Abstract
The aim of this study was to evaluate whether a newly acquired locomotor skill can be transferred to the mirror condition. Subjects were trained to step over an obstacle on a treadmill, the appearance of which was signalled by an acoustic stimulus, while visual information was prevented. Feedback information about foot clearance was provided by acoustic signals. During two successive runs (each consisting of 100 steps over the obstacle) the same leg was leading (i.e. the leg crossing the obstacle first). In the following third run, the leading and trailing legs were changed. During each of the three successive runs the adaptational changes were analysed by recording leg muscle electromyographic (EMG) activity, joint angle trajectories and foot clearance over the obstacle. The training effect gained between the first and second runs and the transfer to the mirror condition (third run) were evaluated. Adaptational changes of all measures, except ankle joint trajectory, could to a significant extent be transferred to the mirror condition. No side-specific differences in the amount of transfer were found, neither from the right to the left side, nor vice versa. These observations are at variance with adaptational changes observed during split-belt walking or one-legged hopping on a treadmill, where no transfer to the mirror condition occurred. It is assumed that this might be due to the specific requirements of the tasks and the leg muscles involved. While in the split-belt and hopping experiments leg extensor muscles are mainly involved, leg flexors predominate in the performance of the present task. It is hypothesised that the learning effects observed in the present experiments are mediated at a higher level (e.g. brainstem) of locomotor control.
Complex skilled movements require different but finely tuned and co-ordinated muscle activation patterns on both sides of the body. The scope of highly trained functional movements might be expanded if motor skills could be transferred to the contralateral side, i.e. the ‘mirror’ condition. It has been shown that a bilateral transfer of training effects occurs for mirror-image movements after intensive training of the original, unilateral movement (Hicks et al. 1982). Also the transfer between the bilateral (e.g. the two arms or the two legs), ipsilateral (e.g. arm and leg on the same side) and diagonal limbs has been examined (Hicks et al. 1983). It became obvious that the amount of bilateral transfer was greater than the ipsilateral or diagonal transfer. These findings are in line with the observation that during learning a unilateral tapping task, this task could be transferred to the other hand (Hicks et al. 1982). It could be shown that the amount of ipsilateral training corresponded to the performance of the contralateral hand. This effect, however, did not occur if the second hand was engaged in unrelated activities during the training of the first hand.
Nevertheless, no transfer of adaptational changes in spatio- temporal and electromyographic (EMG) parameters from the fast to the slow walking leg and vice versa was found when subjects walked with two different speeds on a split-belt treadmill (Prokop et al. 1995). Furthermore, no transfer of adaptational effects to the contralateral side was observed following unilateral hopping on a treadmill (Anstis, 1995). In both studies, it was assumed that adaptation takes place at a spinal level.
A more complex task requiring a finely tuned co-ordination between the two legs is represented by the stepping over an obstacle (Patla & Prentice, 1995; Chou et al. 2001). In a recent study (Erni & Dietz, 2001) the influence of specific afferent information during motor learning in such a complex task was investigated. It has been shown that adaptation to a new locomotor skill occurs during repetitive stepping over an obstacle. A transfer of the newly learned locomotor pattern to different stimulus conditions was found to depend on the sequence of the stimuli. The question emerged as to whether the locomotor skill learned during repetitive stepping over an obstacle, can also be transferred to the situation where the contralateral leg is leading in the step over the obstacle, i.e. the mirror condition.
The aim of this study was to gain a better understanding of the neuronal mechanisms underlying adaptation and learning in automatically performed movements such as locomotion. Therefore it was evaluated: (1) whether a transfer of a newly learned complex locomotor movement pattern to the mirror condition occurs; and (2) whether there is a side-specific difference between the right and the left leg.
Methods
Subjects
Twelve healthy subjects (mean age 29.9, range 24–37 years, eight males and four females; 165 to 180 cm in height) participated in the study that conformed to standards set by the Declaration of Helsinki. The local Ethical Committee approved the study. The subjects were informed about the experiments and gave written informed consent. The subjects had no specific training with the legs and had no experience with treadmill walking.
Experimental set-up
The general experimental set-up, recording techniques and data analysis have been described in detail previously (Erni & Dietz, 2001). In short, a custom-built ‘obstacle-machine’ was placed next to the treadmill in order to study repetitive stepping over an obstacle. The obstacle consisted of a foamed stick located 11 cm over the belt. It was attached to the machine in such a way that it folded back when the subject touched it. After release, the obstacle moved with the same speed as the treadmill (Fig. 1). At the end of the treadmill the obstacle folded up and moved back into its starting position.
Figure 1. Experimental set-up.
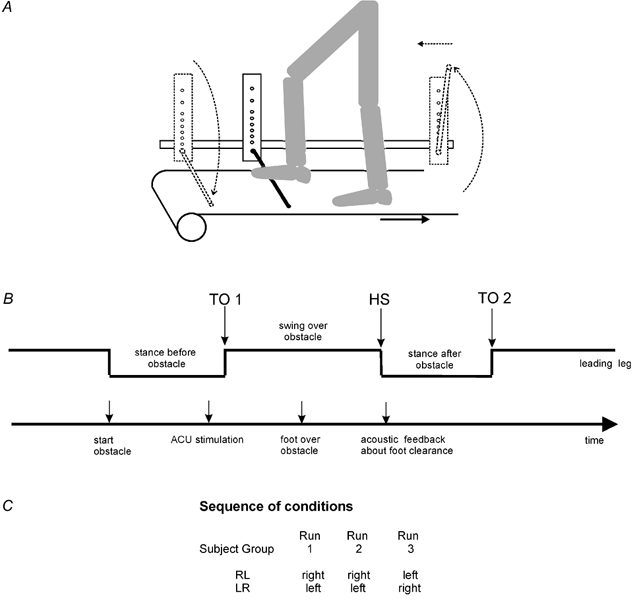
A, subject stepping over the obstacle with the right leg and the left leg, respectively, leading. B, schematic drawing of the timing of all events during one step over the obstacle. C, table of the sequence of conditions with leading right or left leg. Abbreviations, TO1, toe off before onset swing over obstacle; HS, heel strike; TO2, toe off at end stance after obstacle; ACU, acoustic stimulus.
A vertical linear array of eight light-sensitive diodes was attached to the obstacle machine next to the foamed stick (distance between the lower six diodes 2 cm and 3 cm for the upper two diodes), indicating the level of clearance of the subject's foot over the obstacle. The signal of the lowest activated diode was recorded for each step over the obstacle. The subject received an acoustic feedback about foot clearance over the obstacle according to the activated diode. The feedback signal consisted of either a double beep (707 Hz and 1400 Hz sinusoidal signal of 600 ms duration) for the lowest diode (optimal clearance) or a single beep (125, 176, 250, 354, 500, 707 and 1000 Hz rectangle signal of 400 ms duration) for the seven diodes from the second lowest to the highest diode, respectively.
The signal from force plates located underneath the two treadmill belts, which indicated heel strike (HS) of the leading leg (e.g. onset of the stance phase of the right or left leg) was used as a trigger to release the obstacle randomly. The time period between foot fall and the start of the obstacle was chosen in such a way that the leading leg always had to move over the obstacle during the following swing phase without disturbing the rhythmic stepping movements. A possible obstacle hit did not cause a perturbation, but was perceived just as a contact with the stick.
A metal bar positioned over the parallel bars on either side of the treadmill controlled the anterior-posterior position of the subject on the treadmill. Earphones prevented subjects receiving any acoustic information about the moving treadmill or the obstacle machine. Before the experiment started, the volunteers became familiarised with the approach. The subjects performed about four steps over the obstacle with full vision and both an acoustic warning signal and acoustic feedback were provided to enable subjects to become familiarised to the experimental set-up. They were instructed for all three runs to move their leading foot (without shoes) as low as possible over the obstacle, without touching it. The speed of the treadmill was 2.5 km h−1.
Special glasses prevented visual information from the lower visual field, i.e. the treadmill, the obstacle and the legs. The release of the obstacle was indicated by an acoustic signal (2 kHz sinusoidal signal of 100 ms duration), just before the end of the stance phase of the leading leg (at 33 % of the subject's step cycle). The time interval between two steps over the obstacle was randomly varied between 9 s and 16 s, corresponding to 6–11 normal steps.
Recording protocol
The experiment consisted of three successive runs. Each run contained 100 steps over the obstacle. The three runs were interrupted by a break of five minutes. During the first two runs, the subject performed repetitive steps over the obstacle with the same leg leading. In the third run, the subject's leading leg became the trailing one and vice versa. This was called the ‘mirror condition’. Before the first run, the subjects became accommodated to treadmill walking with vision and without the obstacle.
Assessment of side-specific effects
The twelve subjects were randomly divided into two equal sized groups. The first group (RL) performed the condition right (R)-left (L): During the first two runs these subjects stepped over the obstacle with the right leg leading (Fig. 1). During the third run, the left leg was the leading one. The second group (LR) started with the condition left (L)-right (R), i.e. during the first two runs the left and during the third run the right leg was leading.
Recordings of leg muscle EMG and joint movements
EMG recordings were made using surface electrodes from the rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA) and medial gastrocnemius (GM) muscles of the leading leg. Ankle (AN) and knee (KN) joint movements of the leading leg were monitored using mechanical goniometers (Biometrics Ltd, Cwmfelinfach, Gwent, UK) fixed at the lateral aspect of each joint. EMG wires and goniometers did not interfere with gait.
Data analysis
The general recording technique and the data analysis have been described in detail previously (Dietz et al. 1995; Erni & Colombo, 1998). Briefly, the EMG signals were amplified, bandpass filtered (30-300 Hz) and transferred together with the biomechanical signals to a PC microcomputer system via an analog-to-digital converter. All signals were sampled at 1000 Hz. The EMG signals were rectified. The force signal of the leading leg indicating toe off, i.e. before onset of the swing over the obstacle (TO1), was used to trigger EMG and biomechanical signals (Fig. 1). The impact of the leading heel after the obstacle (heel strike, HS) and the toe off after the following stance phase (TO2) was determined. For the evaluation of changes in leg muscle EMG activity from the first to the last step over the obstacle within a run, the signal energy (root mean square RMS) was determined for an interval between first toe off (TO1) and second toe off (TO2) (see Fig. 2). This interval was determined for each step cycle separately using the signals recorded from the force plates located underneath the treadmill belts. The difference between maximal joint flexion and extension was determined for knee and ankle joints for each step over the obstacle for the interval between toe off (TO1) and heel strike (HS).
Figure 2. Adaptational changes of EMG activity during the three successive runs.
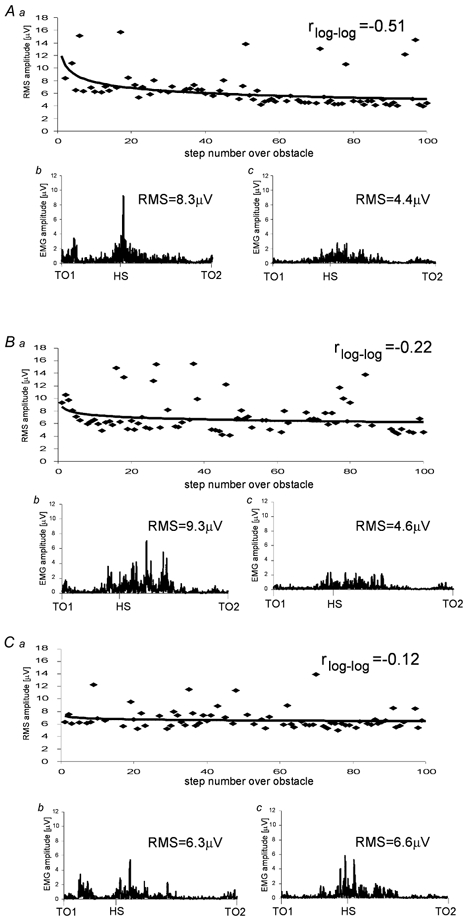
Rectus femoris (RF) EMG activity of one subject during steps over the obstacle during three successive runs: A, right leg leading; B, right leg leading; C, left leg leading. a, course of RF EMG calculated for 100 steps over the obstacle with fitted learning curve (power function). r represents Pearson's correlation coefficient for the normalised and logarithmically transformed data. b and c, rectified raw EMG of RF from toe off at the onset of swing (TO1) over the obstacle to the next toe off (TO2) during the first b and last c, steps over the obstacle. RMS values were calculated for this time interval.
Three changes in performance have been analysed. With the first one, ‘adaptation’, the change in performance has been assessed within an individual trial. The amount of adaptation was reflected in a correlation coefficient (CC). For each subject all data of each signal were normalised to its mean in every run. All measures were logarithmically transformed. For every run and every subject, Pearson's correlation coefficient r was calculated between the number of steps over the obstacle and the measures using the normalised and transformed data. Mean CCs were calculated using Fisher's Z-transformation for grouping the four muscles (all muscles). This was done to provide an overview of leg muscle EMG activity. Further, mean CC was calculated for each measure for all subjects.
With the second change in performance, called ‘training effect’, changes between trials with identical conditions have been assessed, i.e. between the first and second run. A training effect occurred if the CC was significantly smaller in the second run than in the first one.
With the third change in performance, the ‘transfer’ between two different conditions has been assessed, i.e. the change between the second run and the third run, where the leading leg became the trailing one, and vice versa. A transfer to this ‘mirror condition’ occurred if the CC of the third run was significantly smaller compared to the CC of the first run and if the CC did not differ between the third and the second runs.
The significance of the training effect of the learned movement from the first to the second run (same leg) as well as the transfer from the first and the second run to the third run (contralateral leg) was assessed using a repeated measure analysis of variance with Bonferroni correction. The covariance structure of the model used was selected using Akaike's Information Criterion and Schwarz's Bayesian Criterion.
Results
None of the subjects included in this study had any problems with performing the required locomotion task. Therefore, obstacle hits were rare (maximal 1–2 hits in one subject within one run, i.e. 100 steps over the obstacle). All first trial effects were included in the analysis, except artifacts and obstacle hits.
Adaptational changes, training effect and transfer: EMG data
Figure 2 shows a representative individual example of the adaptational effect occurring in the RF muscle activity when a subject stepped repetitively over the obstacle during three successive runs. During the first and second runs the right leg was leading and during the third run, i.e. the mirror condition, the left leg was leading. During the first run, the RF EMG decreased with a linear CC of r = −0.51 in a log-log co-ordinate system while in the second run, the RF EMG decrease was less pronounced (r = −0.22). In the mirror condition (third run) again small adaptational changes took place (r = −0.12).
When the CCs were averaged for all subjects, strong adaptational effects were seen during the first run for the muscle EMG activity with a mean CC of r = −0.37 for all muscles (Table 1). During the second run adaptation was significantly (P < 0.01; Fig. 4) less pronounced (mean r = −0.18). During the third run, the mirror condition, the adaptational changes were also low (mean r = −0.21). The difference between the CCs of run one and of run three was significant (P < 0.01; Fig. 4), while there was no significant difference (P > 0.05) between runs two and three. Both training effect and transfer had occurred.
Table 1.
Mean correlation coefficients for all subjects
| All muscles | RF | BF | TA | GM | AN | KN | Foot clearance | |
|---|---|---|---|---|---|---|---|---|
| Run 1 | −0.37 | −0.48 | −0.44 | −0.32 | −0.24 | −0.13 | −0.64 | −0.30 |
| Run 2 | −0.18 | −0.28 | −0.06 | −0.16 | −0.21 | 0.02 | −0.10 | 0.00 |
| Run 3 | −0.21 | −0.27 | −0.18 | −0.29 | −0.11 | −0.06 | −0.32 | −0.03 |
Mean correlation coefficients between the number of steps over the obstacle and root mean square of all muscles and separately for the rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA) and gastrocnemius medialis (GM) muscles, the amplitudes of ankle (AN) and knee (KN) joint trajectories and the foot clearance over the obstacle for the three runs.
Figure 4. Adaptational changes of EMG, knee joint trajectory and foot clearance.
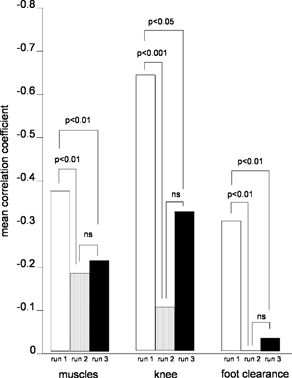
Mean correlation coefficients for the adaptational changes of leg muscles EMG activity, knee joint trajectory and foot clearance during stepping over the obstacle in the three consecutive runs. Data obtained from all subjects are summarised. Correlation coefficients for all three measures were significantly different between runs one and two and between one and three (mirror condition), while they did not differ (ns: not significant) between runs two and run three.
Adaptational changes, training effect and transfer: movement trajectories
Figure 3 shows an individual example of the adaptational effects occurring in the knee and ankle joint movement trajectories when a subject stepped repetitively over the obstacle during three successive runs. In runs one and two the right and in run three (mirror condition) the left leg was leading. The amplitude of the knee joint trajectory strongly decreased during the first run (r = −0.63). During the second run no further adaptational changes took place (r = −0.09). In the third run, however, the adaptational changes were higher (r = −0.38). The amplitude of the ankle joint movement trajectories over the obstacle showed very low adaptational changes in all of the three successive runs.
Figure 3. Knee and ankle joint trajectories during the three successive runs.
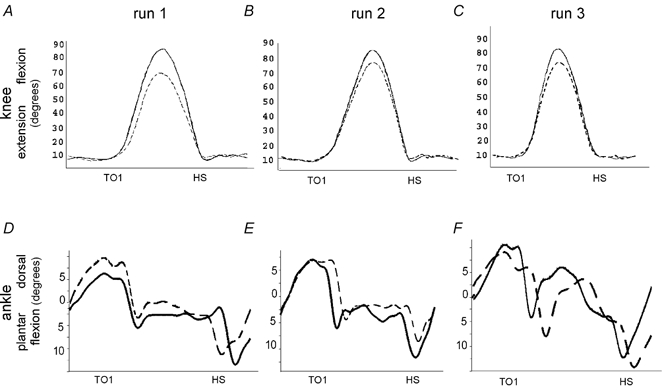
Representative individual example of changes in knee (above) and ankle (below) joint trajectories during run one (A and D, right leg was leading), two (B and E, right leg was leading) and three (C and F, left leg was leading). The solid line represents the first step over the obstacle within the run, the interrupted line the last step (step 100). TO1: toe off before onset of swing over obstacle; HS: heel strike.
When the data obtained from all subjects were averaged, strong adaptational changes were found for the amplitude of the knee joint movement over the obstacle (maximal extension to maximal flexion between toe off and heel strike) during the first run (r = −0.64). During runs two and three, the changes were less pronounced and differed significantly from run one (run two r = −0.10, P < 0.001; run three r = −0.32, P < 0.05). Runs two and three did not differ significantly from each other (P > 0.05), indicating that both training effect and transfer had occurred.
In contrast, the amplitude of the ankle joint movements, showed no clear adaptational changes during all three runs (run one r = −0.13; run two r = 0.02; run three r = −0.06). The CCs of the three runs did not differ significantly from each other (runs one and two P > 0.05; runs one and three P > 0.05, runs two and three P > 0.05) and indicated that neither training effect nor transfer had occurred.
The adaptational changes for the measure ‘foot clearance’ (Table 1) were high during the first run (r = −0.30) and low during the second (r = 0.00) and third runs (r = −0.03). The CC of run one differed significantly from the CC of run two (P < 0.01) as well as from that of run three (P < 0.01). Run two did not differ significantly from run three (P > 0.05). Both training effect and transfer had occurred for the measure ‘foot clearance’.
Table 1 and Fig. 4 summarise the mean correlation coefficients for the three consecutive runs obtained for the measures from all subjects. Three measures, leg muscle EMG activity, knee joint trajectory and foot clearance, showed significant differences between runs one and two as well as between one and three, but not between the runs two and three. These results indicate that overall, a significant training effect and transfer occurred not only to the corresponding condition (run two) but also to the mirror condition. The amount of training effect was more pronounced than the amount of transfer. However, the transfer was also significant for these measures.
Side differences between the two legs
Table 2 shows the CCs between the sequential steps over the obstacle and the movement measures listed for the two groups RL and LR. There were no significant differences between the two groups for any measure (P > 0.05).
Table 2.
Mean correlation coefficients for RL and LR groups
| All muscles | Knee | Foot clearance | |
|---|---|---|---|
| Group RL | |||
| Run 1 (right) | −0.34 | −0.64 | −0.34 |
| Run 2 (right) | −0.20 | −0.10 | −0.02 |
| Run 3 (left) | −0.19 | −0.32 | −0.01 |
| Group LR | |||
| Run 1 (left) | −0.41 | −0.66 | −0.25 |
| Run 2 (left) | −0.16 | −0.10 | −0.04 |
| Run 3 (right) | −0.24 | −0.51 | −0.06 |
Mean correlation coefficients between the number of steps over the obstacle and the root mean square of all muscles, the amplitudes of knee joint trajectory and the foot clearance over the obstacle of the two groups RL and LR are displayed. Group RL performed the first two runs with the right leg leading. In the third run, the left leg was leading. Group LR, vice versa.
Discussion
The aim of this study was to evaluate whether and how far the locomotor skill of stepping over an obstacle, reflected in biomechanical and EMG signals, could be transferred from one leg to the other, i.e. to the mirror condition. The main findings were that (1) a large part of the changes in measures could be transferred to the mirror condition and (2) there was no side preference for the transfer. These observations will be discussed with respect to the changes in other locomotor tasks, where little contralateral transfer was reported.
Adaptational effects within the first run
The adaptation that occurred by stepping repetitively over the obstacle was similar to that described earlier (Erni & Dietz, 2001), i.e. an exponential decrease of leg muscle EMG activity (RMS) and of foot clearance took place during the first run. In line with the literature (Patla et al. 1991; Erni & Colombo, 1998) this adaptation or ‘learning’ is suggested to reflect a change to a more efficient, less energy-consuming movement. The reason for using the present approach for learning was based on an earlier study on obstacle avoidance walking (Erni & Dietz, 2001), which indicated that the learning slope using an acoustic stimulus was more pronounced during the two consecutive runs than when a visual cue was provided. With normal vision the minimum time to implement motor commands for obstacle avoidance is one step cycle (Patla, 1997). One might argue that the adaptational changes could be due to fatigue. However, fatigue would imply a stronger activation of leg muscles and an associated increase of obstacle hits. Data analysis showed that this was not the case. Furthermore, no subject complained about fatigue during or at the end of the experiment. The adaptational changes found in the present study were most pronounced during the first run.
Transfer of motor performance
One major observation of this study was that the adaptational changes in the second and the third runs were less pronounced than in the first run. It was not only the ipsilateral leg that profited from the learning that occurred during the first run (training effect). Hence, even if the contralateral leg became the leading one in the mirror condition, transfer had occurred and performance had profited from the initial training. This was especially true for the measure of foot clearance. Most improvement in performance occurred during the first run. During the second and third runs, optimal foot clearance was already established from the start. The strong early adaptation in the foot clearance might be due to the instruction given to the subjects for all three conditions to move the leading foot as low as possible over the obstacle, i.e. to achieve optimal foot clearance. For the other measures, i.e. leg muscle EMG activity and knee joint trajectory, some further adaptational changes occurred during the third run (mirror condition), usually more than during the second run. Nevertheless the adaptational changes of these measures did not differ between the second and third runs which indicates that transfer had occurred. There was no difference between the CCs, i.e. in the adaptational changes, whether the transfer occurred from the left to the right leg or vice versa.
The lack of adaptational changes for the ankle joint trajectories during all three runs might be due to the great variability and the complexity of ankle joint movement. Furthermore, the analysis used to assess the changes in joint trajectories (movement amplitude) might not be appropriate to assess such changes for the ankle joint trajectory during such a complex task.
A high level of transfer of training effects between the legs is not surprising in the light of the fact that neuronal interleg coupling is substantial for human locomotion (for review see Dietz, 1992,1997). For example, a close co-ordination between the legs exists during unilateral pedalling (Ting et al. 1998). If the contralateral leg changed from pedalling to static activity, muscle co-ordination of the pedalling leg was altered even if its proprioceptive input was not changed. This observation indicates that interleg muscle co-ordination during pedalling depends on the sensorimotor state of the contralateral leg.
In line with these findings a transfer of performance to the contralateral leg was shown for specific afferent inputs (i.e. distinguishing stimuli of different forces or surfaces) to the hands or fingers, respectively (Harris et al. 2001). It was found that the learning effects for discrimination of punctuate pressure or the roughness of a surface can be transferred to the neighbouring fingers of the trained ones and to the corresponding fingers of the contralateral side. In contrast, learning of vibration discrimination could not be transferred to any other finger. Furthermore, transfer was also abolished if the contralateral hand was engaged in unrelated activities like gripping the table (Hicks et al. 1982).
Task specificity of transfer
Our observations of a contralateral transfer, i.e. to the mirror condition, are at variance with other studies on adaptational effects during locomotion. For example, during split-belt locomotion i.e. when subjects walk on a treadmill with different speeds at each leg, adaptation is achieved within 10–15 step cycles (Prokop et al. 1995). After an interruption, learning effects are maintained for the same, but not for the mirror condition. It was concluded that side-specific proprioceptive information about the dynamics of the movement is necessary for the spinal pattern generator for both legs to adapt. Furthermore, one-legged hopping on a treadmill produces an after-effect in the same leg, but not in the other leg (Anstis, 1995). It was concluded that the after-effect is based on a peripheral neural site.
The discrepancy with the observations made here might be explained by the fact that in the locomotor tasks of both studies (Anstis, 1995; Prokop et al. 1995), the leg extensor muscles are predominantly involved in the motor performance and also the main adaptational changes occur in the extensors. In contrast, in the present experiments, the leg flexors play a predominant role in the performance of the task, i.e. stepping over the obstacle. Consequently, most adaptational changes occurred in the flexor muscles.
There exists increasing evidence that leg flexor and extensor muscles are differentially controlled in animals and man (Cheng et al. 1998; for review see Dietz, 1992). For example, leg flexors have a high responsiveness to visual stimuli, but leg extensors are responsive to somatosensory input both in the cat (Beloozerova & Sirota, 1988) and man (Dietz, 1992). In addition, cortico-spinal projections to lower leg motoneurons were shown to be stronger in the tibialis anterior than in the soleus muscles (Brouwer & Ashby, 1992; Schubert et al. 1997). Besides these differences, observations made in the interlimb co-ordination of infant stepping (Pang & Yang, 2001) agree with recent models of locomotor control (Hiebert et al. 1996). These observations indicate that the flexor half centres of homologous limbs reciprocally inhibit each other if assessed during walking, whereas the extensor half centres are not directly coupled with each other. Consequently, one can assume that proprioceptive afferent information continuously modulates the activity of extensors with their antigravity function during gait, whereas the flexor activation is more controlled by central inputs (for review see Dietz, 1992).
The different amount of transfer might be due to different levels of locomotor control involved in the various tasks. In the rather simple task of split-belt locomotion, the adaptation to different speeds at the two legs has been suggested to take place on a spinal level (Jensen et al. 1998). In contrast, it might be hypothesised that the learning effects observed in the present experiments occur at a higher level of locomotor control. Such a higher level of neuronal integration would represent the brainstem. It was suggested that the brainstem centres contribute to the bilateral leg muscle activation during stepping in both cats (Ito, 1984) and humans (Bonnet et al. 1976; Dietz & Berger 1984). Alternatively, we cannot exclude the possibility that the trailing leg learned about the obstacle height before the transfer trial. However, the movement trajectory of the trailing leg, simply following the leading leg, differed basically from that of the leading leg.
The observation that during stepping over an obstacle a considerable transfer of skill to the mirror condition occurs might have consequences for training strategies in sports and rehabilitation.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation (no: 31-64792.01 and 31-62025.00).
References
- Anstis S. After effects from jogging. Experimental Brain Research. 1995;103:476–478. doi: 10.1007/BF00241507. [DOI] [PubMed] [Google Scholar]
- Beloozerova JN, Sirota MB. Role of motor cortex in control of locomotion. In: Gurfinkel VX, Joffe ME, Massion J, Roll JP, editors. Facts and Concepts. New York: Plenum Press; 1988. pp. 163–176. [Google Scholar]
- Bonnet M, Gurfinkel VS, Lipshits M, Popovic KG. Central programming of lower limb muscular activity in the standing man. Agressologie. 1976;17:35–42. [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to lower leg motoneurons in man. Experimental Brain Research. 1992;89:649–654. doi: 10.1007/BF00229889. [DOI] [PubMed] [Google Scholar]
- Cheng JG, Stein RB, Jovanovic K, Yoshida K, Bennet DJ, Han YC. Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus macultus) spinal cord. Journal of Neuroscience. 1998;18:4295–4304. doi: 10.1523/JNEUROSCI.18-11-04295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou L, Kaufmann KR, Brey RH, Draganich LF. Motion of the whole body's center of mass when stepping over obstacles of different heights. Gait and Posture. 2001;13:17–26. doi: 10.1016/s0966-6362(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiological Reviews. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Dietz V. Neurophysiology of gait disorders: present and future applications. Electroencephalography and Clinical Neurophysiology. 1997;103:333–355. doi: 10.1016/s0013-4694(97)00047-7. [DOI] [PubMed] [Google Scholar]
- DietZ V, Berger W. Interlimb dination of posture in patients with spastic paresis. Impaired function of spinal reflexes. Brain. 1984;107:965–978. doi: 10.1093/brain/107.3.965. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Annals of Neurology. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- Erni T, Colombo G. Locomotor training in paraplegic patients: a new approach to assess changes in leg muscle EMG patterns. Electroencephalography and Clinical Neurophysiology. 1998;109:135–139. doi: 10.1016/s0924-980x(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Erni T, DietZ V. Obstacle avoidance during human walking: learning rate and cross-modal transfer. Journal of Physiology. 2001;534:303–312. doi: 10.1111/j.1469-7793.2001.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RE, Frank JM, Kindsbourne M. The locus of bimanual skill transfer. Journal of General Psychology. 1982;107:277–281. doi: 10.1080/00221309.1982.9709935. [DOI] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME. The topography of tactile learning in humans. Journal of Neuroscience. 2001;21:1056–1061. doi: 10.1523/JNEUROSCI.21-03-01056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RE, Gualtieri CT, Schroeder SR. Cognitive and motor components of bilateral transfer. American Journal of Psychology. 1983;96:223–228. [Google Scholar]
- Hiebert GA, Whelan PJ, Prochazka A, Pearson KG. Contribution of hindlimb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of Neurophysiology. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- Jensen L, Prokop T, DietZ V. Adaptational effects during human split-belt walking: Influence of afferent input. Experimental Brain Research. 1998;118:126–130. doi: 10.1007/s002210050262. [DOI] [PubMed] [Google Scholar]
- Pang MY, Yang JG. Interlimb co-ordination in human infant stepping. Journal of Physiology. 2001;533:617–625. doi: 10.1111/j.1469-7793.2001.0617a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patla AE. Understanding the roles of vision in the control of human locomotion. Gait and Posture. 1997;5:54–69. [Google Scholar]
- Patla AE, Prentice SD. The role of active forces and intersegmental dynamics in the control of leg trajectory over obstacles during locomotion in humans. Experimental Brain Research. 1995;106:499–504. doi: 10.1007/BF00231074. [DOI] [PubMed] [Google Scholar]
- Patla AE, Prentice SD, Robinson C, Neufeld J. Visual control of locomotion: strategies of changing direction and for going over obstacles. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:603–634. doi: 10.1037//0096-1523.17.3.603. [DOI] [PubMed] [Google Scholar]
- Prokop T, Berger W, Zijlstra W, DietZ V. Adaptational and learning processes during human split-belt locomotion: interaction between central mechanisms and afferent input. Experimental Brain Research. 1995;106:449–456. doi: 10.1007/BF00231067. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen L, DietZ V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Experimental Brain Research. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- Ting LH, Taasch CC, Brown DA, KautZ SA, Zajac FE. Sensorimotor state of the contralateral leg affects ipsilateral muscle coordination of pedaling. Journal of Neurophysiology. 1998;80:1341–1351. doi: 10.1152/jn.1998.80.3.1341. [DOI] [PubMed] [Google Scholar]


