Abstract
Findings that increased neuromuscular activity significantly reduced sprouting in partially denervated muscles prompted this present study to determine if the converse is true, namely that reduced activity promotes sprouting and motor unit (MU) enlargement. Partial denervation of rat hindlimb muscles by either the L4 or L5 spinal root avulsion resulted in extensive denervation (> 80%) in tibialis anterior (TA) and medial gastrocnemius (MG) muscles, and moderate denervation (∼50%) in soleus (SOL) and plantaris (PL) muscles. The partially denervated muscles were then subjected to a 4 week programme of normal caged activity or TTX-induced neuromuscular inactivity. At 1 month, measurement of MU enlargement and quantification of sprouting were evaluated, respectively, by electrophysiological and histochemical means. Analysis of electrophysiological data showed that MU forces were significantly increased in both extensively and moderately denervated muscles 1 month after partial denervation and normal cage activity and that neuromuscular activity blockade by TTX completely abolished the MU enlargement in these partially denervated muscles. Histochemical analysis of sprouting revealed that the number of sprouts was significantly increased after partial denervation and normal cage activity, particularly after extensive denervation. TTX-induced neuromuscular inactivity dramatically reduced the number of sprouts and increased the number of free endplates in the extensively but not the moderately denervated muscles. These data demonstrate that a reduction in neuromuscular activity mediated by presynaptic blockade of neural action potentials reduces MU enlargement in partially denervated muscles by reducing axonal sprouting.
Denervation syndromes such as poliomyelitis, post-polio syndrome and amyotrophic lateral sclerosis are some of the neuromuscular diseases in which many motoneurones die. Compensatory axonal sprouting increases the number of muscle fibres per motoneurone, thereby enlarging the size of motor units (MUs) to compensate for varying degrees of partial denervation (Brown et al. 1981; Halstead & Wiechers, 1987). A three- to five-fold limit in the sprouting capacity of motoneurones accounts for progressive muscle weakness when partial denervation removes more than 80 % of the innervation of the muscles (Thompson & Jansen, 1977; Brown et al. 1978; Luff et al. 1988; Yang et al. 1990; Rafuse et al. 1992; Rafuse & Gordon, 1996a, b). Even when enlargement of surviving MUs sustains muscle function in denervating syndromes, the muscle weakness experienced by polio patients decades after the acute viral infection, may be associated with an age-related loss of motoneurones (Halstead & Grimby, 1995).
Attempts to optimize muscle function in denervation syndromes has led to investigations of whether neural activation promotes axonal sprouting in animal models of partial denervation (Brown & Holland, 1979; Gardiner et al. 1984; Gardiner & Faltus, 1986; Ribchester, 1988; Michel & Gardiner, 1989; Einsiedel & Luff, 1994; Seburn & Gardiner, 1996). The relatively small effects of neural activity on sprouting that were reported were confirmed by recent findings of relatively little effect of increased neuromuscular activity on sprouting after moderate partial denervation (Tam et al. 2001). However, when the partial denervation of muscles was extensive, increased neuromuscular activity by either exercise or electrical stimulation of the motor nerve significantly reduced sprouting within 1 month of partial denervation (Tam et al. 2001), and compromised the stability of chronically enlarged MUs (Tam et al. 2002). The corollary of these negative effects of neuromuscular activity on sprouting is that reduced neuromuscular activity may be expected to have the opposite effect of increasing sprouting and MU enlargement after partial denervation. Experimental data that short-term muscle paralysis induced either by tetrodotoxin (TTX) or α-bungarotoxin (α-BTX) evoked sprouting in normally innervated muscles (Brown & Ironton, 1977; Holland & Brown, 1980) support this corollary. However, findings that postsynaptic blockade by α-BTX significantly reduced MU enlargement in 2–10 month partially denervated soleus (SOL) muscles, does not (Connold & Vrbová, 1991). Moreover, α-BTX blockade of muscle activity in partially denervated neonatal muscles reduced the size of the enlarged MUs (Connold & Vrbová, 1990). In the light of these conflicting results of the effects of reduced neuromuscular activity and the evidence that high daily neuromuscular activity reduces MU enlargement and sprouting in partially denervated muscles (Tam et al. 2001), the question remains as to whether reduced neuromuscular activity would or would not promote sprouting and MU enlargement in partially denervated adult muscles.
To address this issue, we have undertaken the present study to investigate the effects of neuromuscular activity blockade by TTX on MU enlargement in adult rats during the acute phase of sprouting. We (1) chose to examine the effect of TTX on sprouting on four functionally different muscles: tibialis anterior (TA), medial gastrocnemius (MG), plantaris (PL) and SOL muscles and (2) used complementary force measurement and histochemical methods to respectively quantify MU enlargement and sprouting. We demonstrated that neuromuscular activity blockade by TTX significantly reduced MU enlargement and sprouting within 1 month of extensive partial denervation.
Methods
All surgical and experimental procedures were in compliance with the guidelines issued by the Canadian Council of Animal Care.
Surgery
A total number of 33 female Sprague-Dawley (body weight 180–200 g) rats were used in these studies. Either the L4 or L5 spinal root was avulsed under surgical anaesthesia (sodium pentobarbital administered intraperitoneally as 0.07 ml (g body weight)−1) and using sterile procedure.
Avulsion of the L4 or L5 spinal root (Tam et al. 2001) resulted in extensive denervation of TA and MG muscles, respectively, and a range of partial denervation in the SOL and PL muscles. The partially denervated muscles were then subjected to a four week programme of (1) normal caged activity (n = 20) or (2) neuromuscular activity blockade by TTX (80 μg ml−1) administration via an implanted ALZET osmotic minipump to the sciatic nerve at the rate of 2.5 μl h−1 (n = 13). The unoperated muscles of the left hindlimb of the rats used in the experiment served as contralateral controls. All hindlimb muscles innervated by the sciatic nerve were inactive during the entire month of delivery of the TTX via the osmotic minipump. The controlled delivery was effective in localizing the blockade of the sciatic nerve action potentials since we did not detect any evidence of muscle weakening in the contralateral limbs. We also did not detect any obvious systemic effects, which are readily seen in cats where we detected changes in the stability of the animals despite the localized delivery of TTX via osmotic minipumps (T. Gordon & J. Munson unpublished observations).
MU and muscle force recordings
At 1 month after partial denervation, muscle twitch and tetanic forces were recorded in vivo in the final experiment. Rats were anaesthetized using sodium pentobarbital and hydrated by hourly intravenous injection of saline solution via an external jugular cannula. The trachea was cannulated for mechanical ventilation. TA, MG, SOL and PL muscles were isolated bilaterally by denervating all other hip, tail, and hindlimb muscles (Tam et al. 2001).
Bipolar silver wires were placed on either side of the sciatic nerve for stimulation. Braided silk threads, of size 2 gauge, were tied to the distal muscle tendons for attachment to a force transducer and the skin around the incision was loosely closed. A laminectomy of L3 to L6 spinal processes was performed to isolate the L4 and L5 spinal ventral roots bilaterally. Isometric muscle twitch and tetanic forces were measured in response to suprathreshold (2 × threshold) stimulation of the sciatic nerve and each isolated ventral root (L4 or L5). The ratio of muscle tetanic force elicited by the stimulation of each spinal root to that elicited by stimulation of the sciatic nerve determined on the contralateral side was used to represent the extent of partial denervation of the muscles on the experimental side (Tam et al. 2001).
Isometric MU twitch forces were recorded in response to suprathreshold (2 × threshold) stimulation of teased filaments of L4 and L5 ventral roots. A single MU was isolated by the all-or-none recruitment of a twitch contraction by increasing the stimulation voltage applied to the rootlet. All MUs in each partially denervated muscle, and at least 40 % of MUs in contralateral control muscles were sampled to obtain a representative mean MU twitch force in each case. The total number of MUs in each muscle was estimated by dividing the whole muscle twitch force by the mean MU twitch force. Procedures of muscle and MU force recordings have been previously described in detail (Tam et al. 2001).
Histochemistry
After muscle and MU force recordings, 12 μm thick cryostat cross-sections of fresh TA and MG muscles were stained for acid or alkaline-myosin ATPase (Tötösy de Zepetnek et al. 1992b) and cross-sectional area (CSA) was measured from these sections.
Plantaris and SOL muscles were fixed in 4 % formalin and incubated in gum sucrose solution overnight. They were then cut into 100 μm thick cryostat longitudinal sections on which combined silver-acetylcholinesterase (Ag-AChE) histochemical staining was carried out to visualize the motor axons, sprouts and endplates (Tam et al. 2001). The number of sprouts and free endplates was counted from the sections. After sufficient sprout counts had been obtained from representative numbers of PL and SOL muscles, the remaining PL and SOL muscles were sectioned into 12 μm cross sections and were stained using 5 % toluidine blue solution. Muscle fibre CSA was then measured from these sections.
Normalization of MU forces for muscle fibre CSAs
In the light of the described effect of TTX-induced atrophy of muscle fibres (Spector, 1985; Michel & Gardiner, 1990), we corrected the measured MU forces for the reduced muscle fibre size. Motor unit force is the product of innervation ratio (IR, the number of muscle fibres innervated by one motoneurone), muscle fibre CSA and specific force (force per unit area of muscle fibre). Several studies have shown that MU force varies systematically with IR and CSA in both normal and reinnervated muscles (Kanda & Hashizume, 1992; Tötösy de Zepetnek et al. 1992a) and specific force does not change after reinnervation (Tötösy de Zepetnek et al. 1992a; Fu & Gordon, 1995a, b). Hence, the MU force corrected for muscle fibre CSA, reasonably reflects IR.
Measurements of CSA were made 1 month after TTX administration in randomly sampled innervated muscle fibres (at least 40 % of the total number of innervated muscle fibres) in both the partially denervated and contralateral TA, MG, PL and SOL muscles. The measurements were made using a microcomputer digitizing software program (JAVA, Jandel Scientific). The denervated muscle fibres were obviously atrophic as compared with the innervated muscle fibres despite the concurrent disuse atrophy of the TTX-treated muscle fibres.
Motor unit twitch force of each single MU in each experimental muscle was corrected for changes in CSA by multiplying the recorded MU twitch force by the ratio of the mean muscle fibre CSA of all contralateral control muscles to that of all experimental muscles which belonged to the same experimental condition. The forces corrected by CSA are referred to as ‘corrected motor-unit twitch forces’ throughout the paper.
Sprout quantification
A total of 500 endplates from sections stained for combined Ag-AChE histochemical staining were examined under light microscopy at a total magnification of × 160 or × 400 and classified as one of the following: (1) endplates having no visible axonal attachment (free endplates), (2) axonal outgrowth coming out from the last node of Ranvier to an endplate (intranodal sprouts), (3) axonal outgrowth originating from the myelin-free region of an axon at the entry point to the motor endplates to an endplate (preterminal sprouts) or (4) axonal outgrowth from the myelin-free axons within the motor endplate region to an endplate (ultraterminal sprouts; Tam et al. 2001). We counted the number of endplates reinnervated by each type of sprout and expressed the number as a percentage of the total number of innervated endplates sampled. The number of free endplates was counted and expressed as a percentage of the total number of endplates sampled.
Statistics
Throughout this paper, mean values with standard errors are given. The statistical significance of differences in mean MU numbers and numbers of different sprouts and free endplates between control, partially denervated muscles with and without TTX blockade was determined using one-way analysis of variance (ANOVA) to determine any differences among the mean scores between, and within, experimental groups and subsequently using the Tukey's HSD (Honestly Significant Difference) test to determine the locus of the significance. ANOVA was also used to determine differences between the mean values of the motor unit twitch forces. The Kolmogorov-Smirnov test (Daniel, 1995) was applied to examine the statistical significance of differences in cumulative distribution of MU twitch forces between control and partially denervated muscles with, and without, TTX blockade. For all the above statistical analyses, P < 0.05 was regarded as significant.
Results
No significant differences were found in numbers of sprouts and free endplates, and MU numbers and twitch forces between the contralateral control muscles. Thus, the results from the contralateral control muscles for different experimental groups were grouped and used as overall controls. All MU forces have been corrected for changes in muscle fibre CSA after partial denervation and after both partial denervation and TTX blockade (Fig. 2), as described in Methods.
Figure 2.
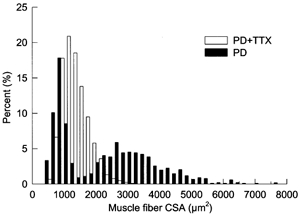
Percentage frequency histogram plotted on a linear scale of innervated muscle fiber CSAs in partially denervated TA muscle of rats experiencing normal caged activity (▪) as compared with partially denervated muscles with TTX treatment (□)
Effect of TTX on MU enlargement in partially denervated muscles
Sectioning of L4 or L5 root denervated > 80 % of MUs in TA and MG muscles, respectively; a mean (± s.e.m.) of 7 ± 2 MUs remained in partially denervated TA and MG muscles as compared with 133 ± 14 and 88 ± 8 MUs in contralateral control TA and MG muscles, respectively (Table 1). As the twitch forces of the few remaining MUs were probably randomly distributed over a 10-fold range of forces as they were in the normally innervated muscles (Tam et al. 2001, 2002), the data of MUs in the partially denervated muscles from different rats were collated and compared with the collated data from the contralateral control muscles as shown in Fig. 1.
Table 1.
Mean (± s.e.m.) MU numbers in contralateral control TA, MG, PL and SOL muscles of rats experiencing normal caged activity and in partially denervated TA, MG, PL and SOL muscles of rats experiencing normal caged activity or with tetrodotoxin (TTX) treatment
| Muscle types | Contralateral control | PD (cage-activity) | PD (with TTX treatment) |
|---|---|---|---|
| TA | 133 ± 14 | 7 ± 2* | 8 ± 1* |
| MG | 88 ± 8 | 7 ± 2* | 9 ± 1* |
| PL | 48 ± 5 | 16 ± 2* | 22 ± 2* |
| SOL | 26 ± 2 | 12 ± 2* | 12 ± 2* |
For each muscle type
denotes MU numbers in partially denervated muscles with either cage activity or TTX treatment which are significantly smaller than those in contralateral control muscles (P < 0.05). There is no significant difference in MU numbers between partially denervated muscles with either cage activity or TTX treatment.
Figure 1. Frequency histograms of twitch force in partially denervated TA and MG muscles.
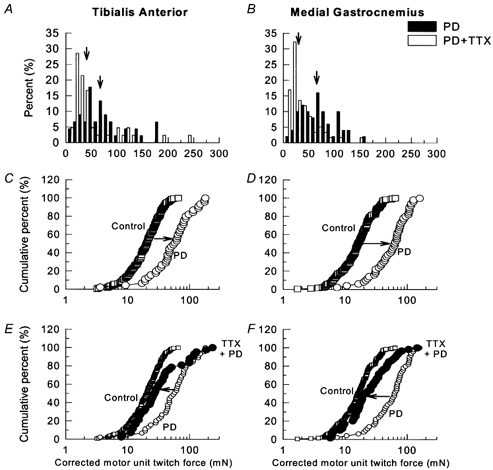
A and B, percentage frequency histograms plotted on a linear scale and C-F, cumulative frequency histograms, plotted on logarithmic scale, of MU twitch force distributions in partially denervated tibialis anterior (TA; A, C and E) and medial gastrocnemius (MG; B, D and F) muscles of rats experiencing normal caged activity (○) as compared with control muscles (▪), and partially denervated muscles with tetrodotoxin (TTX) treatment (•). With TTX treatment, MU twitch forces after normalization for muscle fiber CSA were significantly reduced as shown by the leftward shift of the MU force distributions (P < 0.05). In A and B, the vertical arrows denote the mean values of the motor unit forces of partially denervated muscles (▪) and partially denervated muscles with TTX treatment (□).
As shown in the percentage histograms in Fig. 1A and B, removal of > 80 % of motor innervation to TA and MG muscles shifted the arithmetic distributions of MU forces to the right to significantly larger values. When the data were replotted as cumulative histograms (Fig. 1C and D), it became clear that all MUs were enlarged by the same factor as previously shown in cat muscles (Rafuse et al. 1992) and in rat muscles (Tam et al. 2001). However, after 4 weeks of daily TTX administration to the sciatic nerve via an osmotic minipump, the MU forces in the extensively partially denervated muscles were, on average, the same as the normally innervated control muscles and not increased. In fact, the TTX blockade of neuromuscular activity appeared to completely prevent MU enlargement with the exception of only the very large MUs (Fig. 1E and F). These very large MUs which already have large IRs, normally experience relatively low levels of daily activity because they are only recruited at very high force levels. Because we measured CSAs of the innervated muscles fibres only (Fig. 2), the corrected MU force should reasonably reflect IR.
Even in the moderately denervated (≈50 % partial denervation) PL and SOL muscles, where mean MU twitch forces increased significantly by 2-fold above normal forces, neuromuscular blockade by TTX prevented this increase (Fig. 3).
Figure 3. Frequency histograms of twitch forces in partially denervated plantaris and soleus muscles.
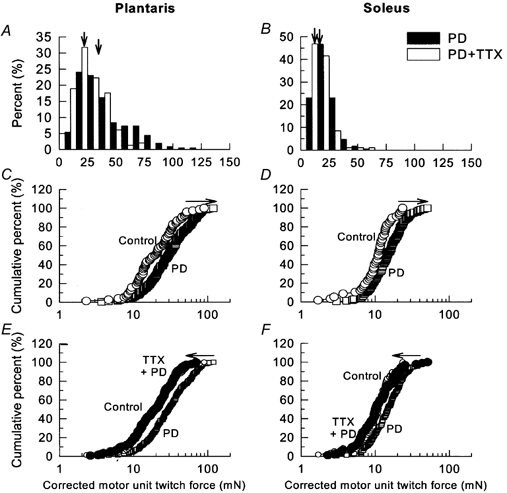
A and B, percentage frequency histograms plotted on a linear scale and C-F, cumulative frequency histograms, plotted on logarithmic scale, of MU twitch force distributions in partially denervated plantaris (PL; A, C and E) and soleus (SOL; B, D and F) muscles of rats experiencing normal caged activity (○) as compared with control muscles (▪), and partially denervated muscles with TTX treatment (•). The detrimental effect of TTX treatment was seen as a leftward shift of the MU force (P < 0.05). In A and B, the vertical arrows denote the mean values of the motor unit forces of partially denervated muscles (▪) and partially denervated muscles with TTX treatment (□).
Thus TTX blockade of neuromuscular activity for 1 month after partial denervation appeared to prevent the normal MU enlargement in both the extensively denervated TA and MG muscles and the moderately denervated PL and SOL muscles. Since MU forces were corrected by the changes in muscle CSAs (a decline due to muscle atrophy), MU forces reflect IRs directly. Hence the effect of TTX in preventing the normal increase in MU force after partial denervation reflects the inability of the inactive MUs to sprout and reinnervate denervated muscle fibres, with the exception of larger and less active MUs.
It must be noted that the number of remaining MUs was the same in the denervated muscles, which were inactivated by TTX blockade, and those partially denervated muscles, which experienced normal caged activity (Table 1). Hence, the effect of TTX blockade in preventing MU enlargement in partially denervated muscles cannot be explained by differences in the extent of partial denervation between TTX-induced inactive partially denervated muscles and the partially denervated muscles which experienced normal caged activity.
Effect of TTX and quantification of sprouting in partially denervated muscles
Since (1) there was no significant differences in the relative proportion of different sprout types between PL and SOL muscles and (2) there was a similar range in the extent of partial denervation in the PL and SOL muscles in each experimental group, we grouped and considered the results from PL and SOL muscles together for each experimental group. We analysed sprouting in PL and SOL muscles that were partially denervated (PD) by either less than 75 % (PD < 75 %: moderate denervation) or more than 75 % (PD > 75 %: extensive denervation) in the light of our unpublished observations that maximum sprouting occurs when the extent of partial denervation exceeds 75 %.
The number of sprouts expressed as a percentage of innervated endplates was significantly increased after partial denervation and normal caged activity, particularly after extensive partial denervation (PD > 75 %; Fig. 4). TTX did not appear to affect the number of sprouts in the moderately denervated muscles (PD < 75 %); but in the extensively denervated muscles (PD > 75 %) the number of sprouts was dramatically reduced to almost normal levels by TTX blockade. This is in agreement with the shift of the cumulative histogram of MU twitch forces back to normal values, as shown for the partially denervated TA and MG muscles (Fig. 1).
Figure 4. Numbers of sprouts and free endplates in partially denervated and control plantaris and soleus muscles.
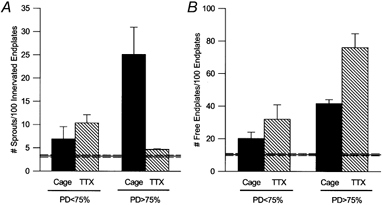
A, mean (± s.e.m.) number of sprouts per 100 innervated endplates, and B, number of free endplates per 100 endplates of contralateral controls (horizontal line; n = 38), moderately denervated (PD < 75 %), and extensively denervated (PD >75 %) PL and SOL muscles after normal caged activity (▪; n = 7 for PD < 75 % and n = 4 for PD>75 %) and TTX treatment ( ; n = 7 for PD < 75 % and n = 5 for PD>75 %). The dotted horizontal lines denote the s.e.m. values for the contralateral control data. TTX treatment did not have a significant effect on sprouting for PD < 75 %, but dramatically reduced the number of sprouts and hence increased the number of free endplates for PD >75 %.
; n = 7 for PD < 75 % and n = 5 for PD>75 %). The dotted horizontal lines denote the s.e.m. values for the contralateral control data. TTX treatment did not have a significant effect on sprouting for PD < 75 %, but dramatically reduced the number of sprouts and hence increased the number of free endplates for PD >75 %.
Despite the significant change in the number of axonal sprouts in the moderately denervated muscles, there were obviously more free endplates in the extensively denervated muscles (Fig. 4). Following TTX blockade, the percentage of free endplates increased to almost 80 %, only ≈10 % being accounted for by a cutting artifact that was evident in the contralateral muscles (Fig. 4). The dramatic effect of TTX was clearly seen in longitudinal sections of the muscles where the majority of endplates remained denervated, the atrophic endplates being free of axonal terminals (Fig. 5).
Figure 5. Higher power combined Ag-AChE histochemical photomicrographs of 100 μm thick cryostat longitudinal sections of partially denervated and control muscles.
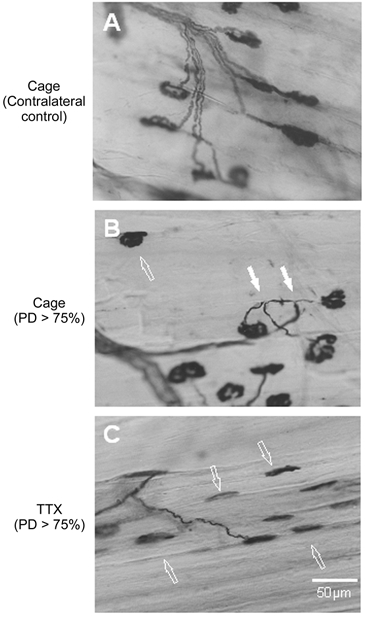
A, contralateral control and B and C, extensively denervated PL muscles (>75 % PD) after normal caged activity (B), and TTX treatment (C). TTX blockade of neuromuscular activity dramatically reduced the incidence of sprouts and further increased the amount of free endplates. Both motor endplates and muscle fibres became atrophic. Filled arrows indicate sprouts and open arrows indicate free endplates.
Discussion
Using both electrophysiological and histochemical techniques, we demonstrate that TTX blockade of neuromuscular activity during the acute phase of sprouting significantly reduces sprouting and almost completely prevents MU enlargement in extensively denervated muscles. Relatively smaller effects of TTX blockade on moderately denervated muscles were detected as reduced MU size. These findings do not support the hypothesis put forward in the Introduction that the negative effects of high daily neuromuscular activity on MU enlargement by sprouting would be reversed by reducing neuromuscular activity. Rather they support the findings of Vrbová and her colleagues that α-BTX-induced postsynaptic blockade of neuromuscular activity reduced MU enlargement in partially denervated adult muscles as well as maintaining the size of enlarged MUs in partially denervated neonatal muscles (Connold & Vrbová, 1990, 1991). However, the mechanisms by which TTX and α-BTX reduce sprouting are likely to be different, in line with their pre- and postsynaptic actions, respectively.
Connold & Vrbová (1991) suggested that α-BTX reduced sprouting and MU enlargement by compromising the ability of axonal sprouts to maintain their newly formed synaptic contacts with muscle fibres. While it is unlikely that neuromuscular activity blockade by either TTX or α-BTX would interfere with the release of sprout-inducing ciliary neurotrophic and insulin-like growth factors from the Schwann cells at denervated endplates (Caroni & Grandes, 1990; Gurney et al. 1992; Kwon & Gurney, 1994; Siegel & English, 1997; Thompson & Kopp, 1996), or the formation of the extended glial processes in the perisynaptic Schwann cells (Son & Thompson, 1995a, b), TTX and not α-BTX, would reduce calcium influx into the nerve terminals by blocking nerve conduction of action potentials. Since adequate intracellular calcium concentrations are critical for neurite outgrowth (Cohan et al. 1987; Mattson & Kater, 1987; Mattson et al. 1988; Kater et al. 1988, 1989; Connor et al. 1990; Collins et al. 1991; Kater & Mills, 1991; Rehder & Kater, 1992), the TTX blockade of nerve terminal electrical activity may be sufficient to prevent or at least, reduce the outgrowth of sprouts from electrically silenced MUs, despite the availability of the sprout-inducing factors and extended glial processes of the perisynaptic Schwann cells. Hence it is likely that TTX prevents, or at least substantially reduces, outgrowth of sprouts rather than reduces the stability of contact between newly formed sprouts and denervated muscle fibres. The latter explanation that was proposed to explain the negative effects of α-BTX on sprouting (Connold & Vrbová, 1990, 1991) remains tenable because it is unlikely that the postsynaptic α-BTX blockade prevents outgrowth of sprouts in partially denervated muscles. This is in contrast to presynaptic blockade with TTX, because α-BTX actually induces the outgrowth of sprouts even in normally innervated muscles (Brown & Ironton, 1977; Holland & Brown, 1980).
Our findings that TTX blockade was less effective in reducing sprouting in moderately, as compared with extensively, denervated muscles could be explained by the short distances over which growth factors from denervated muscles attract axonal sprouts, the distances being 200 μm or less (Brown et al. 1978, 1980; Slack & Pockett, 1981; Pockett & Slack, 1982). The few sprouts that may emerge from the TTX-blocked nerve terminals could respond to the sprout-inducing factors relatively more in moderately denervated muscles where the sprouts negotiate relatively small distances between denervated and innervated endplates. In extensively denervated muscles, the axonal sprouts that emerge from the few intact endplates would access the growth factors within a 200 μm radius but would fail to access the factors at the more distant denervated muscle fibres. Hence the long distances required for axonal sprouts to reinnervate many denervated fibres in the extensively denervated muscles would negate the effectiveness of the short-range diffusible sprout-inducing factors in supporting the few sprouts that do emerge from the inactive nerve terminals.
Irrespective of the mechanism by which TTX or α-BTX blockade of neuromuscular activity reduces sprouting and MU enlargement in partially denervated muscles, our findings that both high levels of neuromuscular activity induced by intensive running exercise or functional electrical stimulation (Tam et al. 2001, 2002) and very low levels of neuromuscular activity (present study and Connold & Vrbová, 1990) interfere with sprouting mechanisms that allow MU enlargement after partial nerve injuries, advocate caution in designing rehabilitation strategies for partial nerve injuries.
Acknowledgments
This work was supported by the Muscular Dystrophy Association of Canada. We thank the Alberta Heritage Foundation of Medical Research for supporting Dr Tessa Gordon as a research scientist, and Rick Hansen, Man in Motion Legacy Fund and Alberta Heritage Foundation Medical Research for supporting Ms Siu Lin Tam as a research fellow. This work partially fulfilled the requirements for Ms Siu Lin Tam's MSc thesis.
References
- Brown MC, Holland RL. A central role for denervated tissues in causing nerve sprouting. Nature. 1979;282:724–726. doi: 10.1038/282724a0. [DOI] [PubMed] [Google Scholar]
- Brown MC, Holland RL, Hopkins WG, Keynes RJ. An assessment of the spread of the signal for terminal sprouting within and between muscles. Brain Research. 1980;210:145–151. doi: 10.1016/0006-8993(81)90891-x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Holland RL, Hopkins WG. Motor nerve sprouting. Annual Review of Neuroscience. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Brown MC, Holland RL, Ironton R. Is the stimulus for motoneurone terminal sprouting localized? Journal of Physiology. 1978;282:7–8P. [PubMed] [Google Scholar]
- Brown MC, Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potential. Nature. 1977;265:459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. Journal of Cell Biology. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan CS, Connor JA, Kater SB. Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. Journal of Neuroscience. 1987;7:3588–3699. doi: 10.1523/JNEUROSCI.07-11-03588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F, Schmidt MF, Guthrie PB, Kater SB. Sustained increase in intracellular calcium promotes neuronal survival. Journal of Neuroscience. 1991;11:2582–2587. doi: 10.1523/JNEUROSCI.11-08-02582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connold AL, Vrbová G. The effect of muscle activity on motor unit size in partially denervated rat soleus muscles. Neuroscience. 1990;34:525–532. doi: 10.1016/0306-4522(90)90161-v. [DOI] [PubMed] [Google Scholar]
- Connold AL, Vrbová G. Temporary loss of activity prevents the increase of motor unit size in partially denervated rat soleus muscles. Journal of Physiology. 1991;434:107–119. doi: 10.1113/jphysiol.1991.sp018461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Kater SB, Cohan C, Fink L. Ca2+ dynamics in neuronal growth cones: regulation and changing patterns of Ca2+ entry. Cell Calcium. 1990;11:233–239. doi: 10.1016/0143-4160(90)90074-5. [DOI] [PubMed] [Google Scholar]
- Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. New York: John Wiley & Sons Inc.; 1995. [Google Scholar]
- Einsiedel LJ, Luff AR. Activity and motor unit size in partially denervated rat medial gastrocnemius. Journal of Applied Physiology. 1994;76:2663–2671. doi: 10.1152/jappl.1994.76.6.2663. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. Journal of Neuroscience. 1995a;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. The Journal of Neuroscience. 1995b;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner PF, Faltus RE. Contractile responses of rat plantaris muscles following partial denervation, and the influence of daily exercise. Pflügers Archiv. 1986;406:51–56. doi: 10.1007/BF00582952. [DOI] [PubMed] [Google Scholar]
- Gardiner PF, Michel RN, Iadeluca G. Previous exercise training influences functional sprouting of rat hindlimb motoneurons in response to partially denervation. Neuroscience Letters. 1984;45:123–1267. doi: 10.1016/0304-3940(84)90086-7. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Yamamoto H, Kwon Y. Induction of motor neuron sprouting in vivo by ciliary neurotrophic factor and basic fibroblast growth factor. Journal of Neuroscience. 1992;12:3241–3247. doi: 10.1523/JNEUROSCI.12-08-03241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead LS, Grimby G. Post-Polio Syndrome. Philadelphia: Hanley and Belfus Inc.; 1995. [Google Scholar]
- Halstead LS, Wiechers DO. Research and Clinical Aspects of the Late Effects of Poliomyelitis. New York: March of Dimes Birth Defects Foundation White Plains; 1987. [Google Scholar]
- Holland RL, Brown MC. Postsynaptic transmission block can cause terminal sprouting of a motor nerve. Science. 1980;207:649–651. doi: 10.1126/science.6243417. [DOI] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius muscle. Journal of Physiology. 1992;448:677–695. doi: 10.1113/jphysiol.1992.sp019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater SB, Mattson MP, Cohan CS, Connor JA. Calcium regulation of the neuronal growth cone. Trends in Neurosciences. 1988;11:315–320. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mattson MP, Guthrie PB. Calcium-induced neuronal degeneration: a normal growth cone regulating signal gone away. Annals of the New York Academy of Sciences. 1989;568:252–261. doi: 10.1111/j.1749-6632.1989.tb12514.x. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mills LR. Regulation of growth cone behavior by calcium. Journal of Neuroscience. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Gurney ME. Systemic injections of ciliary neuromuscular factor induce sprouting by adult motor neurons. NeuroReport. 1994;5:789–792. doi: 10.1097/00001756-199403000-00013. [DOI] [PubMed] [Google Scholar]
- Luff AR, Hatcher DD, Torkko K. Enlarged motor units resulting from partial denervation of cat hindlimb muscles. Journal of Neurophysiology. 1988;59:1377–1394. doi: 10.1152/jn.1988.59.5.1377. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Calcium regulation of neurite elongation and growth cone motility. Journal of Neuroscience. 1987;7:4034–4042. doi: 10.1523/JNEUROSCI.07-12-04034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Taylor-Hunter A, Kater SB. Neurite outgrowth in individual neurons of a neuronal population is differentially regulated by calcium and cyclic AMP. Journal of Neuroscience. 1988;8:1704–1711. doi: 10.1523/JNEUROSCI.08-05-01704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel RN, Gardiner PF. Influence of overload on recovery of rat plantaris from partial denervation. Journal of Applied Physiology. 1989;66:732–740. doi: 10.1152/jappl.1989.66.2.732. [DOI] [PubMed] [Google Scholar]
- Michel RN, Gardiner PF. To what extent is hindlimb suspension a model of disuse? Muscle and Nerve. 1990;13:646–653. doi: 10.1002/mus.880130714. [DOI] [PubMed] [Google Scholar]
- Pockett S, Slack JR. Source of the stimulus for nerve terminal sprouting in partially denervated muscle. Neuroscience. 1982;7:3173–3176. doi: 10.1016/0306-4522(82)90239-1. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. I. Comparisons of the capacity of regenerating nerves to form enlarged motor units after extensive peripheral nerve injuries. Journal of Neurophysiology. 1996a;75:268–281. doi: 10.1152/jn.1996.75.1.268. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. II. Analysis of the mechanisms and significance of fibre type grouping in reinnervated muscles. Journal of Neurophysiology. 1996b;75:282–297. doi: 10.1152/jn.1996.75.1.282. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. Journal of Neurophysiology. 1992;68:1261–1275. doi: 10.1152/jn.1992.68.4.1261. [DOI] [PubMed] [Google Scholar]
- Rehder V, Kater SB. Regulation of neuronal growth cone filopodia by intracellular calcium. Journal of Neuroscience. 1992;12:3175–3186. doi: 10.1523/JNEUROSCI.12-08-03175.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester RR. Activity-dependent and independent synaptic interactions during reinnervation of partially denervated rat muscle. Journal of Physiology. 1988;401:53–75. doi: 10.1113/jphysiol.1988.sp017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seburn KL, Gardiner PF. Properties of sprouted rat motor units: effects of period of enlargement and activity level. Muscle and Nerve. 1996;19:1100–1109. doi: 10.1002/(SICI)1097-4598(199609)19:9<1100::AID-MUS4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Siegel SG, English AW. CNTF is required for denervation-induced sprout formation at the neuromuscular junction. Society for Neuroscience Abstracts. 1997;23:248. [Google Scholar]
- Slack JR, Pockett S. Terminal sprouting of motoneurones is a local response to a local stimulus. Brain Research. 1981;217:368–374. doi: 10.1016/0006-8993(81)90013-5. [DOI] [PubMed] [Google Scholar]
- Spector SA. Trophic effects on the contractile and histochemical properties of rat soleus muscle. Journal of Neuroscience. 1985;5:2189–2196. doi: 10.1523/JNEUROSCI.05-08-02189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995a;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995b;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Gordon T. Effect of exercise on stability of chronically enlarged motor units. Muscle and Nerve. 2002;25:359–369. doi: 10.1002/mus.10057. [DOI] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Jassar B, Gordon T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. Journal of Neuroscience. 2001;21:654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WJ, Jansen JK. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2:523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Kopp DM. Schwann cells accompany nerve sprouts induced in muscle by insulin-like growth factors. Society for Neuroscience Abstracts. 1996;22:297. [Google Scholar]
- TÖTÖsy De Zepetnek JE, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscle. Journal of Neurophysiology. 1992a;67:1385–1403. doi: 10.1152/jn.1992.67.5.1385. [DOI] [PubMed] [Google Scholar]
- TÖTÖsy De Zepetnek JE, Zung HV, Erdebil S, Gordon T. Motor-unit categorization based on contractile and histochemical properties: a glycogen depletion analysis of normal and reinnervated rat tibialis anterior muscle. Journal of Neurophysiology. 1992b;67:1404–1415. doi: 10.1152/jn.1992.67.5.1404. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Annual Review of Neurology. 1990;28:496–502. doi: 10.1002/ana.410280405. [DOI] [PubMed] [Google Scholar]


