Abstract
Members of the ENaC/degenerin family of ion channels include the epithelial sodium channel (ENaC), acid-sensing ion channels (ASICs) and the nematode Caenorhabditis elegans degenerins. These channels are activated by a variety of stimuli such as ligands (ASICs) and mechanical forces (degenerins), or otherwise are constitutively active (ENaC). Despite their functional heterogeneity, these channels might share common basic mechanisms for gating. Mutations of a conserved residue in the extracellular loop, namely the ‘degenerin site’ activate all members of the ENaC/degenerin family. Chemical modification of a cysteine introduced in the degenerin site of rat ENaC (βS518C) by the sulfhydryl reagents MTSET or MTSEA, results in a ∼3-fold increase in the open probability. This effect is due to an 8-fold shortening of channel closed times and an increase in the number of long openings. In contrast to the intracellular gating domain in the N-terminus which is critical for channel opening, the intact extracellular degenerin site is necessary for normal channel closing, as illustrated by our observation that modification of βS518C destabilises the channel closed state. The modification by the sulfhydryl reagents is state- and size-dependent consistent with a conformational change of the degenerin site during channel opening and closing. We propose that the intracellular and extracellular modulatory sites act on a common channel gate and control the activity of ENaC at the cell surface.
The epithelial Na+ channel (ENaC) belongs to the ENaC/degenerin (DEG) family of ion channels. Members of this family are present in nematodes, flies, snails and mammals and are involved in Na+ transport, neurotransmission, mechanotransduction and nociception (Mano & Driscoll, 1999). ENaC is expressed in the apical membrane of epithelial cells of the distal nephron, the colon and the lung, where it mediates vectorial transepithelial Na+ absorption. In the distal nephron, aldosterone and vasopressin regulate ENaC activity, serving to maintain Na+ balance, extracellular volume and blood pressure (Garty & Palmer, 1997). ASICs are proton-gated Na+ channels expressed in the central and peripheral nervous systems although their physiological roles still remain to be clearly defined. Degenerin members of the ENaC/DEG family are found in the nematode Caenorhabditis elegans neurones and are involved in touch sensation.
ENaC/DEG family members form multimeric channels made of homologous subunits characterised by a large extracellular loop between two transmembrane domains (M1 and M2) and cytoplasmic N- and C-termini. Evidence obtained for ENaC support a heteromeric arrangement of four subunits around a central channel pore (Firsov et al. 1998).
It has been shown in C. elegans degenerins, that mutation of a conserved Ala residue in the segment that precedes M2 causes neuronal cell swelling and degeneration. This phenotype was interpreted as being the result of an increased influx of cations into the cell due to a mutation rendering the channel constitutively active (Driscoll & Chalfie, 1991). Later it was found in other ENaC/DEG family members such as ASICs (Waldmann & Lazdunski, 1998), RPK-dGNaC1 (Adams et al. 1998a), BKINaC(Sakai et al. 1999), hINaC (Schaefer et al. 2000) and ENaC (Snyder et al. 2000), that the substitution of the corresponding Ser or Gly residue by large amino acids activates the channel. This external residue involved in channel gating was called the ‘degenerin (DEG) site’. It had been hypothesised that these external DEG mutations prevent the channel from closing effectively, resulting in a residual influx of cations (Driscoll & Chalfie, 1991). Recent studies indeed suggest that the DEG site in ASIC2a and ENaC is involved in a conformational change during channel opening and closing (Adams et al. 1998b; Snyder et al. 2000). In these studies it was reported, that an engineered Cys residue at the DEG position was modified by hydrophilic sulfhydryl reagents only when ASIC2a channels were activated by protons, and only during channel openings in single-channel experiments with ENaC.
The DEG site of ENaC is located in close proximity to the outer pore entrance and the selectivity filter that are formed by the pre-M2/M2 segments of the ENaC subunits. A G/S×S sequence in all ENaC subunits forms the narrowest part of the pore, which determines ionic selectivity and unitary conductance (Kellenberger et al. 1999a, b, 2001; Snyder et al. 1999; Sheng et al. 2000; G587-S589 in αENaC and homologous residues in β and γENaC, see Fig. 1). The binding site of the pore blocker amiloride is located four residues upstream of the selectivity filter in the outer pore entrance, as identified by mutations of residues αS583 and the homologous βG525 and γG537 that disrupt amiloride block (Schild et al. 1997) (Fig. 1). The DEG residue is located seven residues upstream of the amiloride binding site (αS576 and the homologous βS518 and γS530).
Figure 1. Hypothetical model of the ENaC pore.
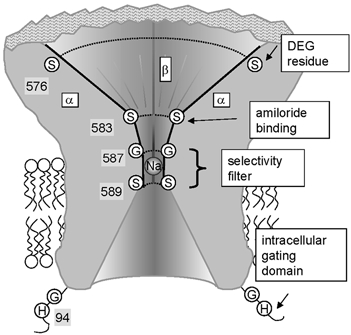
The cross-section shows the extracellular pore opening that narrows down to the amiloride binding site and the selectivity filter (where a Na+ ion is shown) and from there opens towards the transmembrane region and the cytoplasmic side. Experimental evidence indicates that the pre-M2 segments of all three subunits form the extracellular pore entry. Shown are the pre-M2 segments of two α subunits (left and right) and the β subunit in the back. The γ subunit located on the side of the viewer is not shown. The DEG residue is indicated for the α subunits (αS576). Amiloride binds to αS583 and the corresponding Gly residues in the β and γ subunit. The vestibule narrows down to the selectivity filter formed by αG587, βG529 and γS541 residues and the ring of Ser residues (αS589 and analogues). The N-terminal gating domain HG is indicated on the cytoplasmic side of the protein. S, G & H represent the amino acids Ser, Gly and His, respectively.
To elucidate the role of the DEG site in the control of ENaC gating, we have mutated the DEG residue in all three ENaC subunits to Cys and modified the engineered Cys residues chemically to render these channels hyperactive. Using electrophysiological techniques, we have shown that modification of the DEG site of the β subunit alters the gating kinetics of the channel by destabilising the closed state resulting in shorter closings and by slowing the closing of the channel, as indicated by the increase in the proportion of long openings. Analysis of the function of the DEG site in channels that contain inactivating mutations in the cytoplasmic N-terminus suggests that these intracellular and extracellular modulatory sites act on a common channel gate. Modification of the DEG site does not interfere with amiloride binding and has no consistent effect on ion permeation, suggesting that the DEG site may not be localised in the narrow part of the pore entry.
Methods
Site-directed mutagenesis and expression in Xenopus laevis oocytes
Site-directed mutagenesis of the degenerin (DEG) residue was performed on rat ENaC cDNA as described previously (Schild et al. 1997). Complementary RNAs of each α β and γ subunit were synthesised in vitro. Stage V-VI oocytes were surgically removed from the ovarian tissue of female Xenopus laevis which had been anaesthetised by immersion in MS-222 (2 g l−1; Sandoz, Basel, Switzerland). Following surgery, the frogs were allowed to recover in isolation in a shallow tank and, after full recovery had been verified a few hours later, they were returned to the rearing tank. About two months later, the frogs were operated on a second time for the removal of the ovarian lobe on the other side. They were then killed by decapitation under anaesthesia. All procedures were performed in accordance with local institutional animal welfare guidelines (State of Vaud, Switzerland). The oocytes were defolliculated and healthy stage V and VI Xenopus oocytes were pressure-injected with 100 nl of a solution containing equal amounts of α, β and γ ENaC subunits at a total concentration of 100 ng μl−1. For simplicity, mutants were named by the mutated subunit only, although all three subunits (α, β and γ) were always co-expressed.
Electrophysiological analysis
Electrophysiological measurements were taken at 16–48 h after injection. Macroscopic amiloride-sensitive currents, defined as the difference between ionic currents obtained in the presence and absence of 10 μM amiloride (Sigma, Buchs, Switzerland) in the bath were recorded using the two-electrode voltage-clamp technique. All macroscopic currents shown are amiloride-sensitive currents as defined above. Except for I/V curves, whole-cell currents were measured at −100 mV. Currents were recorded with a Dagan TEV-200 amplifier (Minneapolis, MN, USA) equipped with two bath electrodes. The standard bath solution contained 110 mm NaCl or LiCl, 1.8 mm CaCl2, 10 mm Hepes-NaOH, pH 7.35. Pulses for current-voltage curves were applied, and data were acquired using a PC-based data acquisition system (Pulse, HEKA Electronic, Lambrecht/Pfalz, Germany). Single-channel currents were measured in the outside-out configuration of the patch-clamp technique essentially as described previously (Kellenberger et al. 1999b). The bath solution in patch-clamp experiments was the standard bath solution described above, with Li+ or Na+ as the monovalent cation. The pipette solution contained (mm): 75 CsF, 17 N-methyl-d-glucamine (NMDG), 10 EGTA and 10 Hepes (pH 7.35). In patch-clamp experiments that involved extracellular application of the sulfhydryl reagent MTSEA, 20 mm cysteine was included in the pipette solution to prevent intracellular effects of MTSEA. Aqueous stock solutions of MTS reagents MTS-PTrEA ([3-(triethylammonium)propyl] methanethiosulfonate) MTSEA and MTSET (Toronto Research Chemicals, Toronto, Canada) were prepared just prior to the experiment, maintained on ice, and diluted into the bath solution immediately before use. Averaged data are presented as means ± s.e.m. Pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL, USA). In patch-clamp experiments, currents were recorded with a List EPC-9 patch-clamp amplifier (HEKA Electronic, Lambrecht/Pfalz, Germany) and filtered at 100–500 Hz for single-channel analysis. Currents were analysed and duration and amplitude histograms were constructed and fitted using TAC and TACFIT 4.09 (Bruxton Corporation, Seattle, WA, USA). Open and closed times were analysed from the binned data with durations > 0.4 ms (data filtered at 500 Hz: βS518C + MTSEA, βS518C + MTSET, αH94AβS518C) or > 2 ms (100 Hz: βS518C unmodified). In channels with long openings, open duration time constants may have been overestimated due to missed short closings. This error was in all cases less than 3 %, as calculated according to Colquhoun and Hawkes (1995).
Results
Bulky DEG residue side chains in α and βENaC increase channel activity
We have substituted the DEG residue in α, β and γENaC with Cys and expressed these Cys mutants αS576C, βS518C and γS530C individually with the complementing ENaC wild-type (WT) subunits as αβγ channels in Xenopus oocytes. The DEG mutation in the α subunit decreased the whole-cell amiloride-sensitive Na+ current at −100 mV (INa) by 40 % with regard to ENaC WT, while mutation βS518C did not significantly affect INa and γS530C increased INa by (2.0 ± 0.2)-fold (n = 35-42, P < 0.05, Fig. 2A). As shown in a typical experiment with βS518C (Fig. 2B), application of the positively charged sulfhydryl reagent MTSET to these ENaC mutants increased the amiloride-sensitive current. This increase could be reversed by application of dithiothreitol (DTT). This reduction of INa after DTT application was due to reversal of the MTSET modification as indicated by our observation that 10 mm DTT did not affect INa of unmodified βS518C ENaC (98 ± 1 % of control INa, n = 3). As summarised in Fig. 2C, a 5 min incubation with 1 mm MTSET slightly decreased the WT INa and led to a (3.7 ± 0.7)-fold (n = 16) and a (6.0 ± 0.9)-fold increase (n = 21) in the αS576C and the βS518C mutants, respectively. In contrast, no significant increase in INa after MTSET incubation was found with the γS530C mutant. The high initial INa in the γS530C mutant (Fig. 2A) suggests that the lack of an MTSET effect could be due to constitutive hyperactivity of this mutant channel due to the S→C substitution even in the absence of MTSET.
Figure 2. Effects of chemical modification on macroscopic currents.
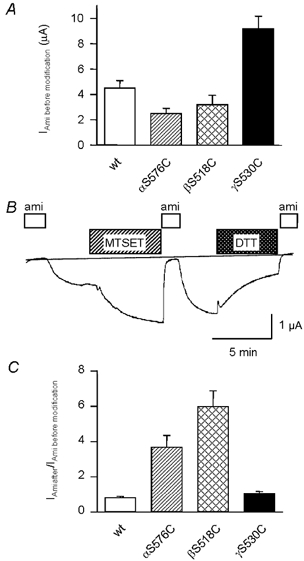
Whole-cell amiloride-sensitive currents (Iami). A, current expression of WT ENaC and the DEG mutants listed. B, trace of an experiment with an oocyte expressing the βS518C mutant. Extracellular application of reagents and drugs is indicated by bars. ami, 10 μM amiloride; MTSET, 1 mm MTSET; DTT, 10 mm dithiothreitol. C, increase in amiloride-sensitive current after 5 min incubation in 1 mm MTSET.
State-dependent modification of βC518
The βS518C mutant was used to investigate the characteristics of the channel activation by MTSET at the single channel level. Single-channel currents were measured in the excised outside-out configuration to allow rapid external application of MTSET, and of amiloride, to block the channels and to determine the zero current level. Figure 3A shows MTSET modification of βS518C and its consequence on channel activity in a patch containing two active channels. MTSET modified the two channels independently and the changes in channel function were characterised by a slight decrease in current amplitude followed by an almost permanent opening of the channels. These changes never occurred spontaneously in the absence of sulfhydryl reagents (data not shown). After modification by MTSET, the channels gated with a high open probability due to long open dwell times and short channel closures. The changes in channel gating and current amplitude induced by MTSET could be reversed by DDT (Fig. 3B). Thus, the high open probability of ENaC after modification can account for the MTSET-induced stimulation of the macroscopic current observed in Fig. 2C.
Figure 3. State-dependent modification of βS518C by MTSET.
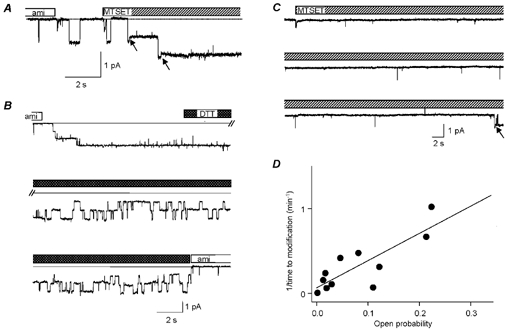
A, trace of an outside-out patch containing two active βS518C channels. Amiloride (10 μM) was first removed from the extracellular solution to allow normal channel opening and the sulfhydryl reagent MTSET (2 mm) was added at the time indicated. During the solution changes, small artefacts in the trace due to the solution change are visible. The arrows point to modification events. Holding voltage −100 mV. B, reversal of sulfhydryl modification of βS518C by DDT. Channels had previously been modified by MTSET. Channel activity appears after switching to the amiloride-free solution. Unitary current amplitude at −80 mV in this experiment was 0.80 pA (unmodified), 0.67 pA (MTSET) and 0.78 pA (DTT). C, outside-out patch containing one βS518C channel that showed only rare, extremely short openings during the time of MTSET application. Modification occurred during the first opening of longer duration (arrow). D, the inverse of the duration of MTSET application until successful modification is plotted versus the open probability (Po) of the channel during this time.
The decrease in single-channel conductance after MTSET modification is evident from Fig. 3A. Assuming that the observed transition to a lower unitary conductance exactly corresponds to the time point of channel modification, we propose that MTSET modification took place when the channels were open. If modification had occurred during a channel closing we should have observed transitions from a zero current level directly to the lower conductance level. Thus the decrease in single-channel conductance after MTSET application tells us whether modification of βS518C occurs in the open or closed state. Snyder and colleagues (Snyder et al. 2000) recently found in cell-attached patches that MTSET (10 μM) in the pipette solution opens ENaC containing a Cys residue at the degenerin site and reduces its unitary current. The transition to the reduced unitary current was observed only during channel openings and therefore the authors concluded that the DEG site is only accessible when the channel is open. In the outside-out configuration of the patch-clamp technique we found in 11 out of 12 MTSET modification events a decrease of the unitary current amplitude during a channel opening, thus confirming the conclusion of Snyder and colleagues.
If the βS518C residue is indeed more easily accessible in the open channel conformation, then the time required for the modification by MTSET is expected to depend on the probability of the channel being in the open conformation. For instance, Fig. 3C illustrates that a 160 s time delay was necessary for the modification of a βS518C channel gating with a low open probability (Po) with only four brief openings of 10–20 ms and long closed dwell times. For different channels with a wide range of open probabilities we observed a significant inverse correlation between channel open probability and the time needed for channel modification after addition of MTSET (Fig. 3D). In other words, ENaC channels with a higher open probability are more rapidly modified by MTSET consistent with a state-dependent modification of βS518C. These experiments also exclude the following alternative possibility: MTSET might modify the sulfhydryl group of βC518 independently of the open or closed conformation of the channel, but the changes in gating kinetics and channel conductance would require opening of the channel after modification to become effective. In this case, the changes in channel gating would invariably be observed following the first channel opening after MTS modification and the delay for the gating changes would not be strictly correlated with the channel open probability. Thus, the decrease in unitary current amplitude indeed coincides with channel modification.
Open channels are rapidly modified by MTSET. The mean time from the opening of the channel in the presence of MTSET to modification, defined as the transition to a lower conductance state, was 170 ± 28 ms (n = 11).
In ASICs, a Cys residue introduced at the position corresponding to βS518C shows a similar state dependence of accessibility for MTS reagents but is accessible independently of the open or closed conformation to the smaller Zn2+ ion (Adams et al. 1998b, 1999). We investigated the possibility of such a size dependence in the case of ENaC βS518C with the smaller reagent MTSEA, which has a volume of ≈130 Å3 compared with ≈180 Å3 for MTSET. Figure 4 illustrates that MTSEA modification, as detected by the appearance of the lower conductance state, can arise either from a closed channel or an open channel. We observed that in eight patches, five modifications occurred while the channel was open and three when the channel was closed. Despite the limited number of observations, these experiments show that as for ASICs, the accessibility of the βS518C is not only state dependent but also depends on the size of the modifying reagent. MTSEA still preferentially modifies open channels, as the time from the beginning of MTSEA exposure to modification was ≤ 4.3 ± 2.4 s (n = 8), whereas after channel opening modification occurred within 134 ± 38 ms (n = 5).
Figure 4. Modification of βS518C by MTSEA.
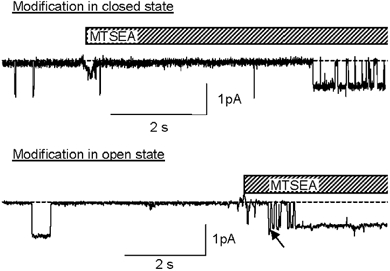
Outside-out patches containing βS518C at holding voltage of −100 mV. Modification occurred while the channel was closed (upper trace) or while it was open (lower trace).
Changes in gating kinetics
The changes in channel open probability due to modification are summarised in Fig. 5A. Open probability (Po) was determined during 1 min before and after modification in patches containing one single active channel. After modification with MTSET, Po reached 1.00 ± 0.00 (n = 4), and 0.98 ± 0.02 with MTSEA suggesting that these modifications of β518C prevent the channel from closing or to remain closed. The analysis of the gating kinetics of single βS518C channels before and after modification gave the open and closed time distributions shown in Fig. 5B and Table 1. The open times distribution in the absence of modification showed two exponential components, with a short and a long time constant of τopen,1 = 100 ms and τopen,2 = 1.5 s, respectively (for the relative weight of the two components τ1 and τ2 see Table 1). The dwell time distribution of the closed state events also showed two components, with a short and a long time constant of τclosed,1 = 79 ms and τclosed,2 = 5.1 s, respectively. Modification by MTSEA or MTSET increased the relative weight of the component of longer open times. More importantly, modification by either of the reagents almost completely abolished long closing events and decreased the duration of the short closures by ≈8-fold.
Figure 5. Effect of modification on βS518C single-channel kinetics.
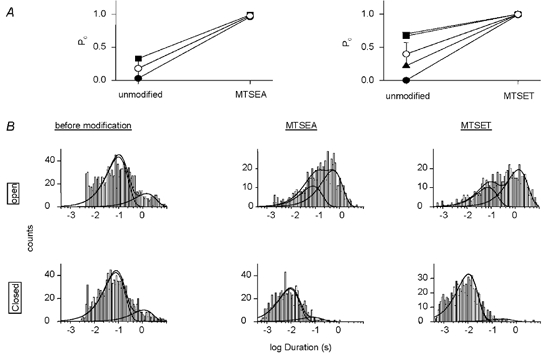
A, changes in single-channel open probability (Po) due to modification by MTSET or MTSEA. Po before and after modification by MTSEA (left panel) and MTSET (right panel). Po was determined during the minute prior to and after, modification, respectively. The Po data are from patches that contained single active channels. Holding potential was −100 mV. Filled symbols represent individual experiments. B, open and closed time distributions of βS518C channels before and after modification. Time distributions were obtained from single-channel excised outside-out patches at −100 mV. The time constants and the relative weights of the components are listed in Table 1. The overall Po of the data used for the kinetic analysis was 0.52 (unmodified), 0.95 (MTSEA-modified) and 0.97 (MTSET-modified). The number of exponential components of the fit was determined according to Colquhoun & Sigworth (1995) by visual inspection of the histograms and by the criterion of whether a given number of components was required to fit histograms derived from individual experiments. Therefore, open times of unmodified and MTSET-modified channels were fitted with two components. The open time distribution of MTSEA-modified channels was then fitted with two components to be better able to compare these data with those obtained in the other two conditions. Closed times were fitted with two exponential components.
Table 1.
Summary of the single-channel kinetics analysis
| βS518C | βS518C + MTSEA | βS518C + MTSET | βH94A-βS518Cβ | βH94A-βS518Cβ+ MTSET | βS530C low Po gating | βS530C high Po gating | |
|---|---|---|---|---|---|---|---|
| Open time β1 (ms) | 100 | 81 | 75 | 3 | 4 | 10 | 693 |
| A1 | 0.79 | 0.36 | 0.34 | 0.53 | 0.30 | 0.88 | 0.88 |
| Open time β2 (ms) | 1470 | 551 | 1400 | 26 | 75 | 136 | 12210 |
| Closed time β1 (ms) | 79 | 9 | 11 | ND | 6 | 244 | 99 |
| A1 | 0.86 | 0.89 | 0.95 | ND | 0.45 | 0.53 | 1 |
| Closed time β2 (ms) | 5068 | 81 | 294 | ND | 36 | 4028 | — |
| A2 | 0.14 | 0.11 | 0.05 | ND | 0.45 | 0.47 | — |
| Closed time β3 (ms) | — | — | — | ND | 1182 | — | — |
| no of patches (n) | 5 | 3 | 4 | 4 | 3 | 1 | 3 |
A1, A2, relative weight of the first/second component of the exponential fit to the dwell time distribution; ND, not determined, because the presence of multiple channels in the patch could not be excluded. The number of exponential components of the fits was determined as described in Fig. 5. —, exponentials not required for fit. Data were obtained at a holding potential of −100 mV from patches that contained a single active channel.
As shown in Fig. 2A, the γS530C mutant had an increased basal INa compared with ENaC WT that was not further increased by application of MTSET. Single-channel analysis of γS530C showed that about half of the channels had a high Po close to 1 (for example, in two single-channel patches the Po was 0.83 and 0.94, respectively), whereas the other half of the channels gated with a Po that was approximately 0.5 or below. The difference between low and high Po-γS530C channels is based mainly on different open time distributions and thus on the rate of channel closing (Table 1). Modification may further increase the Po of γS530C channels gating with a low Po and thus the INa carried by these channels. In contrast, the Po of γS530C channels that already have a Po close to 1 cannot be further increased and consequently, the INa carried by these channels will not be affected by modification. Therefore, the total increase in INa due to modification is reduced in γS530 channels and might be too small to be detected.
Inversely, it has been shown that mutations of a conserved HG motif in the intracellular N-terminus result in channels that are present at the cell surface at a normal density and with normal unitary conductance but with a low open probability (Grunder et al. 1997, 1999), as illustrated by the current trace in Fig. 6A. We investigated in the αH94A mutant, whether modification of the βS518C residue would be able to shorten the long closed states and increase the open probability to values close to 1 as in the wild-type channel. If this were the case, the relative increase after MTSET modification should be greater than in the single βS518C mutant. Measurements of the increase in the macroscopic current due to MTSET modification in αH94A or αG95S co-expressed with βS518C show that the ≈5-fold increase in INa due to MTSET is similar to the control ENaC without the αH94A or αG95S mutation (Fig. 6B). Thus, MTSET modification of the αH94A mutant channel does not lead to a Po near to 1 as in the control ENaC. Typical current traces of a patch containing one active modified αH94AβS518Cγ channel are shown in Fig. 6C. The gating of these channels often produced bursts of high Po that were separated by long closings. The overall Po of αH94AβS518Cγ single-channel activity used for the kinetic analysis was 0.47. The effect of the αH94A mutation on ENaC gating in the context of activation by the DEG site can be best appreciated by comparison of the dwell time histograms of MTSET-modified αH94AβS518Cγ and αβS518Cγ ENaC (Fig. 5B and Fig. 6D, Table 1). The αH94A mutation in the background of the MTSET-modified βS518C shifted both components of the open dwell time distribution to shorter dwell times. In addition, it preserved a substantial proportion of long closed times after modification (Fig. 5B and Fig. 6D, Table 1). The intact external DEG site is important for normal channel closures and the intact internal HG motif is necessary for normal channel openings. Mutation of the internal αH94 and modification of the external βS518C shortens the open and closed states, respectively. Combining the intracellular mutation and the extracellular modification results in a channel that oscillates between short open and closed states and only sometimes closes for a longer period of time.
Figure 6. Dependence of channel gating on the intracellular HG motif and the DEG site.
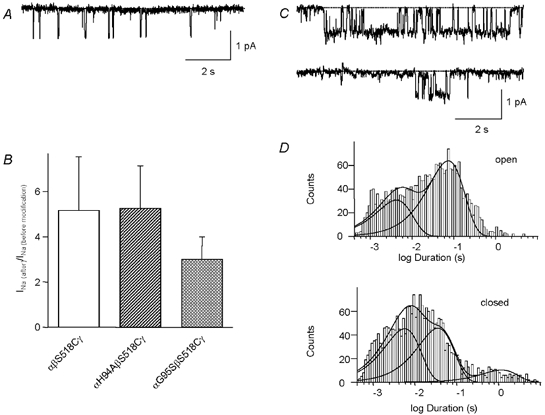
A, outside-out patch containing several αH94A ENaC channels at a holding voltage of −100 mV. B, increase in amiloride-sensitive whole-cell currents due to modification of channels co-expressing βS518C with mutations in the pre-M1 gating domain. Oocytes were incubated for 5 min in 1 mm MTSET. The relative increase in INa was not significantly different between the single and the double mutants (P < 0.05). Initial whole-cell currents of the mutants were 3.8 ± 2.1 μA (βS518C), 0.06 ± 0.02 μA (αH94AβS518C), and 0.14 ± 0.13 μA (αG95SβS518C). C, representative traces from an outside-out patch containing αH94AβS518C after modification by MTSET, at a holding voltage of −100 mV. D, dwell time distributions of αH94AβS518C channels after modification by MTSET. Time distributions were obtained from single-channel excised outside-out patches at −100 mV. The time constants and the relative weights of the components are listed in Table 1.
Localisation of the DEG residue relative to the outer ENaC pore
Modification of βS518C by MTSET resulted in a decrease in the unitary current amplitude with Li+ as the permeant ion (Figs 3, 4 and 7A), which was interpreted as electrostatic interaction between the modified βS518C and the permeant ion in the external channel pore (Snyder et al. 2000). We have investigated how modification of engineered Cys residues at the DEG site interferes with functional parameters of the extracellular pore such as ion permeation and block. Firstly, we observed that the unitary Na+ conductance of the βS518C mutant was not changed by MTSET modification (Fig 6B), indicating that its effect on ion conductance is specific to Li+ ions. Secondly, the conductance effect did not depend on the size of the adduct on βS518C, since MTSEA modification produced a similar decrease in unitary Li+ conductance (Fig. 4 and Fig. 7C). Finally, the reduction in unitary Li+ conductance due to MTSET was not observed for the αS576C modification (Fig. 7D). Thus, the reduction in ion conductance by modification of the βS518C is not a general phenomenon and is restricted to particular experimental conditions. The fact that the change in ion conductance is independent of the size of the modifying reagent makes direct and close interactions of the modified site with the permeant ion quite unlikely. Cd2+ at millimolar concentrations increased the current of the βS518C mutant (Fig. 7E) but not that of ENaC WT (not shown). This effect on βS518C was not voltage dependent (Fig. 7F), indicating that βS518C is not located in the transmembrane electrical field.
Figure 7. Possible involvement of DEG residues in pore functions.
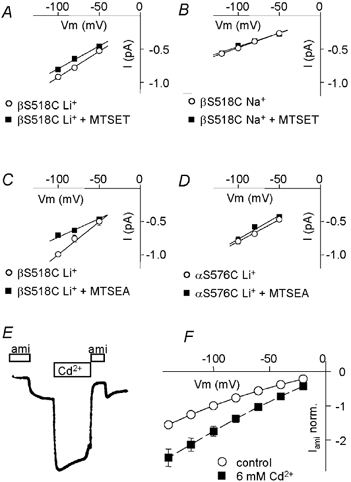
A-D, single-channel current-voltage relation from outside-out patches (n = 3–5 patches per condition). A, Li+ current of βS518C before (○) and after (▪) modification by MTSET (unitary conductance was 7.8 ± 0.9 pS (unmodified) and 6.8 ± 0.5 pS (modified) in direct comparison). B, Na+ current of βS518C before (○) and after (▪) modification by MTSET (4.4 ± 0.2/4.1 ± 0.3 pS). C, Li+ current of βS518C before (○) and after (▪) modification by MTSEA (9.9 ± 1.2/5.6 ± 0.7 pS). D, Li+ current of αS576C before (○) and after (▪) modification by MTSET (6.6 ± 0.6/7.1 ± 1.2 pS). E and F, two-electrode voltage-clamp recordings from oocytes expressing the βS518C mutant. E, current trace at holding voltage of −100 mV, the times of addition of amiloride (10 μM) or Cd2+ (10 mm) are indicated. F, current-voltage relationship of the macroscopic amiloride-sensitive current, Iami norm. in the absence (○) and presence (▪) of 6 mm Cd2+ (n = 4).
Recent studies indicate that outward from the narrow selectivity filter the extracellular entry of the ENaC pore opens to the amiloride binding site and extends farther towards the extracellular side (Schild et al. 1997; Kellenberger et al. 1999a,b, 2001; Snyder et al. 1999; Sheng et al. 2000). Since the degenerin site lies in the amino acid sequence seven residues upstream of the amiloride binding site and may therefore be close to the pore entry, we tested whether modification of the DEG site by large MTS reagents can affect channel blocking by amiloride in the external pore. We have used the large sulfhydryl reagent MTS-PTrEA (diameter of ≈7 Å3) for chemical modification of βS518C and αS576C which resulted in an increase in INa of (2.8 ± 0.4)- and (2.0 ± 0.3)-fold, respectively. Figure 8A and B show that the equilibrium inhibition curves by amiloride of ENaC WT and DEG mutants were similar in unmodified and MTS-PtrEA-modified channels. In addition, the presence of bound amiloride did not affect the modification of the DEG site by sulfhydryl reagents (data not shown), indicating that the two molecules do not interact in the external channel pore. It should be noted, however, that the absence of an apparent change of the amiloride IC50 does not necessarily mean that amiloride block is unchanged. Proportional changes of the association and dissociation rate constants kon and koff would not change the IC50. We have determined the kon for the amiloride analogue benzamil from the open time distribution in the presence of a high concentration of the drug (1 μM) where most of the openings are terminated by a blocking event, as 57 μM−1 s−1 for MTSET-modified βS518C compared with 111 μM−1 s−1 in ENaC WT. The off-rate for amiloride and benzamil was determined in excised outside-out macropatches of βS518C-expressing oocytes by rapidly changing from an extracellular Li+ solution containing 1 μM of the blocker to one free of blocker. The speed of the perfusion change was monitored by changing from a K+ to the Li+ solution. Resulting current traces measured before and after modification by MTSET are shown in Fig. 8C. Currents before and after MTSET incubation were normalized for better comparison. It is clear from Fig. 8C, that modification did not change the off-rate of the blockers. Consequently, the MTSET modification of βS518C does not interfere with the binding of amiloride at its receptor site in the external pore of the channel.
Figure 8. Amiloride block before and after chemical modification of DEG residues.
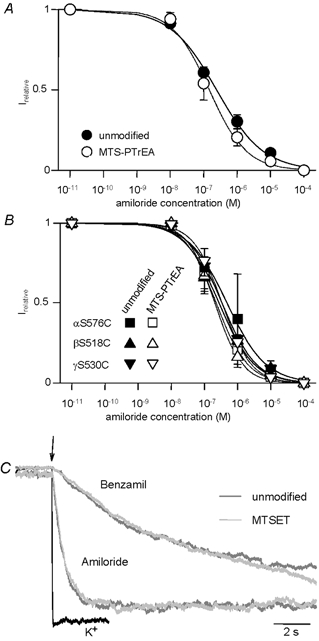
Inhibition curves of Na+ current (Irelative)carried by WT (A) and the individual DEG mutants (B) before (filled symbols) and after modification by MTS-PtREA (open symbols). Continuous lines are fits to the concentration-dependence of inhibition. The inhibition curves were obtained using two-electrode voltage clamp at −100 mV. C, washout of amiloride and benzamil from βS518 channels measured in excised outside-out macropatches at −100 mV. The solution change is indicated by the arrow. The speed of the solution change was tested by changing from K+ to Li+ solution (black trace). Amiloride and benzamil washout was measured before (dark grey traces) and after (light grey traces) modification by MTSET. The maximal currents were normalised for better comparison of the time course. Exponential fits to this and similar experiments determined time constants of current increase of 0.58 ± 0.05 and 0.50 ± 0.15 s due to amiloride washout before and after MTSET incubation, respectively, and the corresponding values were 10.8 ± 4.4 s and 8.7 ± 0.7 s for benzamil washout (n = 2–3 each), respectively.
Discussion
In ENaC, the DEG site consists of a conserved Ser residue that is in the amino acid sequence close to the amiloride binding site at the external entrance of the channel pore. Chemical modification of a Cys residue introduced at the DEG site results in an almost permanently open channel due a dramatic shortening of channel closed times and to a higher proportion of long channel openings. Accessibility of the residues at the DEG site is state and size dependent: the large sulfhydryl reagent MTSET modifies the residue only when the channel is open while the smaller reagent MTSEA can modify this residue in the open and the closed state. Modification does not interfere with amiloride binding or with the permeating ions indicating that the DEG site residue is not oriented towards the narrow part of the external pore lumen.
Large residues at the DEG site activate ENaC/DEG channels
For C. elegans degenerins, it was initially found that the ability of mutations of the DEG residue (Ala in WT degenerins) to induce neurodegeneration correlated with the size of the substituting residue. Mutations with substitutions by Ser and Gly behaved like wild-type, substitution by Cys had an intermediate effect, while substitutions by larger amino acids, regardless of charge, produced neurodegeneration (Driscoll & Chalfie, 1991). One possible interpretation is that a large residue at the DEG site prevents an extracellular gate from closing completely over the outer pore entry. The first direct demonstration that mutation of the DEG site affects channel gating was done in the related ASIC channels. The degenerin site in ASICs is a Gly. In ASIC2a, mutation of this residue to relatively small amino acids, such as Ser and Cys, shifts the pH dependence of channel activation to higher pH values and slows the kinetics of inactivation. Only mutations to larger amino acids induce a constitutive activity in addition to these effects on pH-dependent gating (Champigny et al. 1998). ENaC is activated by mutation of the DEG site to Cys or larger residues independently of their charge (Snyder et al. 2000). The comparison of different ENaC/degenerin family members thus shows that introduction of residues at the DEG site that are larger than Cys - thus > 90 Å3 in volume - induce channel hyperactivity.
Interestingly, the DEG residues of different ENaC subunits are not functionally equivalent. Differences include the following: firstly, substitution by large residues or chemical modification induces a greater current increase in the case of the β compared with the α subunit and modification of the engineered Cys residue of γENaC does not increase the current (Fig. 2 and Snyder et al. 2000). Secondly, our single-channel experiments show that modification reduces the unitary Li+ conductance if the Cys residue has been introduced in the βENaC DEG site but not for the analogous αENaC mutant. Thirdly, systematic cysteine scanning and modification of residues of the α and γENaC pore entry shows that in αENaC, several residues can affect channel gating (residues αV572, S576, N577, S580) and in γENaC this is restricted to residues immediately surrounding the DEG site (Snyder et al. 1999; Sheng et al. 2001). In βENaC, the increase appears to be restricted to the DEG site, similarly to what has been shown for MEC-4 (Hong et al. 2000; Snyder et al. 2000). These differences between ENaC subunits illustrate potentially different roles of the individual subunits in ENaC gating, as has been suggested by studies of ENaC channels formed by only two types of subunits, αβ or αγ (Fyfe & Canessa, 1998; Fyfe et al. 1999).
Our kinetic analysis of βS518C ENaC before and after sulfhydryl modification elucidates the mechanism of the current increase: MTS modification of the DEG residue βC518 shortens channel closed times and increases the relative weight of the component of long open times. Channel gating can be viewed as transitions of the channel protein between conformational states with defined energy levels, the open and closed states. In this view, ENaC WT or the unmodified βS518C mutant have open and closed states of approximately equal energy levels, because they spend a similar part of their time in the open versus the closed state. The energy barrier between the two states is relatively high as reflected by the long residency times. After modification of the βS518C mutant the energy level of the closed state increases dramatically - the closed state is ‘destabilised’ - so that the rate of leaving the closed state increases. In addition, the energy barrier between the two states also increases, which is reflected in an increase in the mean open time.
Epithelial sodium channels carrying the N-terminal αH94A mutation show short open times and long closed times and as a consequence have very low Po values. Thus, in these channels the open state is destabilised and has a higher energy level than the closed state. The βS518C modification in the background of the αH94A mutation yields channels that display short open and closed times with a Po close to 0.5. Thus, open and closed states of these channels are of equally high energy and the barrier between the two states is low.
Is βS518 part of an external gate?
The small reduction in Li+ conductance after channel modification allowed determination of whether modification occurs in the open or closed conformation. The MTSET modification of the DEG site in βS518C ENaC occurs almost exclusively in the open state of the channel. As a possible mechanism of the effect of DEG mutations, it has been proposed that they prevent an extracellularly located gate from closing completely over the pore entry (Tavernarakis & Driscoll, 1997; Snyder et al. 2000). The different accessibility of the DEG residue in open and closed channels would then be explained by the orientation of the DEG residue towards the lumen of the pore entry, at the position over which this putative gate closes. Such a model, however, is not compatible with our finding that the smaller reagent MTSEA still has access from the extracellular solution to the DEG site in closed channels. If this putative gate allowed passage of MTSEA in the closed state of the channel, the smaller Na+ and Li+ ions would also have access to the pore and an ionic current would still flow in the ‘closed’ state, which is not the case. This, and the indications that the DEG site does not face the narrow pore lumen, suggests that the DEG site is not directly involved in the closing of a lid over the channel pore.
This and previous work (Snyder et al. 2000) provide clear evidence for a critical involvement of the DEG site in ENaC gating. Alternatively to a lid that closes over the external pore, channel closing may be induced by subtle conformational changes that impair ion transport through the narrow region of the pore. Conformational changes in the outer pore have been reported for C-type inactivation of K+ channels and it has been proposed that changes in the narrow pore region may occur after ligand binding in ligand-gated channels, as is the case with the acetylcholine receptor (Liu et al. 1996; Unwin 2000). We propose the following model for the involvement of the DEG site in ENaC gating: in the closed channel, the DEG site is partially hidden from the extracellular solution. When the channel opens, conformational changes occur in this region which fully expose the DEG site to an extracellular surface. Mutations of the DEG site to larger residues interfere with these conformational changes during channel closing thereby increasing the proportion of long openings. In addition they make the closed state of the channel energetically less favourable, as reflected by the short duration of channel closings. The slight decrease in unitary Li+ conductance in the modified βS518C channel might be due to conformational changes in the pore region.
It is interesting to note, that the size limits for disrupting the stability of the closed state and for the accessibility of the DEG site in the closed state are different. While amino acid residues at the DEG site with a volume > 90 Å3 disrupt the stability of the closed state, MTSEA (≈130 Å3) can still reach the DEG residue when the channel is closed.
It has been proposed that ENaC WT can exist in different gating modes (Palmer & Frindt, 1996). A possible mechanism for the DEG mutants to increase the Po would be to switch ENaC gating completely to a high Po gating mode that is rare in WT ENaC. Our kinetic analysis shows that high Po gating with long open times separated only by very short closings, as seen after modification, is never observed in unmodified ENaCs, indicating that modification does not induce a preference for a gating mode that already exists in the ENaC before modification.
Relation to the HG gating domain in the cytoplasmic N-terminus
Mutations in the HG motif in the intracellular N-terminus decrease the channel Po, essentially by destabilising the open state as shown by the brief channel openings. (Grunder et al. 1999; Fig. 6A). When combining mutations in the intracellular HG domain and modification of the extracellular DEG site βS518C, we observed that after the increase in channel activity due to the modification, the Po remained substantially lower than 1, in contrast to channels with an intact HG motif.
The HG motif and the DEG site might control two independent gating mechanisms, similar to channel closing and inactivation in voltage-dependent K+ and Na+ channels. Indeed, closed time distributions of the unmodified βS518C mutant show two exponential components which might correspond to closed states conferred by two independent gates (Fig. 5B, Table 1). However, modification of the external DEG site affects both closed time components, indicating that they both depend on this site and that the DEG site does not control a single independent extracellular gate. Our observations are consistent with one gate controlled by both ‘gating domains’ in the extracellular pre-M2 and the intracellular pre-M1 structures. We propose that the intact intracellular gating domain is required for normal channel openings since the αH94A mutation destabilises the open state, and that the extracellular DEG site is critical for normal channel closures since binding of external ligands at βS518C reduces dramatically the channel closed dwell times. The dual mutant αH94A/MTSET-modified βS518C oscillates between short (unstable) open and closed states.
Interestingly, intracellular factors such as increasing Ca2+ or Na+ concentration are known to reduce channel activity. The feedback inhibition by intracellular Na+ probably controls Na+ entry into the cell and prevents intracellular Na+ from rising above certain levels that would impair cell function. On the other hand, extracellular factors such as proteases are known to increase channel activity at the cell surface by increasing the channel open probability. The target site on ENaC for these proteolytic enzymes has not yet been identified but could well involve the degenerin site.
Possible physiological role of the DEG site
Comparison of the structure-function relationship of ENaC/DEG family members shows that although these channels have very different roles and are activated by different stimuli they share a number of conserved functional domains, e.g. the DEG site. Together with previous studies, our study indicates that at least some aspects of the gating machinery are the same in different ENaC/DEG channels. Presently, it is not known whether activating stimuli, such as mechanical stimuli in the case of degenerins or proton binding in ASICs, directly couple to the DEG site for channel activation. ENaC cell surface expression is regulated mainly by the hormones aldosterone and vasopressin, while non-hormonal factors predominantly control channel open probability. Extracellular factors such as proteases and Na+ - by a mechanism called self-inhibition - regulate ENaC activity (Kroll et al. 1991; Garty & Palmer, 1997; Vallet et al. 1997; Chraibi et al. 1998; Palmer et al. 1998; Vuagniaux et al. 2000). Regulation of ENaC activity at the cell surface by these extracellular factors might involve the DEG site.
Acknowledgments
We thank Aude Bachelard and Carole Chappuis for doing some of the experiments during their undergraduate studies. This work was supported by a grant from the Swiss National Foundation for Scientific Research (to L.S., no. 3100-059217.99). We thank J.-D. Horisberger and Marc A. Thomas for critical reading of the manuscript.
References
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila Deg/Enac subunits expressed in early development and in mechanosensory neurons. Journal of Cell Biology. 1998a;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CM, Snyder PM, Price MP, Welsh MJ. Protons activate brain Na+ channel 1 by inducing a conformational change that exposes a residue associated with neurodegeneration. Journal of Biological Chemistry. 1998b;273:30204–30207. doi: 10.1074/jbc.273.46.30204. [DOI] [PubMed] [Google Scholar]
- Adams CM, Snyder PM, Welsh MJ. Paradoxical stimulation of a DEG/ENaC channel by amiloride. Journal of Biological Chemistry. 1999;274:15500–15504. doi: 10.1074/jbc.274.22.15500. [DOI] [PubMed] [Google Scholar]
- Champigny G, Voilley N, Waldmann R, Lazdunski M. Mutations causing neurodegeneration in Caenorhabditis elegans drastically alter the pH sensitivity and inactivation of the mammalian H+-gated Na+ channel MDEG1. Journal of Biological Chemistry. 1998;273:15418–15422. doi: 10.1074/jbc.273.25.15418. [DOI] [PubMed] [Google Scholar]
- Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. Journal of General Physiology. 1998;111:127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion-channel mechanisms. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. pp. 453–457. [Google Scholar]
- Colquhoun D, Sigworth F. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO Journal. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe GK, Canessa CM. Subunit composition determines the single channel kinetics of the epithelial sodium channel. Journal of General Physiology. 1998;112:423–432. doi: 10.1085/jgp.112.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe GK, Zhang P, Canessa CM. The second hydrophobic domain contributes to the kinetic properties of epithelial sodium channels. Journal of Biological Chemistry. 1999;274:36415–36421. doi: 10.1074/jbc.274.51.36415. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels-function, structure and regulation. Physiological Reviews. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Grunder S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO Journal. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder S, Jaeger NF, Gautschi I, Schild L, Rossier BC. Identification of a highly conserved sequence at the N-terminus of the epithelial Na+ channel alpha subunit involved in gating. Pflugers Archiv. 1999;438:709–715. doi: 10.1007/s004249900119. [DOI] [PubMed] [Google Scholar]
- Hong K, Mano I, Driscoll M. In vivo structure- function analyses of Caenorhabditis elegans MEC-4, a candidate mechanosensory ion channel subunit. Journal of Neuroscience. 2000;20:2575–2588. doi: 10.1523/JNEUROSCI.20-07-02575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Auberson M, Gautschi I, Schneeberger E, Schild L. Permeability properties of ENaC selectivity filter mutants. Journal of General Physiology. 2001;118:679–692. doi: 10.1085/jgp.118.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Schild L. A single point mutation in the pore region of the epithelial Na+ channel changes ion selectivity by modifying molecular sieving. Proceedings of the National Academy of Sciences of the USA. 1999a;96:4170–4175. doi: 10.1073/pnas.96.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Hoffmann-Pochon N, Gautschi I, Schneeberger E, Schild L. On the molecular basis of ion permeation in the epithelial Na+ channel. Journal of General Physiology. 1999b;114:13–30. doi: 10.1085/jgp.114.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll B, Bremer S, Tummler B, Kottra G, Fromter E. Sodium dependence of the epithelial sodium conductance expressed in Xenopus laevis oocytes. Pflugers Archiv. 1991;419:101–107. doi: 10.1007/BF00373753. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Mano I, Driscoll M. DEG/ENaC channels: a touchy superfamily that watches its salt. Bioessays. 1999;21:568–578. doi: 10.1002/(SICI)1521-1878(199907)21:7<568::AID-BIES5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. Journal of General Physiology. 1996;107:35–45. doi: 10.1085/jgp.107.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L, Sackin H, Frindt G. Regulation of Na+ channels by luminal Na+ in rat cortical collecting tubule. Journal of Physiology. 1998;509:151–162. doi: 10.1111/j.1469-7793.1998.151bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Lingueglia E, Champigny G, Mattei MG, Lazdunski M. Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. Journal of Physiology. 1999;519:323–333. doi: 10.1111/j.1469-7793.1999.0323m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Sakai H, Mattei MG, Lazdunski M, Lingueglia E. Molecular cloning, functional expression and chromosomal localization of an amiloride-sensitive Na+ channel from human small intestine. FEBS Letters. 2000;471:205–210. doi: 10.1016/s0014-5793(00)01403-4. [DOI] [PubMed] [Google Scholar]
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the α, β, γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. Journal of General Physiology. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng SH, Li JQ, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelial sodium channel. Journal of Biological Chemistry. 2000;275:8572–8581. doi: 10.1074/jbc.275.12.8572. [DOI] [PubMed] [Google Scholar]
- Sheng SH, Li JQ, McNulty KA, Kieber-Emmons T, Kleyman TR. Epithelial sodium channel pore region-structure and role in gating. Journal of Biological Chemistry. 2001;276:1326–1334. doi: 10.1074/jbc.M008117200. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Bucher DB, Olson DR. Gating induces a conformational change in the outer vestibule of ENaC. Journal of General Physiology. 2000;116:781–790. doi: 10.1085/jgp.116.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Bucher DB. A pore segment in DEG/ENaC Na+ channels. Journal of Biological Chemistry. 1999;274:28484–28490. doi: 10.1074/jbc.274.40.28484. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Molecular modeling of mechanotransduction in the nematode Caenorhabditis elegans. Annual Review of Physiology. 1997;59:659–689. doi: 10.1146/annurev.physiol.59.1.659. [DOI] [PubMed] [Google Scholar]
- Unwin N. The Croonian Lecture (2000). Nicotinic acetylcholine receptor and the structural basis of fast synaptic transmission. Philosophical Transactions of the Royal Society of London B. 2000;355:1813–1829. doi: 10.1098/rstb.2000.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. Journal of the American Society of Nephrology. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels - neuronal acid sensors in the NaC/DEG family of ion channels. Current Opinion in Neurobiology. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]


