Abstract
Several lines of evidence show that the preoptic area (POA) of the hypothalamus is critically implicated in the regulation of sleep. Functionally heterogeneous cell groups with sleep-related discharge patterns are located both in the medial and lateral POA. Recently a cluster of neurons showing sleep-related c-Fos immunoreactivity was found in the median preoptic nucleus (MnPN). To determine the specificity of the state-related behaviour of MnPN neurons we have undertaken the first study of their discharge patterns across the sleep-waking cycle. Nearly 76% of recorded cells exhibited elevated discharge rates during sleep. Sleep-related units showed several distinct types of activity changes across sleep stages. Two populations included cells displaying selective activation during either non-rapid eye movement (NREM) sleep (10%) or REM sleep (8%). Neurons belonging to the predominant population (58%) exhibited activation during both phases of sleep compared to wakefulness. Most of these cells showed a gradual increase in their firing rates prior to sleep onset, elevated discharge during NREM sleep and a further increase during REM sleep. This specific sleep-waking discharge profile is opposite to that demonstrated by wake-promoting monoaminergic cell groups and was previously found in cells localized in the ventrolateral preoptic area (vlPOA). We hypothesize that these vlPOA and MnPN neuronal populations act as parts of a GABAergic/galaninergic sleep-promoting (‘anti-waking’) network which exercises inhibitory control over waking-promoting systems. MnPN neurons that progressively increase activity during sustained waking and decrease activity during sustained sleep states may be involved in homeostatic regulation of sleep.
An important role of the preoptic area (POA) in the mechanisms of sleep was initially proposed on the basis of neuropathological studies in humans (von Economo, 1930). This hypothesis has been repeatedly verified using a wide variety of experimental approaches, including surgical, electrolytic and neurotoxic lesions (Nauta, 1946; McGinty & Sterman, 1968; Szymusiak & McGinty, 1986; Sallanon et al. 1989; John & Kumar, 1998; Lu et al. 2000); functional inactivation (Alam & Mallick, 1990); and electro-, chemo- and thermostimulation (Sterman & Clemente, 1962; Hernandez-Peon & Chavez-Ibarra, 1963; Heuser et al. 1967; Obal et al. 1982; Mendelson et al. 1989; Ticho & Radulovacki, 1991; Matsumura et al. 1994). Collectively these studies suggest the existence of ‘hypnogenic’ neuronal populations within the preoptic region. Unit activity recordings confirmed the presence of cell groups exhibiting activation during either non-rapid eye movement sleep (NREM) or REM sleep within the lateral and medial POA of cats, rats and rabbits (Kaitin, 1984; Burikov & Suntsova, 1989; Koyama & Hayaishi 1994; Alam et al. 1995; Suntsova & Burikov, 1995). The investigation of c-Fos-protein immunoreactivity in asleep and awake rats revealed a discrete cluster of sleep-related cells in the ventral lateral POA (vlPOA) (Sherin et al. 1996). Electrophysiological studies showed that vlPOA neurons display a unique sleep-waking discharge pattern (Suntsova & Burikov, 1995; Szymusiak et al. 1998; Suntsova & Dergachyova, 2002) opposite to that exhibited by putative waking-promoting monoaminergic cell groups (Hobson et al. 1975; McGinty & Harper, 1976; Aston-Jones & Bloom, 1981; Vanni-Mercier et al. 1984; Lydic et al. 1987; Gervasoni et al. 1998, 2000; Steininger et al. 1999; Guzman-Marin et al. 2000). The findings that vlPOA neurons give rise to GABAergic/ galaninergic descending projections to monoaminergic cell groups (Sherin et al. 1998; Luppi et al. 1999; Gervasoni et al. 2000; Steininger et al. 2001) and that cells exhibiting sleep-related c-Fos immunoreactivity are GABAergic or galanin-positive (Gaus & Saper, 1999; Gong et al. 2001a) suggested that they may exercise inhibitory control over multiple monoaminergic arousal systems (Sherin et al. 1998; Szymusiak et al. 1998; Gallopin et al. 2000; McGinty & Szymusiak, 2000; Suntsova & Dergachyova, 2000; Saper et al. 2001).
Recently, an additional aggregate of cells exhibiting sleep-related c-Fos immunoreactivity was found in the median preoptic nucleus (MnPN) of rats (Gong et al. 2000). Fos-immunocytochemistry has been found to be a valid method to show the occurrence of neuronal activation during sleep as a whole, but this technique has insufficient temporal resolution to determine changes of neuronal activity within sleep cycles, stages and state transition periods. Therefore we studied the details of the MnPN neuronal discharge patterns across the sleep-waking cycle.
Methods
Experiments were conducted on male Sprague-Dawley rats (300-350 g) in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. All animal use protocols were reviewed and approved by the Internal Animal Care and Use Committee of the V.A. Greater Los Angeles Healthcare System.
Surgical procedures
Under ketamine/xylazine anaesthesia (80/10 mg kg−1, respectively, i.p.) and aseptic conditions, animals were surgically prepared for chronic recordings of MnPN neuronal activity and for assessment of sleep-waking state. For polygraphic behavioural state monitoring, four screw electrodes were symmetrically placed into the skull over the frontal and parietal cortex to record the electroencephalogram (EEG) and two Teflon-coated wires were implanted into the dorsal neck muscles to record the electromyogram (EMG). For extracellular single unit activity recording and temperature monitoring, a preassembled construction was stereotaxically implanted. It consisted of ten Formvar-insulated microwires, a copper-constantan microthermocouple, a mechanical microdrive with attached 23-gauge guide cannulae, and a miniature electric plug. The microdrive was anchored to the plug with dental acrylic using stereotaxic apparatus in order to fix the guide cannulae in a vertical position. Microwires and thermocouple wires were soldered to the plug's electrical connectors and inserted into the guide cannulae such that their tips were inside the latter. A 2 mm × 2 mm hole was trephined in the skull, centred at bregma. The dura mater was incised 0.5 mm lateral and parallel to a sagittal sinus. During assembly implantation the guide cannula was used to first displace the sagittal sinus and was then lowered in the midline to a site 3 mm above the target. Stereotaxic coordinates for rostral and caudal MnPN were, respectively: AP, 0.0 to −0.11; ML, 0.0; H, 5.8 to 7.0 and AP, −0.26 to −0.46; ML, 0.0; H, 4.0 to 6.5 (Swanson, 1998). After fixation of the entire assembly to the skull with dental acrylic, the microwires and thermocouple were additionally advanced through the guide cannulae to a site 0.5 mm above the MnPN dorsal margin. The tip of the thermocouple was positioned 0.3-0.5 mm dorsal to the tips of microwires. In order to manipulate local brain temperature, a water-perfused thermode (21-gauge outer tube) was implanted 1.5-2 mm rostral to microdrive guide cannulae. All the implanted devices, except the thermocouple, were stainless steel.
Recording and data analysis
The rats were allowed to recover for 7 days before recordings. During this period they were adapted to the experimental conditions. Throughout recovery and recording sessions animals were housed individually in Plexiglas cages placed inside electrically shielded, sound-attenuated temperature-controlled incubators. Animals were kept under a 12 : 12 h light : dark cycle with lights on at 08.00 h (illumination intensity about 100 lux) and had ad libitum access to food and water.
All electrophysiological recordings were performed on unanaesthetized, unrestrained rats. EEG and EMG activity were recorded bipolarly (amplifier passbands, 1–30 Hz and 100–1000 Hz, respectively; model 78 Polygraph, Grass Instrument, USA). For ambient and brain temperature recordings, signals from thermocouples were measured by Thermalert monitoring thermometers (Physiotemp Instruments, Inc., USA) and through its analog output transmitted to a Polygraph DC amplifier.
Neuronal activity was recorded using bipolar derivations from microwires (electrode impedance at 1000 Hz, 500–700 kΩ) and amplified by a differential AC amplifier (model 1700, A-M Systems, USA) with low and high cut-off filters of 10 Hz and 10 kHz, respectively. Signals were continuously monitored on a digital storage oscilloscope (Hitachi, VC-6024, Japan). During recording sessions the microwires were advanced in 25–30 μm steps. Only units with signal-to-noise ratios ≥ 2.5 were recorded.
Bioelectrical signals were analog-to-digital converted and stored on a hard drive for off-line analysis using a Micro 1401 data acquisition interface and the Spike2 software package (Cambridge Electronic Design, Cambridge, UK). Polygraphic data were digitized at a sampling rate of 256 Hz and unit activity data at 10 kHz or 25 kHz for waveform and wavemark data channels, respectively.
The mean firing rates of recorded cells were calculated for three to ten 30–60 s-duration artefact-free periods of wakefulness (W), NREM sleep and REM sleep, which were identified on the basis of EEG and EMG parameters using standard criteria (Timo-Iaria et al. 1970).
In order to classify the recorded cells into groups with different firing rate patterns across the sleep-waking cycle, cluster analysis (k-means clustering procedure) was performed. Analysis of variance (one-way repeated measures ANOVA) followed by Tukey's honestly significant difference (HSD) post hoc test was used to examine the inter-stage differences in mean firing rates for statistical significance. State-relatedness for each cell was determined according to the criteria described elsewhere (Steininger et al. 1999). Neurons exhibiting greater than 100 %, 50–100 % or 25–50 % differences in the mean discharge rates between states were classified, respectively, as strongly, moderately or weakly state-related.
In neurons showing activation during both phases of sleep the firing rate changes during the time course of NREM and REM sleep were determined. In the sequence of NREM sleep episodes interrupted by short (less than 20 s) arousals we examined the differences in discharge rate between the first episode following at least a 3 min period of W and the last episode preceding REM sleep or the next sustained W episode (Student's paired t test). For the records including several such sequences, the successive episodes of NREM sleep were numbered and the correlation between episode number and mean firing rate during the episode was calculated. Additionally, each NREM and REM sleep episode was divided into four segments and the differences in mean firing rates between the first and last quarters or between all the quarters were tested for statistical significance using Student's paired t test or the analysis of variance (one-way repeated measures ANOVA), respectively. In addition during sustained W (more than 5 min in duration) the discharge rates during the first and the last minute were compared (Student's paired t test).
Histology
Following the completion of all recordings, under deep pentobarbital anaesthesia (100 mg kg−1, i.p.), microlesions were made at the tip of two or three microwires by passing DC current (20 μA, 15–20 s) at the most ventral recorded site. The animals were then injected with heparin (500 U, i.p.), and perfused transcardially with phosphate-buffered saline (PBS; pH 7.4) followed by 4 % paraformaldehyde and 10 % sucrose; all in PBS. The brains were removed and equilibrated in 30 % sucrose. Serial sections (40 μm) were made on a freezing microtome through the coronal plane and stained for Nissl (Cresyl violet). Reconstructions of microwire tracts were made with the aid of a Neurolucida imaging system (Microbrigthfield, Colchester, VT, USA) guided by the rat brain atlas of Swanson (1998). According to reconstructions (Fig. 1), 89 cells were recorded within the rostral to caudal MnPN of five rats across the sleep-waking cycle. These neurons were used in the present study for the analysis of sleep-waking discharge patterns.
Figure 1. Locations of different types of recorded neurons within the median preoptic nucleus and their distribution among subjects.
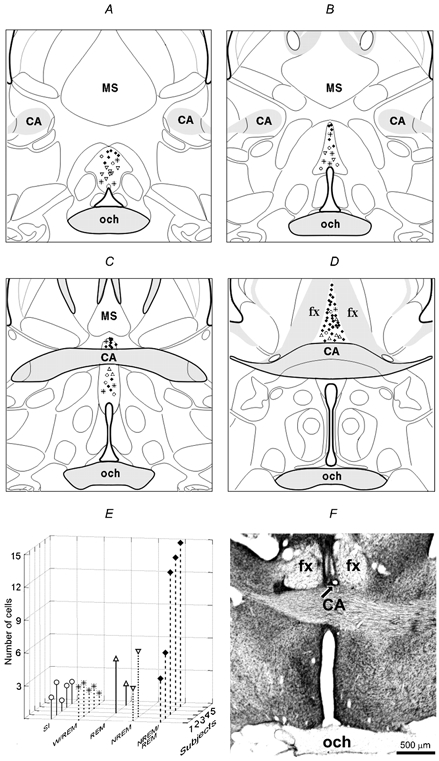
A and B, rostral MnPN; C and D, caudal MnPN. Note that NREM sleep-related neurons (▿) were predominantly recorded in the rostral MnPN whereas the vast majority of NREM/REM-(♦) and REM sleep-related cells (▵) were found within the caudal portion of this structure. W/REM sleep-related (*) and state-indifferent (○) cells did not have predominant locations. E, distribution of different cell types among subjects. F, photomicrograph of a coronal section through the MnPN showing the microwire tracts. Arrow indicates the site of electrolytic lesion at the site of the most ventral recording. SI, state-indifferent neurons; CA, commissure anterior; MS, medial septum; och, optic chiasm; fx, fornix.
Results
The overall statistical characteristics of discharge frequency of MnPN neurons during W, NREM, and REM sleep are shown in Table 1. The variability of firing rates was high in each state. The distribution of discharge frequency had significant (P < 0.01) right-side skewness. The mean firing rate was the lowest during W and significantly changed in the course of the sleep-waking cycle (F(2,176) = 37.3, P < 0.001), increasing in NREM sleep compared to W (P < 0.001) and increasing again in REM sleep compared to NREM sleep (P < 0.001).
Table 1.
Statistical characteristics of discharge rate (spikes s−1) of MnPN neurons (n = 89) during different stages of the sleep-waking cycle
| Mean ±s.e.m. | Median | Range(%) | Coefficient of variation | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|
| Wakefulness | 8.3 ± 0.7 | 6.9 | 0.1−34.9 | 80.7 | 1.3 | 2.1 |
| NREM sleep | 10.6 ± 0.8 | 9.0 | 1.4−42.7 | 74.5 | 1.8 | 4.4 |
| REM sleep | 12.3 ± 1.0 | 9.5 | 1.6−40.1 | 76.4 | 1.4 | 1.3 |
MnPN neurons were functionally heterogeneous with respect to sleep-waking discharge pattern. The classification carried out with the aid of cluster analysis was the most appropriate (in terms of both correspondence with the types of firing rate dynamics and within/between-cluster variability) when the number of clusters was specified as ten. The sleep-wake profiles of mean discharge frequency in each cluster are displayed in Fig. 2. According to the similarities in the dynamics of firing rate, some clusters were joined into larger taxonomic units. As a result, the recorded cells were subdivided into sleep-related, W/REM sleep-related and state-indifferent neuronal groups. The reconstructed locations of neurons showing different types of state-related behaviour and the distribution of cell types among subjects are shown in Fig.1.
Figure 2. Classification of MnPN neurons based on the firing rate dynamics across the sleep-waking cycle.
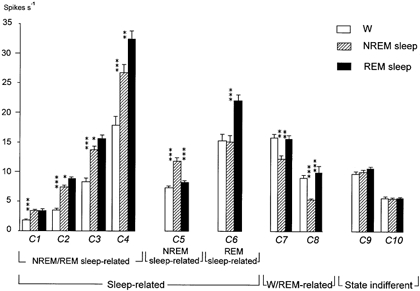
According to the results of cluster analysis, neurons were subdivided into 10 clusters (C1-10). The clusters with the same state-related discharge profile but with different firing rates were identified as belonging to the same taxonomic units. All cells were classified into three main groups: (1) sleep-related neurons exhibiting activation during sleep compared to W; (2) W/REM sleep-related cells displaying elevated firing rates during both W and REM sleep compared to NREM; (3) state-indifferent neurons. The group of sleep-related cells included three populations: NREM/REM-related neurons exhibiting activation during both phases of sleep compared to W, and NREM and REM sleep-related neurons showing selective activation during NREM and REM sleep, respectively. In all the clusters except that belonging to the state-indifferent group the changes of firing rates across the sleep-waking cycle were statistically significant (ANOVA, P < 0.001). Evaluation of inter-stage differences in discharge frequency for each cluster was done using the Tukey HSD post hoc test: *P < 0.05; **P < 0.01; ***P < 0.001.
Sleep-related neurons
This group included the majority of recorded cells (n = 68; 76.4 %). They exhibited higher firing rates during one or both phases of sleep compared to W. According to the specificity of discharge changes during sleep, neurons were identified as NREM/REM, NREM, or REM sleep-related.
NREM/REM sleep-related neurons
This population included 52 cells grouping into four clusters (n1 = 20, n2 = 4, n3 = 0, n4 = 8) with different mean firing rates (Fig. 2, C1-4).
Within each cluster the mean discharge frequency showed significant (P < 0.001) changes in the course of the sleep- waking cycle (F1(2,38) = 4.6; F2(2,26) = 83.7; F3(2,18) = 113.8; F4(2,14) = 32.6).
During NREM sleep compared to W the mean firing rate significantly increased in each cluster (P < 0.001). Every cell exhibited the same trend of changes across states, whereas the range of discharge elevation varied considerably (range1 = 9–1300 %, range2 = 34–680 %, range3 = 3–104 %, range4 = 25–91 %).
During REM in comparison with NREM sleep the mean firing rate changed insignificantly in the first cluster (P1 > 0.05), but increased within the others (P2 < 0.01, P3 < 0.05, P4 < 0.01). Twenty-four NREM/REM sleep-related cells exhibited a greater than 25 % change in discharge frequencies: 20 units displayed elevated (range = 25–108 %) firing rates, whereas four neurons exhibited reduced (range = 31–41 %) discharge in REM compared to NREM sleep, but continued to fire 33–300 % faster than during W.
According to the percentage of firing rate inter-stage differences, 29 NREM/REM sleep-related cells were classified as strongly, 19 as moderately, and 4 as weakly state-related. An example of the sleep-waking discharge pattern of a strongly state-related cell is shown in Fig. 3.
Figure 3. The discharge pattern of an individual NREM/REM sleep-related MnPN neuron across the sleep-waking cycle.
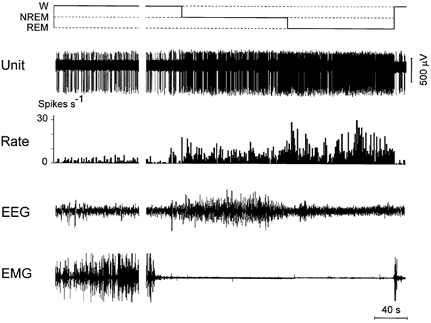
In this figure and in Figs 4–6 the hypnogram (top), the extracellularly recorded unit activity (unit), firing rate (rate), electroencephalogram (EEG) and neck electromyogram (EMG) are displayed. Note the increase in discharge rate in NREM sleep compared to W and in REM sleep compared to NREM.
In addition to between-state differences, NREM/REM sleep-related neurons displayed changes in their firing rates that were correlated with the time course of each particular state.
Within a sustained period of W, neurons exhibited minimal firing rates or even ceased discharge for periods ranging up to 150 s following awakening. Phasic decreases in EMG amplitude during W were accompanied by increased firing rate (Fig. 4A). The discharge frequency increased further within the later portions of W during the epochs characterized by moderate EMG activity and the appearance of partial EEG synchronization, but not patterns characteristic of NREM sleep. In several cases the firing rates during such epochs immediately preceding sleep onset were even higher than during subsequent stable NREM sleep (Fig. 4A). Several cells (n = 8) gradually increased their firing rates during sustained W without correlation with EMG amplitude changes (Fig. 4B). Overall, the discharge frequency of 49 cells during the first minute after awakening was significantly lower compared with the last minute of W preceding the next sustained sleep episode (P < 0.001). This difference exceeded 25 % in 30 neurons.
Figure 4. The discharge pattern of NREM/REM sleep-related neurons exhibiting gradual increase in their firing rate prior to sleep onset.
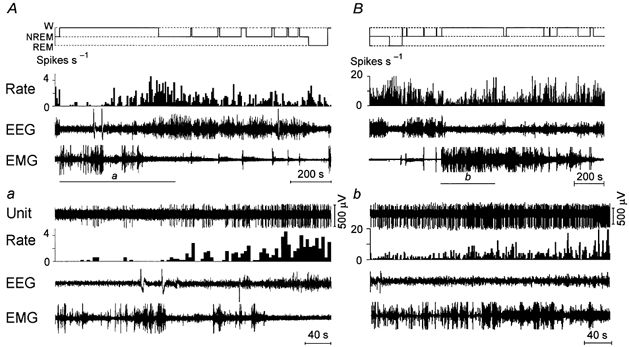
A and B, recordings showing the discharge of NREM/REM sleep-related neurons across the sleep-waking cycle. a and b, expanded tracing from the section shown by bars in A and B showing changes of neurons’ activity during W and on transition to NREM sleep. Note that during W, the neuron shown in panel A was silent during periods with locomotor activity (phasic bursts in EMG channel), then exhibited an increase of firing rate during periods with decreased muscle tone, and had a maximal firing rate during the 15 s period prior to NREM sleep onset. However, in general, the changes of cell activity across the sleep-waking cycle were not correlated with alterations in muscle tone level. The neuron shown in B exhibited a gradual increase in firing rate during a sustained period of W with no correlation with changes in muscle tone. Note that in panel A the histogram of discharge rate shows the mean firing rate within 5 s bins.
In rats, REM sleep usually appears after several periods of NREM sleep interrupted by short arousals. The mean firing rate of NREM/REM sleep-related cells calculated for the first NREM sleep episode in the sequence was significantly higher than during the last one (P < 0.001). In 35 of 48 cells showing the decrease in discharge frequency across a series of NREM sleep episodes this decline exceeded 25 % (Fig. 5). In cells recorded during several such sequences a statistically significant negative correlation between the firing rate and the NREM sleep episode number was found (Fig. 5C). During each distinct sustained NREM sleep episode there also was a decline in mean firing rate of NREM/REM sleep-related cells during the last NREM sleep quarter in comparison with the first one (P < 0.001). For several cells the ANOVA confirmed the gradual decrease (Fig. 5D).
Figure 5. Discharge pattern of a NREM/REM sleep-related neuron, which gradually decreases its firing rate during individual NREM sleep episodes and their sequences and during REM sleep.
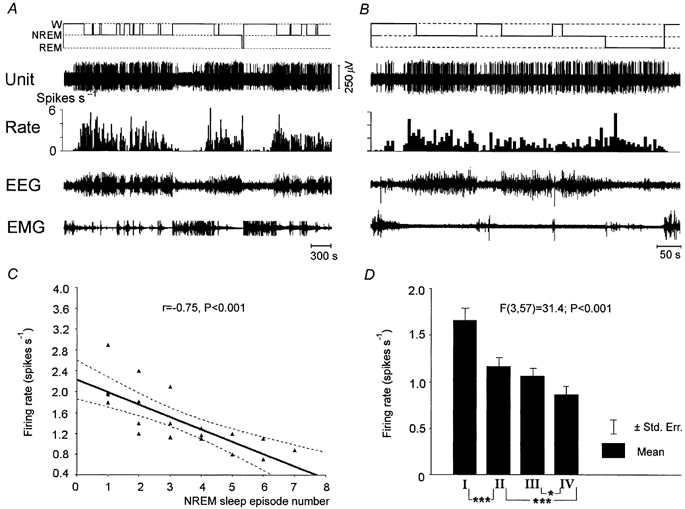
A and B, 66 and 10 min continuous recordings showing the discharge of a NREM/REM sleep-related neuron across the sleep-waking cycle. C, regression function and correlation between the firing rate of the cell and NREM sleep episode number in the sequence of episodes. D, difference in the firing rate of the same cell in different NREM sleep quarters. Note that in A and B the histogram of discharge rate shows the mean firing rate within 5 s bins. *P < 0.05; ***P < 0.001, Tukey's HSD post hoc test.
Within REM sleep, as in NREM sleep, there was a significant decrease in the mean firing rate of NREM/REM sleep-related cells during the last quarter of REM sleep episodes compared to the first one (P < 0.01). Thirty-four neurons exhibited a more than 25 % decline (Fig. 5B) and only three cells showed an opposite trend of changes.
All cells belonging to this population had a tonic firing mode during each stage of the sleep-waking cycle. They did not show any changes in the pattern of their activity timed to the appearance of sleep spindles or delta waves during NREM sleep and theta activity in REM sleep.
NREM sleep-related neurons
Firing rates of neurons belonging to this population (n = 9) were at their maximal values during NREM sleep (Fig. 6). The population included a cluster of cells (n = 8) firing during W at moderate rates (Fig. 2, C5) and one high-frequency neuron (24.8, 42.7 and 33.2 spikes s−1 during W, NREM sleep and REM sleep, respectively). Within the cluster a mean discharge frequency in the course of the sleep-waking cycle changed significantly (F(2,14) = 46.6, P < 0.001). During NREM sleep it was significantly higher than during both W (P < 0.001) and REM sleep (P < 0.001), whereas the difference between W and REM sleep was insignificant (P > 0.05).
Figure 6. The discharge pattern of a neuron showing selective activation during NREM sleep across the sleep-waking cycle.
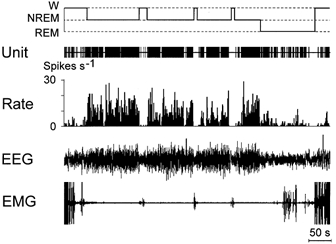
Note that firing rate dramatically decreases during W and REM sleep compared to NREM. Discharge rate changes do not precede state transitions.
In this population two cells were strongly state-related, six were moderately state-related, and one was weakly state-related.
These neurons exhibited tonic firing during all states of the sleep-waking cycle and did not show advanced changes in their activity during transitions. In NREM sleep they did not show any changes in the frequency or pattern of pulse activity that coincided with EEG phenomena characteristic for this stage of the sleep-waking cycle.
REM sleep-related neurons
This population included seven cells which fired at approximately the same rates during W and NREM sleep, and increased their frequency of discharge in REM sleep (Fig. 2, C6). The changes of mean firing rate across the sleep-wakefulness cycle were statistically significant (F(2,12) = 7.4, P < 0.001). The increase during REM sleep was significant in comparison with both W and NREM sleep (P < 0.001). The difference between W and NREM sleep was insignificant (P > 0.05).
Among the cells belonging to this population two cells were moderately and five cells were weakly REM sleep-related neurons.
The changes of firing rates on transition to REM sleep were strictly timed to the appearance of the electrographic picture of this functional state.
Each cell showed distinct changes in the pattern of activity in parallel with the appearance of theta activity in the EEG during W or REM sleep (Fig. 7), generating bursts in phase with the positive components of theta waves. The burst duration as well as the intraburst pulse frequency were maximal during REM sleep, reaching, respectively, 100 ms and 250–300 Hz.
Figure 7. The discharge pattern of a REM-related neuron during different stages of the sleep-waking cycle.
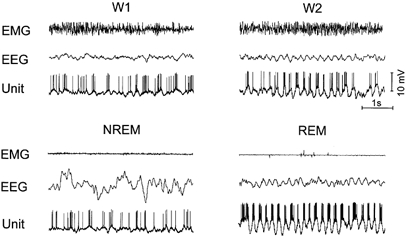
W1, wakefulness with desynchronized frontoparietal EEG,; W2, wakefulness with theta rhythm in the EEG. Note the increased mean firing rate and intraburst frequency during REM sleep. In states with theta rhythm in the EEG (W2 and REM sleep) bursts are phase-locked to the surface-positive component of theta waves.
Most of the cells belonging to this population were found in the same rat. In order to identify their location a lesion was made where the last such unit was recorded on the electrode track (Fig. 1F). The reconstruction of REM-related neurons showed that all were localized within the caudal MnPN (Fig. 1D).
W/REM sleep-related neurons
The cells belonging to this group (n = 11) exhibited minimal firing rates during NREM sleep. The group consisted of two clusters (n1 = 6, n2 = 5) with different levels of firing (Fig. 2, C7 and 8). In each cluster, state transitions were accompanied by significant changes in the mean firing frequency (F1(2,8) = 18.2; P < 0.001 and F2(2,10) = 37.9; P < 0.001). Specifically, a decrease in discharge during NREM sleep was statistically significant as compared to both W (P1 < 0.01; P2 < 0.001) and REM sleep (P1 < 0.01; P2 < 0.001). The difference in mean discharge rate between W and REM sleep was insignificant in both clusters (P > 0.05). Neurons belonging to the first cluster were weakly W/REM sleep-related, whereas the second cluster included two strongly and three moderately state-related units.
On transition from one stage of the sleep-waking cycle to another these neurons exhibited changes in their firing rates only after the appearance of electropolygraphic correlates of the new functional state.
W/REM sleep-related cells discharged tonically during all sleep-wake stages.
State-indifferent neurons
This group consisted of two clusters (n1 = 6, n2 = 4; Fig. 2, C9 and 10) without statistically significant state-dependent changes in the mean frequency of pulse activity (F1(2,10) = 0.34, P > 0.05; F2(2,6) = 4.6, P > 0.05).The mean firing rate of neurons belonging to both clusters also did not show reliable changes across the sleep-waking cycle (F(2,18) = 1.22, P > 0.05), and did not exceed 15 % in individual neurons. We considered all these units as state-indifferent. During both wakefulness and sleep they exhibited a tonic (continuously arrhythmic) pattern of activity. Both firing rate and firing mode were unchanged during state transitions or phasic alterations in the EEG patterns.
Discussion
The present study is the first investigation of MnPN neuronal activity across the sleep-waking cycle. Nearly 75 % of sampled MnPN neurons exhibited increased discharge rates during sleep. These results are consistent with the demonstration of sleep-related c-Fos protein expression in the rat MnPN (Gong et al. 2000).
The most important finding of the present study was that the predominant cell type (58 %) found within MnPN of each rat exhibited activation during both phases of sleep, compared to wakefulness. During transitions to sleep, the majority of these units increased discharge rate prior to sleep onset. Neurons exhibiting similar state-related discharge patterns have been previously described in the vlPOA of rats (Szymusiak et al. 1998) and cats (Suntsova & Burikov, 1995; Suntsova & Dergachyova, 2002). During sleep, a majority of rat MnPN cells displayed increased firing rates in REM compared to NREM sleep. This pattern was typical of NREM/REM sleep-active neurons recorded in the cat vlPOA (Suntsova & Dergachyova, 2002). The remaining rat MnPN NREM/REM sleep-related cells showed no change or a modest decline during REM compared to NREM sleep, but activity levels during REM sleep remained above waking values. This latter pattern was more typical of vlPOA neurons recorded in rats (Szymusiak et al. 1998).
The MnPN sleep-active neuronal group also included small populations of cells exhibiting selective activation during either NREM (10 %) or REM sleep (8 %). Both of these cell types were found only in two animals (Fig. 1E) and will not be discussed further.
The MnPN has been prominently implicated in the regulation of several autonomic functions related to osmotic, cardiovascular and temperature homeostasis (Scammel et al. 1993; Travis & Johnson, 1993; Oka et al. 2000; Budzikowski & Leenen 2001). It is possible, therefore, that the changes in MnPN neuronal activity across the sleep-waking cycle described here, reflect responses to changes in autonomic variables or alterations in controlling influences of this structure upon some parameters of autonomic or neuroendocrine system functioning. We consider this assumption to be unlikely, at least with respect to the NREM/REM sleep-related neuronal population. First, our preliminary data indicate that these cells are unresponsive to changes in peripheral and/or brain temperature (authors’ unpublished observations). Second, the changes in activity of NREM/REM sleep-related neurons across the sleep-waking cycle do not correspond in terms of direction and timing to alterations in osmotic pressure and rat arterial blood pressure (Junqueira & Krieger, 1976; Rubin et al. 1978; Mancia & Zanchetti, 1980). Third, lesion experiments indicated the involvement of rostroventral portions of the MnPN in body fluid balance (Gardiner et al. 1985; Cunningham et al. 1991). Neurons responsive to angiotensin and osmotic stimuli were also found in the same part of the MnPN (Travis & Johnson, 1993), whereas the majority of NREM/REM sleep-related cells were recorded in the dorsocaudal portions of this nucleus. Although the relation of NREM/REM sleep-active neurons to osmotic and cardiovascular regulation remains to be determined, their behaviour more strongly supports a role in sleep-waking state control.
The specific pattern of enhanced discharge in vlPOA and MnPN neurons prior to sleep and during both its phases suggests that they are involved in promoting sleep onset and maintenance, rather than in executive mechanisms of either NREM or REM sleep specifically. Only these cell groups exhibit a state-related discharge profile opposite to those demonstrated by putative monoaminergic, wake-promoting neuronal populations (Hobson et al. 1975; McGinty & Harper, 1976; Aston-Jones & Bloom, 1981; Vanni-Mercier et al. 1984; Lydic et al. 1987; Gervasoni et al. 1998; Steininger et al. 1999; Guzman-Marin et al. 2000) and by wake-active, REM-off neurons located in the hypocretin-immunoreactive neuronal field of the rat perifornical lateral hypothalamus (Alam et al. 2002). The factors responsible for the gradual decrease in wake-promoting network neuronal activity during the wake- NREM-REM cycle still remain controversial (Steriade, 2001). Despite the appearance of several additional arguments in favour of GABA-mediated suppression of wake-promoting monoaminergic elements during sleep (Sallanon et al. 1989; Nitz & Siegel, 1997; Yang & Hatton, 1997; Gervasoni et al. 2000), the idea of active inhibition has lacked firm support at the cellular level (Steriade, 2001). In recent years this idea has been strengthened by the discovery of vlPOA neuronal elements in rats (Szymusiak et al. 1998) and cats (Suntsova & Burikov, 1995; Suntsova & Dergachyova, 2002) with state-related discharge patterns that confirm their possible role as the morphological substratum of inhibitory control of monoaminergic systems. Retrograde and anterograde tracer studies have identified the vlPOA as a source of afferents to all monoaminergic waking-promoting cell groups (Sherin et al. 1996, 1998; Steininger et al. 2001). The vast majority of retrogradely labelled vlPOA neurons contain the neurotransmitters GABA and galanin (Sherin et al. 1998). These transmitters cause inhibitory action upon some waking-promoting cell groups (Seutin et al. 1989; Schonrock et al. 1991). Recently, sleep-related c-Fos protein immunoreactivity in vlPOA has been shown to co-localize with markers of GABAergic (Gong et al. 2001a) and galaninergic (Gaus & Saper, 1999) neurons.
However, excitotoxic lesions of the vlPOA (Lu et al. 2000) cause only partial sleep loss. These data suggest that hypothesized hypnogenic vlPOA neurons ‘do not seem necessary and sufficient for sleep induction’ (Steriade, 2001). The results presented here show that cells increasing their functional activity in parallel with a decrease of arousal level are not unique to the vlPOA. Several lines of experimental evidence support the hypothesis that the MnPN can function as another important source of inhibitory modulation of arousal systems. Sleep-related Fos activation is extensively co-localized with markers for GABAergic neurons in the rat MnPN (Gong et al. 2001a). Projections from the MnPN to the pontine monoaminergic cell groups have been documented (Zardetto-Smith & Johnson, 1995). The MnPN is also a source of afferents to putative arousal-regulatory cell groups in the perifornical lateral hypothalamic area and to cholinergic regions of the magnocellular basal forebrain (Gong et al. 2001b). Therefore, MnPN neurons could work in conjunction with vlPOA neurons to regulate excitability of some monoaminergic cell groups, and may exert inhibitory control over arousal systems not strongly regulated by vlPOA, possibly including hypocretin- and/or melanin-concentrating hormone-containing neurons of the perifornical lateral hypothalamus and cholinergic neurons in the basal forebrain. Further studies are required to confirm that MnPN neurons that project to arousal-related neuronal groups are GABAergic and NREM/REM sleep-related.
In addition, the MnPN projects heavily to vlPOA (Chou et al. 2002) and could, therefore, participate in regulating the state-dependent excitability of vlPOA sleep-active neurons.
Thus, the MnPN and vlPOA neuronal populations have similar anatomical, physiological and neurochemical properties, and can be hypothesized to be components of a GABAergic/galanergic network that exercises inhibitory control over multiple arousal systems. Since within existing nomenclature used to characterize components of the sleep-wake control apparatus this network has not been specified, elsewhere (McGinty & Szymusiak, 2000; Suntsova & Dergachyova, 2000) we have argued that this inhibitory network may be designated as sleep-promoting or ‘anti-waking’ (Valatx, 1996).
There is anatomical (Chou et al. 2002) and physiological (Gallopin et al. 2000) evidence that sleep-promoting elements within the vlPOA are under inhibitory control from some monoaminergic cell groups. Mutual inhibitory relationships between the vlPOA and arousal systems has been hypothesized to function as a ‘sleep switch’ (McGinty & Szymusiak, 2000; Saper et al. 2001; Chou et al. 2002) producing a bistable pattern of sleep-wake state distribution, with a tendency to yield stable states of either waking or sleep, and to avoid intermediate states. Our data concerning the details of MnPN and vlPOA neurons state-related behaviour allow us to propose that these mutually inhibitory relationships underlie the continuous opposite changes in the activity of putative sleep- and waking-promoting cell groups across the wake-NREM-REM cycle. Some recent findings may elucidate the role of these neuronal activity patterns. There is extensive evidence that monoaminergic systems both directly and indirectly, through activation of mesopontine or basal forebrain cholinergic neurons (Lin, 2000), exert inhibitory control over the thalamocortical synchronizing network (Steriade, 1999) and the brainstem REM sleep-generating network (McCarley et al. 1995; Valatx, 1996; Lin, 2000). A gradual decrease of monoaminergic neuronal activity has been hypothesized to be ‘permissive’ for expression of NREM and then REM sleep mechanisms. On the other hand, the MnPN and vlPOA sleep-promoting system inhibits the monoaminergic cell groups and can be considered as ‘permissive’ for waking. If so, the expression of each sleep-waking state is possible only if the functional activity of the corresponding ‘permissive’ system is within a certain range. Therefore, the continuous reciprocal interdependent changes in the activity of sleep- and waking-promoting systems may underlie all sequential transitions from one stage of the sleep-waking cycle to another, including switching between wakefulness and sleep.
Another finding of interest in the data presented here is that a significant number of MnPN NREM/REM sleep-related neurons showed an increase of functional activity in the time course of wakefulness and a decline during sustained NREM sleep episodes as well as in the time course of successive NREM episodes (Fig. 5). As such, this neuronal population is the first whose activity has been shown to be correlated with the homeostatic drive for sleep (process S), which rises during waking and declines during sleep (Borbely, 1982). In accordance with a two-process model, sleep propensity at any point is determined by interactions between process S and the circadian process (Borbely, 1982). The suprachiasmatic nucleus has been shown to have indirect projections to the MnPN (Deurveilher et al. 2002). Therefore, the MnPN may be the site of a neuronal sleep-promoting mechanism upon which the homeostatic and circadian determinants of sleep-wake propensity converge.
There is evidence that endogenous somnogenic substances, such as adenosine, cytokines and prostaglandin D2 are involved in the homeostatic regulation of sleep (Porkka-Heiskanen et al. 2000; Hayaishi, 2002). Administration of these agents or their agonists to the subarachnoid space just anterior to the vlPOA and ventral to the MnPN or by intracerebroventricular infusion increases NREM sleep and causes activation of vlPOA neurons (Satoh et al. 1996; Scammell et al. 1998, 2001; Terao et al. 1998) The ability of these endogenous somnogens to modulate MnPN neuronal activity remains to be determined.
Acknowledgments
The authors thank Feng Xu for excellent technical assistance. This research was supported by the US Department of Veterans Affairs Medical Research Service and US National Institutes of Health grants MH 47480, MH 04708 and HL 60296.
References
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. Journal of Physiology. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. American Journal of Physiology. 1995;269:R1240–1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- Alam MN, Mallick BN. Differential acute influence of medial and lateral preoptic areas on sleep-wakefulness in freely moving rats. Brain Research. 1990;525:242–248. doi: 10.1016/0006-8993(90)90870-h. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. Journal of Neuroscience. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. A two-process model of sleep. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Budzikowski AS, Leenen FH. ANG II in median preoptic nucleus and pressor responses to CSF sodium and high sodium intake in SHR. American Journal of Physiology - Heart and Circulatory Physiology. 2001;281:H1210–1216. doi: 10.1152/ajpheart.2001.281.3.H1210. [DOI] [PubMed] [Google Scholar]
- Burikov AA, Suntsova NV. The neuronal impulse activity of the preoptic area in the rabbit during electrocorticographic correlates of wakefulness and slow-wave sleep. Zhurnal vysshej nervnoj deiatelnosti im. I. P. Pavlova. 1989;39:1146–1148. [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. Journal of Neuroscience. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Sullivan MJ, Edwards GL, Farinpour R, BeltZ TG, Johnson AK. Dissociation of experimentally induced drinking behavior by ibotenate injection into the median preoptic nucleus. Brain Research. 1991;554:153–158. doi: 10.1016/0006-8993(91)90183-v. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Burns J, Semba K. Indirect projections from the suprachiasmatic nucleus to the median preoptic nucleus: a dual tract-tracing study in rat. Sleep. 2002;25:A308. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- Gardiner TW, Verbalis JG, Stricker EM. Impaired secretion of vasopressin and oxytocin in rats after lesions of nucleus medianus. American Journal of Physiology. 1985;249:R681–688. doi: 10.1152/ajpregu.1985.249.6.R681. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Saper CB. Sleep-active neurons in the ventrolateral preoptic nucleus (VLPO) are galaninergic. Society for Neuroscience Abstracts. 1999;25:625. [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurones of the rat locus coeruleus are tonically inhibited by GABA during sleep. European Journal of Neuroscience. 1998;10:964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, McGinty D, Szymusiak R. Projections from the median preoptic nucleus to forebrain arousal systems in rats. Society for Neuroscience Abstracts. 2001a;27:522. [Google Scholar]
- Gong H, Szymusiak R, King J, Shin SM, McGinty DJ. Co-localization of c-Fos protein and GABA in preoptic area neurons following sleep. Sleep. 2001b;24(suppl.):A155. [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. American Journal of Physiology - Renal Physiology. 2000;279:R2079–2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Alam MN, Szymusiak R, Drucker-Colin R, Gong H, McGinty D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: effects of preoptic/basal forebrain warming. Brain Research. 2000;875:23–34. doi: 10.1016/s0006-8993(00)02561-0. [DOI] [PubMed] [Google Scholar]
- Hayaishi O. Molecular genetic studies on sleep-wake regulation, with special emphasis on the prostaglandin D(2) system. Journal of Applied Physiology. 2002;92:863–868. doi: 10.1152/japplphysiol.00766.2001. [DOI] [PubMed] [Google Scholar]
- Hernandez-Peon R, Chavez-Ibarra G. Sleep induced by electrical or chemical stimulation of the forebrain. Electroencephalography and Clinical Neurophysiology. 1963;24:188. [PubMed] [Google Scholar]
- Heuser G, Ling G, Kluver M. Sleep induction by progesterone in the pre-optic area in cats. Electroencephalography and Clinical Neurophysiology. 1967;22:122–127. doi: 10.1016/0013-4694(67)90151-4. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- John J, Kumar VM. The effects of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep. 1998;21:587–598. doi: 10.1093/sleep/21.6.587. [DOI] [PubMed] [Google Scholar]
- Junqeira FL, Jr, Krieger EM. Blood pressure and sleep in the rat in normotension and in neurogenic hypertension. Journal of Physiology. 1976;259:725–735. doi: 10.1113/jphysiol.1976.sp011491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI. Preoptic area unit activity during sleep and wakefulness in the cat. Experimental Neurology. 1984;83:347–357. doi: 10.1016/S0014-4886(84)90103-1. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Hayaishi O. Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep. Neuroscience Research. 1994;19:31–38. doi: 10.1016/0168-0102(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Lin JS. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Medicine Reviews. 2000;4:471–503. doi: 10.1053/smrv.2000.0116. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. Journal of Neuroscience. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Peyron C, Rampon C, Barbagli B, Boissard R, Fort P. Norepinephrine and REM sleep. In: Mallick BN, Inoué S, editors. Rapid Eye Movement Sleep. New Delhi: Narosa Publishing House; 1999. pp. 107–122. [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. Serotonin neurons and sleep. II. Time course of dorsal raphe discharge, PGO waves, and behavioral states. Archives Italiennes de Biologie. 1987;26:1–28. [PubMed] [Google Scholar]
- McCarley RW, Greene RW, Rainnie DG, Portas CM. Brainstem neuromodulation and REM sleep. Seminars in Neuroscience. 1995;7:341–345. [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Research. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. The sleep-wake switch: A neuronal alarm clock. Nature Medicine. 2000;6:510–511. doi: 10.1038/74988. [DOI] [PubMed] [Google Scholar]
- Mancia G, Zanchetti A. Cardiovascular regulation during sleep. In: Orem J, Barnes CD, editors. Physiology in Sleep. New York: Academic Press; 1980. pp. 1–55. [Google Scholar]
- Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, Kantha SS, Kasahara K, Hayaishi O. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proceedings of the National Academy of Sciences of the USA. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV, Perlis M, Wagner R. Enhancement of sleep by microinjection of triazolam into the medial preoptic area. Neuropsychopharmacology. 1989;2:61–66. doi: 10.1016/0893-133x(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Hypothalamic regulation of sleep in rats. An experimental study. Journal of Neurophysiology. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997b;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal F, Benedek G, Obal F, Dibo G, Jancso-Gabor A. Basal forebrain sleep mechanisms activated by electrical and thermal stimulations. In: Lissak K, editor. Recent Developments of Neurobiology in Hungary. Budapest: Akademiai Kiado; 1982. pp. 159–175. [Google Scholar]
- Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. Relationship of EP(1–4). prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. Journal of Comparative Neurology. 2000;428:20–32. doi: 10.1002/1096-9861(20001204)428:1<20::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Gouin PR, Tower BB. Secretion of hormones influencing water and electrolyte balance (antidiuretic hormone, aldosterone, prolactin) during sleep in normal adult men. Psychosomatic Medicine. 1978;40:44–59. doi: 10.1097/00006842-197802000-00006. [DOI] [PubMed] [Google Scholar]
- Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends in Neurosciences. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proceedings of the National Academy of Sciences of the USA. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proceedings of the National Academy of Sciences of the USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Price KJ, Sagar SM. Hyperthermia induces c-fos expression in the preoptic area. Brain Research. 1993;618:303–307. doi: 10.1016/0006-8993(93)91280-6. [DOI] [PubMed] [Google Scholar]
- Schonrock B, Busselberg D, Haas H. Properties of tuberomammillary histamine neurones and their response to galanin. Agents Actions. 1991;33:135–137. doi: 10.1007/BF01993148. [DOI] [PubMed] [Google Scholar]
- Seutin V, Verbanck P, Massotte L, Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. European Journal of Pharmacology. 1989;164:373–376. doi: 10.1016/0014-2999(89)90481-0. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. Journal of Neuroscience. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Research. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. Journal of Comparative Neurology. 2001;429:638–653. [PubMed] [Google Scholar]
- Steriade M. Cellular substrates of oscillations in corticothalamic systems during vigilance. In: Lydic R, Bahdoyan HA, editors. Handbook of Behavioral State Control: Cellular and Molecular Mechanisms. Boca Raton, FL, USA: CRC Press; 1999. pp. 327–347. [Google Scholar]
- Steriade M. Active neocortical processes during quiescent sleep. Archives Italiennes de Biologie. 2001;139:37–51. [PubMed] [Google Scholar]
- Sterman MB, Clemente CD. Forebrain inhibitory mechanisms, sleep pattern induced by basal forebrain stimulation in behaving cat. Experimental Neurology. 1962;6:103–117. doi: 10.1016/0014-4886(62)90081-x. [DOI] [PubMed] [Google Scholar]
- Suntsova NV, Burikov AA. The restructuring of the neuronal activity of the lateral hypothalamic preoptic area during the development of sleep. Zhurnal vysshej nervnoj deiatelnosti im. I. P. Pavlova. 1995;45:948–956. [PubMed] [Google Scholar]
- Suntsova NV, Dergachyova OY. On the existence of anti-waking system in the ventrolateral preoptic area. Journal of Sleep Research. 2000;9(suppl. 1):124. [Google Scholar]
- Suntsova NV, Dergachyova OY. Dynamics of neuronal activity in the lateral preoptic area of hypothalamus in the course of sleep-waking cycle. Zhurnal vysshej nervnoj deiatelnosti im. I. P. Pavlova. 2002;52 in the Press. [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty DJ. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Research. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep suppression following kainic acid-induced lesions of the basal forebrain. Experimental Neurology. 1986;566:598–614. doi: 10.1016/0014-4886(86)90240-2. [DOI] [PubMed] [Google Scholar]
- Terao A, Matsumura H, Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. Journal of Neuroscience. 1998;18:6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacology, Biochemistry and Behaviour. 1991;40:33–40. doi: 10.1016/0091-3057(91)90317-u. [DOI] [PubMed] [Google Scholar]
- Timo-Iaria C, Negrao N, Schmidek W, Hoshino K, Demenezes C, Darocha TL. Phases and states of sleep in the rat. Physiology and Behaviour. 1970;5:1057–1062. doi: 10.1016/0031-9384(70)90162-9. [DOI] [PubMed] [Google Scholar]
- Travis KA, Johnson AK. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. American Journal of Physiology. 1993;264:R1200–1205. doi: 10.1152/ajpregu.1993.264.6.R1200. [DOI] [PubMed] [Google Scholar]
- Valatx JL. Mechanisms of dream-sleep-wakefulness cycle. La Revue du Praticien-Medecine Generale. 1996;46:2404–2410. [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Salvert D, Jouvet M. Waking-state specific neurons in the caudal hypothalamus of the cat. Comptes Rendus de l'Academie des Sciences. 1984;298:195–200. [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. Journal of Nervous and Mental Disease. 1930;71:249–259. [Google Scholar]
- Yang QZ, Hatton GI. Excitatory and inhibitory inputs to histaminergic tuberomammillary nucleus. Brain Research. 1997;773:162–172. doi: 10.1016/s0006-8993(97)00932-3. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Johnson AK. Chemical topography of efferent projections from the median preoptic nucleus to pontine monoaminergic cell groups in the rat. Neuroscience Letters. 1995;199:215–219. doi: 10.1016/0304-3940(95)12003-m. [DOI] [PubMed] [Google Scholar]


