Abstract
The vanilloid receptor protein (VR1) is a well-characterised integrator of noxious stimuli in peripheral sensory neurones. There is evidence for the presence of VR1 in the central nervous system, but little information as to its role there. In this study we have examined the actions of agonists for VR1 receptors in the rat locus coeruleus (LC), using whole-cell patch-clamp recordings from acutely isolated neurones and neurones in slices. Superfusion with capsaicin resulted in a concentration-dependent increase in the frequency of isolated miniature excitatory postsynaptic currents (mEPSCs) in LC neurones. The mean amplitude of the mEPSCs was not affected by capsaicin. The effects of capsaicin (1 μM) were abolished by the VR1 receptor antagonists capsazepine (10 μM) and iodoresiniferatoxin (300 nm). Removal of extracellular Ca2+ abolished the capsaicin-induced increase in frequency of mEPSCs. Capsaicin superfusion had no consistent effects on evoked excitatory postsynaptic currents. Capsaicin superfusion also resulted in the release of an adrenoceptor agonist in the LC but did not affect the membrane currents of acutely isolated LC neurones. These data demonstrate that the VR1 receptor appears to be located presynaptically on afferents to the LC, and that activation of VR1 may serve to potentiate the release of glutamate and adrenaline/noradrenaline in this brain region.
The protein vanilloid receptor 1 (VR1) is involved in sensory transduction in the peripheral nerve endings of sensory neurones. It can be activated by elevations in temperature, decreases in pH or by exogenous ligands such as capsaicin (Caterina et al. 1997; Tominaga et al. 1998). In addition to environmental stimuli, VR1 can be activated by endogenous fatty-acid-derived mediators such as 12-hydroperoxyeicosatetraenoic acid and anandamide (Zygmunt et al. 1999; Hwang et al. 2000). Capsaicin acting at VR1 has also been demonstrated to modulate synaptic transmission at the primary afferent synapse in the spinal dorsal horn and in visceral ganglia (Tsunoo et al. 1982; Urban et al. 1985). At the primary afferent synapse, capsaicin causes a large increase in spontaneous glutamate and substance P release, but paradoxically blocks electrically stimulated neurotransmitter release (Urban et al. 1985; Urban & Dray, 1992; Yang et al. 1999). In myenteric neurones capsaicin also causes a release of substance P from sensory fibres that is independent of electrical stimulation. (Tsunoo et al. 1982; Dun & Kiraly, 1983). Capsaicin has not been reported to exert direct effects on intrinsic spinal dorsal horn neurones, although immunohistochemical evidence for postsynaptic VR1 receptors in the spinal cord has been reported. (Valtschanoff et al. 2001).
It was originally reported that there was no VR1 mRNA in the brain (Caterina et al. 1997), but subsequent studies have found widespread expression (Sasamura et al. 1998; Mezey et al. 2000). VR1 immunoreactivity and the binding of the VR1-selective radioligand [3H]resiniferatoxin have also been reported to occur in several brain regions, including the hypothalamus and locus coeruleus (LC; Acs et al. 1996; Mezey et al. 2000; Szabo et al. 2002). Several physiological studies of these regions have also provided tantalising evidence for functional activity of VR1 in the brain. Local application of capsaicin can directly excite and/or inhibit hypothalamic-preoptic neurones in vivo (Hori et al. 1988) and capsaicin will stimulate the release of glutamate from hypothalamic slices (Sasamura et al. 1998). Systemic capsaicin has been shown to produce robust activation of LC neurones. This action was not abolished by neonatal capsaicin treatment, indicating that it was independent of sensory neurone activation (Hajos et al. 1987). In light of the robust innervation of the LC by the hypothalamus (Luppi et al. 1995; Steininger et al. 2001), as well as the reported presence of VR1 protein in the nucleus, we became curious as to what the consequences of VR1 receptor activation in the LC might be. Using capsaicin as a pharmacological activator of VR1, we found that VR1 activation results in a large increase in glutamate release in the LC, indicating that the VR1 receptor may have a role as a modulator of nerve terminal excitability in the central nervous system, beyond its potential role at the primary afferent synapse.
Methods
Brain slice recordings
All procedures were carried out under protocols approved by the University of Sydney Animal Ethics Committee. Sprague-Dawley rats (12-22 days old), of either sex, were anaesthetised with halothane, decapitated and coronal brain slices containing the LC were cut (250 μm) in ice-cold artificial cerebrospinal fluid (aCSF). Slices were superfused continuously (2 ml min−1) with aCSF (32 °C) of composition (mm): NaCl 126, KCl 2.5, NaH2PO4 1.4, MgCl2 1.2, CaCl2 2.4, glucose 11, NaHCO3 25, as described previously (Vaughan et al. 2000). In experiments using Cd2+, NaH2PO4 was omitted from the aCSF. LC neurones were visualised using infrared Nomarski optics on an upright microscope.
A potassium-gluconate-based internal solution containing (mm): potassium gluconate 95, KCl 30, NaCl 15, MgCl2 1, Hepes 10, EGTA 11, MgATP 2, NaGTP 0.25, pH 7.3 was used for experiments on postsynaptic membrane currents. Experiments on synaptic currents utilised a caesium-chloride-based internal solution, which contained (mm): CsCl 140, EGTA 10, Hepes 5, CaCl2 2, MgATP 2, pH 7.3. Voltage-clamp recordings (holding potential −60 mV) were made in the whole-cell configuration using an Axopatch 200B (Axon Instruments, Union City, CA, USA) with borosilicate electrodes (2-5 MΩ). Series resistance (< 20 MΩ) was compensated by 80 % and monitored continuously during experiments. Liquid junction potentials of −12 mV for potassium-gluconate-based, and −4 mV for CsCl-based internal solutions were corrected. Postsynaptic membrane current recordings were filtered (100 Hz low-pass filter) and sampled (200 Hz) for off-line analysis (Axograph 4, Axon Instruments).
Electrically evoked excitatory postsynaptic currents (eEPSCs) were elicited in the presence of picrotoxin (100 μM) and strychnine (3 μM) via a theta-glass stimulating electrode placed within the LC 50–100 μm dorsolateral to the recording electrode (rate 0.083-0.033 Hz, stimuli: 50–80 V, 100–400 μs). Spontaneous miniature EPSCs (mEPSCs) obtained in the presence of tetrodotoxin (TTX, 300 nm), strychnine (3 μM), and bicuculline (100 μM) or picrotoxin (100 μM) were filtered (2 kHz low-pass filter) and sampled at 10 kHz. TTX (300 nm) completely abolished the EPSCs evoked by focal electrical stimulation in LC cells (Fig. 6A). EPSCs were filtered (2 kHz low-pass filter) and sampled at 10 kHz for on-line and later off-line analysis (Axograph 4, Axon Instruments). mEPSCs above a preset threshold (3.4-4.2 standard deviations above baseline noise) were automatically detected by a sliding template algorithm (Axograph 4), then manually checked off-line. mEPSCs were counted in 4 s epochs every 6 s to construct plots of event frequency vs time. In the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), miniature inhibitory postsynaptic currents were only encountered infrequently (less than 0.1 Hz), so the actions of capsaicin were not studied further under these conditions.
Figure 6. Capsaicin has little effect on evoked glutamatergic synaptic currents.
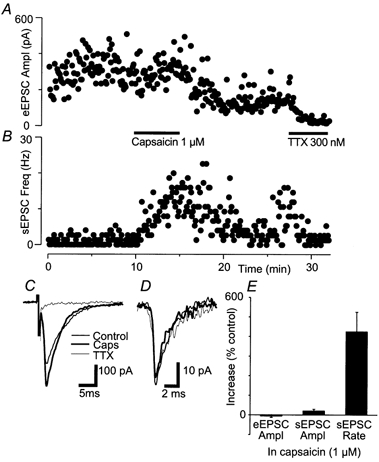
A, time course of the amplitude of evoked EPSCs (eEPSCs) during superfusion of capsaicin (1 μM) and TTX (300 nm). B, time course of the frequency of spontaneous EPSCs measured over a 1 s epoch subsequent to each eEPSC. C, averaged traces of eEPSCs before (thin line) and during superfusion with capsaicin (thick line) and then during superfusion with TTX (hairline). D, averaged traces of spontaneous EPSCs before (thin line) and during superfusion with capsaicin (thick line) and then during superfusion with TTX (hairline). E, bar charts summarising the effects of 1 μM capsaicin on eEPSC amplitude, spontaneous EPSC (sEPSC) amplitude and sEPSC rate (n = 6). A and B are taken from the same neurone, which was clamped at −70 mV in the presence of picrotoxin (100 μM) and strychnine (3 μM). These results are typical of the 10 cells superfused with capsaicin concentrations between 1 μM and 10 μM.
Recordings from isolated neurones
LC neurones were dissociated as follows. Horizontal brainstem slices (320 μm thick) containing the LC were prepared from rats (28-35 days old) as described above. Slices were incubated in a modified aCSF (as above except for the addition of an extra 10 mm MgCl2) for 15 min, then transferred to a modified Hepes-buffered saline (HBS) containing 20 units ml−1 papain, pH 7.3 and incubated for 2 min at 35 °C. The modified HBS consisted of the HBS described below, diluted by 1 part in 15, and supplemented with 10 mm MgCl2. The digestion was terminated with fresh dissociation buffer containing 1 mg ml−1 bovine serum albumin (BSA) and 1 mg ml−1 trypsin inhibitor. The LC region was subdissected from each slice with a fine tungsten wire and the cells dissociated by trituration.
Recordings of currents in isolated neurones were made at room temperature (22-24 °C) using standard whole-cell patch-clamp techniques, as described previously (Connor et al. 1999). Cells were superfused with a buffer of composition (mm): NaCl 140, KCl 2.5, CaCl2 2.5, MgCl2 1.5, Hepes 10, glucose 10, pH 7.3 (HBS). For Ca2+ channel current (ICa) recordings, cells were then superfused with a solution containing (mm): tetraethylammonium chloride 140, BaCl2 2, MgCl2 1, CsCl 2.5, Hepes 10, glucose 10, BSA 0.05 %, pH 7.3. For recordings of VR1 and glutamate-activated currents, cells were again superfused with HBS. Recordings were made with fire-polished pipettes of resistance 1–2 MΩ filled with solution containing (mm): CsCl 120, MgATP 5, Na2GTP 0.2, EGTA 10, CaCl2 2 and Hepes 10, pH 7.3. Cell capacitance and series resistance were compensated manually by nulling the capacitive transient evoked by a 20 mV pulse from −90 mV. Series resistance (1.5-5 MΩ) was compensated by at least 80 % in all experiments. Currents were sampled at 5–10 kHz and recorded for later analysis with pCLAMP and Axograph (Axon Instruments). Cells were exposed to drugs via a series of flow pipes positioned above the cells.
All data are expressed as means ± s.e.m., unless otherwise indicated. Statistical significance was determined by using an unpaired Student's t test, unless otherwise stated.
Drugs and chemicals
Methionine-enkephalin (ME) was from Auspep (Melbourne, Australia). CNQX, capsaicin, resiniferatoxin, iodoresiniferatoxin and capsazepine were from Tocris Cookson (Bristol, UK). Prazocin was from Research Biochemicals International (Natick, MA, USA). Idazoxan was from Reckitt and Coleman (Kingston upon Hull, UK). Papain was from Worthington Biochemical (Freehold, NJ, USA). TTX was from Alomone Laboratories (Jerusalem, Israel). Picrotoxin, bicuculline methiodide, strychnine hydrochloride, BSA and trypsin inhibitor (Type II-O) were from Sigma Australia.
Results
Capsaicin increases glutamatergic synaptic transmission in the LC
In the presence of the GABAA receptor antagonists bicuculline (100 μM) or picrotoxin (100 μM), and the glycine receptor antagonist strychnine (3 μM) and TTX (300 nm), mEPSCs were readily observed that were blocked by the non-NMDA receptor antagonist CNQX (3 μM, n = 5). Superfusion of capsaicin (1 μM) produced an increase in the frequency of mEPSCs (1700 ± 630 %, n = 10), but did not significantly affect their amplitude (6 ± 5 %) or kinetics (Fig. 1). This indicates that capsaicin increased glutamate release. Following washout of capsaicin, there was a slow return of mEPSC frequency to baseline levels that was often incomplete at 15 min post-washout (Fig. 1A).
Figure 1. Capsaicin produces a presynaptic increase in glutamatergic synaptic transmission.
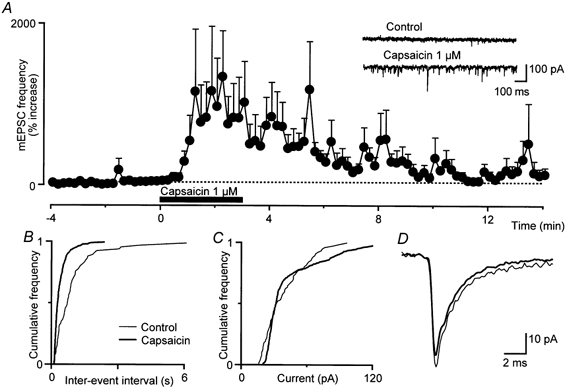
Capsaicin increased the frequency of glutamatergic synaptic currents, without modulating their amplitude or kinetics. A, summary time course of the increase in miniature EPSC (mEPSC) frequency during superfusion with capsaicin (1 μM). The inset shows averaged traces of mEPSCs before (control) and during capsaicin superfusion. B and C, cumulative distribution plots of mEPSC inter-event interval (B) and mEPSC amplitude (C) before (thin line) and during superfusion with capsaicin (thick line). D, averaged raw traces of mEPSCs before (thin line) and during capsaicin superfusion (thick line). In the time plot of A, the mEPSC frequency for each neurone (n = 10) is normalised against the mean of the 4 min baseline period prior to capsaicin application, and data are expressed as the mean ± s.e.m. percentage increase above the baseline level (dotted line). B-D are taken from the same neurone.
The increase in mEPSC frequency produced by capsaicin was concentration dependent over the concentration range 100 nm to 3 μM (Fig. 2, n = 5-10). The increase in mEPSC frequency was maintained during prolonged exposure to lower concentrations of capsaicin (100-300 nm, Fig. 3A) and declined during prolonged exposure to capsaicin (1-3 μM). At higher concentrations of capsaicin (10 μM) there was a variable increase in mEPSC frequency that usually desensitised rapidly, within 1–4 min of application (n = 5, Fig. 3B).
Figure 2. The capsaicin-induced increase in glutamatergic synaptic transmission is mediated by the vanilloid receptor protein 1 (VR1) receptor.
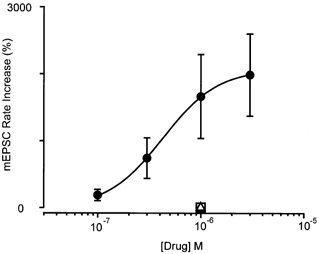
Concentration-response relationship for percentage increase in mEPSC rate produced by capsaicin (•). The responses to capsaicin (1 μM) in the presence of capsazepine (10 μM, ▪) and iodoresiniferatoxin (300 nm, ▵) are also shown. Each point shows the mean ± s.e.m. of responses of several different neurones (n = 5-10). A log function was fitted to determine the EC50 for the capsaicin-induced increase in mEPSC frequency (520 ± 240 nm, slope factor = 1.1 ± 0.4).
Figure 3. The capsaicin-induced increase in glutamatergic synaptic transmission desensitises at higher concentrations.
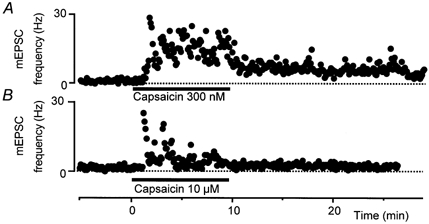
Time course of the increase in mEPSC frequency during superfusion of capsaicin at concentrations of 300 nm (A) and 10 μM (B). The time plots of mEPSC frequency are taken from different neurones and the results are typical of at least five neurones per concentration.
The increase in mEPSC frequency produced by capsaicin (1 μM) was abolished by the VR1 antagonists capsazepine (10 μM, n = 7) and iodoresiniferatoxin (300 nm, n = 5; Wahl et al. 2001). The mean increase in mEPSC frequency was 6 ± 11 and 22 ± 31 % in capsazepine and iodoresiniferatoxin, respectively (Fig. 2). The VR1 agonist resiniferatoxin (1-100 nm) also produced a variable increase in mEPSC frequency that usually desensitised rapidly, within 1 min of application (n = 10, Fig. 4).
Figure 4. Resiniferatoxin (Rtx) also produces a presynaptic increase in glutamatergic synaptic transmission.
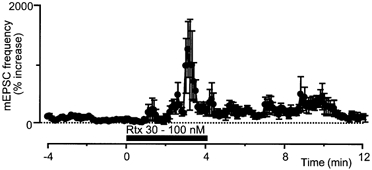
Summary time course of the increase in mEPSC frequency during superfusion of Rtx (30-100 nm). The time plots of mEPSC frequency are taken from different neurones. The time plot of mEPSC frequency has been normalised and averaged (n = 4), as in Fig. 1B.
Capsaicin (1 μM) did not produce a significant increase in mEPSC frequency (6 ± 11 % increase) in calcium-free external solutions (0 mm Ca2+, 10 mm Mg2+, n = 8; Fig. 5A). In contrast, capsaicin (1 μM) produced an increase in mEPSC frequency of 730 ± 180 % in normal-calcium/-magnesium solutions, which also contained Cd2+ (30 μM) to prevent Ca2+ entry through voltage-activated Ca2+ channels (Fig. 5A). Cd2+ (30 μM) superfusion abolished inward Ca2+ currents evoked from −90 to 0 mV in LC cells in slices (Fig. 5B).
Figure 5. The capsaicin-induced increase in glutamatergic synaptic transmission is mediated via Ca2+ entry, in part through VR1.
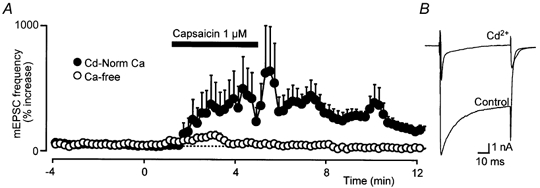
A, summary time course of the increase in mEPSC frequency during superfusion with capsaicin (1 μM) in Cd2+ (30 μM) containing normal Ca2+ artificial cerebrospinal fluid (aCSF; •) and in calcium-free (Ca2+ 0 mm, Mg2+ 10 mm) aCSF (○). The time plots of mEPSC frequency have been normalised and averaged, as in Fig. 1B, and data from eight cells are represented for each condition. B, representative raw traces of membrane currents evoked by stepping a locus coeruleus (LC) neurone from −90 to 0 mV before (Control) and during superfusion of Cd2+. This response is typical of the five neurones tested.
In the presence of picrotoxin (100 μM) and strychnine (3 μM), eEPSCs were readily observed, and they were blocked by CNQX (3 μM, n = 2) or TTX (300 nm, Fig. 6A, n = 5). Superfusion with capsaicin (1 μM) had a variable effect on the amplitude of eEPSCs, producing an increase in amplitude in 2/6 neurones (Fig. 6). However, this increase in eEPSC amplitude was not significant when averaged over all neurones (-5 ± 8 %, range 19 to −29 %, n = 6, P = 0.9). In the same experiments, capsaicin produced a robust increase in the frequency of spontaneous EPSCs (Fig. 6B, n = 6, P = 0.03). Superfusion with higher concentrations of capsaicin (3-10 μM) did not significantly stimulate or inhibit eEPSCs (n = 4 for each, data not shown). Capsaicin did not have a significant effect on the amplitude (P = 0.9) or kinetics of spontaneous EPSCs (Fig. 6B).
Capsaicin produces both inward and outward currents
When LC neurones were recorded using a potassium-gluconate-based internal solution, the cells responded to capsaicin (10 μM) with transient inward (-18 ± 1 pA, n = 7/7) and/or outward currents (11 ± 1 pA, n = 2/7). The inward and/or outward currents could only be obtained with one superfusion of capsaicin per slice (not shown). The capsaicin-induced inward (-24 ± 10 pA, n = 3/7) and/or outward currents (34 ± 7 pA, n = 7/7) were also observed in the combined presence of CNQX (3 μM), bicuculline (100 μM), strychnine (3 μM) and TTX (300 nm, Fig. 7A). However, the capsaicin-induced currents were abolished in the additional presence of the α1- and α2-adrenoceptor antagonists prazocin (3 μM) and idazoxan (3 μM, Fig. 7B, n = 7). Methionine-enkephalin (ME; 10 μM) produced an outward current in all neurones examined, confirming that the characteristic opioid activation of K+ channels in these cells remained intact following capsaicin treatment.
Figure 7. Capsaicin produces inward and outward currents, an effect that is mediated by adrenergic receptors.
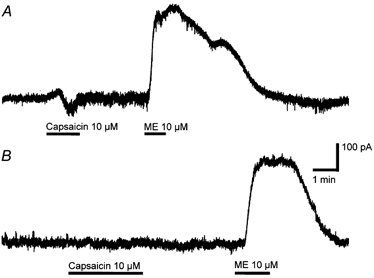
Membrane current traces of LC neurones during superfusion of capsaicin (10 μM) and met-enkephalin (ME, 10 μM) in the absence (A) and the presence (B) of prazocin (3 μM) plus idazoxan (3 μM). A and B are taken from different neurones, clamped at - 60 mV in aCSF containing TTX (300 nm), CNQX (3 μM) and bicuculline (30 μM). The scale bars are the same for both traces.
Capsaicin does not affect acutely isolated LC neurones
Isolated LC neurones were identified as previously described (Connor et al. 1999), utilising the criteria of size (28 ± 3 pF) and sensitivity to opioid agonists. ICa evoked by stepping the neurones from a holding potential of −90 mV to −10 mV was reversibly inhibited by ME (10 μM, 43 ± 3 %, n = 18). When the LC neurones were voltage clamped at +40 mV and perfused with HBS, application of capsaicin (10 μM) did not produce a significant current in any neurone (mean current = 1 ± 3 pA, n = 16; Fig. 8A). In contrast, application of glutamate (100 μM) produced a mean outward current of 1200 ± 400 pA at +40 mV (n = 4). Under these recording conditions, it has been shown that VR1 activation produces large outward currents in sensory neurones (Piper et al. 1999; Roberts et al. 2002). When the membrane currents of isolated LC neurones were examined over a range of membrane potentials by means of a slow ramping of the membrane potential from +80 to −60 mV, capsaicin application failed to produce a current at any potential (Fig. 8B). Glutamate superfusion produced currents over the full range of membrane potentials (Fig. 8B), indicating that both inward and outward currents could be measured in LC neurones in this recording configuration.
Figure 8. Capsaicin does not affect membrane currents in isolated LC neurones.
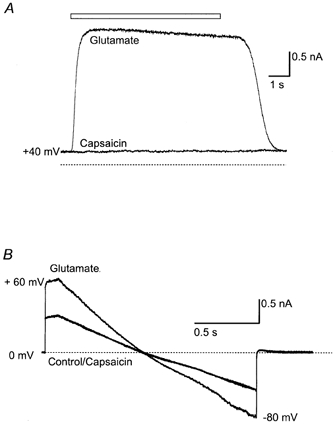
A, an isolated LC neurone was voltage clamped at +40 mV, and superfused with Hepes-buffered saline. Application of capsaicin (10 μM) had no effect on the membrane current of the LC neurone, whereas a subsequent application of glutamate (100 μM) produced a robust outward current. B, another neurone was voltage clamped at 0 mV and then ramped from +60 mV to −80 mV. Capsaicin did not produce a current at any potential, while glutamate (100 μM) superfusion produced a current that reversed at about 0 mV. These results are typical of the responses of 18 LC cells examined.
Discussion
This study demonstrates that capsaicin, acting via VR1 receptors, stimulates the spontaneous release of glutamate and adrenaline/noradrenaline in the LC in vitro. These results are consistent with the study of Hajos et al. (1987), in which it was demonstrated that the intravenous injection of capsaicin produced a robust excitation of LC neurones in rat. This effect persisted in rats treated with capsaicin neonatally, suggesting that it was not the result of activation of capsaicin-sensitive primary afferent fibres. In the present study, capsaicin did not directly affect the membrane currents of isolated LC neurones, suggesting that intravenous capsaicin can act on VR1 in the glutamatergic terminals in the LC (but see below). Importantly, direct innervation of the LC by primary afferent neurones has not, to our knowledge, been reported, and the characteristic capsaicin-induced degeneration of central primary afferent terminals was not observed in the LC following neonatal treatment of rats with high-dose capsaicin (Jansco & Kiraly, 1980). Thus it is likely that the effects of capsaicin observed in this study were on glutamatergic terminals innervating the LC from another structure within the brain. The LC receives afferent innervation from many structures, and which of these give rise to the capsaicin-sensitive glutamatergic terminals in LC is unknown, although candidate regions with strong glutamatergic projections to the LC and robust expression of VR1 (Mezey et al. 2000) include the anterior hypothalamic/preoptic areas (Steininger et al. 2001), the nucleus tractus solitarius (Van Bockstaele et al. 1999) and the central amygdala (Van Bockstaele et al. 1998).
Capsaicin also caused a small release of adrenaline and/or noradrenaline in the LC. The currents produced by the adrenoceptor agonist(s) were too small to be able to study accurately their ionic basis. However, the outward current produced by activation of α2-adrenoceptors may have been mediated by activation of a K+ conductance, whilst the inward current produced by activation of α1-receptors may be mediated by inhibition of a K+ conductance (Finlayson & Marshall, 1984; Williams & Marshall, 1987). The source of the adrenaline/noradrenaline released by capsaicin is not known, and there are a number of adrenergic nuclei that project to the LC. One attractive possible source for the capsaicin-induced adrenoceptor agonist is the terminals of the noradrenergic projection from the relatively VR1-rich nucleus tractus solitarius (Van Bockstaele et al. 1999; Mezey et al. 2000). Another possible source of noradrenaline is the axon collaterals of the LC neurones themselves. Although we found no direct effects of capsaicin on isolated LC neurones, or on LC neurones exposed to capsaicin in a cocktail of synaptic transmission blockers, if VR1 was selectively trafficked to the axon terminals of LC neurones, then direct effects on the cell soma and dendrites may not be apparent. This possibility is consistent with the reported expression of VR1 in the LC (Mezey et al. 2000).
VR1 immunoreactivity and mRNA have been identified in the rat LC, although details of its cellular localisation have not been reported (Mezey et al. 2000). On the basis of that report, one might have expected to see clear actions of capsaicin on LC cell bodies, but there are several possible explanations as to why we saw no such effects. Firstly, it is possible that the VR1 immunoreactivity reported by Mezey and colleagues (2000) represents labelling of presynaptic structures in the LC, and our results would be fully consistent with this explanation. It is also possible that the VR1 protein is made in LC neurones but trafficked exclusively to distant nerve terminals. Finally, the riboprobe used to identify VR1 mRNA in the LC (Mezey et al. 2000) is likely to amplify several transcripts including the SIC channel (Suzuki et al. 1999) and the recently identified N-terminal splice variants of VR1, VR.5′sv and VR1L2 (Xue et al. 2001).
The potency of capsaicin with regard to the stimulation glutamate release was similar to that reported for capsaicin-induced activation of the native (Marsh et al. 1987; Roberts et al. 2002) and cloned (Caterina et al. 1997) VR1 protein. The potent VR1 agonist resiniferatoxin mimicked the effects of capsaicin, but its effects were difficult to quantify because of profound desensitisation. The effects of capsaicin (1 μM) were abolished by the well-characterised VR1 antagonist capsazepine (10 μM) and the recently synthesised potent VR1 antagonist iodoresiniferatoxin (300 nm; Wahl et al. 2001). These data indicate that the VR1 receptor in the glutamatergic nerve terminals in the LC exhibits a pharmacological profile indistinguishable from the receptor in sensory neurones (Szabo et al. 2002).
Previously, capsaicin-mediated activation of VR1 in the central or visceral ganglionic terminals of primary afferent neurones has been shown to have a profound stimulatory effect on spontaneous synaptic transmission (Tsunoo et al. 1982; Dun & Kiraly, 1983; Urban et al. 1985; Urban & Dray, 1992; Yang et al. 1998, 1999). In these studies application of capsaicin caused a large release of either glutamate (Urban & Dray, 1992; Yang et al. 1998) or substance P (Tsunoo et al. 1982; Dun & Kiraly, 1983; Urban et al. 1985; Urban & Dray, 1992) that was accompanied by a persistent inhibition of electrically evoked release (Yang et al. 1999). In the present study, capsaicin also caused spontaneous glutamate release, but it had no consistent effects on electrically evoked glutamate release. The actions of capsaicin in the LC were likely to be occurring at least in part at VR1 located in the nerve terminals themselves, because the effects of capsaicin on glutamate release persisted in the presence of TTX. TTX-insensitive effects of capsaicin on the frequency of spontaneous glutamatergic events in the dorsal horn have also been reported in studies utilising whole-cell patch-clamp techniques (Yang et al. 1998; Nakatsuka et al. 2002). Earlier studies with high-resistance microelectrodes reported TTX-insensitive release of substance P by capsaicin in the inferior mesenteric ganglion (Dun & Kiraly, 1983), but only TTX-sensitive actions of capsaicin in the spinal dorsal horn (Urban et al. 1985; Urban & Dray, 1992)
The increase in mEPSC frequency observed in the presence of capsaicin presumably results from an increase in intraterminal Ca2+ concentration resulting from Ca2+ influx into the nerve terminal, as it was abolished by removal of extracellular Ca2+. Interestingly, the ICa blocker Cd2+ did not abolish the effects of capsaicin in normal aCSF, suggesting that the increase in transmitter release resulted at least in part from Ca2+ entry directly through VR1, and not solely Ca2+ entry through ICa activated as a consequence of VR1-mediated depolarisation of the nerve terminal.
Capsaicin stimulated spontaneous glutamate release in the LC, but had no consistent effects on electrically evoked transmitter release. The reasons for this are not known, but the most likely explanation is that the terminals being activated by electrical stimulation are a different population to those being activated by capsaicin. It is not possible to stimulate identified afferent pathways in the LC slice, although the major excitatory input to the LC originates in the nucleus paragigantocellularis reticularis (PGi, Ennis & Aston-Jones, 1988), and terminals from these cells are the most likely terminals to be excited by focal electrical stimulation in the LC slice. It is not known if neurones in the PGi express VR1, although only scattered VR1 immunoreactivity was reported in the reticular formation as a whole (Mezey et al. 2000). Interestingly, capsaicin activation of VR1 at the primary afferent synapse abolishes rather than potentiates evoked neurotransmitter release, although the reasons for this remain obscure (Urban et al. 1985; Urban & Dray, 1992; Yang et al. 1999).
We can only speculate about how VR1 receptors in afferents to the LC may be activated. Pathological changes in brain temperature or pH, for example after a severe stroke (Schwab et al. 1997), may influence VR1 activity, but normal brain pH (≈7.3) and temperature (≈38°C) are unlikely to result in VR1 activation per se (Tominaga et al. 1998). However, VR1 can be activated by endogenously generated compounds such as anandamide and the products of lipoxygenase enzymes (Zygmunt et al. 1999; Hwang et al. 2000). We were, however, unable to activate VR1 in the LC with concentrations of anandamide up to 30 μM (C. W. Vaughan, unpublished observations). At the primary afferent synapse in the dorsal horn of the spinal cord, exogenously applied anandamide stimulates glutamate release only at concentrations above 10 μM (Jennings et al. 2001; Morisset et al. 2001; Luo et al. 2002), which is well above the concentration needed to activate CB1 receptors on the same population of terminals (< 1 μM, Morisset & Urban, 2001; Luo et al. 2002). Similarly, only at a concentration of 10 μM or above did anandamide modulate synaptic transmission in the hippocampus through VR1-dependent mechanisms (Al-Hayani et al. 2001). Anandamide is subject to efficient uptake and degradation in brain tissue, and so superfusion of brain slices may not readily deliver enough anandamide to reach the concentrations necessary to activate VR1 receptors in terminals in the LC. It remains an intriguing possibility that eicosanoids generated within VR1-expressing nerve terminals (Vaughan et al. 1997; Manzoni & Williams, 1999) or in neuronal cell bodies near to these terminals (Krietzer & Regehr, 2001; Ohno-Shosaku et al. 2001; Wilson et al. 2001) may act to stimulate neurotransmitter release.
Recently, it was reported that activators of VR1 including capsaicin and anandamide increased the paired-pulse depression of electrically evoked population responses in rat CA1 pyramidal neurones in vitro (Al-Hayani et al. 2001). These effects were sensitive to capsazepine, indicating the involvement of VR1, and it was suggested that the effects arose from an increase in GABAergic synaptic transmission in the hippocampal slice. To our knowledge there are no other reports of VR1 activation influencing central nervous system synaptic transmission other than at a the primary afferent synapse. Nevertheless, the results of the present study and those of Al-Hayani and colleagues (2001) indicate that VR1 receptor activation may influence the release of diverse neurotransmitters in many parts of the CNS.
Acknowledgments
This study was supported by NH and MRC of Australia Project Grants to C.W.V. (no. 153844) and M.C. (no. 153911) and by The Medical Foundation of the University of Sydney (MJC). We thank Dr Janet Keast, Dr Peregrine Osborne and Dr Elena Bagley for useful comments on these studies, and Dr Darren Smart for the discussion that inspired these experiments.
References
- Acs G, Palkovits M, Blumberg PM. Specific binding of [3H]resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sciences. 1996;59:1899–1908. doi: 10.1016/s0024-3205(96)00537-1. [DOI] [PubMed] [Google Scholar]
- Al-Hayani A, Wease KN, Ross RA, Pertwee RG, Davies SN. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. doi: 10.1016/s0028-3908(01)00145-9. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Connor M, Borgland SL, Christie MJ. Continued morphine modulation of calcium channel currents in acutely isolated locus coeruleus neurons from morphine-dependent rats. British Journal of Pharmacology. 1999;128:1561–1569. doi: 10.1038/sj.bjp.0702922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Kiraly M. Capsaicin causes release of a substance P-like peptide in guinea-pig inferior mesenteric ganglia. Journal of Physiology. 1983;340:107–120. doi: 10.1113/jphysiol.1983.sp014752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. Journal of Neuroscience. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Marshall KC. Hyperpolarizing and age-dependent depolarizing responses of cultured locus coeruleus neurons to noradrenaline. Brain Research. 1984;317:167–175. doi: 10.1016/0165-3806(84)90094-4. [DOI] [PubMed] [Google Scholar]
- Hajos M, Jansco G, Engberg G. Capsaicin-induced excitation of locus coeruleus neurons. Acta Physiologica Scandanavia. 1987;129:415–420. doi: 10.1111/j.1748-1716.1987.tb08086.x. [DOI] [PubMed] [Google Scholar]
- Hori T, Shibata M, Kiyohara T, Nakashima T, Asami A. Responses of anterior hypothalamic-preoptic thermosensitive neurons to locally applied capsaicin. Neuropharmacology. 1988;27:135–142. doi: 10.1016/0028-3908(88)90162-1. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee S-Y, Kang C-J, Jung J, Cho S, Min KH, Suh Y-G, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proceedings of the National Academy of Sciences of the USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansco G, Kiraly E. Distribution of chemosensitive primary sensory afferents in the central nervous system of the rat. Journal of Comparative Neurology. 1980;190:781–792. doi: 10.1002/cne.901900409. [DOI] [PubMed] [Google Scholar]
- Jennings EA, Connor M, Vaughan CW, Christie MJ. Presynaptic actions of anandamide on rat medullary dorsal horn neurons. Society for Neuroscience Abstracts. 2001;27:805.19. [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Luo C, Kumamoto E, Furue H, Chen J, Yoshimura M. Anandamide inhibits excitatory transmission to rat substantia gelatinosa neurones in a manner different from that of capsaicin. Neuroscience Letters. 2002;321:17–20. doi: 10.1016/s0304-3940(01)02471-5. [DOI] [PubMed] [Google Scholar]
- Luppi P-H, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. Journal of Neuroscience. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortwright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proceedings of the National Academy of Sciences of the USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Ahluwahlia J, Nagy I, Urban L. Possible mechanisms of cannabinoid-induced antinociception in the spinal cord. European Journal of Pharmacology. 2001;429:93–100. doi: 10.1016/s0014-2999(01)01309-7. [DOI] [PubMed] [Google Scholar]
- Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. Journal of Neurophysiology. 2001;86:40–46. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor 1 receptors and αβ-methylene-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. Journal of Neuroscience. 2002;22:1228–1237. doi: 10.1523/JNEUROSCI.22-04-01228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarised postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Piper AS, Yeats JC, Bevan S, Docherty RJ. A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. Journal of Physiology. 1999;528:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. Anandamide behaves as a partial agonist at native VR1 receptors in trigeminal ganglion neurons. Proceedings of the Australian Neuroscience Society. 2002;13:164. [Google Scholar]
- Sasamura T, Sasaki M, Tohda C, Kuraishi Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. NeuroReport. 1998;9:2045–2048. doi: 10.1097/00001756-199806220-00025. [DOI] [PubMed] [Google Scholar]
- Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48:762–767. doi: 10.1212/wnl.48.3.762. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. Journal of Comparative Neurology. 2001;429:638–653. [PubMed] [Google Scholar]
- Suzuki M, Sato J, Kutsuwada K, Ooki G, Imai M. Cloning of a stretch-inhibitable nonselective cation channel. Journal of Biological Chemistry. 1999;274:6330–6335. doi: 10.1074/jbc.274.10.6330. [DOI] [PubMed] [Google Scholar]
- Szabo T, Biro T, GonzaleZ AF, Palkovits M, Blumberg PM. Pharmacological characterization of vanilloid receptor located in the brain. Molecular Brain Research. 2002;98:51–57. doi: 10.1016/s0169-328x(01)00313-8. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tsunoo A, Konishi S, Otsuka M. Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience. 1982;7:2025–2037. doi: 10.1016/0306-4522(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Urban L, Dray A. Synaptic activation of dorsal horn neurons by selective C-fibre excitation with capsaicin in the mouse spinal cord in vitro. Neuroscience. 1992;47:693–702. doi: 10.1016/0306-4522(92)90177-4. [DOI] [PubMed] [Google Scholar]
- Urban L, Willets J, Randic M, Papka RE. The acute and chronic effects of capsaicin on slow excitatory transmission in rat dorsal horn. Brain Research. 1985;330:390–396. doi: 10.1016/0006-8993(85)90705-x. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. Journal of Comparative Neurology. 2001;436:225–235. [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotrophin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. Journal of Neuroendocrinology. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in the rat brain: evidence for a monosynaptic pathway. Journal of Comparative Neurology. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal grey neurons in vitro. Molecular Pharmacology. 2000;57:288–295. [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABAergic neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Molecular Pharmacology. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. Journal of Neuroscience. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Xue Q, Yu Y, Trilk SL, Jong BE, Schumacher MA. The genomic organization of the gene encoding the vanilloid receptor: evidence for multiple splice variants. Genomics. 2001;76:14–20. doi: 10.1006/geno.2001.6582. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Li Y-Q, Yoshimura M. Actions of capsaicin on dorsal root-evoked synaptic transmission to substantia gelatinosa neurons in adult rat spinal cord slices. Brain Research. 1999;830:268–273. doi: 10.1016/s0006-8993(99)01408-0. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neuroscience Letters. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang HH, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


