Abstract
Many freshwater turtles in temperate climates may experience winter periods trapped under ice unable to breathe, in anoxic mud, or in water depleted of O2. To survive, these animals must not only retain function while anoxic, but they must do so for extended periods of time. Two general physiological adaptive responses appear to underlie this capacity for long-term survival. The first is a coordinated depression of metabolic processes within the cells, both the glycolytic pathway that produces ATP and the cellular processes, such as ion pumping, that consume ATP. As a result, both the rate of substrate depletion and the rate of lactic acid production are slowed greatly. The second is an exploitation of the extensive buffering capacity of the turtle's shell and skeleton to neutralize the large amount of lactic acid that eventually accumulates. Two separate shell mechanisms are involved: release of carbonate buffers from the shell and uptake of lactic acid into the shell where it is buffered and sequestered. Together, the metabolic and buffering mechanisms permit animals to survive for 3–4 months at 3 °C with no O2 and with circulating lactate levels of 150 mmol l−1 or more.
It is axiomatic that O2 is essential for vertebrate life. Anaerobic energy sources can only temporarily supply the requisite ATP and maintain cellular function before substrate depletion, energy shortfall, or endproduct poisoning threaten survival. In most vertebrates, the limits of anoxia tolerance are short, of the order of minutes, because of the urgent dependence of the heart and central nervous system on a continuous supply of O2. Remarkably, however, certain species are able to survive for periods lasting several months in the absence of O2 and can recover full function at the end of this time when O2 is restored. A well-studied example is the freshwater turtle, Chrysemys picta, a widely distributed resident of ponds and streams in the northern United States and southern Canada. When anoxic, this animal, commonly known as the painted turtle, relies on anaerobic glycolysis for energy and must therefore cope with the intrinsic inefficiency of this pathway, and must face the dual challenges of depletion of substrate and accumulation of acid metabolites. Nonetheless, it spends long periods during the winter in ice-covered ponds without access to the surface, often in water or mud with little or no O2 (Ultsch, 1989). In simulated hibernation in the laboratory, these animals can survive continuous submergence in nitrogen-equilibrated water at 3 °C for more than 4 months (Ultsch & Jackson, 1982; Jackson et al. 2000). The purpose of this paper is to discuss the physiological characteristics of the painted turtle that permit this extraordinary resistance to anoxia. Recent reviews dealing with aspects of this topic include: Ultsch (1989), Hochachka et al. (1996), Storey (1996), Lutz & Nilsson (1997), Jackson (2000a, b) and Jackson et al. (2001).
Mechanisms underlying anoxic tolerance
Metabolic depression
Organismal and organ-system response
A low metabolic rate is central to the turtle's ability to tolerate long-term anoxia. As an ectothermic reptile, its energy metabolism is only 10–20 % that of a mammal of similar size even at the same body temperature (Bennett & Ruben, 1979). At lower temperatures, metabolism falls still further in the thermally conforming ectotherm, typically at a rate of 2- to 3-fold per 10 °C decrease in temperature (Q10 = 2–3). Moreover, the painted turtle, like other reptiles (Bennett & Dawson, 1976), exhibits an exaggerated Q10 effect at temperatures below 10 °C (Herbert & Jackson, 1985b), so that at 3 °C aerobic metabolism is depressed to about 0.1 % of the euthermic mammalian level. Finally, the anoxic state is characterized by a further sharp fall in metabolism by about 90 % (Jackson, 1968; Buck et al. 1993), so that the metabolic rate of the anoxic turtle at its usual hibernating temperature is over 10 000 times lower than that of a similarly sized mammal resting at its normal body temperature. Values of metabolic rate towards the end of a long submergence, estimated on the basis of lactate accumulation in the body fluids (Jackson et al. 2000), are estimated to be about 0.01 cal (0.04 J) kg−1 min−1 (0.7 mW kg−1). To illustrate how slowly the metabolic fires are burning in this situation, consider that if all of the turtle's metabolic heat was stored, it would take almost 7 days for its body temperature to rise by 0.1 °C. Figure 1 depicts the pattern of metabolic depression that occurs during anoxia at 3 and 24 °C.
Figure 1. Metabolic rate depression during anoxic submergence at 24 °C (top) and 3 °C (bottom).
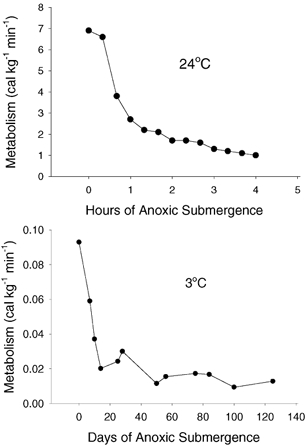
Note the similarity in pattern but the time scale difference and the 100-fold difference in the final metabolic values. Data adapted from Jackson (1968), Jackson & Heisler (1982) and Jackson et al. (2000).
The low rate of metabolism is functionally important to the turtle because it drastically delays the depletion of stored substrate and slows the build-up of acid endproducts; nevertheless, the depressed metabolism must still supply all the cellular energy requirements of the animal during its long submergence. Clearly, these requirements are profoundly depressed. The animals are responsive to stimuli and periodically move about but are generally extremely sluggish. Systolic arterial blood pressure is only 10 cmH2O and heart rates average one beat in 2–3 min, and in extreme cases only every 5–10 min (Herbert & Jackson, 1985b). Cardiac output has not been measured under these circumstances, but flow must essentially stop during the long diastolic pauses. Blood stasis in euthermic mammals can result in clot formation, but clotting rates at low temperature are greatly slowed (Valeri et al. 1995). A practical advantage this affords the experimenter is that chronic catheters remain patent with minimal attention. At the low prevailing blood pressures, renal function is presumably minimal (Warburton & Jackson, 1995; Jackson et al. 1996), although Ca2+ and lactate concentrations are both elevated in urine collected from the urinary bladder after 3–4 months of anoxia at 3 °C (Jackson & Ultsch, 1982).
Cellular responses
Experimental evidence from anoxic turtles indicates a coordinated downregulation in the rates of both ATP utilization and ATP production. The mechanisms involved are still being resolved, but it is clear that cellular ATP levels remain stable during long periods of anoxia (Kelly & Storey, 1988).
ATP utilization
Major consumers of cellular energy are the ion pumps that maintain transmembrane ionic gradients and protein synthesis. Evidence exists for sharp reductions in both these cellular functions during anoxia. The energy requirement for maintaining transmembrane ionic gradients is reduced by slowing the passive flux of ions through membrane channels, via so-called ‘channel arrest’ (Hochachka, 1986), although the mechanisms whereby this occurs are not well understood. Channel arrest potentially has general importance in circumstances involving reduced metabolism. In a warm reptile, for example, transmembrane ion gradients are similar to those in a mammal of the same size, yet the metabolic rates of these animals are greatly different (Hulbert & Else, 1981). Assuming that the cost of maintaining these gradients requires a significant fraction of each animal's total metabolism, then the cost to the reptile must be less. Furthermore, when the reptile's temperature falls, so does its metabolic rate, but ion concentrations remain essentially unchanged (Herbert & Jackson, 1985a). The interpretation is that membrane ion leakage through ion channels is less in the reptile, and that it falls further when the animal's temperature falls. Anoxia induces a further reduction in ion channel activity.
Studies of channel arrest in the anoxic turtle have focused largely on the brain because of the critical importance of maintaining this organ's function and because of the brain's normally large commitment of energy to ionic regulation. In addition, the susceptibility of the mammalian brain to hypoxic damage is linked to the collapse of ion gradients, and destructive Ca2+ influx (Hochachka, 1986; Sattler & Tymianski, 2000). Evidence exists for reduced channel function for Na+, K+ and Ca2+ in the turtle brain, although the evidence for the first two ions is indirect. Reduced Na+ channel density was found in isolated cerebellum exposed to anoxic perfusion for 4 h at room temperature using the channel ligand brevetoxin (Pérez-Pinzón et al. 1992). The magnitude of the reduction (42 %) is much less than the estimated fall in brain metabolism, so it is possible that passive Na+ flux is further lowered by decreasing the open probability of remaining channels. Downregulation of K+ channels was inferred from a slowing of K+ efflux from anoxic neurones, compared to normoxic neurones, in anaesthetized animals treated with ouabain to poison Na+–K+-ATPase (Chih et al. 1989). The mammalian brain responds to anoxia and the attendant fall in ATP by opening ATP-sensitive K+ channels (KATP channels). The resultant hyperpolarization reduces electrical activity and serves as a short-term defence mechanism, but persistent anoxia leads in minutes to massive failure due to a rapid increase in extracellular K+, membrane depolarization, rapid influx of Ca2+ through voltage-dependent channels and Ca2+-induced cellular damage. In contrast, the turtle brain apparently lacks KATP channels and instead reduces activity in other K+ channels. Coupled with the similar effect on Na+ leakage, the energy required for Na+–K+-ATPase can thereby be significantly reduced.
Direct evidence for inactivation of Ca2+ channels in anoxic slices of turtle cortex has been obtained by Bickler and colleagues (Bickler & Buck, 1998). Initial studies demonstrated that the intracellular Ca2+ concentration ([Ca2+]i) of cortical brain slices of turtle fell slightly during anoxia in contrast to a rapid and large increase in [Ca2+]i in rat brain slices (Bickler, 1992). Inhibition of glycolysis with iodoacetate caused rapidly increased [Ca2+]i in the acutely anoxic turtle brain, but this effect was greatly attenuated after prolonged anoxic exposure, suggesting a time-dependent arrest of Ca2+ channels. Because glutamate receptors are implicated in mediating lethal neuronal Ca2+ fluxes in anoxia-sensitive animals (Bickler & Hansen, 1994), these receptors were postulated as potential sites of downregulation in turtle neurones. Patch-clamp studies of NMDA receptors from turtle cortex revealed a 65 % decrease in the open probability of Ca2+ channels and further established that the downregulation was mediated by adenosine (Buck & Bickler, 1998), a molecule previously implicated in turtle brain anoxia tolerance (Nilsson & Lutz, 1992). Furthermore, normal [Ca2+]i persisted in turtle brain slices removed from animals after 6 weeks of experimental anoxia at 2–3 °C, even in the presence of elevated solution [Ca2+] simulating the in vivo state (Bickler, 1998). In recent studies, time-dependent mechanisms for NMDA-receptor downregulation have been identified, including an acute reduction of channel open probability mediated by phosphatase 1 or 2A, a delayed suppression of receptors associated with elevated [Ca2+]i and controlled by calmodulin, and a longer term removal of NMDA receptors from the cell membrane (Bickler et al. 2000). Of particular significance is that prevention of NMDA-receptor downregulation eliminated the anoxia tolerance of the brain tissue.
Utilization of ATP for protein synthesis is also suppressed during anoxia in turtle hepatocytes (Land et al. 1993) and in the heart (Bailey & Driedzic, 1996), although increased synthesis of selected proteins has been reported (Brooks & Storey, 1993; Hochachka et al. 1996; Chang et al. 2000).
ATP production
During anoxia, ATP production occurs via glycolysis, and modulation of the flux rate is considered to be via control of key enzymes in this pathway. The reduced rate goes counter to the usual hypoxia-induced activation and has been termed the ‘reverse Pasteur effect’ (Hochachka, 1986). Storey and co-workers (Storey & Storey, 1990; Storey, 1996) suggest three effects of anoxia on glycolytic enzymes: alteration of activity via phosphorylation and dephosphorylation, reversible binding of enzymes to macromolecules or organelles and allosteric regulation via specific metabolites.
The low ATP yield of this pathway requires a large commitment of substrate in the form of glucose or glycogen to supply the energy needs of prolonged anoxia. However, the extremely low rate of glycolysis in the anoxic turtle and the large initial stores of glycogen in liver and muscle (Daw et al. 1967) probably prevent this from being a limiting factor for the animal.
Ionic and acid-base responses
A major challenge for an anoxia-tolerant animal experiencing extended anaerobiosis is the accumulation of high concentrations of lactate and the associated burden of protons. Although certain species of fish circumvent this problem by producing ethanol as the principal anaerobic endproduct (Shoubridge & Hochachka, 1980), this strategy has not been adopted by vertebrate tetrapods, which all produce lactate as the glycolytic endproduct. When ATP synthesis equals ATP hydrolysis (see above), ATP hydrolysis produces protons in a 1:1 stoichiometry with lactate production (Hochachka & Mommsen, 1983), so that anoxic turtles produce, in effect, lactic acid. After 3–5 months of experimental submergence at 3 °C, despite profound metabolic depression, circulating lactate levels can reach 150–200 mmol l−1. The acid load that this amount of lactate represents greatly exceeds normal body fluid buffering capacity and must require substantial supplemental buffering to prevent fatal acidosis. As will be discussed, the shell and skeleton of the turtle serve as the sources of this additional buffering as well as a sink for lactic acid, and these roles for the turtle's most distinctive anatomical feature may be the primary bases for its remarkable durations of anoxic tolerance.
Extracellular buffering
Because the submerged anoxic turtle has no pulmonary ventilation and only minimal kidney function (Warburton & Jackson, 1995), the primary defence against an acid load is its endogenous buffering. Initially this is restricted to the buffers within its intracellular and extracellular fluids. Intracellular buffering is not exceptional in turtles compared to other vertebrates (Shi et al. 1997), but extracellular buffering is enhanced by unusually high concentrations of HCO3− ([HCO3−]) (Smith, 1929), of the order of 40 mmol l−1, in the painted turtle (Herbert & Jackson, 1985a). In addition, the turtle possesses specialized pericardial and peritoneal fluids with [HCO3−] values of about 120 and 80 mmol l−1, respectively (Smith, 1929). Blood pH is also high in this animal at 3 °C, at about 8.0, but this is in line with vertebrate blood pH-temperature relationships in general, in accordance with alphastat regulation (Reeves, 1972). In short-term anoxic exposures, extracellular buffers, including HCO3− and plasma proteins, are exploited as the major sink for protons (Robin et al. 1964; Jackson & Silverblatt, 1974). As anoxia continues, however, and lactate levels rise, supplemental buffering is required and a characteristic pattern of plasma ion change develops (Fig. 2) that includes increases in the concentrations of K+, Ca2+ and Mg2+ and decreases in the concentration of Cl−. [Na+] generally decreases, but not always significantly. Available information on the exchange mechanisms involved and how these exchanges contribute to supplementing extracellular buffering will now be discussed.
Figure 2. Ion balance of turtles (Chrysemys picta picta) at 3 °C.

Measured data (from Ultsch et al. 1999) were collected from normoxic animals (control) and from animals after 125 days of submergence (anoxic observed). The hypothetical result of the same increase in lactic acid with no supplemental buffering response is also shown (anoxic uncompensated), in which much of the acid is unbuffered.
Calcium and magnesium
The rise in plasma lactate concentration ([lactate]) in anoxic turtles is paralleled by increased concentrations of both Ca2+ and Mg2+. In extreme cases, in which plasma [lactate] reached 150 mmol l−1 or more, total [Ca2+] was 40–50 mmol l−1 and total [Mg2+] 15–20 mmol l−1 (Ultsch & Jackson, 1982; Jackson et al. 2000). Normoxic [Ca2+] is 2–3 mmol l−1, similar to other vertebrates. Much of the total Ca2+, as much as two-thirds of the total at the highest anoxic levels, however, was complexed with lactate (Cannan & Kilbrick, 1938) so that free Ca2+ only rose to a maximum of about 12.5 mmol l−1 (Jackson & Heisler, 1982). Skeletal muscle and cardiac muscle continue to function despite the severe hypercalcaemia, albeit at a reduced level consistent with the torpid state of the animals. We hypothesized, based on earlier work documenting the depressant effect of anoxia and acidosis on heart muscle from turtle and other vertebrates (Bing et al. 1972; Williamson et al. 1975; Gesser & Jorgensen, 1982; Orchard & Kentish, 1990), that elevated [Ca2+] may help protect the heart muscle against the severe acidosis, and studies testing this have shown a small ameliorative effect (Wasser et al. 1990; Shi et al. 1999). As noted above, Bickler (1998) observed normal [Ca2+]i activity after 6 weeks of anoxic submergence at 2–3 °C, despite elevated extracellular levels.
The source of Ca2+ and Mg2+, based on both indirect and direct evidence, is considered to be the bony tissues of the turtle, the skeleton and shell. The indirect evidence is the fact that the vast bulk (> 99 %) of the animal's stores of these two elements is in bone. Direct evidence is that shell Mg2+ concentration falls during anoxia (Jackson et al. 2000), and that both Ca2+ and Mg2+ are released from the shell in vitro, as a direct function of the incubating solution acidity, and reach levels in vitro that approximate those observed at the same pH in vivo (Jackson et al. 1999). The accompanying anion, based on the stoichiometry of CO2 evolution during in vitro experiments, is carbonate. No phosphate was released from shell in vitro down to an incubating pH of 6.0 and no increase in plasma phosphate has been observed during anoxia in vivo (Jackson et al. 2000). Thus the principal mineral molecule of shell and bone, calcium phosphate, is apparently not broken down during anoxic acidosis, but calcium and magnesium carbonates are mobilized as extracellular buffers.
Sodium and potassium
Plasma [Na+] generally falls during anoxic submergence, although not always significantly. Plasma [K+], on the other hand, rises predictably to levels as high as 12 mequiv l−1 (Ultsch et al. 1999). The reciprocal change in these ions may represent a partial failure of Na+–K+-ATPase function in cell membranes (Jackson & Heisler, 1983), although the fall in Na+ may also be attributed in part to the diluting effect of water that enters the animal and increases body weight by about 5 % on average during anoxia at 3 °C (Jackson et al. 2000). However, a significant reduction occurred in both shell and skeletal [Na+], which presumably added both Na+ and carbonate to the extracellular fluid. The magnitude of the shell and bone loss was such that a significant rise in plasma [Na+] should have occurred; because it did not, we hypothesize that Na+ may be lost to the surrounding water and that the release from shell and bone serves to defend extracellular fluid [Na+] (Jackson et al. 2000).
The effect of elevated [K+] on turtle cardiac muscle mechanical and electrical activity has been investigated (Nielsen & Gesser, 2001), and decreases in contractile force, resting membrane potential, action potential duration and action potential amplitude were all observed when [K+] was increased from 2.5 to 10 mmol l−1. When hyperkalaemia was combined with anoxia, the force generated by ventricular strips declined drastically, but addition of adrenaline (10 μmol l−1) restored normal force. In vivo, the elevated plasma [K+] of anoxic turtles is associated with significant elevations of both adrenaline and noradrenaline (Wasser & Jackson, 1991).
Chloride
Plasma [Cl−] falls consistently during anoxia from the normal value of 80–85 mequiv l−1 to 50 mequiv l−1 or less after 3–4 months of anoxic submergence at 3 °C (Jackson et al. 2000). The cause of Cl− decline is uncertain, but may be the result of the same mechanisms affecting [Na+]: dilution by water uptake from the environment and possible loss of Cl− to the surrounding water. On this basis, the greater fall in plasma [Cl−] than in [Na+] can be attributed to the absence of a compensatory release of Cl− from shell and bone. If this is the case, then the acid-base relevant change is the release of Na+ from shell and bone in association with carbonate and the loss of Cl− is an acid-base neutral event involving equivalent losses of Na+ and Cl− to the surrounding water.
Lactic acid uptake by shell and bone
During anoxia, lactate enters shell and bone in parallel with the rise in plasma [lactate]. After long-term submergence anoxia at 3 and 10 °C, lactate concentrations in shell and bone (mmol (kg wet weight)−1) approximated plasma concentrations (mmol l−1). Because of the large mass of shell and skeletal bone in these animals (over 35 % of body mass), an estimated 40–45 % of the total body lactate resided within these mineralized structures (Jackson, 2000). During recovery from anoxia, lactate levels fell in the shell, again in parallel with the restoration of plasma lactate.
Observations on shell incubated in vitro reveal this to be an acid-base relevant mechanism (Jackson et al. 1999). Uptake of lactate by shell powder caused an alkalinization of the bathing solution, and release of lactate from lactate-loaded shell acidified the solution. In effect, this indicates that lactate exchanges occur accompanied by a proton. Within the shell, again based on in vitro experiments, the acid is buffered by carbonate, releasing CO2 to the surrounding fluid. In vivo, this CO2 can be lost to the environment via extrapulmonary avenues, so that the lactic acid sequestered within the shell has no effect whatever on blood acid-base status.
The physical state of the lactate anion within the shell is not known, but indirect evidence suggests that much of it is in combined form, probably associated with Ca2+. Because the water content of shell is only about 30 %, the [lactate] would have to be several times higher than in the plasma if it were in simple solution. In addition, it is known that a calcium-lactate complex forms in the plasma of turtles under prolonged anoxic conditions (Jackson & Heisler, 1982).
It is striking that this role of bone, so central to the anoxic turtle's acid buffering, has apparently not been considered to be important in other organisms. The explanation is due to special features of the turtle and its anoxic response. Because of the turtle's large mass of bone, its extremely high circulating levels of lactate, and the long time available for an otherwise slow exchange process, this mechanism can be expressed to an exaggerated extent in this animal. There is no reason to think that turtle bone and shell are otherwise special in this regard, except for a relatively high concentration of carbonate (Biltz & Pellegrino, 1969).
Conclusions
The capacity to survive periods of anoxia is not unusual amongst the lower vertebrates; many species can support all living processes anaerobically for brief periods. What is exceptional in the turtle is the length of time it can continue in this state. Certainly specialized features of vulnerable organs such as the heart and brain enable function without O2, but for the organism to continue in this state for months requires additional adaptations. The key mechanisms that underlie this extended capacity are the extreme reduction in energy metabolism and the buffering contributions of its shell and bone; without these features, the turtle would not be able to survive a long winter of O2 deprivation.
Acknowledgments
This work was supported by the USA National Science Foundation (current grant IBN01-10322).
References
- Bailey JR, Driedzic WR. Decreased total ventricular and mitochondrial protein synthesis during extended anoxia in turtle heart. American Journal of Physiology. 1996;271:R1660–1667. doi: 10.1152/ajpregu.1996.271.6.R1660. [DOI] [PubMed] [Google Scholar]
- Bennett AF, Dawson WR. Metabolism. In: Gans C, Dawson WR, editors. Biology of the Reptilia, Physiology A. Vol. 5. New York: Academic Press; 1976. pp. 127–223. [Google Scholar]
- Bennett AF, Ruben JA. Endothermy and activity in vertebrates. Science. 1979;206:649–654. doi: 10.1126/science.493968. [DOI] [PubMed] [Google Scholar]
- Bickler PE. Cerebral anoxia tolerance in turtles: regulation of intracellular calcium and pH. American Journal of Physiology. 1992;263:R1298–1302. doi: 10.1152/ajpregu.1992.263.6.R1298. [DOI] [PubMed] [Google Scholar]
- Bickler PE. Reduction of NMDA receptor activity in cerebrocortex of turtles (Chrysemys picta) during 6 wk of anoxia. American Journal of Physiology. 1998;275:R86–91. doi: 10.1152/ajpregu.1998.275.1.R86. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Buck LT. Adaptations of vertebrate neurons to hypoxia and anoxia: maintaining critical Ca2+ concentrations. Journal of Experimental Biology. 1998;201:1141–1152. doi: 10.1242/jeb.201.8.1141. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Donohoe PH, Buck LT. Hypoxia-induced silencing of NMDA receptors in turtle neurons. Journal of Neuroscience. 2000;20:3522–3528. doi: 10.1523/JNEUROSCI.20-10-03522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Hansen BM. Causes of calcium accumulation in rat cortical brain slices during hypoxia and ischemia: role of ion channels and membrane damage. Brain Research. 1994;665:269–276. doi: 10.1016/0006-8993(94)91347-1. [DOI] [PubMed] [Google Scholar]
- Biltz RM, Pellegrino ED. The chemical anatomy of bone. I. A comparative study of bone composition in sixteen vertebrates. Journal of Bone and Joint Surgery. 1969;51A:456–465. [PubMed] [Google Scholar]
- Bing OHL, Brooks WW, Inamdar AN, Messer JV. Tolerance of isolated heart muscle to hypoxia: turtle vs. rat. American Journal of Physiology. 1972;223:1481–1485. doi: 10.1152/ajplegacy.1972.223.6.1481. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. De novo protein synthesis and protein phosphorylation during anoxia and recovery in the red-eared turtle. American Journal of Physiology. 1993;265:R1380–1386. doi: 10.1152/ajpregu.1993.265.6.R1380. [DOI] [PubMed] [Google Scholar]
- Buck LT, Bickler PE. Adenosine and anoxia reduce N-methyl-d-aspartate response open probability in turtle cerebrocortex. Journal of Experimental Biology. 1998;201:289–297. doi: 10.1242/jeb.201.2.289. [DOI] [PubMed] [Google Scholar]
- Buck LT, Land SC, Hochachka PW. Anoxia-tolerant hepatocytes: model system for study of reversible metabolic suppression. American Journal of Physiology. 1993;265:R49–56. doi: 10.1152/ajpregu.1993.265.1.R49. [DOI] [PubMed] [Google Scholar]
- Cannan RK, Kilbrick A. Complex formation between carboxylic acids and divalent metal cations. Journal of the American Chemical Society. 1938;60:2314–2320. [Google Scholar]
- Chang J, Knowlton AA, Wasser JS. Expression of heat shock protein in turtle and mammalian hearts: relationship to anoxia tolerance. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278:R209–214. doi: 10.1152/ajpregu.2000.278.1.R209. [DOI] [PubMed] [Google Scholar]
- Chih CP, Rosenthal M, Sick TJ. Ion leakage is reduced during anoxia in turtle brain: a potential survival strategy. American Journal of Physiology. 1989;255:R338–343. doi: 10.1152/ajpregu.1989.257.6.R1562. [DOI] [PubMed] [Google Scholar]
- Daw JC, Wenger DP, Berne RM. Relationship between cardiac glycogen and tolerance to anoxia in the western painted turtle, Chrysemys picta bellii. Comparative Biochemistry and Physiology. 1967;22:69–73. doi: 10.1016/0010-406x(67)90167-3. [DOI] [PubMed] [Google Scholar]
- Gesser H, Jorgensen E. pHi, contractility and Ca-balance under hypercapnic acidosis in the myocardium of different vertebrate species. Journal of Experimental Biology. 1982;96:405–412. doi: 10.1242/jeb.96.1.405. [DOI] [PubMed] [Google Scholar]
- Herbert CV, Jackson DC. Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii. I. Blood acid-base and ionic changes during and following anoxic submergence. Physiological Zoology. 1985a;58:655–669. [Google Scholar]
- Herbert CV, Jackson DC. Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii. II. Metabolic rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiological Zoology. 1985b;58:670–681. [Google Scholar]
- Hochachka PW. Defence strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxic tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proceedings of the National Academy of Sciences of the USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Mommsen TP. Protons and anaerobiosis. Science. 1983;219:1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL. Comparison of the ‘mammal machine’ and the ‘reptile machine’: energy use and thyroid activity. American Journal of Physiology. 1981;241:R350–356. doi: 10.1152/ajpregu.1981.241.5.R350. [DOI] [PubMed] [Google Scholar]
- Jackson DC. Metabolic depression and oxygen depletion in the diving turtle. Journal of Applied Physiology. 1968;24:503–509. doi: 10.1152/jappl.1968.24.4.503. [DOI] [PubMed] [Google Scholar]
- Jackson DC. Living without oxygen: lessons from the freshwater turtle. Comparative Biochemistry and Physiology A Molecular and Integrative Physiology. 2000a;125:299–315. doi: 10.1016/s1095-6433(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Jackson DC. How a turtle's shell helps it survive prolonged anoxic acidosis. News in Physiological Sciences. 2000b;15:181–185. doi: 10.1152/physiologyonline.2000.15.4.181. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Crocker CE, Ultsch GR. Bone and shell contribution to lactic acid buffering of submerged turtles Chrysemys picta bellii at 3 °C. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278:R1564–1571. doi: 10.1152/ajpregu.2000.278.6.R1564. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Crocker CE, Ultsch GR. Mechanisms of homeostasis during long-term diving and anoxia in turtles. Zoology. 2001;103:150–156. [Google Scholar]
- Jackson DC, Goldberger Z, Visuri S, Armstrong RN. Ionic exchanges of turtle shell in vitro and their relevance to shell function in the anoxic turtle. Journal of Experimental Biology. 1999;202:513–520. doi: 10.1242/jeb.202.5.513. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Heisler N. Plasma ion balance of submerged anoxic turtles at 3 °C: The role of calcium lactate formation. Respiration Physiology. 1982;49:159–174. doi: 10.1016/0034-5687(82)90071-8. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Heisler N. Intracellular and extracellular acid-base and electrolyte status of submerged anoxic turtles at 3 °C. Respiration Physiology. 1983;53:187–201. doi: 10.1016/0034-5687(83)90066-x. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Silverblatt H. Respiration and acid-base status of turtles following experimental dives. American Journal of Physiology. 1974;226:903–909. doi: 10.1152/ajplegacy.1974.226.4.903. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Toney VI, Okamoto S. Lactate distribution and metabolism during and after anoxia in the turtle, Chrysemys picta bellii. American Journal of Physiology. 1996;271:R409–416. doi: 10.1152/ajpregu.1996.271.2.R409. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Ultsch GR. Long-term submergence at 3 °C of the turtle, Chrysemys picta bellii, in normoxic and severely hypoxic water. II. Extracellular ionic responses to extreme lactic acidosis. Journal of Experimental Biology. 1982;96:29–43. doi: 10.1242/jeb.97.1.87. [DOI] [PubMed] [Google Scholar]
- Kelly DA, Storey KB. Organ-specific control of glycolysis in anoxic turtles. American Journal of Physiology. 1988;255:R774–779. doi: 10.1152/ajpregu.1988.255.5.R774. [DOI] [PubMed] [Google Scholar]
- Land SC, Buck LT, Hochachka PW. Response of protein synthesis to anoxia and recovery in anoxia-tolerant hepatocytes. American Journal of Physiology. 1993;265:R41–48. doi: 10.1152/ajpregu.1993.265.1.R41. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Nilsson GE. Contrasting strategies for anoxic brain survival - glycolysis up or down. Journal of Experimental Biology. 1997;200:411–419. doi: 10.1242/jeb.200.2.411. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, Gesser H. Effects of high extracellular [K+] and adrenaline on force development, relaxation and membrane potential in cardiac muscle from freshwater turtle and rainbow trout. Journal of Experimental Biology. 2001;204:261–268. doi: 10.1242/jeb.204.2.261. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Lutz PL. Adenosine release in the anoxic turtle brain: a possible mechanism for anoxic survival. Journal of Experimental Biology. 1992;162:345–351. [Google Scholar]
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. American Journal of Physiology. 1990;258:C967–981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Pérez-Pinzón MA, Rosenthal M, Sick TJ, Lutz PL, Pablo J, Mash D. Downregulation of sodium channels during anoxia: a putative survival strategy of turtle brain. American Journal of Physiology. 1992;262:R712–715. doi: 10.1152/ajpregu.1992.262.4.R712. [DOI] [PubMed] [Google Scholar]
- Reeves RB. An imidazole alphastat hypothesis for vertebrate acid-base regulation: tissue carbon dioxide and body temperature in bullfrogs. Respiration Physiology. 1972;14:219–236. doi: 10.1016/0034-5687(72)90030-8. [DOI] [PubMed] [Google Scholar]
- Robin ED, Vester JW, Murdaugh HV, Millen JE. Prolonged anaerobiosis in a vertebrate: anaerobic metabolism in the freshwater turtle. Journal of Cellular and Comparative Physiology. 1964;63:287–297. doi: 10.1002/jcp.1030630304. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. Journal of Molecular Medicine. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Shi H, Hamm PH, Lawler RG, Jackson DC. Different effect of simple anoxic lactic acidosis and simulated in vivo anoxic acidosis on turtle heart. Comparative Biochemistry and Physiology A Molecular and Integrative Physiology. 1999;122:173–180. doi: 10.1016/s1095-6433(98)10163-0. [DOI] [PubMed] [Google Scholar]
- Shi H, Hamm PH, Meyers RS, Lawler RG, Jackson DC. Intracellular pH regulation of isolated turtle heart during normoxic and anoxic acidosis: a 31P-NMR study. American Journal of Physiology. 1997;272:R6–15. doi: 10.1152/ajpregu.1997.272.1.R6. [DOI] [PubMed] [Google Scholar]
- Shoubridge EA, Hochachka PW. Ethanol: novel endproduct in vertebrate anaerobic metabolism. Science. 1980;209:308–309. doi: 10.1126/science.7384807. [DOI] [PubMed] [Google Scholar]
- Smith HW. The inorganic composition of the body fluids of the Chelonia. Journal of Biological Chemistry. 1929;82:541–661. [Google Scholar]
- Storey KB. Metabolic adaptations supporting anoxia tolerance in reptiles: recent advances. Comparative Biochemistry and Physiology B Biochemistry and Molecular Biology. 1996;113:23–35. doi: 10.1016/0305-0491(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Quarterly Review of Biology. 1990;65:145–174. doi: 10.1086/416717. [DOI] [PubMed] [Google Scholar]
- Ultsch GR. Ecology and physiology of hibernation and overwintering among freshwater fishes, turtles, and snakes. Biological Reviews. 1989;64:435–516. [Google Scholar]
- Ultsch GR, Carwile ME, Crocker CE, Jackson DC. The physiology of hibernation among painted turtles: the eastern painted turtle, Chrysemys picta picta. Physiological and Biochemical Zoology. 1999;72:493–501. doi: 10.1086/316687. [DOI] [PubMed] [Google Scholar]
- Ultsch GR, Jackson DC. Long-term submergence at 3 °C of the turtle, Chrysemys picta bellii, in normoxic and severely hypoxic water: I. Survival, gas exchange and acid-base status. Journal of Experimental Biology. 1982;96:11–28. doi: 10.1242/jeb.97.1.87. [DOI] [PubMed] [Google Scholar]
- Valeri CR, MacGregor H, Cassidy G, Tinney R, Pompei F. Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Critical Care Medicine. 1995;23:698–704. doi: 10.1097/00003246-199504000-00019. [DOI] [PubMed] [Google Scholar]
- Warburton SJ, Jackson DC. Turtle (Chrysemys picta bellii) shell mineral content is altered by exposure to prolonged anoxia. Physiological Zoology. 1995;68:783–798. [Google Scholar]
- Wasser JS, Freund EV, Gonzalez LA, Jackson DC. Force and acid-base state of turtle cardiac tissue exposed to combined anoxia and acidosis. American Journal of Physiology. 1990;259:R15–20. doi: 10.1152/ajpregu.1990.259.1.R15. [DOI] [PubMed] [Google Scholar]
- Wasser JS, Jackson DC. Effects of anoxia and graded acidosis on the levels of circulating catecholamines in turtles. Respiration Physiology. 1991;84:363–377. doi: 10.1016/0034-5687(91)90130-b. [DOI] [PubMed] [Google Scholar]


