Abstract
The ATP-sensitive K+ (KATP) channels are regulated by intracellular H+ in addition to ATP, ADP, and phospholipids. Here we show evidence for the interaction of H+ with ATP in regulating a cloned KATP channel, i.e. Kir6.2 expressed with and without the SUR1 subunit. Channel sensitivity to ATP decreases at acidic pH, while the pH sensitivity also drops in the presence of ATP. These effects are more evident in the presence of the SUR1 subunit. In the Kir6.2 + SUR1, the pH sensitivity is reduced by about 0.4 pH units with 100 μM ATP and 0.6 pH units with 1 mm ATP, while a decrease in pH from 7.4 to 6.8 lowers the ATP sensitivity by about fourfold. The Kir6.2 + SUR1 currents are strongly activated at pH 5.9-6.5 even in the presence of 1 mm ATP. The modulations appear to take place at His175 and Lys185 that are involved in proton and ATP sensing, respectively. Mutation of His175 completely eliminates the pH effect on the ATP sensitivity. Similarly, the K185E mutant-channel loses the ATP-dependent modulation of the pH sensitivity. Thus, allosteric modulations of the cloned KATP channel by ATP and H+ are demonstrated. Such a regulation allows protons to activate directly the KATP channels and release channel inhibition by intracellular ATP; the pH effect is further enhanced with a decrease in ATP concentration as seen in several pathophysiological conditions.
The KATP channels play an important role in controlling membrane potential and cellular excitability (Quayle et al. 1997; Ashcroft & Gribble, 1998; Yokoshiki et al. 1998). Activity of these channels is regulated by several cytosolic factors such as ATP, phospholipids and nucleotide diphosphates (Noma, 1983; Lederer & Nichols, 1989; Larsson et al. 1993; Fan & Makielski, 1997; Gribble et al. 1997; Baukrowitz et al. 1998; Shyng & Nichols, 1998). In addition to these KATP channel regulators, there is evidence indicating that the KATP channels in several cell types are sensitive to intracellular pH (Lederer & Nichols, 1989; Misler et al. 1989; Davies, 1990; Koyano et al. 1993; Fan et al. 1994; Proks et al. 1994; Allard et al. 1995; Vivaudou & Forestier, 1995). Thereby, detailed studies of the molecular mechanisms underlying the pH sensitivity may lead to an understanding of KATP channel function in regulating cellular excitability under various physiological and pathophysiological conditions when pH or both pH and ATP are low.
The cloned KATP channels are particularly suitable for these studies. It is known that the KATP channels are made of the pore-forming Kir6 subunit and the sulfonylurea receptor (SUR) subunit (Inagaki et al. 1995). Truncation of 24–36 amino acids at the C-terminal end (Kir6.2ΔC24, Kir6.2ΔC36) allows the channels to be expressed without the SUR subunit (Tucker et al. 1997). Indeed, we have recently shown that Kir6 channels are activated by hypercapnic acidosis (Piao et al. 2001; Xu et al. 2001a). The pH sensitivity as well as the availability of genetic materials makes it now possible to understand (1) whether protons and ATP act on the same or different binding sites; (2) whether the pH effect results from titration of ATP; and (3) how these KATP channel modulators interact with each other in controlling the channel activity. These are questions that have been asked since the pH sensitivity was first demonstrated in the KATP channels (Lederer & Nichols, 1989; Davies et al. 1992; Koyano et al. 1993; Fan et al. 1994; Proks et al. 1994; Allard et al. 1995; Vivaudou & Forestier, 1995). Our previous studies have indicated that a C-terminal histidine residue (His175) is likely to be the protonation site in Kir6.2 and be responsible for the pH-dependent channel activation (Xu et al. 2001b). However, this result should not rule out the possibility that proton sensing is affected by ATP, and vice versa. In fact, Vivaudou & Forestier (1995) have shown that ATP and pH sensitivities are reciprocally modulated by each other in frog muscular KATP channels, whereas Proks et al. (1994) suggested that titration of ATP was the mechanism underlying the pH sensitivity. In addition, it is unclear whether protons compete with ATP for binding to the Kir6.2 channel protein, as recently demonstrated for ATP and phospholipid binding (MacGregor et al. 2002). The above three questions are still open, and we intended to address them in the present study. Our results showed that intracellular H+ and ATP allosterically modulated the Kir6.2 channel. Such modulations depended on His175 and Lys185, respectively, were greatly enhanced by the SUR1 subunit and allowed the Kir6.2 channels to be activated in the presence of physiological concentrations of ATP.
Methods
Kir6.2 (Genbank no. D50581, a gift from Dr S. Seino) and SUR1 (Genebank no. L40623, a gift from Dr L. Bryan) cDNAs were used in the present study. The cDNAs were cloned to a eukaryotic expression vector (pcDNA3.1, Invitrogen Inc., Carlsbad, CA, USA) and expressed in mammalian cell line and frog oocytes.
The human embryonic kidney cells (HEK293, CRL-1573, Batch no. 2187595, ATCC, Rockville, MD, USA) were chosen, as these cells possess the mammalian expression machineries, and show little inward rectifying K+ current (Zhu et al. 1998). Cells were cultured as monolayers in minimum essential medium - Eagle (MEM-E; Sigma, St Louis, MO, USA) with 10 % fetal bovine serum with penicillin/streptomycin added and incubated at 37 °C with 5 % CO2 in atmospheric air. The cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) with 5 μg wild-type or mutant Kir6.2 cDNA per Petri dish (35 mm) and split twice weekly. When the cells were cotransfected with SUR1, a 5 μg mixture of Kir6.2 and SUR1 cDNAs in a 1: 2 ratio was used. To facilitate the identification of positively transfected cells, 0.5 μg of green fluorescent protein (GFP) cDNA (pEGFP-N2, Clontech, Palo Alto, CA, USA) were added to the cDNA mixture. Cells were disassociated from the monolayer using 0.01 % trypsin 24–48 h post-transfection. A few drops of the cell suspension were added to a Petri dish, and then incubated at 37 °C for at least 2 h before experiments.
To validate the data obtained from the HEK293 cells, frog (Xenopus laevis) oocytes were used in a few experiments. The oocytes were obtained as described previously (Piao et al. 2001; Xu et al. 2001;), and treated with 2 mg ml−1 of collagenase (Type I, Sigma Chemicals, St Louis, MO, USA) in OR2 solution (82 mm NaCl, 2 mm KCl, 1 mm MgCl2 and 5 mmN-2-hydroxyethyl-piperazine-N‘-2-ethanesulfonic acid or Hepes, pH 7.4) for 90 min at the room temperature. After three washes (10 min each) of the oocytes with the OR2 solution, cDNAs were injected into the oocytes (20- 50 ng in 50 nl double distilled water). The oocytes were then incubated at 18 °C in an ND-96 solution containing (mm): NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8, Hepes 5, and sodium pyruvate 2.5 with 100 mg l−1 geneticin added (pH 7.4).
Whole-cell currents were studied on the oocytes 2–4 days after injection to determine channel expression. Two electrode voltage clamp was performed using an amplifier (Geneclamp 500, Axon Instruments Inc., Foster City, CA, USA) at room temperature (23- 25 °C). The extracellular solution contained (mm): KCl 90, MgCl2 3, and Hepes 5 (pH 7.4).
Patch clamp experiments were performed at room temperature as described previously (Zhu et al. 1999; Yang et al. 2000). In brief, fire-polished patch pipettes were made from 1.2 mm borosilicate capillary glass (Sutter Instruments, Novato, CA, USA). Giant inside-out patches were employed to study macroscopic currents in a cell-free condition using recording pipettes of 0.5-1.0 MΩ. Current records were low-pass filtered (2 kHz, Bessel, 4-pole filter, −3 dB), digitized (20 kHz, 16-bit resolution), and stored on computer disk for later analysis (pCLAMP 8, Axon Instruments). Recordings were performed using solutions containing equal concentrations of K+ applied to the bath and recording pipettes. The bath solution contained (mm): KCl 10, potassium gluconate 105, KF 5, potassium pyrophosphate 5, sodium vanadate 0.1, EGTA 5, glucose 5, and Hepes 10 (pH 7.4). It is known that pyrophosphate and vanadate can alleviate channel rundown. We examined the current profile and channel responses to pH and ATP in the presence versus absence of pyrophosphate and vanadate, and did not find any difference. The pH sensitivity presented is identical to our previous observations in the absence of pyrophosphate and vanadate (Xu et al. 2001a, b), and the ATP sensitivity is comparable to those documented previously (Quayle et al. 1997; Ashcroft & Gribble, 1998; Yokoshiki et al. 1998). Also, the concentration of vanadate in the patch solution has been shown to have no effect on the Kir6.2 channels (Shyn et al. 1997b; Proks et al. 1999a). When high concentrations of ATP (K+ salt) was used, K+ gluconate was replaced in a 1:2 (K2-ATP : K-gluconate) ratio. The pipette was filled with the same solution. The same intra- and extracellular solutions were also used for patch recording from the Xenopus oocytes. The oocyte vitelline membranes were mechanically removed after being exposed to a hypertonic solution (400 mosmol l−1) for 5 min. Patch clamp was performed on the stripped oocytes in a 35 mm Petri dish.
Low pH exposures were carried out using the same bath solutions that were titrated to various pH levels as required by experimental protocols. For ATP exposures, pH levels were adjusted after appropriate ATP concentrations were made in each solution. To avoid ATP degradation, all ATP-containing solutions were made immediately before experiments and only used for 3 h. Since the variation of Cl− concentrations in solutions was rather small, the resultant liquid junction potential was less than 1 mV according to the Henderson equation (Barry & Lynch, 1991), and was not corrected.
Data analysis was done using the Clampfit 6 software (Axon Instruments Inc.). Leak currents were removed from the data by subtracting the residue currents when the Kir6.2 channels were completely inhibited by ATP or acidic pH. The current-pH relationship was described using a sum of two Hill equations: y = {m/[1 + (pH/pK1)h1]} + {m/[1 + (pK2/pH)h2]} - m, where pK1 = the pH level for mid-point channel activation, h1 = the Hill coefficient for channel activation, pK2 = the pH level for mid-point channel rundown, h2 = the Hill coefficient for channel rundown, and m = 2 - percentage peak activation reached at the intersection of the activation and rundown curves (Xu et al. 2001a). The activation and rundown curves were first fitted individually using a single Hill equation. The value of percentage peak activation was then determined at the intersection of the activation and rundown curves, which ranged from 70 to 75 %, yielding an m value of 1.25-1.30. The m value would be 1 if the currents reached 100 % peak activation when the two curves meet. We have chosen to use a sum of two Hill equations because (1) there is no evidence for the mutual dependence of the activation and rundown; (2) a sum of two Hill equations conserves most of the channel activation and rundown as they are shown by fitting the data individually; and (3) we have used the same fitting in our previous publications (Xu et al. 2001a,b). The current-ATP relationship was expressed with the regular Hill equation: y = 1/(1 + ([ATP]/IC50)h), where [ATP] = ATP concentration, and IC50 = the [ATP] at mid-point channel inhibition. Data are presented as means ± s.e.m. Differences in means were tested with the ANOVA or Student's t test and were accepted as significant if P ≤ 0.05.
Results
Baseline channel properties
Wild-type (WT) Kir6.2 + SUR1 and homomeric Kir6.2ΔC36 currents were studied in inside-out patches using symmetric concentrations of K+ (145 mm) on both sides of the plasma membrane. Membrane potential was held at 0 mV, and ramp command potentials from −100 to 100 mV (-150 to 150 mV in some patches) were applied to the patches through the recording pipette. Under such a condition, inward rectifying currents with single channel conductance of ≈70 pS were recorded from cells receiving both the Kir6.2 and SUR1 cDNAs (Kir6.2 + SUR1) or the Kir6.2ΔC36 alone. Exposure of the internal membranes to perfusates containing ATP (K+ salt) produced concentration-dependent inhibitions of the inward rectifying currents with the ATP concentration at mid-point channel inhibition (IC50) being ≈10 μM in the Kir6.2 + SUR1 currents and ≈100 μM in the Kir6.2ΔC36.
Effects of ATP on the pH Sensitivity of Kir6.2 currents
The pH sensitivity was studied by exposures of the internal surface of patch membranes to perfusates with various pH levels. Consistent with our previous studies, moderate acidification augmented the macroscopic Kir6.2 + SUR1 currents. This was observed in all patches, no matter whether or not there was ATP in the internal solution (Fig. 1). In the absence of ATP, the peak activation occurred at pH 6.5-6.8. Further decrease in pH caused rapid current inhibition (Fig. 1A). This inhibition appeared to be related to channel rundown because channel activity showed little or no recovery with washout at pH 7.4. This is consistent with previous reports on both cell-endogenous and cloned KATP channels (Davies et al. 1992; Koyano et al. 1993; Fan et al. 1994; Baukrowitz et al. 1999; Xu et al. 2001a,b). Although a similar current-pH relationship was observed in the presence of ATP (100 μM or 1 mm), the maximal activation was reached at more acidic pH levels (pH 5.9-6.2), and the channel rundown did not occur until pH 5.4-5.6 (Fig. 1B and C). When the currents were expressed as a function of pH, a bell-shaped channel activation followed by rundown was seen. This biphasic response can be described using a sum of two Hill equations (Fig. 2A). In the absence of ATP, the regression curve was produced with Hill coefficients pK1 7.07 for activation and pK2 6.35 for rundown (n = 5). The current-pH relationship was shifted by 0.35 and 0.57 pH units toward lower pH levels in the presence of 100 μM ATP (pK1 = 6.72, pK2 = 6.02, n = 5) and 1 mm ATP (pK1 = 6.50, pK2 = 5.75, n = 7) in the internal solution, respectively.
Figure 1. Effects of pH on Kir6+SUR1 currents.

The Kir6.2 + SUR1 currents were expressed in the HEK293 cell line using lipofectamine together with an expression vector containing the green fluorescent protein (GFP) gene. Currents were recorded from an HEK293 cell that showed GFP fluorescence. Symmetric concentrations of K+ (145 mm) were applied to both sides of the patch membrane. Ramp command potentials from 100 to −100 mV were applied to the patches from a holding potential of 0 mV. A, current response to acidic pH was studied in the absence of ATP by exposing the internal surface of the patch membrane to perfusates with various pH levels as indicated above each panel. An increase in the current amplitude started at pH 7.1. The maximal activation occurred at pH 6.5. Further decrease in pH caused a rapid inhibition of the currents. The currents did not recover after ≈3 min washout. B, in the presence of 100 μM ATP, the Kir6.2 + SUR1 currents were inhibited. Maximal activation of the currents was seen at a more acidic pH level (pH 6.2), although similar channel activation followed by inhibition was observed. C, the channels were strongly activated at pH 5.9 even in the presence of 1 mm ATP. Note different calibrations. Eight superimposed traces are shown in each panel.
Figure 2. Reciprocal effects of ATP and H+ on the channel sensitivities to each other.

A, relationship of pH versus inward rectifying currents. In the absence of ATP, the Kir6.2 + SUR1 currents show a biphasic response to acidic pH. Currents increase at pH 7.1-6.5, and then decrease at pH 6.2. The current-pH relationship (open diamond and dashed line) is expressed with a sum of two Hill equations (see Methods), in which pK1 = 7.07, h1 = 1.6, pK2 = 6.35, and h2 = 4.7 (n = 5). Although a similar biphasic current-pH relationship is seen in the presence of 100 μM (continuous line and open triangle) and 1 mm ATP (continuous line and open circle), these concentrations of ATP shift the curves toward lower pH levels (100 μM: pK1 = 6.72, h1 = 1.7, pK2 = 6.02, h2 = 4.2, n = 5; 1 mm: pK1 = 6.50, h1 = 1.7, pK2 = 5.75, h2 = 4.0, n = 7). The channel activation followed by inhibition is also observed in the Kir6.2ΔC36 expressed without SUR (filled diamond and dashed line; pK1 = 7.15, h1 = 1.5, pK2 = 6.40, h2 = 4.7, n = 4). The current-pH relationship is shifted by 0.15 pH units toward more acidic pH levels in the presence of 1 mm ATP (continuous line and filled circle; pK1 = 7.0, h1 = 1.5, pK2 = 6.31, h2 = 4.7, n = 5), while 100 μM ATP had very little effect (filled triangle). B, effect of pH on the ATP sensitivity of Kir6.2 channels. Macroscopic Kir6.2 currents were studied in giant inside-out patches. Exposure of the internal surface to ATP produced a concentration-dependent inhibition of these currents. The relationship of ATP with Kir6.2 + SUR1 currents is expressed using the Hill equation with IC50 (ATP concentration at mid-point inhibition) 7 μM and h 1.2 (n = 5) at pH 7.4 (filled diamond and dashed line) and IC50 30 μM and h 1.2 (n = 4) at pH 6.8 (continuous line and filled triangle). The Kir6.2ΔC36 currents (open diamond and dashed line) show a much lower ATP sensitivity (IC50 95 μM, h 1.3, n = 3; at pH 7.4) than the Kir6.2 + SUR1. The ATP sensitivity is further reduced at pH 6.8 (IC50 205 μM, h 1.3, n = 4; at pH 7.4; open triangle and line). Abbreviation: I, current.
The bell-shaped current-pH relationship was also observed in the Kir6.2ΔC36 (Fig. 2A and Fig. 3). In the presence of 100 μM ATP, however, the current-pH relationship did not show evident difference from that without ATP (Fig. 2A). When the ATP concentration was increased to 1 mm, the current-pH curve was displaced by 0.15 pH units toward more acidic pH levels (Fig. 2A and Fig. 3). These results thus indicate that ATP reduces the pH sensitivity of the Kir6.2 + SUR1 and Kir6.2ΔC36 currents, and this effect is enhanced by the SUR1 subunit.
Figure 3. Kir6ΔC36 current response to acidic pH.

The Kir6.2DC36 currents were expressed in Xenopus oocytes and studied under the same conditions as Fig 1. Channel activation followed by inhibition is seen in the absence (A) and presence of 1 mm ATP (B). Note that changes in the pH sensitivity by ATP are not as obvious as the Kir6.2 + SUR1 shown in Fig 1. Eight superimposed traces are shown in each panel.
Effects of pH on the ATP sensitivity
To elucidate whether protons also affect the channel sensitivity to ATP, we studied the current-ATP relationship at pH 7.4 and 6.8 in inside-out patches. As documented previously, exposures of the internal patches membranes to ATP produced a concentration-dependent inhibition of the Kir6.2 currents (Fig 4). The Kir6.2 + SUR1 currents were highly sensitive to ATP. At pH 7.4, the currents were half inhibited by ≈10 μM ATP and strongly suppressed by 100 μM ATP (Fig 4A). At pH 6.8, the ATP sensitivity was markedly reduced (Fig 4B). The current-ATP relationship is described using the Hill equation with the IC50 = 7 μM (n = 5) at pH 7.4 and 30 μM (n = 4) at pH 6.8 (Fig 2B). The Kir6.2ΔC36 currents had lower ATP sensitivity with IC50 = 100 μM (n = 4) at pH 7.4 as demonstrated previously (Tucker et al. 1997). The ATP sensitivity was further reduced at pH 6.8 (IC50 = 205 μM, n = 4; Fig 2B). Thus, acidic pH shifts the current-ATP relationship curves toward higher ATP levels, which is more evident in the presence of the SUR1 subunit.
Figure 4. Dose-dependent inhibition of Kir6.2 + SUR1 currents by ATP.
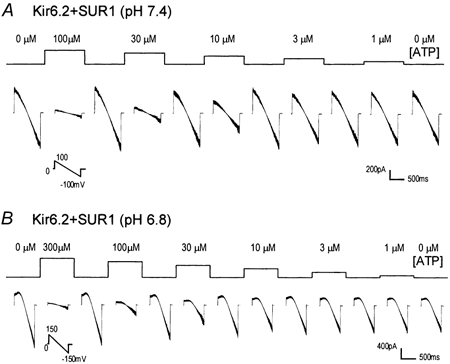
Kir6.2 + SUR1 channels were expressed in the HEK293 cells and studied in the same condition as in Fig 1. A, at pH 7.4, exposures of the internal surface of the patch membrane to various concentrations of ATP produce a concentration-dependent inhibition of these currents with mid-point inhibition at about 10 μM. B, at pH 6.8, the ATP sensitivity of the Kir6.2 + SUR1 currents becomes lower with the mid-point inhibition at about 30 μM. Note different command potentials. Eight superimposed traces are shown in each panel.
Dependence of the allosteric effects on His175 and Lys185
The reciprocal effects of H+ and ATP on channel activity suggest that these two KATP channel modulators interact with each other. Such an interaction could be due to their competitive binding to the channel protein, or could result from allosteric effects. The availability of Kir6.2 cDNA offers a readily accessible approach to these potential mechanisms, as it is possible to differentiate these effects by working on specific sites that are involved in ATP and pH sensing. The channel is likely to be modulated through the allosteric mechanism, if the reciprocal effects are related to ATP and pH binding to the channel protein rather than to their existence in the recording solution; H+ and ATP might share the same binding site, if mutation of a residue affects both ATP and pH sensitivity.
Several residues are involved in the ATP sensitivity of the Kir6.2 channel (Shyng et al. 1997a; Drain et al. 1998; Trapp et al. 1998; Tucker et al. 1998; Koster et al. 1999; Proks et al. 1999b; Reimann et al. 1999). Of these Lys185 is special and is believed to influence ATP binding electrostatically (Reimann et al. 1999; Tanabe et al. 1999). Therefore, we studied the pH sensitivity and the effect of ATP on the pH sensitivity using the K185E mutant. In the absence of ATP, the K185E-mutant Kir6.2ΔC36 channel showed a pH sensitivity similar to the Kir6.2ΔC36 (Fig. 5A and Fig. 6A), suggesting this residue is not involved in proton sensing. ATP (1 or even 10 mm) failed to shift the current-pH relationship in the K185E-mutant Kir6.2ΔC36 (Fig. 5B,C and Fig. 6A). Because the Kir6.2 + SUR1 with the K185E mutation showed high baseline activity with Popen 0.7-0.8, this mutant was only slightly stimulated at pH 6.6-7.0. Consistently, the K185E mutation abolished the effect of ATP (1 and 10 mm) on the current-pH relationship in the Kir6.2 + SUR1 (Fig 6A). Thus, the pH sensitivity of the Kir6.2 channel is modulated by the ATP binding to the channel protein rather than the molecular ATP per se.
Figure 5. The effect of the K185E mutation on the pH sensitivity.
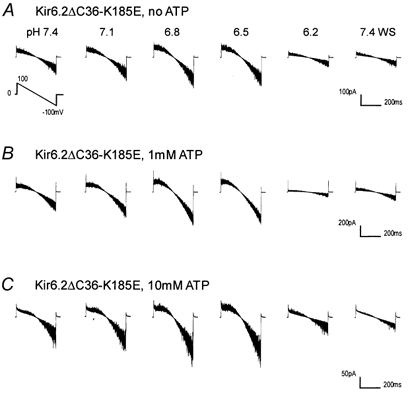
Under the same experimental conditions as in Fig. 3, ATP (1 mm in B; 10 mm in C) failed to change the pH sensitivity of the Kir6.2ΔC36-K185E channels. Patches were obtained from Xenopus oocytes. Eight superimposed traces are shown in each panel.
Figure 6. Elimination of the allosteric effects of H+ and ATP by mutations of His175 and Lys185.

A, in the absence of ATP, the K185E-mutant Kir6.2ΔC36 channel showed pH sensitivity similar to WT channels. The K185E mutation, however, completely eliminated the effect of ATP (1 and 10 mm) on the pH sensitivity of the Kir6.2ΔC36 channel. Data are presented as means ± s.e.m. (n = 4-6), and fitted as described in Fig 2A: pK1 = 7.17, h1 = 1.2, pK2 = 6.33, and h2 = 4.3. The biphasic effect of pH on the K185E-mutant Kir6.2 plus SUR1 was not as evident as on the Kir6.2ΔC36. Despite of this, ATP failed to affect the pH sensitivity in the K185E-mutant channels. B, similarly, the effect of pH on the channel sensitivity to ATP is abolished with the H175A or H175K mutation. The ATP sensitivity of the H175K remained almost the same at pH 6.8 as at pH 7.4 (IC50 = 7 μM and h = 1.1 for Kir6.2 + SUR1 and its mutants; IC50 = 100 μM and h = 1.1 for Kir6.2ΔC36 and its mutants; n = 4- 6). Abbreviation: I, current.
We have previously shown that His175 is the only protonation site for the Kir6.2 channel activation at acidic pH (Xu et al. 2001b). Interestingly, the H175A- and H175K-mutant Kir6.2 channels (Kir6.2 + SUR1 and Kir6.2ΔC36) showed ATP sensitivity identical to the WT Kir6.2 + SUR1 and Kir6.2ΔC36 (Fig. 6B and Fig. 7). When the ATP sensitivity was studied at pH 6.8 and 7.4, we found that the pH-induced shift in the ATP sensitivity was completely eliminated (Fig 6B). These results therefore strongly suggest that the ATP sensitivity is modulated by protonation of the His175 in the Kir6.2 subunit.
Figure 7. Loss of effect of pH on the ATP sensitivity.

His175 was mutated to alanine in Kir6.2. This mutant Kir6.2 was coexpressed with SUR1 in HEK293 cells, and studied in the same condition as Fig 4. A, at pH 7.4, the H175A-mutant channel is still highly sensitive to ATP with mid-point inhibition at about 10 μM. B, acidic pH (pH 6.8) failed to change the ATP sensitivity of the H175A-mutant channel. Eight superimposed traces are shown in each panel.
Discussion
One important property of the KATP channels is pH sensitivity. In the presence of ATP, low pH enhances KATP channel activity in several tissues and cell types (Cuevas et al. 1991; Davies et al. 1992; Proks et al. 1994; Allard et al. 1995). In the absence of ATP, however, reports on the pH effect are rather inconsistent (Cuevas et al. 1991; Davies et al. 1992; Koyano et al. 1993; Proks et al. 1994; Allard et al. 1995; Vivaudou & Forestier, 1995), which may result from the biphasic response of these channels to acidic pH. It is very likely that the channels are stimulated by a brief exposure to moderate acidification but inhibited with a longer exposure to lower pH as shown in the present study. In the presence of ATP, the channel rundown becomes less severe (Quayle et al. 1997) and occurs at more acidic pH (see Fig. 1 and Fig. 2A). Therefore, the likelihood of seeing channel activation increases. A similar biphasic response has been observed in the cell-endogenous KATP channels (Koyano et al. 1993; Fan et al. 1994), further indicating that the Kir6.2 responds to acidic pH similarly to the cell-endogenous KATP channels.
The pH sensing is distinct from ATP sensing in the Kir6.2 channel. We have previously demonstrated His175 to be the only protonation site for activation of the channel at acidic pH (Xu et al. 2001b). Our results from the present study show that mutation of the His175 abolishes the pH sensitivity, but has no effect on the ATP sensitivity. Similarly, our studies indicate that ATP sensing is also separate from pH sensing: the pH sensitivity of the K185E mutants remains the same as the WT channels, whereas the ATP sensitivity of the same mutant has been previously shown to be greatly diminished (Reimann et al. 1999). These results as well as our previous observations (Xu et al. 2001b) indicate that ATP sensing is separate from pH sensing in the Kir6.2 channels.
Although the pH sensing does not rely on ATP, ATP can dramatically change the pH sensitivity of KATP channels. Our data show that ATP displaces the pH-current relationship curve toward lower pH levels without changing the Hill coefficient. This is consistent with a previous study by Vivaudou & Forestier (1995) showing that the pK value for KATP channel activation shifts from pH 6.25 (with 30 μM ATP) to pH 5.30 (with 1 mm ATP) in frog skeletal muscles. We realize that such low pK values were not observed in the Kir6.2 + SUR1 and Kir6.2ΔC36 channels in the present study. It is possible that there are other KATP channel species in frog skeletal muscles. In addition to the effect on channel activation, ATP attenuates the KATP channel rundown in several native tissues (Koyano et al. 1993; Fan et al. 1994), which is also consistent with the leftward shift in the pH-current relationship curve, as the rundown apparently occurs at more acidic pH when ATP is present in the cytosol.
The pH sensitivity enables the Kir6.2 channels to play a role in regulating membrane excitability even in the presence of physiological concentrations of ATP. Our data show that Kir6.2 + SUR1 channels are strongly activated at pH 5.9-6.5 even in the presence of 1 mm ATP, although they are almost completely suppressed by such a concentration of ATP at pH 7.4. Since these pH levels are seen in several pathophysiological conditions, protonation should be considered as an important KATP channel regulator similar to ATP, ADP and phospholipids.
The ATP sensitivity of the KATP channels is also modulated by pH. Results from the present study on the Kir6.2 and from those on the cell-endogenous KATP channels indicate that protons produce a parallel shift in the ATP-current relationship curve. At pH range 6.4-6.8, the IC50 ATP concentration is doubled from that at pH 7.4 (Lederer & Nichols, 1989; Koyano et al. 1993; Proks et al. 1994). An even larger increase in the IC50 was reported with lower pH levels (Davies et al. 1992; Vivaudou & Forestier, 1995).
There are four possible ways that ATP and protons can modulate the channel sensitivity to each other: (1) these two KATP channel regulators may compete for the same binding site; (2) ATP may be protonated affecting its inhibition of the channel; (3) in the presence of ATP, the channel rundown is reduced which may affect channel sensitivity and (4) ATP and protons may affect the channel sensitivity to each other via the allosteric mechanism. Based on our studies, protons do not seem to compete with ATP for binding to the channel protein, as mutation of His175 and Lys185 selectively interrupts channel sensitivity to pH and ATP, respectively. The inability of the His175 mutation to affect the ATP sensitivity also suggests that His175 does not contribute to the unidentified ATP binding site. Similarly, our data indicate that Lys185 is not a proton sensor. Thus, H+ is a unique KATP channel modulator distinct from ADP and PIP2. The latter two compete with ATP for binding to the Kir6.2 subunit (Tucker et al. 1998; MacGregor et al. 2002). The second possibility can be largely excluded as well, because our results show that the shift in current-ATP relationship depends on the protonation of His175 rather than acidification or titration of intracellular factors such as ATP. Therefore, these results are in disagreement with a previous report by Proks et al. (1994). Thirdly, ATP and ADP are known to reduce channel rundown in KATP channels, which is consistent with the leftward shift in the titration curve for channel inhibition. However, the activation curve is also shifted to a lower pH level in the presence of ATP, which should not be related to channel rundown. Thus, the improvement of channel rundown cannot account for the shift in the pH-dependent channel activation by ATP. Finally, our studies support the idea that channel sensitivities to intracellular protons and ATP are allosterically modulated by these two KATP channel regulators. This is based on the following: (1) ATP and H+ affect channel sensitivity to each other, consistent with reciprocal effects that occur in all allosteric modulations; (2) ATP and H+ bind to distinct sites in the channel protein, i.e. proton binding to His175 and Lys185 contributes to ATP binding (Reimann et al. 1999; Tanabe et al. 1999); and (3) the parallel shifts in the pH and ATP sensitivities are totally abolished when the reciprocal binding site is mutated. Therefore, it is very likely that proton binding to His175 allosterically reduces the binding affinity of ATP to its receptor, and vice versa, a mechanism that is consistent with the typical allosteric modulation described in haemoglobins and a number of other molecules (Monod et al. 1965).
It is worth noting that the shift in the ATP sensitivity at acidic pH does not seem to be produced by His175 protonation alone, as the H175K mutant shows ATP sensitivity comparable to the WT channels. It is possible that protonation of the His175 changes the tertiary structure of the C-terminus where the ATP binding site is likely to be located (Drain et al. 1998; MacGregor et al. 2002), and in turn affects the ATP sensitivity.
Another interesting phenomenon that we have found in these studies is that the allosteric effects are significantly enhanced by the SUR1 subunit. The pH sensitivity is shifted 0.57 pH units by 1 mm ATP in the Kir6.2 + SUR1, while such a concentration of ATP only shifts the sensitivity by 0.15 pH units in the Kir6.2ΔC36. Since the SUR1 markedly reduces the IC50 concentrations of ATP, these results further support the idea that the allosteric effect requires ATP-channel interaction. The pH effect on the ATP sensitivity is also improved in the presence of SUR1. A change in pH from 7.4 to 6.8 doubles the IC50 (from 100 to 205 μM) in the Kir6.2ΔC36, whereas it increases the IC50 fourfold (from 7 to 30 μM) in the Kir6.2 + SUR1. The SUR1 subunit has very little effect on the pH sensitivity in the absence of ATP (see Fig 2A), and thus does not seem to play a role in protonation of the His175. It is possible that the interaction of the SUR1 with Kir6.2 is compromised at acidic pH, leading to a decrease in the ATP sensitivity.
Coupling of the metabolic state to cellular activity, the most important property of KATP channels, was believed to be produced by the channel sensitivity to intracellular ATP concentration. Studies over the past few years, however, have indicated that other KATP channel regulators such as ADP and phospholipids also play important roles in controlling channel activity. The ATP sensitivity decreases in the presence of phospholipids, facilitating the activation of these channels under physiological conditions (Fan & Makielski, 1997; Baukrowitz et al. 1998; Shyng & Nichols, 1998). In the present study, we have shown that protons interact with ATP in regulating channel activity. A decrease in intracellular pH reduces the ATP sensitivity, leading to a relief of the channel inhibition by ATP. A drop in ATP concentration, on the other hand, increases the pH sensitivity and enhances the activatory effect of protons on the channel. With these effects, the KATP channels can be activated more readily than with a fall in the ATP or pH level alone. Since the reduction in intracellular ATP concentration is often accompanied by intracellular acidosis as seen in a number of metabolic stresses including hypoxia, hypoglycaemia, and ischaemia, the demonstration of the allosteric modulations of KATP channels by ATP and protons may have a major impact on the understanding of KATP channel regulation and their function in a number of physiological and pathophysiological conditions.
Acknowledgments
The authors are grateful to Dr S. Seino for the Kir6.2 cDNA and Dr L. Bryan for the SUR1 cDNA. We are also grateful to Mr Asheebo Rojas for his critical reading of the manuscript. This work was supported by the NIH (HL58410), the American Diabetes Association (1-01-RA-12). C.J. is a Career Investigator of the American Lung Association.
References
- Allard B, Lazdunski M, Rougier O. Activation of ATP-dependent K+ channels by metabolic poisoning in adult mouse skeletal muscle: role of intracellular Mg2+ and pH. Journal of Physiology. 1995;485:283–296. doi: 10.1113/jphysiol.1995.sp020730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends in Neurosciences. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Tucker SJ, Schulte U, Benndorf K, Ruppersberg JP, Fakler B. Inward rectification in KATP channels: a pH switch in the pore. EMBO Journal. 1999;18:847–853. doi: 10.1093/emboj/18.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Bassett AL, Cameron JS, Furukawa T, Myerburg RJ, Kimura S. Effect of H+ on ATP-regulated K+ channels in feline ventricular myocytes. American Journal of Physiology. 1991;261:H755–761. doi: 10.1152/ajpheart.1991.261.3.H755. [DOI] [PubMed] [Google Scholar]
- Davies NW. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990;343:375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Davies NW, Standen NB, Stanfield PR. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. Journal of Physiology. 1992;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proceedings of the National Academy of Sciences of the USA. 1998;95:13953–13958. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Tokuyama Y, Makielski JC. Modulation of ATP-sensitive K+ channels by internal acidification in insulin-secreting cells. American Journal of Physiology. 1994;267:C1036–1044. doi: 10.1152/ajpcell.1994.267.4.C1036. [DOI] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. Journal of Biological Chemistry. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. Journal of Physiology. 1997;504:35–45. doi: 10.1111/j.1469-7793.1997.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, GonzaleZ G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Koster JC, Sha Q, Shyng S, Nichols CG. ATP inhibition of KATP channels: control of nucleotide sensitivity by the N-terminal domain of the Kir6. 2 subunit. Journal of Physiology. 1999;515:19–30. doi: 10.1111/j.1469-7793.1999.019ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano T, Kakei M, Nakashima H, Yoshinaga M, Matsuoka T, Tanaka H. ATP-regulated K+ channels are modulated by intracellular H+ in guinea-pig ventricular cells. Journal of Physiology. 1993;463:747–766. doi: 10.1113/jphysiol.1993.sp019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Ammala C, Bokvist K, Fredholm B, Rorsman P. Stimulation of the KATP channel by ADP and diazoxide requires nucleotide hydrolysis in mouse pancreatic beta-cells. Journal of Physiology. 1993;463:349–365. doi: 10.1113/jphysiol.1993.sp019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. Journal of Physiology. 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor GG, Dong K, Vanoye CG, Tang L, Giebisch G, Hebert SC. Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proceedings of the National Academy of Sciences of the USA. 2002;99:2726–2731. doi: 10.1073/pnas.042688899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S, Gillis K, Tabcharani J. Modulation of gating of a metabolically regulated, ATP-dependent K+ channel by intracellular pH in B cells of the pancreatic islet. Journal of Membrane Biology. 1989;109:135–143. doi: 10.1007/BF01870852. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. Journal of Molecular Biology. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Piao H, Cui N, Xu H, Mao J, Rojas A, Wang R, Abdulkadir L, Li L, Wu J, Jiang C. Requirement of multiple protein domains and residues for gating KATP channels by intracullular pH. Journal of Biological Chemistry. 2001;276:36673–36680. doi: 10.1074/jbc.M106123200. [DOI] [PubMed] [Google Scholar]
- Proks P, Ashfield R, Ashcroft FM. Interaction of vanadate with the cloned beta cell KATP channel. Journal of Biological Chemistry. 1999a;274:25393–25397. doi: 10.1074/jbc.274.36.25393. [DOI] [PubMed] [Google Scholar]
- Proks P, Gribble FM, Adhikari R, Tucker SJ, Ashcroft FM. Involvement of the N-terminus of Kir6. 2 in the inhibition of the KATP channel by ATP. Journal of Physiology. 1999b;514:19–25. doi: 10.1111/j.1469-7793.1999.019af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Takano M, Ashcroft FM. Effects of intracellular pH on ATP-sensitive K+ channels in mouse pancreatic beta-cells. Journal of Physiology. 1994;475:33–44. doi: 10.1113/jphysiol.1994.sp020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiological Reviews. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Reimann F, Ryder TJ, Tucker SJ, Ashcroft FM. The role of lysine 185 in the Kir6. 2 subunit of the ATP-sensitive channel in channel inhibition by ATP. Journal of Physiology. 1999;520:661–669. doi: 10.1111/j.1469-7793.1999.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S, Ferrigni T, Nichols CG. Control of rectification and gating of cloned KATP channels by the Kir6. 2 subunit. Journal of General Physiology. 1997a;110:141–153. doi: 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. Journal of General Physiology. 1997b;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Tucker SJ, Matsuo M, Proks P, Ashcroft FM, Seino S, Amachi T, Ueda K. Direct photoaffinity labeling of the Kir6. 2 subunit of the ATP-sensitive K+ channel by 8-azido-ATP. Journal of Biological Chemistry. 1999;274:3931–3933. doi: 10.1074/jbc.274.7.3931. [DOI] [PubMed] [Google Scholar]
- Trapp S, Proks P, Tucker SJ, Ashcroft FM. Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. Journal of General Physiology. 1998;112:333–349. doi: 10.1085/jgp.112.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO Journal. 1998;17:3290–3296. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6. 2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Vivaudou M, Forestier C. Modification by protons of frog skeletal muscle KATP channels: effects on ion conduction and nucleotide inhibition. Journal of Physiology. 1995;486:629–645. doi: 10.1113/jphysiol.1995.sp020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P, Jiang C. Direct activation of cloned KATP channels by intracellular acidosis. Journal of Biological Chemistry. 2001b;276:12898–12902. doi: 10.1074/jbc.M009631200. [DOI] [PubMed] [Google Scholar]
- Xu H, Wu J, Cui N, Abdulkadir L, Wang R, Mao J, Giwa LR, Chanchevalap S, Jiang C. Distinct histidine residues control the acid-induced activation and inhibition of the cloned KATP channel. Journal of Biological Chemistry. 2001a;276:38690–38696. doi: 10.1074/jbc.M106595200. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xu H, Cui N, Qu Z, Chanchevalap S, Shen W, Jiang C. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4. 1-Kir5.1 channels by CO2 and pH. Journal of General Physiology. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. American Journal of Physiology. 1998;274:C25–37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2. 3 currents. Journal of Physiology. 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Zhang Y, Xu H, Jiang C. Identification of endogenous outward currents in the human embryonic kidney (HEK 293). cell line. Journal of Neuroscience Methods. 1998;81:73–83. doi: 10.1016/s0165-0270(98)00019-3. [DOI] [PubMed] [Google Scholar]


