Abstract
Studies of W/WV mice, which lack intramuscular interstitial cells of Cajal (IC-IM), have suggested that IC-IM act as mediators of enteric motor neurotransmission in the gastrointestinal tract. We have studied Sl/Sld mice, which lack the ability to make membrane-bound stem cell factor, to determine the consequences of inappropriate stem cell factor expression on IC-IM populations and on enteric motor neurotransmission. IC-IM were found within the circular and longitudinal muscles of the gastric fundus of wild-type mice. IC-IM were intimately associated with motor nerve terminals and nerve varicosities formed synaptic structures with these cells. IC-IM were also connected with neighbouring smooth muscle cells via gap junctions. Immunohistochemistry and electron microscopy showed that IC-IM were absent from fundus muscles of Sl/Sld mice, but the density of excitatory and inhibitory nerves was not significantly different than in wild-type muscles. Loss of IC-IM was associated with decreased membrane noise (unitary potentials) and significant reductions in post-junctional excitatory and inhibitory enteric nerve responses. Reductions in neural responses were not due to defects in smooth muscle cells as responses to exogenous ACh and K+-induced depolarization were normal in Sl/Sld mice. Responses to neurally released ACh were revealed in Sl/Sld mice by inhibiting ACh breakdown with the acetylcholinesterase inhibitor neostigmine. Inhibitory nerve stimulation elicited inhibitory junction potentials (IJPs) and relaxations in wild-type mice. IJPs were reduced in amplitude and relaxation responses were absent in Sl/Sld mice. These observations suggest that membrane-bound stem cell factor is essential for development of IC-IM and that the close, synaptic-like relationship between nerve terminals and IC-IM may be the primary site of innervation by enteric motor neurons in gastric muscles.
In the proximal stomach the mechanical response to ingestion of food is an increase in volume due to the gastric accommodation reflex. This response and subsequent reduction in volume as the stomach contracts back to its resting dimensions after gastric emptying is complete are dependent upon inhibitory and excitatory motor control by the enteric nervous system. The major neurotransmitter involved in gastric accommodation is nitric oxide (NO; e.g. Desai et al. 1991) and cholinergic neurotransmission provides the major excitatory neural regulation. In recent years the idea that enteric motor neurons directly innervate smooth muscle cells in an ‘en passant’ fashion has been questioned (Burns et al. 1996; Ward et al. 2000), and it is likely that an intermediate cell type receives and transduces neural signals in gastrointestinal muscles. These intermediate cells are interstitial cells of Cajal (ICC).
Evidence suggesting that ICC are involved in neurotransmission include the observations that these cells are closely associated with varicose nerve terminals of enteric motor neurons (Daniel & Posey-Daniel, 1984; Burns et al. 1996; Wang et al. 1999; Vanderwinden, 1999; Ward et al. 2000), express receptors for neurotransmitters (Lavin et al. 1998; Epperson et al. 2000), respond to neurotransmitters (Publicover et al. 1993) and also that animals lacking intramuscular ICC (IC-IM) have abnormal enteric excitatory and inhibitory responses (Burns et al. 1996; Ward et al. 1998, 2000). However, a recent study questioned this hypothesis, and reported that nitrergic relaxations of the lower oesophageal sphincter in response to swallowing in W/Wv mutant mice (that lack IC-IM) were normal (Sivarao et al. 2001). These authors concluded that ICC are not necessary for normal enteric inhibitory responses, and suggested that defects noted in sphincter pressure might be due to developmental defects in the smooth muscle cells of these animals.
In the present study we have investigated the regulation of IC-IM development by stem cell factor in the murine gastric fundus. We used Sl/Sld mice which lack membrane-bound stem cell factor but continue to express soluble stem cell factor (Brannan et al. 1991). After observing loss of IC-IM in these animals, we tested whether loss of IC-IM affects responses to enteric neural inputs. We also tested whether differences in post-junctional responses in Sl/Sld mice could be due to loss of motor neural fibres within the muscle layers, loss of responsiveness to the enteric neurotransmitters, nitric oxide (NO) and acetylcholine (ACh), and whether enteric excitatory neurons release less transmitter in response to field stimulation of intrinsic neurons. The study confirms that loss of IC-IM results in seriously impaired neural responses and suggests that these defects are not the result of changes in mechanical performance by the smooth muscle cells.
METHODS
Animals
Compound heterozygote Steel-Dickie (Sl/Sld; white coats) mutants and their wild-type siblings (black coats) were obtained from the Jackson Laboratory (Bar Harbor, MN, USA). Animals between the ages of 30 and 60 days postpartum (aged-matched) were anaesthetized by inhalation with isoflurane (Baxter, Deerfield, IL, USA) and killed by cervical dislocation. The use and treatment of animals was approved by the Institutional Animal Use and Care Committee at the University of Nevada.
Stomachs, including portions of the oesophagus and duodenum, were removed and opened along the lesser curvature from the most proximal regions of the fundus to the corpus. Gastric contents were washed with Krebs solution (KRB) and the mucosa was removed.
Morphological studies
Immunohistochemistry
After removing the mucosa and submucosa by sharp dissection, gastric fundus muscles from wild-type and Sl/Sld animals were fixed in acetone (10 min at 4 °C). Following fixation tissues were incubated in bovine serum albumin for 1 h (1 % in phosphate-buffered saline; PBS). For double-label immunostaining, tissues were then incubated sequentially with two primary antibodies, each raised in a different animal species (see Table 1). The first incubation was carried out for 24–48 h at 4 °C. Following 3–4 h of washing with 0.01 m PBS, tissues were incubated in the second primary antiserum for 24–48 h. The two combinations of antibodies used were rat/sheep and rat/goat (see Table 1). The labelled secondary antibodies were conjugated to fluorescein isothiocyanate (FITC) or Texas Red (TR). Secondary antibodies were purchased from Vector Laboratories (Burlingame, CA, USA), and diluted to 1:100 in PBS. Secondary incubations were performed sequentially, each for 1 h at room temperature. Control tissues were prepared by either omitting primary or secondary antibodies from the incubation solutions. All antisera were diluted with 0.3 % Triton X-100 in 0.01 m PBS (pH 7.4). Tissues were examined with a Biorad MRC 600 confocal microscope (Hercules, CA, USA) with an excitation wavelength appropriate for FITC (494 nm) and Texas Red (595 nm). Confocal micrographs are digital composites of Z-series scans of 3–20 optical sections through a depth of 1.68–20 μm. Final images were constructed with Bio-Rad ‘Comos’ software.
Table 1.
Details of antibodies used for immunohistochemistry
| Combined antibodies | Resource | Mono-or polyclonal antibodies | Host | Dilution |
|---|---|---|---|---|
| ACK2/anti-vAChT | Gibco-BRL, Gaithersburg, MD, USA/Chemicon Inc. Temecula, CA, USA | Mono-/Poly- | Rat/Goat | 1:200/1:200 |
| ACK2/anti-NOS | Gibco BRL, Gaithersburg, MD, USA/Piers Emson, Mol. Sci. Group, Cambridge, UK | Mono-/Poly- | Rat/Sheep | 1:200/1:200 |
ACK2, anti Kit; NOS, nitric oxide synthase; vAChT, vesicular acetylcholine transporter.
Electron microscopy
Stomachs of wild-type and Sl/Sld animals were removed and fixed with 4 % paraformaldehyde and 3 % glutaraldehyde in 0.1 m PBS (pH 7.4). After fixation, tissues were further processed for conventional electron microscopy as previously described (Torihashi et al. 1993). Sections were examined using a Philips CM10 transmission electron microscope.
Physiological studies
Electrical responses
After removing the mucosa, strips of fundus muscle (6 mm × 5 mm), with the circular muscle layer facing upwards, were impaled and transmembrane potentials measured with a high impedance electrometer (WPI Intra 767, World Precision Instruments, Sarasota, FL, USA). Intrinsic nerves were stimulated using electrical field stimulation (0.5 ms duration, 1–50 Hz, supramaximal voltage; Grass S48 stimulator; Quincy, MA, USA). Electrical signals were recorded on videotape (A.R. Vetter Co., Rebersburg, PA, USA) for analysis purposes.
Mechanical responses
Mechanical studies were performed on muscle strips approximately 6 mm × 2.0 mm using standard organ bath techniques and isometric force measurements as previously described (Ward et al. 2000). Mechanical responses to electric field stimulation and to application of agonists were recorded on a PC, using the software Acqknowledge 3.2.6 (BIOPAC Systems, Inc., Santa Barbara, CA, USA).
Transmitter release studies
Gastric fundus muscles from wild-type and Sl/Sld animals were prepared as in the electrophysiological studies. Tissues were incubated at 37 °C in a modified Hepes-buffered solution containing [14C]choline chloride (1.33 μCi ml−1) for 40 min while continuously stimulating with electric field stimulation (EFS; 1 ms pulses at 1 Hz, 70 V). After an appropriate equilibration and washout period (Ward et al. 2000), the tissues were stimulated at 40 min intervals (S2, 95th min; S3, 135th min; S4, 175th min) for 1 min (5 Hz, 0.3 ms duration pulses). To test the effects of the sodium channel blocker tetrodotoxin, the drug was added 20 min before stimulation. Samples taken after stimulation were counted in a Beckman LS60001C scintillation counter. Overflow of 14C was calculated as a fractional release from the tissue. This technique has been employed to study the release of acetylcholine (ACh) from a variety of neuroeffector preparations such as guinea-pig gastrointestinal (GI) tissues (Alberts et al. 1982).
Data are expressed as means ± s.e.m. from wild-type and Sl/Sld mutant animals. Differences in the data were evaluated by Student's paired t test or two-way analysis of variance (two-way ANOVA) for multiple comparisons (where stated). P values less than 0.05 were taken as statistically significant. The n value reported in the text refers to the number of muscle strips used for each protocol. One muscle strip from each animal was used, unless otherwise specified in the text. For membrane noise analysis the variance was calculated as the mean-square deviation from the mean resting membrane potential.
A total of 15 wild-type and 15 Sl/Sld animals were used for physiological experiments, 6 wild-type and 6 Sl/Sld animals for morphological studies, and 10 wild-type and 10 Sl/Sld animals for transmitter release studies.
Solutions and drugs
For electrophysiological and mechanical experiments muscles were maintained in KRB (37.5 ± 0.5 °C; pH 7.3–7.4) containing (mm): Na+ 137.4, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 134, HCO3− 15.5, H2PO4− 1.2 and dextrose 11.5; gassed with 97 % O2-3 % CO2. The modified Hepes-buffered solution used for [14C]choline release experiments had the following composition (mm): NaCl 140, KCl 5.0, MgCl2 1.0, CaCl2 1.5, Hepes 5, glucose 10, and 1.33 μCi ml−1 [14C]choline chloride (PerkinElmer Life Sciences, Boston, MA, USA). Acetylcholine, neostigmine bromide, atropine sulphate, tetrodotoxin and Nω-nitro-l-arginine (l-NA) were dissolved in distilled water at 0.1–0.01 m. Nifedipine was dissolved in ethanol at 0.01 m. All drugs were then diluted in KRB to the stated final concentrations (all obtained from Sigma, St Louis, MO, USA).
RESULTS
Close morphological appositions between enteric motor neurons and intramuscular ICC (IC-IM)
Labelling with an anti-Kit antibody (ACK2) revealed the distribution of interstitial cells of Cajal (ICC) in the murine gastric fundus of wild-type animals. Kit-immunopositive ICC were evenly distributed within the circular and longitudinal muscle layers throughout the gastric fundus. These cells possessed a spindle-shaped morphology and ran parallel to the smooth muscle cells of the respective muscle layers (Figs 1A, 2A and 2B). The distribution and morphology of Kit-immunopositive cells identified them as intramuscular interstitial cells of Cajal (IC-IM; Burns et al. 1996). IC-IM had a density of 6.3 ± 0.2 cells per 100 μm cross-section, perpendicular to the circular muscle layer. IC-IM were absent from the gastric fundus of nine out of 12 Sl/Sld animals (Fig. 1B). In the other three Sl/Sld animals only 2–4 IC-IM were observed in the circular and longitudinal muscle layers of the entire gastric fundus.
Figure 1. Distribution of IC-IM within the circular and longitudinal muscle layers of wild-type and Sl/Sld murine gastric fundus muscles.
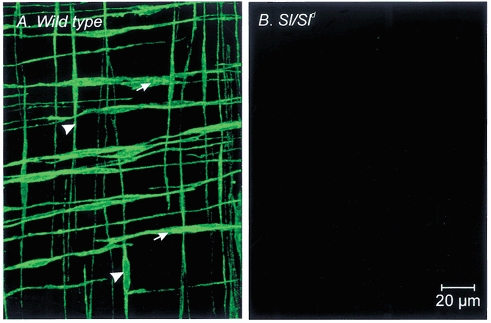
A, Kit-like immunopositive IC-IM within the circular (arrows) and longitudinal muscle layers (arrowheads) of a wild-type control animal. IC-IM are elongate spindle shaped cells that run parallel to the muscle fibres of each layer. Kit-like immunohistochemistry revealed an absence of IC-IM in the circular and longitudinal muscle layers of age-matched Sl/Sld mutant siblings (B). Scale bar shown in B also applies to A.
Figure 2. Confocal microscopy revealing the relationship between enteric motor neurons and ICC within the circular muscle layer (IC-IM).
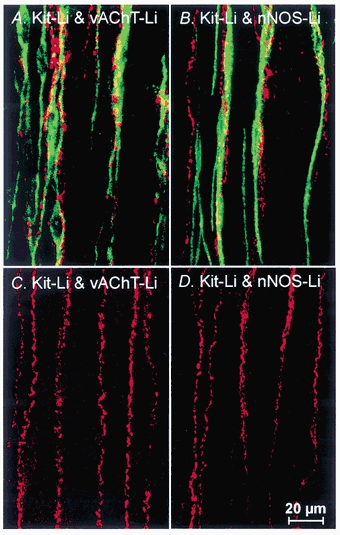
A, an overlay of confocal fluorescence micrographs showing Kit-like immunopositive IC-IM (Kit-Li; green) and vesicular acetylcholine transporter-like immunoreactivity (vAChT-Li; red) to label excitatory motor neurons within the circular muscle layer of wild-type fundus. Note the close anatomical relationship between excitatory enteric nerves and IC-IM. B, an overlay of fluorescence micrographs showing the close association between IC-IM (Kit-Li; green) and neuronal nitric oxide synthase-like immunoreactivity (nNOS-Li; red) to label inhibitory motor neurons in wild-type fundus. Note the close apposition of IC-IM with varicose terminals of inhibitory motor neurons. C and D are from an Sl/Sld animal. Despite the absence of Kit-Li IC-IM in the circular muscle layer of Sl/Sld mutants, both vAChT-Li and nNOS-Li enteric motor nerves (red) are distributed in a manner similar to that of controls (C and D, respectively). Images shown are digital reconstructions taken from 3 × 0.56 μm scans. Scale bar shown in D is for all panels.
Labelling with antibodies against vesicular acetylcholine transporter (vAChT) revealed varicose nerve fibres within the circular and longitudinal muscle layers of wild-type animals that ran parallel to the smooth muscle fibres. The density of innervation of vAChT immunopositive nerve fibres was (6.1 ± 0.2 fibres per random 100 μm transecting line within the circular muscle layer). Double labelling for vAChT and Kit showed that nerve fibres containing vAChT were closely apposed to IC-IM (Fig. 2A). Nerve fibres with vAChT-like immunoreactivity were associated with IC-IM for distances > 200 μm, and some fibres were observed to be associated with more than one IC-IM.
Double-labelling with antibodies against neuronal nitric oxide synthase (nNOS) and Kit (Fig. 2B) also showed a close relationship between inhibitory enteric nerves and IC-IM. Nerves with nNOS-like immunoreactivity (spatial density = 5.9 ± 0.2 fibres per random 100 μm transecting line within the circular muscle layer) were closely associated with IC-IM for distances > 200 μm (Fig. 2B) and often tracked more than one IC-IM.
Double labelling experiments using vAChT and Kit antibodies or nNOS and Kit antibodies confirmed the absence of IC-IM in Sl/Sld fundus muscles but revealed an apparently normal distribution of excitatory and inhibitory motor nerves in the circular muscle layer (i.e. vAChT-like and nNOS-like immunopositive fibres had a spatial density of 6.0 ± 0.1 and 6.5 ± 0.4 fibres per random 100 μm transecting line in the circular muscle layer, respectively; Fig. 2C and D). These values were not statistically different from values from wild-type animals. The absence of IC-IM in the Sl/Sld mutant provides a useful model to assess the functional role of these cells in gastric motility.
Ultrastructural relationships between enteric nerves, IC-IM and smooth muscle cells
To more closely investigate the apparent close spatial relationship between enteric motor nerves and IC-IM, transmission electron microscopy was performed on the circular and longitudinal muscle layers of the murine gastric fundus of wild-type and Sl/Sld animals. IC-IM were observed throughout the circular muscle layer. These cells were typically interposed between nerve processes and smooth muscle cells (Fig. 3A and B). The cytoplasm of IC-IM was characteristically electron dense compared to that of adjacent smooth muscle cells and possessed an abundance of mitochondria (Fig. 3B and C). Numerous free ribosomes, a well-developed endoplasmic reticulum and prominent Golgi were also observed within the cytoplasm of IC-IM (Fig. 3A, D and E). IC-IM lacked dense bodies and contractile filaments. A discontinuous basal lamina was associated with the plasma membrane that distinguished these cells from macrophages or fibroblasts (Fig. 3E and F).
Figure 3. Transmission electron microscopy reveals the morphology of IC-IM and their ultrastructural relationship with enteric motor nerve endings.
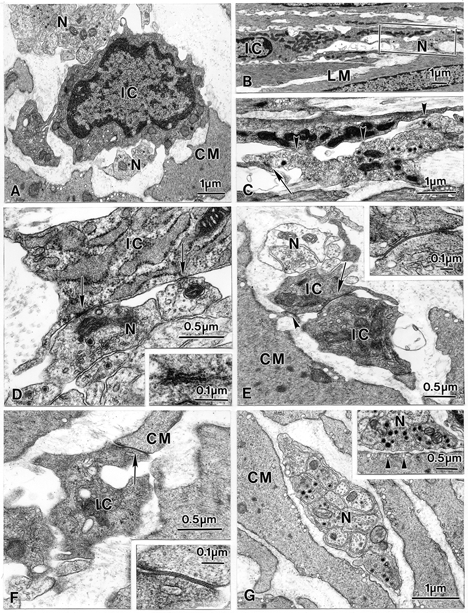
A, an IC-IM (IC) within the circular muscle layer interposed between nerve processes (N) and circular smooth muscle cells (CM). The cytoplasm of IC-IM were characteristically electron dense compared to smooth muscle cells and possessed an abundance of mitochondria (B; frame in B is magnified in C). Free ribosomes, a prominent Golgi apparatus and a well-developed endoplasmic reticulum were also observed within the cytoplasm of IC-IM (A, D and E). A discontinuous basal lamina was associated with the plasma membrane that distinguished these cells from macrophages or fibroblasts, and the lack of dense bodies and contractile filaments distinguish the IC-IM from smooth muscle cells (E and F). Varicose nerve fibre endings possessed large dense cored and smaller electron-lucent vesicles and formed morphological appositions with IC-IM of distances < 10 nm. Close contacts (arrowheads) and synaptic-like specializations (arrows) appeared to exist between the membranes of enteric nerve varicosities and IC-IM (C and D; inset in D is a higher power image of the contact indicated by the left-hand arrow). IC-IM formed gap junctions with one another (arrow in E and inset) and with neighbouring smooth muscle cells (arrowhead in E, and arrow in F; shown at greater magnification in inset). IC-IM within the longitudinal muscle layer had a similar ultrastructural appearance to IC-IM in the circular layer and also formed close associations with enteric nerves, adjacent IC-IM and neighbouring smooth muscle cells (B and C). Cells with the ultrastructual features of IC-IM were absent in Sl/Sld mutants (G). Enteric nerves of Sl/Sld mutants (N) contained both large dense cored and smaller electron-lucent vesicles, but these nerves never formed the synaptic-like membrane specializations with smooth muscle cells (CM) (inset in G, arrowheads).
IC-IM formed close contacts (< 10 nm) with nerve varicosities containing large dense core and smaller, electron-lucent vesicles. At points of close apposition, the membranes of IC-IM and nerve varicosities had an electron dense appearance relative to the surrounding plasma membranes. The increased electron density at these points suggests synaptic-like specializations between the two cells (Fig. 3C and D). We were unable to find close contacts (i.e. < 25 nm) and synaptic-like membrane specializations between neurons and smooth muscle cells. IC-IM formed prominent gap junctions with each other (Fig. 3E and inset) and with neighbouring smooth muscle cells (Fig. 3F and inset). Thus, IC-IM and smooth muscle cells are part of an electrical syncytium. IC-IM in the longitudinal muscle had a similar ultrastructural appearance and also formed close associations with enteric nerves, adjacent IC-IM and neighbouring smooth muscle cells (Fig. 3B and C).
The absence of Kit-like immunopositive IC-IM in the circular muscle layer of the fundus of Sl/Sld mice was confirmed by electron microscopy. IC-IM were not present in any section examined (20 sections from two Sl/Sld mutants). Although we found no IC-IM, enteric nerve fibres with normal ultrastructural appearance were present in fundus muscles of Sl/Sld mice. The varicosities of these neurons contained numerous dense core and small, electron-lucent vesicles (Fig. 3G and inset). The absence of IC-IM was accompanied by the disappearance of the close apposition and membrane specializations between varicosities and post-junctional cells (Fig. 3G and inset). Intercellular spaces of at least 40 nm were observed between nerve varicosities and smooth muscle cells in Sl/Sld tissues.
Comparison of noise analysis of intracellular recordings from wild-type and Sl/Sld mutants
Intracellular microelectrode recordings were made from circular muscle cells of the gastric fundus. This region lacked electrical slow waves in both wild-type and Sl/Sld animals. In fundus muscles of wild-type animals resting membrane potentials averaged -41.6 ± 1.3 mV (n = 10). The resting membrane potentials of Sl/Sld animals were more hyperpolarized, averaging -48.0 ± 1.5 mV (n = 12; P < 0.05 compared to wild-type tissues). Intracellular recordings revealed a marked difference in the electrical activity in the fundus of wild-type and Sl/Sld animals. Wild-type muscles displayed irregular fluctuations in membrane potential that were not blocked by the addition of tetrodotoxin (TTX, 1 μM) or the Ca2+ channel blocker nifedipine (1 μM), suggesting that they were non-neurogenic in nature and did not require the opening of voltage-dependent (L-type) calcium channels (Fig. 4A). In wild-type muscles the variance from the mean resting membrane potential was 1.05 ± 0.29 mV2. In comparison Sl/Sld fundus muscles were more quiescent with small fluctuations in membrane potential that were barely distinguishable above the electrical noise of the recording system. In Sl/Sld fundus muscles the variance from the mean resting membrane potential was 0.12 ± 0.01 mV2 (P < 0.05 when compared to wild-type animals). These events were also insensitive to TTX or nifedipine (Fig. 4B). Spectral density analysis, which reflects the waveform energy at each frequency, was performed on electrical recordings of wild-type and Sl/Sld fundus muscles (Fig. 4C). Spectral density curves of wild-type and Sl/Sld muscles showed that the energy of the membrane noise was predominately concentrated at frequencies less than 10 Hz and had a plateau at frequencies less than 2 Hz. The power spectra for both wild-type and Sl/Sld muscles had similar temporal characteristics and displayed a steep inverse dependency on frequency suggesting that a similar common electrical event underlies the membrane fluctuations in both groups (Fig. 4C). Data from wild-type muscles revealed that the membrane noise was more powerful in the 0.3–10 Hz range than in Sl/Sld muscles (Fig. 4C). Thus the spontaneous activity appears to be similar to the ‘unitary potentials’ recorded from ICC in the guinea-pig stomach (Edwards et al. 1999).
Figure 4. Intracellular recordings of wild-type and Sl/Sld fundus muscles reveals differences in the basal resting membrane potential.
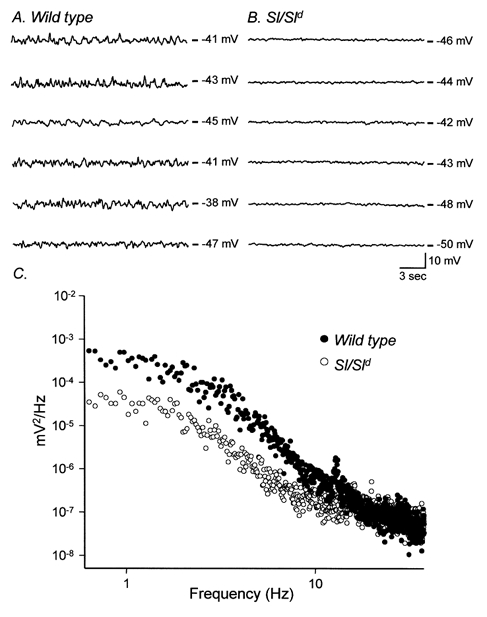
A, the typical activity recorded from six wild-type animals (20 s traces) revealing membrane potential fluctuations. B shows recordings from six age-matched Sl/Sld mutant siblings. The membrane potential fluctuations observed in wild-type controls were greatly reduced in these tissues. C shows differences in power spectral densities of the recordings from wild-type (•) and Sl/Sld mutants (○). Membrane fluctuations of wild-type fundus muscles had greater power in the 0.3–10 Hz range compared to Sl/Sld mutants.
Post-junctional neural responses are attenuated in muscles of Sl/Sld animals
In wild-type controls, electric field stimulation (EFS) of enteric neurons using single pulses (0.5 ms duration, supramaximal voltage) produced biphasic, tetrodotoxin-sensitive (0.5 μM; data not shown), post-junctional neural responses in circular muscles. These responses were characterized by a rapid excitatory junction potential (EJP) with an average amplitude and duration of 6.5 ± 1.0 mV and 358 ± 34 ms, respectively (n = 10), followed by an inhibitory junction potential (IJP) with an average amplitude and duration of 8.1 ± 1.7 mV and 950 ± 72 ms, respectively (n = 10; Fig. 5A). The nitric oxide synthase inhibitor, Nω-nitro-l-arginine (l-NA; 100 μM) depolarized the resting membrane potential (from -42.1 ± 1.2 mV to -39.6 ± 1.3 mV; P < 0.05; n = 8) in wild-type tissues. The EJP component of the response to field stimulation was potentiated by l-NA (i.e. by 48 % from 6.5 ± 1.0 mV to 9.7 ± 1.2 mV; P < 0.05 compared to control), and the IJP component was reduced by 83 % in amplitude (i.e. from 8.1 ± 1.7 mV to 1.4 ± 0.3 mV; P < 0.05; n = 9; Fig. 5B). These results suggest that release of nitric oxide from inhibitory nerves is responsible for a significant portion of the IJP. EJPs were cholinergic in nature, and were completely blocked by atropine (1 μM; EJPs decreased from 9.7 ± 1.2 mV before addition of atropine and were not resolved after atropine; n = 9; Fig. 5C). Data for wild-type animals are summarized in Fig. 5G.
Figure 5. Differences in neural responses to electrical field stimulation (EFS) in wild-type and Sl/Sld mutant animals.
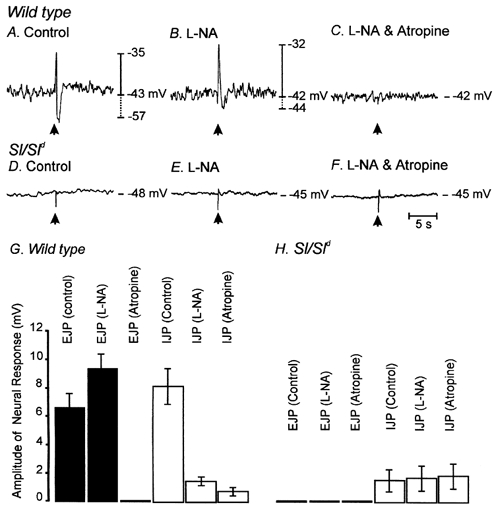
EFS (arrow; 0.5 ms pulse at supramaximal voltage) produced a bi-phasic electrical response in wild-type muscles, characterized by a rapid excitatory junction potential (EJP) that was followed by an inhibitory junction potential (IJP; A). Nω-Nitro-l-arginine (l-NA; 100 μM) reduced the amplitude of IJPs and potentiated the amplitudes of the EJPs (B). After addition of l-NA, atropine (1 μM) completely blocked the EJPs, providing evidence that the EJP response was cholinergic in nature. In Sl/Sld mutant animals, EJPs were absent and IJPs were greatly reduced in amplitude (D). l-NA had little or no effect on EJPs and failed to reduce the attenuated IJPs (E). In the presence of l-NA, atropine had no effect (F). The effects of l-NA and atropine on EJPs and IJPs on 10 wild-type (▪) and 10 Sl/Sld mutant animals (□) is summarized in G and H, respectively.
In contrast to the responses of wild-type fundus muscles, neural responses to EFS (single pulses, 0.5 ms duration) of fundus muscles from Sl/Sld mice were greatly reduced (Fig. 5D). Excitatory junctional potentials were not observed, and IJPs (resolved in five of eight Sl/Sld mice) were of greatly reduced amplitude (i.e. 1.7 ± 0.6 mV; n = 8; P < 0.05 compared to wild-type animals; Fig. 6D). Addition of l-NA (100 μM) depolarized Sl/Sld circular muscle cells (-48 ± 1.5 mV to -44.1 ± 1.2 mV; P < 0.05 in comparison to control conditions) but did not decrease IJPs (1.8 ± 0.9 mV after addition of l-NA; n = 8) or reveal excitatory responses (Fig. 5E). Atropine (1 μM) did not affect either resting potential or neural responses in Sl/Sld muscles (Fig. 5F). Data for Sl/Sld animals are summarized in Fig. 5H.
Figure 6. Multiple pulses of EFS (1–20 Hz; 0.5 ms duration for 1 s) did not reveal significant excitatory or inhibitory responses in Sl/Sld mutants.
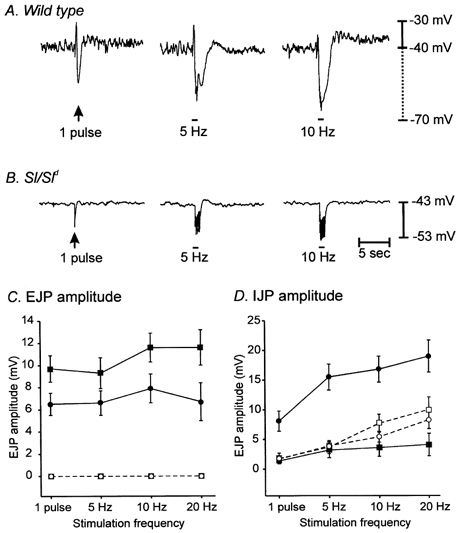
A shows neural responses of a wild-type animal to EFS (1 pulse, 5 and 10 Hz for 1 s) delivered at the time indicated by the arrow and bars. B shows the neural responses of an Sl/Sld mutant to similar EFS parameters. C and D show the summarized data for EJP (C) and IJP responses (D) to EFS recorded from wild-type and Sl/Sld mutants before (circles; filled for wild-type and open for Sl/Sld) and after (squares; filled for wild-type and open for Sl/Sld) the addition of l-NA (100 μM).
We also compared responses of wild-type and Sl/Sld muscles to multiple pulses of electrical field stimulation (5, 10 and 20 pulses; 0.5 ms pulse duration delivered in a 1 s time period). These stimulation parameters evoked large amplitude excitatory and inhibitory junctional potentials in wild-type muscles (Fig. 6A). l-NA (100 μM) decreased IJP amplitude and potentiated EJP amplitude at all stimulation frequencies tested (Fig. 6C and D).
In Sl/Sld muscles, fast excitatory junction potentials were not evoked by multiple pulse stimulation (1–20 Hz), and IJPs were of significantly smaller amplitude than those evoked in wild-type preparations (Fig. 6B and summarized in Fig. 6C and D). Excitatory junction potentials were not revealed by treatment with l-NA (100 μM), and inhibition of nitric oxide synthesis did not significantly affect IJPs in Sl/Sld muscles (Fig. 6C and D).
A second multiple pulse protocol in which even higher frequencies of stimulation were delivered, was used in an attempt to evoke excitatory responses in Sl/Sld muscles. Stimuli consisted of three and five pulses of EFS delivered at frequencies of 30 and 50 Hz, respectively, in a 100 ms time period. EJPs reached a maximum with three pulses at 30 Hz in wild-type muscles (i.e. 8.3 ± 0.6 mV; n = 7). At these higher frequencies, EJPs were partially masked by large overlapping IJPs. Application of l-NA significantly attenuated the IJPs (from 12.1 ± 1.4 mV to 4.6 ± 1.0 mV at three pulses and 14.0 ± 1.8 mV to 6.1 ± 1.3 mV at five pulses; P < 0.05 for both) and increased EJPs at both frequencies (from 8.3 ± 0.6 mV to 11.8 ± 1.4 mV at three pulses and 8.0 ± 0.8 mV to 12.4 ± 1.6 mV at five pulses; P < 0.05 for both). High frequency stimulation failed to evoke fast EJPs in Sl/Sld muscle cells and IJPs were of smaller amplitude than those evoked in wild-type preparations (3.5 ± 0.6 mV and 6.1 ± 2.1 mV at three and five pulses, respectively; P < 0.05 compared to IJPs in wild-type animals).
Mechanical responses to nerve stimulation are attenuated in muscles of Sl/Sld animals
Mechanical responses to EFS were also compared in wild-type and Sl/Sld muscles. In wild-type muscle strips a single pulse (0.5 ms) stimulation increased tone by 0.2 ± 0.1 mN mg−1 (seven muscle strips from five animals). Administration of l-NA (100 μM) did not significantly increase the response of wild-type muscles (i.e. 0.5 ± 0.1 mN mg−1; P = 0.08). In the continued presence of l-NA, addition of atropine (1 μM) abolished the contractile responses to EFS. A single pulse stimulation failed to evoke contractile responses in seven of eight Sl/Sld muscle strips (eight muscle strips from five animals). In one Sl/Sld muscle strip a single pulse of EFS evoked a small contraction (0.07 mN mg−1). l-NA did not unmask contractile responses with single pulse EFS in Sl/Sld tissues.
EFS at 1 Hz (0.5 ms pulse duration; delivered in 30 s evoked contractile responses averaging 2.2 ± 0.9 mN mg−1 at the onset of stimulation in wild-type muscles (n = 7, from five animals). In three of these muscles, higher frequency EFS produced larger contractile responses; with amplitudes of 6.7 ± 2.5, 9.0 ± 2.3 and 9.2 ± 2.3 mN mg−1 at 5, 10 and 20 Hz, respectively. In another three muscle strips, multiple pulse EFS of 5, 10 and 20 Hz evoked biphasic mechanical responses consisting of a relaxation followed by a rebound contraction (Fig. 7A). In the remaining muscle strip contractions were evoked by 1–10 Hz EFS and a biphasic mechanical response was produced with 20 Hz. Addition of l-NA (100 μM) abolished the relaxations and potentiated the contractile responses, confirming that the relaxations were mediated via nitric oxide (Fig. 7B). The amplitude of contractile responses evoked by EFS in the presence of l-NA (100 μM) were 4.2 ± 0.9, 7.4 ± 1.3, 9.2 ± 1.2 and 8.4 ± 1.3 mN mg−1 at 1, 5, 10 and 20 Hz, respectively (mean amplitudes are for all seven wild-type muscle strips). In the continued presence of l-NA, addition of atropine (1 μM) abolished contractions at 1 Hz and significantly attenuated contractions evoked by all other stimulus frequencies tested (i.e. to 2.1 ± 0.8, 3.3 ± 1.2 and 2.8 ± 1.1 mN mg−1 at 5, 10 and 20 Hz, respectively; Fig. 7C and summarized in Fig. 7G).
Figure 7. EFS revealed differences in the mechanical responses to nerve stimulation of wild-type and Sl/Sld mutant muscles.
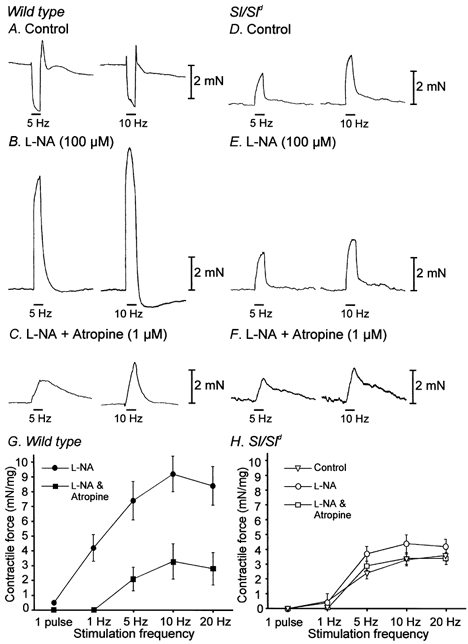
A shows mechanical responses to EFS (5 Hz and 10 Hz of EFS, 0.5 ms duration for 30 s delivered at the time indicated by the bars using supramaximal voltage) of wild-type fundus muscle strips. l-NA (100 μM) abolished relaxations induced by EFS and unmasked large contractile responses (B). In the continued presence of l-NA, atropine (1 μM) significantly attenuated the amplitude of contractions in response to EFS (C). D shows mechanical responses of an Sl/Sld mutant in control conditions using the same EFS stimulus parameters. In Sl/Sld muscle strips relaxations were not evoked and contractions induced by EFS were significantly smaller compared to wild-type animals. l-NA (100 μM) and atropine (1 μM) had little effect on the amplitude of Sl/Sld fundus muscle contractions (E and F). G shows a summary of the data of wild-type muscles in the presence of l-NA to reveal excitatory responses, before (•) and after (▪) the addition of atropine. A significant portion of the contractile responses of wild-type muscles was blocked by atropine. H shows a summary of the data from Sl/Sld muscles. Contractions evoked in Sl/Sld muscles in control conditions (▿) were enhanced by l-NA (○) but remained significantly smaller in amplitude than those evoked in wild-type muscles in identical conditions. Sl/Sld muscle responses in the presence of l-NA and atropine (□) were similar in amplitude to wild-type muscle responses in these conditions.
Multiple pulses (1–20 Hz) evoked contractile responses in all Sl/Sld muscle strips. Relaxation responses were never observed in these tissues. The contractile responses in Sl/Sld muscle strips tended to be of smaller amplitude than in wild-type muscles; however, the mixed responses of the wild-type muscles made it difficult to make direct comparisons. The contractile responses of Sl/Sld muscles averaged 0.5 ± 0.1, 2.4 ± 0.4, 3.3 ± 0.4 and 3.6 ± 0.4 mN mg−1 with 1, 5, 10 and 20 Hz stimulation, respectively (n = 8, from five animals). Following addition of l-NA (100 μM), the contractile responses of Sl/Sld muscles at 1 Hz were not increased in amplitude (i.e. 0.4 ± 0.1 mN mg−1; P < 0.05). l-NA had little effect on contractions elicited by higher frequencies of stimulation (i.e. to 3.7 ± 0.5, 4.4 ± 0.6 and 4.2 ± 0.5 mN mg−1 at 5, 10 and 20 Hz, respectively; Fig. 7E; P < 0.05. The responses of Sl/Sld muscles to EFS after addition of l-NA were smaller than the responses in wild-type muscles under the same conditions (tested using two-way ANOVA). Amplitudes of Sl/Sld contractions in the presence of l-NA (conditions in which cholinergic responses should be maximal) were comparable to the amplitudes of contractions evoked in wild-type muscles in the presence of l-NA and atropine (conditions in which cholinergic responses should be minimal), demonstrating that only a small component of Sl/Sld muscle contraction was due to muscarinic receptor activation (P < 0.05 for all stimulation frequencies). Furthermore, in the presence of both l-NA (100 μM) and atropine (1 μM) the mean amplitude of contractions evoked by 5, 10 and 20 Hz in Sl/Sld fundus muscles were 2.9 ± 0.3, 3.4 ± 0.4 and 3.4 ± 0.4 mN mg−1, respectively, which were not statistically significant from contractions evoked in the presence of l-NA alone (P < 0.05; Fig. 7F and summarized in Fig. 7G). Amplitudes of non-cholinergic contractions (i.e. in the presence of l-NA and atropine) were not statistically different in Sl/Sld muscle strips compared to wild-type muscles for any of the stimulus frequencies tested (P < 0.05).
Effects of excitatory and inhibitory agonists
We tested whether the reduction in excitatory and inhibitory neural responses in Sl/Sld mice was due to loss of sensitivity to neurotransmitters. Responses to exogenous acetylcholine (1 μM) and sodium nitroprusside (SNP; 1 μM) were obtained. We also compared the mechanical responses of wild-type and Sl/Sld muscles to depolarization induced by increased external potassium concentration ([K+]o = 36 mm). Wild-type muscles depolarized by 11.7 ± 1.9 mV in response to the bath application of ACh (1.0 μM; Fig. 8A and C), and Sl/Sld muscles depolarized by 17.7 ± 2.6 mV in response to ACh (1.0 μM; Fig. 8B and C; P < 0.1; n = 5 for both wild-type and Sl/Sld). The increase in contractile force elicited by ACh (1 μM) was similar with wild-type and Sl/Sld muscles (Fig 8D, E and F). Tonic contraction increased in wild-type muscles by 3.6 ± 0.4 mN mg−1 and in Sl/Sld muscles by 2.5 ± 0.5 mN mg−1 (P < 0.1; 11 muscle strips from five animals for wild-types and 12 strips from five animals for Sl/Sld mice).
Figure 8. Electrical and mechanical responses of wild-type and Sl/Sld muscles to exogenous acetylcholine (ACh) and sodium nitroprusside (SNP).
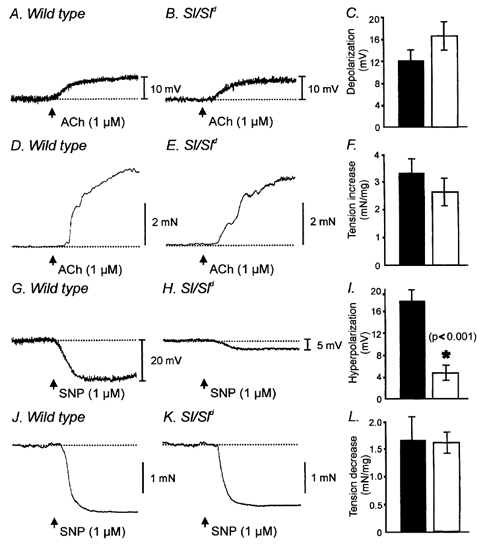
ACh (1.0 μM) depolarized muscles from wild-type and Sl/Sld mutants in a similar manner. A and B show depolarization responses of wild-type and Sl/Sld muscles to ACh (1.0 μM, arrows), respectively. C is the summarized data for depolarizations to ACh in wild-type (▪) and Sl/Sld mutants (□). Summarized data were not statistically significant between wild-type and Sl/Sld mutants. D and E show the contractile responses of wild-type and Sl/Sld muscles to ACh (1.0 μM, arrows). F shows the summarized contractile responses for wild-type (▪) and Sl/Sld mutants (□) in response to acetylcholine. Contractile responses were not statistically different between the two groups. G and H show hyperpolarization responses of wild-type and Sl/Sld muscles in response to addition of the nitric oxide donor sodium nitroprusside (SNP, 1 μM, arrows), respectively. Wild-type muscles hyperpolarized following addition of SNP but Sl/Sld mutants showed little or no response (H shows one of the five muscle strips, from a total of six, hyperpolarized slightly in response to SNP). Summarized data of the hyperpolarization responses of wild-type (▪) and Sl/Sld mutants (□) are shown in I. * Significant difference (P < 0.001) between wild-type and Sl/Sld mutants in response to SNP. J and K show relaxations in response to SNP (1 μM) for wild-type and Sl/Sld mutants, respectively. No statistical difference was observed in the relaxation responses between these two groups (L).
In response to application of SNP (1 μM) wild-type muscles hyperpolarized by 18.0 ± 2.1 mV (Fig. 8G and I). The membrane hyperpolarization elicited by addition of SNP (1 μM) was significantly reduced in Sl/Sld mutants. In one out of six animals no change in membrane potential was produced and in the remaining five animals the average hyperpolarization was 4.8 ± 1.4 mV (P < 0.001 compared to wild-type animals; Fig. 8H and I). Despite the attenuated electrical responses, Sl/Sld muscle strips relaxed normally in response to addition of SNP, compared to wild-type muscles (1.6 ± 0.4 mN mg−1 for wild-type versus1.6 ± 0.2 mN mg−1 for Sl/Sld; P < 0.5; 11 muscle strips from five wild-type animals and 12 strips from five Sl/Sld animals; Fig. 8J, K and L).
Elevating external potassium concentration from 5.9 to 36 mm produced a large sustained contraction in both wild-type and Sl/Sld muscle strips. Wild-type muscles produced an average contractile force of 4.2 ± 0.2 mN mg−1 in response to the elevated [K+]o. In comparison, muscles of Sl/Sld mice produced similar contractions of 5.5 ± 0.9 mN mg−1 in response to the elevated [K+]o. These responses were not statistically different (P < 0.05; 11 muscle strips from five animals for wild-types and 12 strips from five animals for Sl/Sld mutants; data not shown).
Comparison of ACh release in wild-type and Sl/Sld mutants in response to nerve stimulation
Steel is expressed in a sub-population of enteric nerves (Young et al. 1998) and it is possible that a mutation in this ligand could affect transmitter release from enteric motor nerves or that developmental loss of IC-IM could affect the ability of enteric neurons to release transmitter. To evaluate this possibility we measured release of [14C]choline in response to nerve stimulation in wild-type and Sl/Sld muscles. EFS (60 V; 0.3 ms pulse duration at 5 Hz for 1 min) of fundus tissues from wild-type animals produced approximately a 430 % increase in the fractional release of [14C]choline (n = 10; Fig. 9A). Release of [14C]choline by EFS was greatly reduced in the presence of tetrodotoxin (1 μM; peak release of choline in TTX was only 124 % of basal release) and this was reversed upon washout of the TTX (1 μM; n = 10; Fig. 9A). EFS (identical stimulus parameters as wild-type) of Sl/Sld muscles produced approximately a 320 % increase in [14C]choline release (n = 10) that was also tetrodotoxin sensitive (Fig. 9B). The differences in [14C]choline release from Sl/Sld mutants were statistically significant compared to wild-type animals (P = 0.015).
Figure 9. [14C]Choline release from wild-type and Sl/Sld muscles in response to EFS.
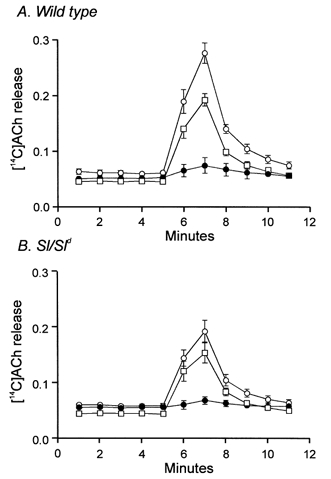
Tissues were stimulated using EFS at 40 min intervals for 1 min (5 Hz, 0.3 ms duration pulses; 60 V) and [14C]choline overflow was assayed from superfusion samples. [14C]Choline overflow is plotted as a percentage of fractional release with time of collection. A shows a summary of [14C]choline overflow, plotted as a percentage of total [14C]choline from wild-type fundus muscles (n = 10), in response to EFS under control conditions (○). [14C]Choline overflow in response to EFS was inhibited by TTX (1 μM, •) and recovered after washout of TTX (□). B shows a summary of [14C]choline overflow from Sl/Sld mutant tissues (n = 10) in response to EFS under control conditions (○). This release was also inhibited by TTX (1 μM, •) and recovered after washout (□).
Nerve evoked excitatory responses are revealed in Sl/Sld mutant tissues in the presence of cholinesterase inhibition
Acetylcholine released from presynaptic cholinergic nerves is rapidly hydrolysed by acetylcholinesterase (AChE) in GI muscles. We employed the use of an acetylcholinesterase inhibitor neostigmine bromide to determine if excitatory responses could be evoked in Sl/Sld muscles when ACh breakdown was reduced. In the continued presence of l-NA, addition of neostigmine (0.5 μM) did not produce any significant changes in resting membrane potential (i.e. in l-NA, -40.0 ± 1.7 mV; in l-NA + neostigmine, -39.3 ± 1.5 mV; P < 0.1; n = 5). In wild-type muscles, addition of neostigmine potentiated EJP amplitude (from 7.7 ± 1.2 mV to 10.6 ± 1.4 mV with 0.5 ms pulse durations; P < 0.05, n = 5) and caused a secondary, sustained depolarization that persisted long after the stimulus (Fig. 10). With single pulse stimulation (0.5 ms) the average amplitudes and durations of the secondary depolarization were 7.2 ± 1.2 mV and 15.2 ± 4.1 s, respectively (n = 5).
Figure 10. Inhibition of endogenous acetylcholinesterase with neostigmine potentiated the fast EJP and revealed a slowly developing membrane depolarization in wild-type tissues and revealed a slowly developing depolarization in Sl/Sld animals.
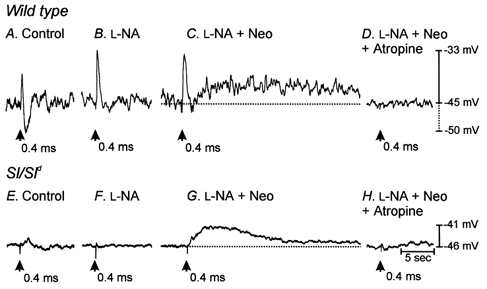
A shows the electrical responses of wild-type muscles to EFS (single pulses 0.4 ms duration, supramaximal voltage, delivered at time indicated by arrow). B shows responses to EFS after the addition of l-NA (100 μM). l-NA greatly reduced or abolished electrical responses at all stimulus durations. C shows recordings in the presence of l-NA and the acetylcholinesterase inhibitor neostigmine (Neo, 1 μM). Neostigmine produced a slowly developing membrane depolarization at all pulse durations tested. Both fast EJPs and slowly developing depolarizations were completely blocked by atropine (D) at all pulse durations, suggesting that muscarinic receptors mediated these responses. Electrical recordings in A-D were all from the same cell. E shows the lack of response to Sl/Sld muscles to single pulse (0.4 ms duration) EFS. After the addition of l-NA (100 μM) Sl/Sld muscles still showed no responses to EFS (F). In the presence of l-NA and neostigmine a slowly developing membrane depolarization occurred in response to EFS (G) that was completely inhibited by the addition of atropine (1 μM, H). Electrical recordings in E-H were all from the same cell.
With Sl/Sld muscles, single pulses of EFS failed to elicit fast EJPs in the presence of neostigmine (0.5 μM), but under these conditions EFS evoked slow depolarizations that were similar in waveform to the secondary depolarizations observed in wild-type muscles (Fig. 10). The average amplitude and duration of these secondary depolarizations were 7.1 ± 2.0 mV and 20.1 ± 3.4 s, respectively. These values are not significantly different when compared to slow depolarizations in wild-type muscles after addition of neostigmine (P = 0.98 for amplitude and P = 0.38 for duration; n = 7). Addition of neostigmine had no significant effect on resting membrane potential in the presence of l-NA in Sl/Sld muscles (mean resting membrane potential in l-NA, -45.9 ± 1.9 mV; in l-NA + neostigmine, -44.8 ± 1.6 mV; P = 0.4; n = 6).
Discussion
The gastric fundus of Sl/Sld mice was found to lack intramuscular ICC (IC-IM). This is the only class of ICC found in the murine fundus, so the loss of this population of cells provides an excellent opportunity to characterize the role of these cells in gastric electro-mechanical coupling. In wild-type mice, very close associations between both excitatory and inhibitory motor neurons and IC-IM were demonstrated using immunohistochemistry and electron microscopy. Ultrastructural examination using electron microscopy revealed the presence of pre- and post-junctional densifications at the junctions between nerve varicosities and IC-IM. Similar synaptic densifications have been described at neuro-neuro junctions in the central nervous system (e.g. Kennedy, 2000). The tight associations and membrane specializations between enteric motor neurons and IC-IM suggest that IC-IM are innervated. Previous studies have demonstrated that receptors for excitatory and inhibitory neurotransmitters are expressed by IC-IM (Epperson et al. 2000). We were unable to find close associations and ultrastructural features indicative of innervation between nerve varicosities and smooth muscle cells. Electron microscopy also demonstrated gap junctions between IC-IM and smooth muscle cells, and these are likely to provide low resistance electrical connections through which neural responses of IC-IM can be conveyed to the smooth muscle syncytium. The morphological observations suggested that loss of IC-IM in Sl/Sld mice might reduce the responses to enteric nerve stimulation. Both excitatory and inhibitory motor neurotransmission were reduced in fundus muscles of Sl/Sld mice. We tested the possibilities that the loss of neurotransmission could have been due to reduced numbers of nerve fibres in the muscle layers, loss of post-junctional responsiveness of the remaining smooth muscle to transmitters, changes in the electro-mechanical coupling in smooth muscle cells, or loss of transmitter release. The major findings of this study support the hypothesis that IC-IM are a necessary element interposed between enteric motor nerve terminals and smooth muscle effects.
Stem cell factor is expressed by a sub-population of enteric neurons (Young et al. 1998). Although its function in enteric neurons is not understood, it is possible that loss of this factor could affect enteric nerve activity. We noted a 25 % reduction in [14C]choline overflow in Sl/Sld tissues in response to EFS. This could explain some loss of the post-junctional cholinergic response; however, we noted nearly a total loss of cholinergic responses in Sl/Sld muscles. For example, the magnitude of contractions in wild-type muscles after addition of l-NA and atropine (i.e. conditions in which little or no muscarinic response would be left) was not significantly different from the contractile response in Sl/Sld muscles after addition of l-NA alone (i.e. condition where the cholinergic response should be maximal). Our data show that most, if not all, of the cholinergic response is absent in Sl/Sld muscles, suggesting that the loss of excitatory innervation is more extensive than can be explained by the reduction in transmitter release.
Previous studies have shown that loss of IC-IM in the fundus of W/Wv mice decreases enteric motor neurotransmission (Burns et al. 1996; Ward et al. 2000). We performed the present study as a means of further testing the significance of IC-IM in enteric neural responses, because others have suggested that loss of enteric nerve responses in W/Wv mice is due to developmental changes that compromise the performance of the smooth muscle (Sivarao et al. 2001). However, these studies seemed to ignore the observation that post-junctional electrical and mechanical responses to exogenous ACh were essentially identical in wild-type and W/Wv mice. In the present study we found that the mechanical performance of fundus muscles is not compromised by loss of IC-IM. Muscles lacking IC-IM due to a deficit in membrane-bound stem cell factor have normal electrical and mechanical responses to exogenous ACh and normal contractions in the presence of high extracellular K+. Furthermore, Sl/Sld muscles lacking IC-IM had normal mechanical responses to bath application of SNP. These findings do not support the suggestion that muscles lacking IC-IM have changes in smooth muscle performance.
We suggest that reduction in excitatory neurotransmission in Sl/Sld mice resulted from breakdown in the normally tight synaptic relationship between varicose nerve terminals and post-junctional cells. Normally, the neurally evoked excitatory response in fundus muscles is a fast EJP due to release of ACh and binding of the transmitter to post-junctional muscarinic receptors. Smooth muscle cells express muscarinic receptors, yet this fundamental post-junctional response was lost in Sl/Sld mice lacking IC-IM and the close synaptic-like contacts between nerve terminals and these cells. However, with more vigorous stimulation (i.e. 5–20 Hz for 30 s), post-junctional mechanical responses could still be initiated, suggesting that if enough ACh is released, some of it can reach smooth muscle receptors. This point was also demonstrated by experiments in which the breakdown of ACh was inhibited. Neostigmine elicited a secondary, slow depolarization in wild-type muscles that was also present in Sl/Sld tissues. We interpret these observations to mean that the first line of innervation by excitatory motor neurons is via IC-IM. Neurotransmission is fast through narrow (< 20 nm) synaptic clefts and elicits fast EJPs. Neurally released ACh is rapidly metabolized by acetylcholinesterase (AChE) at or near synaptic contacts between varicosities and IC-IM, but strong stimulation can produce enough overflow of ACh to elicit weakened post-junctional responses. Blocking the breakdown of ACh in Sl/Sld muscles enhances resolution of post-junctional responses due to direct stimulation of smooth muscle cells, but cannot restore the fast EJP response since this response depends upon the close synaptic relationship between motor nerve terminals and IC-IM.
However, one interesting change was noted in the post-junctional responsiveness of Sl/Sld mice: the hyperpolarization response to exogenous SNP was greatly diminished in these animals. Similar observations were made in gastric muscles of W/Wv mice (Burns et al. 1996). This suggests that the cellular elements required to cause hyperpolarization in response to SNP (i.e. second messenger systems or ion channels) are predominantly expressed by IC-IM. For example, IC-IM could have higher expression of soluble guanylyl cyclase (the post-junctional receptor for NO; Katsuki et al. 1977; Gruetter et al. 1979) or cGMP-dependent protein kinase, or the K+ channels that are responsive to NO-dependent mechanisms could be expressed more densely in these cells. Recent studies of the rat small intestine show that transduction mechanisms necessary for the actions of NO in GI muscles (e.g. soluble guanylyl cyclase and type I cGMP-dependent protein kinase) are abundantly expressed by intramuscular ICC (which are found primarily in the region of the deep muscular plexus in the small intestine) relative to neighbouring smooth muscle cells (Salmhofer et al. 2001). Loss of the electrical responses in Sl/Sld mice did not reduce the relaxation elicited by bath application of SNP. If NO released from enteric neurons freely diffused to nearby smooth muscle cells, then normal inhibitory mechanical responses would have been noted in Sl/Sld mice. However the prominent mechanical relaxation following EFS in muscles of wild-type mice did not occur in Sl/Sld mice, suggesting that loss of IC-IM led to a significant interruption in inhibitory neurotransmission. These data, therefore, indicate that the inhibitory effects of neurally released NO are strongly dependent upon transduction by IC-IM, and that the short half-life of nitric oxide necessitates close physical association between nitrergic nerves and post-junctional effector cells. The data also demonstrate that relaxation in response to exogenously applied NO occurs via different mechanisms (i.e. non-electrical) than relaxation in response to neurally released NO.
Another post-junctional difference in the electrical activity of smooth muscle cells in Sl/Sld mice was the reduction in random membrane potential noise compared to recordings from wild-type tissues. Previous studies have described membrane potential noise of this type in guinea-pig and murine gastric muscles and suggested that it results from a discharge of discrete depolarizing events termed ‘unitary potentials’ (Edwards et al. 1999; Dickens et al. 1999). Gastric smooth muscles from W/Wv animals also lack unitary potentials (Burns et al. 1996; Dickens et al. 2001). The reduced membrane noise in Sl/Sld muscles tends to confirm the previous suggestion that unitary potentials may emanate from IC-IM in gastric muscles (Dickens et al. 2001). Others have suggested that these events may be important in regeneration (or amplification) of slow waves (Edwards et al. 1999; Dickens et al. 2001). However, the role of these events in the fundus is unclear since slow waves do not propagate into this region of the stomach.
Sl/Sld mice lack the ability to make membrane-bound stem cell factor (steel), but cells that normally express stem cell factor can still produce a soluble form of the growth factor that lacks both transmembrane and cytoplasmic domains (Brannan et al. 1991). Production of a truncated form of stem cell factor may explain why these mice are viable. This animal model is extremely interesting because it shows that the membrane-bound form of stem cell factor is critical for the development of gastric IC-IM, and soluble stem cell factor is not an appropriate stimulus of the Kit receptor tyrosine kinase-regulated pathway. This study suggests that loss of membrane-bound stem cell factor produces no obvious changes in the density or pattern of neural elements and no changes in the electro-mechanical responsiveness of the smooth muscle cells that develop in the absence of IC-IM. IC-IM are in close proximity to smooth muscle cells in the circular and longitudinal muscle layers. Since smooth muscle cells express stem cell factor in the fundus (Epperson et al. 2000), it is possible that loss of expression of membrane-bound stem cell factor by smooth muscle cells is the primary defect that leads to loss of IC-IM. Our data also suggest that soluble stem cell factor replacement would not be of therapeutic benefit for the loss of ICC resulting from reduced expression of membrane-bound stem cell factor.
Although IC-IM appear to serve as the primary site of innervation in the murine fundus (Burns et al. 1996; Ward et al. 2000) and in the lower oesophageal and pyloric sphincters (Ward et al. 1998), overflow of transmitter due to more intense neural stimulation may still elicit post-junctional responses. We also found that post-junctional responses due to non-cholinergic excitatory neurotransmission were not significantly affected in Sl/Sld mice. Thus, loss of IC-IM does not result in a complete loss of neural responses, but disappearance of these cells would tend to attenuate the amplitude of neural responses. Loss of ICC has been noted in a number of human motility disorders (Faussone-Pellegrini & Cortesini, 1985; Vanderwinden et al. 1996; Isozaki et al. 1997). Intramuscular ICC in human GI muscles share similar close associations with the terminals of enteric motor neurons (Vanderwinden, 1999). Some motility disorders have been linked to loss of neural responsiveness (Enck & Frieling, 1997), but the present study suggests that this may not only be due to neuropathies or myopathies. Loss of intramuscular ICC (or ICC within the deep muscular plexus of the small intestine) could result in symptoms and motility changes similar to those due to loss or defects in the enteric nervous system. Future experiments should continue to characterize the loss of ICC in human motility disorders and attempt to discover the reasons why ICC populations are lost under specific pathological conditions.
Acknowledgments
The authors are extremely grateful to Dr Frank Edwards, University of Melbourne, for performing the Fourier analysis of non-stationary membrane noise (Fig. 4C) and Dr James Kenyon for helpful discussion. The authors are also extremely grateful to Julia R. Bayguinov for excellent technical assistance and would like to acknowledge Dr P. C. Emson of the Molecular Neuroscience Group, Cambridge, UK for providing the NOS antibody. This work was supported by NIH DK57236 and NIH DK40569 grants. The Morphology Core Laboratory supported by Program Project Grant DK41315 was used for the immunohistochemical studies. Choline release studies and M. Khoyi were supported by NIH HL38126 grant to Professor D. P. Westfall.
References
- Alberts P, Bartfai T, Stjarne L. The effects of atropine on [3H]-acetylcholine secretion from guinea-pig myenteric plexus evoked electrically or by high potassium. Journal of Physiology. 1982;329:93–112. doi: 10.1113/jphysiol.1982.sp014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan CI, Lyman SD, Williams DE, Eisenman J, Anderson DM, Cosman D, Bedell MA, Jenkins NA, Copeland NG. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proceedings of the National Academy of Sciences of the USA. 1991;88:4671–4674. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proceedings of the National Academy of Sciences of the USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. American Journal of Physiology. 1984;246:G305–315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GD. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. Journal of Physiology. 2001;531:827–833. doi: 10.1111/j.1469-7793.2001.0827h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. Journal of Physiology. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. Journal of Physiology. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enck P, Frieling T. Pathophysiology of diabetic gastroparesis. Diabetes. 1997;46:S77–S81. doi: 10.2337/diab.46.2.s77. [DOI] [PubMed] [Google Scholar]
- Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. American Journal of Physiology - Cell Physiology. 2000;279:C529–539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. Journal of Submicroscopic Cytology. 1985;17:673–685. [PubMed] [Google Scholar]
- Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. Journal of Cyclic Nucleotide Research. 1979;5:211–224. [PubMed] [Google Scholar]
- Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. American Journal of Gastroenterology. 1997;92:332–334. [PubMed] [Google Scholar]
- Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. Journal of Cyclic Nucleotide Research. 1977;3:23–35. [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Lavin ST, Southwell BR, Murphy R, Jenkinson KM, Furness JB. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochemical Cell Biology. 1998;110:263–271. doi: 10.1007/s004180050288. [DOI] [PubMed] [Google Scholar]
- Publicover NG, Hammond EM, Sanders KM. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proceedings of the National Academy of Sciences of the USA. 1993;90:2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmhofer H, Neuhuber WL, Ruth P, Huber A, Russwurm M, Allescher HD. Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell and Tissue Research. 2001;305:331–340. doi: 10.1007/s004410100410. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(-/-) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Kobayashi S, Gerthoffer WT, Sanders KM. Interstitial cells in deep muscular plexus of canine small intestine may be specialized smooth muscle cells. American Journal of Physiology. 1993;265:638–645. doi: 10.1152/ajpgi.1993.265.4.G638. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM. Role of interstitial cells of Cajal and their relationship with the enteric nervous system. European Journal of Morphology. 1999;37:250–256. doi: 10.1076/ejom.37.4.250.4728. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279–288. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell and Tissue Research. 1999;295:247–256. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. Journal of Neuroscience. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang X-Y, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Young HM, Torihashi S, Ciampoli D, Sanders KM. Identification of neurons that express stem cell factor in the mouse small intestine. Gastroenterology. 1998;115:898–908. doi: 10.1016/s0016-5085(98)70262-8. [DOI] [PubMed] [Google Scholar]


