technique to human skeletal muscle samples. To achieve this goal we explored middle-aged volunteers from the truly sedentary to the regularly trained level. Indeed, a wide range of fitness levels exists in humans, starting from completely sedentary to extreme endurance capacity, depending on degree, duration, and type of physical activity. The results show that, in addition to increased oxidative capacity, high fitness levels are accompanied by a tighter control of respiration by phosphate acceptors and an increased role of the CK system ensuring an efficient servo-control of energy production to energy expenditure.
Part of this study has been presented at the 49th Meeting of the American College of Cardiology.
Methods
Subject groups and exercise testing
Twenty-nine normal human subjects (4 females, 25 males) were enrolled in the study. All subjects gave written informed consent and were fully informed about the potential risks of the study. All experiments were approved by our institution's ethics committee (Comité Consultatif pour la Protection des Personnes participant à la Recherche Biomédicale) and conformed to the Declaration of Helsinki. Following a preliminary interview, the subjects were classified into three groups: (i) sedentary if they had no regular professional or recreational physical activity, (ii) physically active if they performed physical activity regularly for professional or recreational purposes but followed no regular training programme, and (iii) athletic if they were involved in a training programme with endurance sessions on a regular basis. For the determination of the subgroups, all the subjects filled in a modified Baecke physical activity questionnaire (Baecke et al. 1982; Bigard et al. 1992). As a rule, the Baecke index of total activity was below 6 in sedentary, between 6 and 8 in active and above 8 in athletic subjects. All subjects underwent a cyclo-ergometric incremental symptom-limited maximal exercise test while measuring V̇O2, carbon dioxide production (V̇CO2) and minute ventilation (V̇E) breath by breath by means of an open circuit metabolic cart (Sensor Medics Vmax229, Yorba Linda, CA, USA). The exercise test increments were designed (15-25 W min−1) to exhaust the subject within 10–15 min, and took place within 1 week of their skeletal muscle biopsy. In all subjects the point of aerobic-anaerobic transition was determined by the ventilatory threshold (VT) as defined by the V-slope analysis (Wasserman et al. 1990). These tests yielded the rest, VT and peak V̇O2, respiratory exchange ratio (RER = V̇CO2/V̇O2) and heart rate (HR). As the subjects stopped exercise when they were no longer able to sustain a 50–60 r.p.m. pedalling rate and all had a peak RER largely above 1.1 we assumed that their peak V̇O2 reasonably approached the V̇O2,max.
Skeletal muscle biopsy
Vastus lateralis muscle biopsies were taken by the percutaneous Bergström technique after local anaesthesia (lidocaine/ lignocaine) within 1 week of the maximal exercise test. No complications occurred following biopsies in any subject. The muscle tissue retrieved was rinsed in ice-cold saline, one part was immediately frozen in liquid nitrogen for enzymatic and myosin heavy chain (MHC) isoforms determinations and another part served for the in situ respiration studies. Briefly, thin bundles (diameter, 100–200 μm) were dissected in ice-cold relaxing solution containing (mm): EGTA-calcium buffer 10 (free Ca2+ concentration 100 nmol l−1), MgCl2 1, taurine 20, DTT 0.5, imidazole 20 (pH 7.1), MgATP 5, PCr 15, at ionic strength 160 (potassium methanesulfonate), and permeabilized for 30 min with 50 μg ml−1 saponin, as previously described (De Sousa et al. 1999).
Mitochondrial respiration
Respiratory parameters of the total mitochondrial population were studied in situ as previously described (Veksler et al. 1987; Mettauer et al. 2001) using a Clark electrode (Strathkelvin Instruments, Glasgow, Scotland) in a water-jacketed oxygraphic cell containing 3 ml respiration solution (see below) at 22 °C with continuous stirring. Respiration rates were expressed in micromoles of O2 per minute per gram dry weight. The respiration solution was of the same composition as the relaxing solution except that MgATP and PCr were replaced by 5 mmol l−1 glutamate, 2 mmol l−1 malate as substrates, and 3 mmol l−1 phosphate and 2 mg ml−1 fatty acid-free bovine serum albumin. ADP-stimulated respiration (V̇ADP) above basal oxygen consumption (V̇0) was measured by stepwise addition of ADP (from 2.5 to 2000 μM) with or without creatine (20 mm). The apparent Km values for ADP, which are inversely proportional to ADP sensitivity, and V̇ADP were calculated using a non-linear mono-exponential fitting of the Michaelis-Menten equation. Maximal respiration rate (V̇max) was calculated as (V̇ADP + V̇0). Acceptor control ratio (ACR) was calculated as V̇max/V̇0.
Enzyme analysis
Part of the frozen tissue samples were weighed, homogenised into ice-cold buffer (30 mg ml−1) containing 5 mm Hepes, 1 mm EGTA, 5 m MgCl2 and 0.1 % Triton X-100 (pH 8.7) and incubated for 60 min at 0 °C to ensure complete enzyme extraction. Creatine kinase (CK), and lactate dehydrogenase (LDH) were assayed (30 °C, pH 7.5) using coupled enzyme systems as previously described (Bigard et al. 1998). CK, isoenzymes (MM-CK, MB-CK, mi-CK) and LDH isoenzymes were separated using agarose gel (1 %) electrophoresis performed at 200 V for 90 min. Individual isoenzyme composition was resolved either by incubating the gels with a coupled enzyme system (CK) or a commercial revelation system (Sigma LDH reagent kit) followed by image analysis.
Myosin heavy chains
In other frozen tissue samples, myosin was crudely extracted in a high ionic strength buffer as previously described (Bigard et al. 1998). Myosin heavy chains were separated in 8 % acrylamide-bis (50 : 1) slab gels, at constant voltage (70 V) for 30–31 h and silver stained (Agbulut et al. 1996), then scanned by laser densitometry (Biorad GS700).
Statistical analysis
The values were expressed as means ± standard error of the mean (s.e.m.). One-way analysis of variance (ANOVA) was used to determine the global effect of physical activity level. When appropriate, differences between groups were tested with a Newman-Keuls post hoc test. Within each group the comparison of the Km values with and without creatine was performed using Student's paired t test. The significance level was taken to be P < 0.05.
Results
Subjects' characteristics
Subjects' characteristics are given in Table 1. By design, their total activity index from the Baecke questionnaire differed among groups (P < 0.001). The peak V̇O2 was below 110 % of the predicted V̇O2,max as calculated by Hansen's formulas (Wasserman et al. 1990) in sedentary, between 110 and 125 % of predicted V̇O2,max in active and above 125 % of predicted V̇O2,max in athletic subjects. While ages and heights were similar among groups, weight of the athletic group was lower than for the active and sedentary subjects (P < 0.05 and P < 0.01, respectively).
Table 1.
Group characteristics
| Sedentary | Active | Athletic | |
|---|---|---|---|
| Male/female | 8/0 | 6/2 | 11/2 |
| Age (years) | 46.7 ± 2.8 | 45.6 ± 3.0 | 40.5 ± 2.5 |
| Weight (kg) | 84.4 ± 3.1 | 78.1 ± 4.4 | 68.2 ± 2.3**† |
| Height (cm) | 178 ± 2 | 173 ± 2 | 174 ± 2 |
| % predicted V̇o2,max | 86.2 ± 3.5 | 117.2 ± 4.0*** | 146.5 ± 9.1***††† |
| Total activity index | 5.8 ± 0.2 | 7.0 ± 0.4* | 9.2 ± 0.3***††† |
Values are presented as means ± s.e.m.
P < 0.05
P < 0.01
P < 0.001 versus sedentary.
P < 0.05
P < 0.001 versus active. %predicted V̇o2,max, peak oxygen uptake as a percentage of the predicted maximum oxygen uptake for sedentary.
Exercise tests
Table 2 reports the parameters of general aerobic performance during the incremental symptom-limited exercise test. At rest, gas exchange was similar among groups. As expected for a similar absolute work load, heart rate (HR) decreased with elevation of the activity level, being significantly lower in the athletic group compared with the other two groups (P < 0.01). At the ventilatory threshold, the V̇O2 expressed in absolute and relative to peak V̇O2 values was significantly higher in athletic than in active and sedentary subjects. These two latter groups disclosed no significant V̇O2 difference at VT. At peak exercise, the peak V̇O2 was largely different in the order sedentary < active < athletic by design according to the fitness differences, but the RER remained similar and largely over 1.1 in all groups, indicating that the subjects approached exhaustion during their incremental exercise test.
Table 2.
Gas exchanges during the incremental exercise test
| Sedentary | Active | Athletic | ||
|---|---|---|---|---|
| Rest | V̇o2 | 318 ± 26 | 358 ± 34 | 351 ± 12 |
| RER | 0.82 ± 0.02 | 0.85 ± 0.02 | 0.80 ± 0.02 | |
| HR | 85 ± 6 | 83 ± 3 | 67 ± 3 **†† | |
| VT | V̇o2 kg−1 | 15.2 ± 1.1 | 19.3 ± 1.0 | 32.7 ± 2.1 ***††† |
| RER | 0.92 ± 0.03 | 0.95 ± 0.01 | 0.94 ± 0.01 | |
| HR | 127 ± 4 | 127 ± 3 | 136 ± 4 | |
| %peak V̇o2 | 53.5 ± 3.0 | 53.4 ± 1.6 | 65.1 ± 2.1 **†† | |
| PEAK | V̇o2 kg−1 | 28.5 ± 1.3 | 36.3 ± 1.7 ** | 50.1 ± 1.8 ***††† |
| EFFORT | RER | 1.17 ± 0.03 | 1.22 ± 0.02 | 1.17 ± 0.02 |
| HR | 173 ± 5 | 184 ± 3 | 177 ± 3 |
Values are presented as means ± s.e.m.
P < 0.01
P < 0.001 versus sedentary.
P < 0.01
P < 0.001 versus active. V̇o2, oxygen uptake (expressed in ml min−1); V̇o2 kg−1, oxygen uptake per unit body weight (expressed in ml min−1 kg−1); RER, respiratory exchange ratio (V̇co2/V̇o2); HR, heart rate (expressed in beats min−1); VT, ventilatory threshold; %peak V̇o2, V̇o2 at the VT expressed as a percentage of the individual peak V̇o2.
Mitochondrial function
To establish oxidative capacities of mitochondria in muscles in situ as well as control of the respiratory activity by the principal regulator, ADP, we studied the oxygen consumption rates of saponin-permeabilized fibres at various ADP concentrations. Representative data for each group are presented in Fig. 1 in the absence (A) or presence (B) of creatine. Mean basal respiration rates in the absence of the phosphate acceptor ADP (V̇0), and mean maximal respiration rates at saturating ADP concentration (V̇max) are presented in Fig. 2A. V̇0 was similar in all groups (1.2 ± 0.2, 1.5 ± 0.2 and 1.5 ± 0.1 μmol O2 min−1 (g dry weight)−1 in sedentary, active and athletic subjects, respectively). However, V̇max, which characterises the muscle oxidative capacity, was significantly different among groups in the order sedentary < active < athletic (3.7 ± 0.3, 5.9 ± 0.9 and 7.9 ± 0.5 μmol O2 min−1 (g dry weight)−1, respectively, overall effect P < 0.001). To check if the global oxygen uptake at exercise was related to the muscle oxidative capacity, we studied the correlation between these two parameters. As can be seen in Fig. 3A, V̇max was linearly and significantly related to peak V̇O2 (P < 0.01, r = 0.63). Moreover, a significant correlation (P < 0.01, r = 0.57) existed also between the muscle oxidative capacity and the oxygen uptake at submaximal exercise, i.e. at the ventilatory threshold (VT), a parameter more closely related to the subject's endurance capacity (Fig. 3B).
Figure 1. Oxygen consumption rates of skinned fibres plotted as a function of ADP concentration.
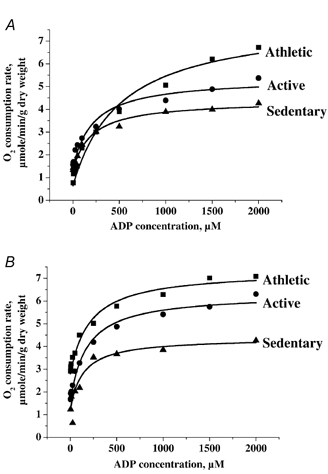
Respiration of fibres taken from athletic (▪), active (•) and sedentary (▴) individuals was measured in the absence (A) or presence (B) of 20 mm creatine. Data obtained were fitted with a Michaelis-Menten equation.
Figure 2. Basal and maximal mitochondrial respiratory rate in saponin-treated fibres.
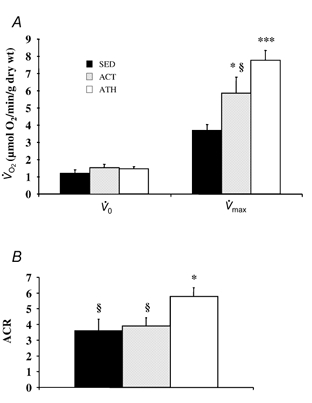
Muscle fibre bundles were taken from the vastus lateralis muscle of sedentary (SED), active (ACT) and athletic (ATH) subjects. Basal (V̇0) and maximal (V̇max) ADP-stimulated respiration (expressed in μmol O2 min−1 (g dry weight)−1) was measured in saponin-skinned fibres (A); the acceptor control ratio (ACR, V̇max/V̇0) represents the coupling between oxidation and phosphorylation (B). Data are presented as means ± s.e.m. Significantly different from SED: * P < 0.05, *** P < 0.001; significantly different from ATH: § P < 0.05.
Figure 3. Relation between oxidative capacities of skeletal muscle and exercise oxygen uptake.
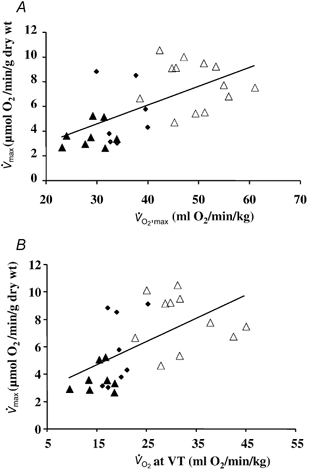
Maximal mitochondrial respiration (V̇max) was significantly correlated with peak oxygen uptake (peak V̇O2) (A, P < 0.01, r = 0.63) and with oxygen uptake at the ventilatory threshold (V̇O2 at VT; B, P < 0.01, r = 0.57) in normal subjects. Symbols indicating data points from individuals in each group: sedentary, ▴; active, ♦; athletic, ▵.
Figure 2B also shows the acceptor control ratio (V̇max/V̇0), a parameter representing the degree of coupling between oxidation and phosphorylation. Although being similar in sedentary and active subjects, this parameter was significantly higher in athletic subjects than in both other groups (3.7 ± 0.8, 3.9 ± 0.5 and 5.9 ± 0.5, respectively, P < 0.05).
In our experiments, the mitochondrial respiration was measured for increasing ADP concentrations and the apparent Km values for ADP were calculated using a non-linear fit of the Michaelis-Menten equation. It has previously been shown in rodents that this constant is high, hundreds of molar (thus sensitivity to ADP is low) in oxidative muscles and low, 10–15 μM, in glycolytic muscles (Veksler et al. 1995; Kuznetsov et al. 1996). Mean Km values for the different groups are presented in Fig. 4. In the absence of creatine, the Km was extremely high in athletic subjects and lower physical activity was associated with significantly diminished Km (P < 0.01). Interestingly, the difference in ADP sensitivity between sedentary and active groups was quite small and insignificant. Addition of creatine produced a decrease in Km values due to mi-CK activity that ensured an efficient ADP regeneration near the inner mitochondrial membrane. Though the creatine addition induced a non-significant decrease in Km in both sedentary and active groups (Fig. 3), mi-CK activation in athletic subjects was followed by a dramatic drop in Km. This threefold increase in ADP sensitivity occurring in athletic subjects indicates a very efficient mi-CK coupling with oxidative phosphorylation and suggests that the mi-CK system in trained skeletal muscle plays a very important role in mitochondrial respiration control. There was no difference in the Km values with creatine among the three groups.
Figure 4. ADP sensitivity of mitochondrial respiration in the presence or the absence of creatine in skeletal muscle.
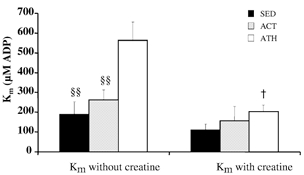
ADP-stimulated respiration was measured as a function of [ADP] with or without creatine (20 mm) in skinned fibres from the vastus lateralis muscle of sedentary (SED), active (ACT) and athletic subjects (ATH). The apparent Km for ADP was calculated using a non-linear fitting of the Michaelis-Menten equation. Data are means ± s.e.m. Significantly different from ATH, §§ P < 0.01; significantly different from Km without creatine for ATH, †P < 0.05.
Enzyme activities and MHC
Enzyme activities and MHC isoforms are presented in Table 3. The total CK and cytosolic MM-CK activities remained almost identical in all three groups whereas both MB-CK and mi-CK demonstrated considerably higher values in the athletic group (+146 and +49 % higher, respectively, in athletic when compared with sedentary subjects). These differences, however, did not reach the level of significance due to much higher data scattering in athletic subjects. Activity of a Krebs cycle enzyme, citrate synthase (CS), showed qualitatively similar distribution between the three groups. Namely, CS activities were fairly similar in sedentary and active groups and significantly higher in the athletic group. Slight differences in adenylate kinase activities which somewhat followed the fitness levels were not significantly different. Analysis of lactate dehydrogenase showed that the total activities were similar in all groups, whereas the athletic group had a significantly higher activity of the H-isoform suggesting a re-arrangement of the ratio between oxidative and glycolytic fibres in highly trained muscles. Such a shift towards a more oxidative metabolic profile in athletic subjects was in line with the modifications of the contractile profile in the same group. As can be seen in Table 3, the athletic subjects had a significantly higher MHC I percentage and a significantly lower MHC IIx percentage than sedentary subjects (P < 0.05), whereas the active group showed intermediate values in the distribution of MHC isoforms.
Table 3.
Enzymes and myosin heavy chains
| Parameters | Sedentary | Active | Athletic | |
|---|---|---|---|---|
| Creatine kinase | Total-CK | 1837 ± 119 | 2062 ± 200 | 1965 ± 119 |
| MM-CK | 1815 ± 117 | 2040 ± 200 | 1918 ± 118 | |
| MB-CK | 12.7 ± 3.2 | 12.8 ± 2.8 | 31.1 ± 9.8 | |
| mi-CK | 10.6 ± 0.7 | 9.4 ± 0.8 | 15.8 ± 3.1 | |
| Citrate synthase | 13.7 ± 2.01 | 17.9 ± 0.7 | 23.6 ± 1.9 **† | |
| Adenylate kinase | 264 ± 40 | 286 ± 36 | 327 ± 43 | |
| LDH | Total LDH | 135 ± 16 | 134.1 ± 17 | 154 ± 18 |
| H-LDH | 27.6 ± 2.6 | 38.1 ± 4.9 | 46.3 ± 3.7* | |
| M-LDH | 107.9 ± 16.7 | 96.1 ± 14.2 | 107.8 ± 15.7 | |
| H-/M-LDH | 0.30 ± 0.06 | 0.42 ± 0.05 | 0.53 ± 0.09 | |
| Myosin heavy chains | MHC-1 | 39.5 ± 4.0 | 48.4 ± 6.7 | 56.3 ± 3.4* |
| MHC-2A | 38.8 ± 5.3 | 36.8 ± 3.0 | 35.1 ± 3.1 | |
| MHC-2X | 21.7 ± 3.5 | 14.8 ± 5.4 | 8.6 ± 1.9* |
Values are presented as means ± s.e.m.
P < 0.05
P < 0.01 versus sedentary.
P < 0.05, versus active. CK,creatine kinase. LDH, lactate dehydrogenase. Enzymatic activities are expressed in international units per gram wet weight. H-/M-LDH = (H-LDH)/(M-LDH) with H = H4+¾H3M +½ H2M2+¼HM3 where H4, M4, H3M, H2M2, HM3 are homo-and hetero-tetrameters of LDH.
Discussion
The results of the present study can be summarised as follows. (1) The maximal in situ oxidative capacity of the vastus lateralis muscle of sedentary, active and athletic volunteers increased with the level of physical activity. Moreover, it was linearly correlated with the peak V̇O2 as well as with the V̇O2 at the ventilatory threshold assessed by an incremental exercise test on an ergocycle. (2) This increase in oxidative capacity was associated with a significant improvement in the coupling between oxidation and phosphorylation in athletic individuals as suggested by the increasing ACR values. (3) Mitochondria in trained skeletal muscle demonstrated a considerably lower ADP sensitivity as evidenced by an almost threefold higher Km for ADP in athletic than in sedentary individuals in the absence of creatine. (4) The addition of creatine decreased the elevated Km for ADP in the athletic group, showing the increased participation of mitochondrial creatine kinase system in the regulation of highly trained skeletal muscle respiration.
Accordingly, our study shows in human muscle, that in addition to the well-established quantitative changes in maximal oxidative capacity, modifications in the qualitative coupling and regulatory properties of mitochondrial respiration are linked to the level of physical activity, especially when a given threshold of activity is reached. This suggests that muscle aerobic performance is not only set by the density of mitochondria as previously thought, but results also from qualitative changes that optimise the control of the re-phosphorylation process and the intracellular distribution of the high energy phosphates.
Mitochondrial oxidative capacity and fitness level
Our observations on mitochondrial respiration measured in situ and CS activity among the three groups are in accordance with previous studies measuring oxidative capacities (quantitative changes) as a function of the fitness level or endurance training, using ultrastructural (Turner et al. 1997; Demirel et al. 1999), biochemical (Gollnick, 1986), and functional techniques (Walsh et al. 2001a). Moreover, the significant correlations observed between muscle oxidative capacity and V̇O2,max or even V̇O2 at the ventilatory threshold are in line with the idea that muscle oxidative capacity is a determinant of endurance capacity. Interestingly, no correlation was found between V̇max and MHC expression, suggesting that muscle oxidative capacity is more related to training status than fibre type composition.
The maximal muscle oxidative capacity is likely to be related to the mitochondrial density, and, although contributing to improve exercise capacity with increasing activity, may not be the ultimate limiting factor of endurance performance. Indeed, a limitation in V̇O2,max involves the succession of many steps controlling the delivery and utilisation of oxygen from lung capacity, cardiac pump function, vascular bed and ultimately mitochondrial respiration. It is generally accepted that during exercise where a large muscle mass is involved, the absolute limit to V̇O2,max depends on oxygen delivery rather than utilisation, and V̇O2 at ventilatory threshold more clearly reflects the endurance capacity (Richardson et al. 1999; Bassett & Howley, 2000). Our results are in line with the symmorphosis concept, a model showing that variations in muscle mitochondrial content are proportional to variations in whole-body aerobic capacity among species on one hand, and among subjects within a given species on the other hand (Hoppeler et al. 1985).
Increased coupling between oxidation and phosphorylation in well-trained subjects
It was observed that the acceptor control ratio was significantly increased in the athletic subjects, suggesting an improvement in the electron transport to phosphorylation coupling. Because V̇0 in our preparation depends on mitochondrial transmembrane proton leak, and on the number of mitochondria, we expected the increase in mitochondrial mass with physical activity to result in an increase in V̇0. This was obviously not the case since V̇max increased among groups with no change in V̇0. This suggests that an increase in the coupling between oxidation and phosphorylation has occurred from the sedentary to the athletic group. Such an increased V̇max with unchanged basal respiration rate was also observed on isolated mitochondria after a 6 week endurance training programme in humans, although the changes in ACR did not reach significance (Tonkonogi et al. 2000). This suggests that long-term training is probably necessary to significantly improve mitochondrial efficiency. It has been demonstrated that regular exercise training per se influences the phospholipid fatty acid composition of muscle membranes (Mataix et al. 1998; Helge et al. 1999, 2001). In this case, the increase in oxidative efficiency of well-trained subjects could be explained by a change in the permeability of the inner mitochondrial membrane due to changes in the phospholipid composition (Brand et al. 1994).
Modification of the regulation of respiration by phosphate acceptor with the level of physical activity
An important and original observation of this study was that mitochondrial regulation by ADP was dramatically changed with increased levels of activity. Indeed, the sensitivity of mitochondria to external ADP was approximately three times lower in athletic individuals than in both other groups, providing evidence that the respiratory control of mitochondria by ADP during muscular contraction differs between sedentary and active individuals on one hand and athletic individuals on the other hand. In the former, mitochondrial regulation by external ADP compares more with the one prevailing in the typically fast glycolytic muscle of rodents, whereas in the latter, it approaches that existing in typically slow oxidative muscle (Veksler et al. 1995; Kuznetsov et al. 1996; Bigard et al. 1998; Saks et al. 1998; Ventura-Clapier et al. 1998). In humans, a decrease in mitochondrial-ADP sensitivity with training has been indirectly suggested to occur in isolated mitochondria (Tonkonogi & Sahlin, 1997), and in skinned fibres (Walsh et al. 2001a). We have here demonstrated this observation by Michaelis-Menten analysis and for increasing levels of physical activity. As the human vastus lateralis muscle has a mixed fibre type I and II composition, the Km for ADP is an integrated parameter of the different fibre types and most probably from the mitochondrial population. In athletic subjects, the higher Km value for ADP is in line with the change in the fibre type composition towards a higher proportion of slow fibres, although it is highly possible that all mitochondria, independently of fibre type, participate in these regulation changes. Indeed, it has been clearly established that the oxidative capacity of all fibre types is increased in trained muscles (Baldwin et al. 1972). This decrease in sensitivity of the muscle mitochondria to external ADP, when the oxidative efficiency increases is at first surprising. However, if one assumes Michaelis-Menten kinetics and if the Km is low (in the 10–50 μM range), resting mitochondrial respiration would be 25–65 % of maximal, not permitting the fine tuning between energy demand and energy production as already discussed by Tonkonogi et al. (1998). Thus the higher Km in athletic individuals may contribute to a fine tuning of mitochondrial respiration to the energy needs of the cell in this group.
Moreover, we showed that the apparent Km for ADP significantly decreases with the addition of creatine only in the athletic group but not in the active group, this contradicts the findings of another study which showed that creatine increases respiration in moderately trained subjects also (Walsh et al. 2001a). However, this may simply reflect the age, training level and sex differences between the two studies. Nevertheless, results imply that the mi-CK becomes functionally coupled to ATP production only in those subjects who sustain important and regular levels of physical activity. It therefore suggests that, in these subjects, the muscle oxidative capacity not only depends on the amount of mitochondria, but also on the mechanisms of respiratory control. An additional support to this conclusion was recently given by Walsh et al. (2001b) who showed that phosphocreatine was also a strong regulator of mitochondrial respiration. Therefore, although it has been long believed that free cytosolic ADP or the ATP/ADP + Pi ratio governs the rate of mitochondrial respiration (Chance & Williams, 1955), the intracellular signal triggering mitochondrial respiration in the vastus lateralis muscle of athletic subjects might not be the cytoplasmic ADP level but the PCr/Cr ratio in the immediate vicinity of the mitochondria (Greenhaff, 2001; Walsh et al. 2001b). Thus, it can be inferred from our observations that high endurance capacity necessitates a close coupling between energy production and energy utilisation on a ‘pay as you go’ basis. In other words, mitochondrial respiration would be under the tight control of cellular energy utilisation through an efficient signalling pathway. It has long been suggested that the creatine kinase system, with isoenzymes specifically located at sites of energy production and utilisation is able to locally control the adenylate pools, to transfer energy from mitochondria to the sites of utilisation and to transfer back the signal to mitochondria, through near-equilibrium creatine kinase reactions in the cytosol (Wallimann et al. 1992; Saks et al. 1994). In athletic individuals, the increased dependency of mitochondrial respiration towards creatine reflects the need for a servo control of mitochondria by energy utilisation and the increasing importance of the creatine kinase system in this process. Our observations show that muscles respond to higher levels of physical activity not only by increasing the amount of mitochondria but also by improvements in the coupling of the respiratory chain. This takes place by changes in the control mechanisms of the mitochondrial respiration and by modifications in the intracellular channelling of the high-energy phosphates. Thus, in trained subjects, mitochondria behave not only as building blocks but modify the way they interact with surrounding subcellular structures by more subtle changes in muscle design.
In addition to CK, other kinases or even morphological changes can take part in increased coupling between energy production and utilisation. Indeed, in the heart, taken as an example of a highly oxidative striated muscle, it has recently been demonstrated that newly synthesised high energy phosphates are directly channelled to the close sites of utilisation (the myosin and sarcoplasmic reticulum ATPases). In the same way, the signal for respiration is channelled back to neighbouring mitochondria, determining a cross-talk between organelles (Kaasik et al. 2001; Saks et al. 2001). These mechanisms of control might also be operating in the skeletal muscle of athletic subjects.
As a significant change in Km with creatine appears only in the athletic group, whereas the quantitative changes in maximal oxidative capacity linearly increases with activity, it is also suggested that differing mechanisms of mitochondrial biogenesis might be triggered and result in quantitative and qualitative mitochondrial changes with physical activity (Hood, 2001). In these regularly trained subjects, we can thus hypothesise that a more efficient coupling between energy production and utilisation may decrease perturbations of the cell homeostasis during exercise, and contribute to the increase of endurance capacity. Interestingly, the percentage peak V̇O2 at VT is higher only in the athletic subjects, corresponding to the fact that V̇O2maintained during an endurance run is more closely linked to the level of V̇O2 at the VT (Bassett & Howley, 2000). It remains that we cannot completely rule out that genetic predisposition may have partially biased our results. Indeed, Park et al. (1988) suggested that there is a greater oxidative capacity relative to glycolytic capacity in untrained skeletal muscles of world-class runners compared to sedentary control subjects reflecting a genetic endowment for physical endurance in these subjects. As we included regularly trained humans in our athletic group, but excluded subjects reaching the elite competitive level, it is unlikely that our observations are only explained by the subjects' genetic profile.
Conclusion
Although the underlying mechanisms remain to be further investigated, our observations concur with a new understanding of muscle energetics where not only the quantitative aspects are taken into account but also the way that energy fluxes are controlled within the cell in a dynamic perspective.
Acknowledgments
We thank E. Boehm for careful reading of the manuscript, and R. Fischmeister and J. Lonsdorfer for continuous support. We are indebted to our volunteers for their enthusiastic participation to the study. R.V.-C. is supported by CNRS. This study was supported by the INSERM PROGRES program and Fondation de France.
References
- Agbulut O, Li Z, Mouly V, Butler-Browne GS. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biology of the Cell. 1996;88:131–135. [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Nutrition. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. American Journal of Physiology. 1972;222:373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Medicine and Sciences in Sports and Exercise. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Bigard AX, Boehm E, Veksler V, Mateo P, Anflous K, Ventura-Clapier R. Muscle unloading induces slow to fast transitions in myofibrillar but not mitochondrial properties. Relevance to skeletal muscle abnormalities in heart failure. Journal of Molecular and Cellular Cardiology. 1998;30:2391–2401. doi: 10.1006/jmcc.1998.0798. [DOI] [PubMed] [Google Scholar]
- Bigard AX, DuforeZ FPP, Guezennec CY. Determination de l'activité physique par questionnaire: validation du questionnaire autoadministrable de Baecke. Science et Sport. 1992;7:215–221. [Google Scholar]
- Brand MD, Chien LF, Ainscow EK, Rolfe DF, Porter RK. The causes and functions of mitochondrial proton leak. Biochimica et Biophysica Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. Journal of Biological Chemistry. 1955;217:409–427. [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations. Implications in heart failure. Circulation Research. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Naito H, Hughes M, Coombes JS. Exercise-induced alterations in skeletal muscle myosin heavy chain phenotype: dose-response relationship. Journal of Applied Physiology. 1999;86:1002–1008. doi: 10.1152/jappl.1999.86.3.1002. [DOI] [PubMed] [Google Scholar]
- Gollnick PD. Metabolic regulation in skeletal muscle: influence of endurance training as exerted by mitochondrial protein concentration. Acta Physiologica Scandinavica. 1986;556(suppl.):53–66. [PubMed] [Google Scholar]
- Greenhaff PL. The creatine-phosphocreatine system: there's more than one song in its repertoire. Journal of Physiology. 2001;537:657. doi: 10.1111/j.1469-7793.2001.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Ayre KJ, Hulbert AJ, Kiens B, Storlien LH. Regular exercise modulates muscle membrane phospholipid profile in rats. Journal of Nutrition. 1999;129:1636–1642. doi: 10.1093/jn/129.9.1636. [DOI] [PubMed] [Google Scholar]
- Helge JW, Wu BJ, Willer M, Daugaard JR, Storlien LH, Kiens B. Training affects muscle phospholipid fatty acid composition in humans. Journal of Applied Physiology. 2001;90:670–677. doi: 10.1152/jappl.2001.90.2.670. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. Journal of Applied Physiology. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. Journal of Applied Physiology. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P, Weibel ER. Endurance training in humans: aerobic capacity and structure of skeletal muscle. Journal of Applied Physiology. 1985;59:320–327. doi: 10.1152/jappl.1985.59.2.320. [DOI] [PubMed] [Google Scholar]
- HorowitZ JF, Klein S. Lipid metabolism during endurance exercise. American Journal of Clinical Nutrition. 2000;72:558S–563S. doi: 10.1093/ajcn/72.2.558S. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circulation Research. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- Kay L, Nicolay K, Wieringa B, Saks V, Wallimann T. Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. Journal of Biological Chemistry. 2000;275:6937–6944. doi: 10.1074/jbc.275.10.6937. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. American Journal of Physiology. 1992;263:C598–606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. European Journal of Biochemistry. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- Mataix J, Quiles JL, Huertas JR, Battino M, Manas M. Tissue specific interactions of exercise, dietary fatty acids, and vitamin E in lipid peroxidation. Free Radical Biology and Medicine. 1998;24:511–521. doi: 10.1016/s0891-5849(97)00288-8. [DOI] [PubMed] [Google Scholar]
- Mettauer B, Zoll J, SancheZ H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. Journal of the American College of Cardiology. 2001;38:947–954. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- Park JH, Brown RL, Park CR, Cohn M, Chance B. Energy metabolism of the untrained muscle of elite runners as observed by 31P magnetic resonance spectroscopy: evidence suggesting a genetic endowment for endurance exercise. Proceedings of the National Academy of Sciences of the USA. 1998;1088:80–84. doi: 10.1073/pnas.85.23.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O2 consumption in trained human skeletal muscle. Journal of Applied Physiology. 1999;87:325–331. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, RegitZ-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochemistry Journal. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Khuchua ZA, Vasilyeva EV, Belikova O, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration – a synthesis. Molecular and Cellular Biochemistry. 1994;133–134:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, KunZ WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Molecular and Cellular Biochemistry. 1998;184:81–100. [PubMed] [Google Scholar]
- Tonkonogi M, Harris B, Sahlin K. Mitochondrial oxidative function in human saponin-skinned muscle fibres: effects of prolonged exercise. Journal of Physiology. 1998;510:279–286. doi: 10.1111/j.1469-7793.1998.279bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiologica Scandinavia. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. Journal of Physiology. 2000;528:379–388. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Hoppeler H, Claassen H, Vock P, Kayser B, Schena F, Ferretti G. Effects of endurance training on oxidative capacity and structural composition of human arm and leg muscles. Acta Physiologica Scandinavica. 1997;161:459–464. doi: 10.1046/j.1365-201X.1997.00246.x. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Anflous K, Mateo P, Van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. Journal of Biological Chemistry. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochimica et Biophysica Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Kuznetsov A, Veksler V, Boehm E, Anflous K. Functional coupling of creatine kinases in muscles: species and tissue specificity. Molecular and Cellular Biochemistry. 1998;184:231–247. [PubMed] [Google Scholar]
- Wagner PD. Gas exchange and peripheral diffusion limitation. Medicine and Science in Sports and Exercise. 1992;24:54–58. [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemistry Journal. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflügers Archiv. 2001a;442:420–425. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. Journal of Physiology. 2001b;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K, Beaver WL, Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation. 1990;81:1114–1130. [PubMed] [Google Scholar]


