Abstract
This study was conducted to investigate the role of splanchnic and adipose tissue in the regulation of fatty acid (FA) metabolism at rest, during 1 h of semi-recumbent cycle exercise at 60 % of maximal power output and 3 h of recovery. In six post-absorptive healthy volunteers catheters were placed in a radial artery, hepatic vein and a subcutaneous vein on the anterior abdominal wall. Whole body, and regional splanchnic and adipose tissue FA metabolism were measured by a constant infusion of the stable isotopes [U-13C] palmitate and [2H5] glycerol and according to Fick's principle. The whole body rate of extracellular FA reesterification was similar at rest and during exercise (≈290 μmol min−1) and increased during recovery to a plateau of 390 μmol min−1. FA and triacylglycerol (TAG) uptake by adipose tissue was undetectable, but a constant but small glycerol uptake of ≈25 nmol (100 g)−1 min−1 was observed. From the FA taken up by the splanchnic area, 13 % was oxidized, 5–11 % converted to ketone bodies, and ≈35 % incorporated in TAG released both at rest and at the third hour of recovery from exercise. Splanchnic FA reesterification could account for 51 % and 58 % of whole body extracellular FA reesterification, of which half was accounted for by TAG released from the splanchnic area, at rest and in recovery, respectively. In conclusion, in the post-absorptive state, adipose tissue contributes very little to extracellular FA reesterification and splanchnic reesterification can account for 50–60 %, implying that FA reesterification in other tissues is important. The extracellular FA reesterification rate does not change with exercise but is higher during recovery. Furthermore, the uptake of glycerol by adipose tissue indicates that adipose tissue can metabolize glycerol.
Fat oxidation supplies much of the body's energy needs, particular in fasting and exercise. Therefore, the regulation of fat deposition and mobilization plays an important role in energy homeostasis. A substrate cycle exists when opposing non-equilibrium reactions are active simultaneously (Newsholme & Crabtree, 1976). The triglyceride-fatty acid cycle is such a cycle where fatty acids (FA) are released during the process of triacylglycerol (TAG) hydrolysis and subsequently reesterified into TAG. The FA can be directly reesterified in the cell of origin, referred to as intracellular FA reesterification, or released into the circulation with subsequent oxidation or reesterification in tissues, the latter being referred to as FA extracellular reesterification. Adipose tissue is considered to be the major site of intracellular FA reesterification, and the liver is considered to be the major site for extracellular FA reesterification. However, evidence has been presented that most of the extracellular FA reesterification occurs in extrahepatic tissues (Diraison & Beylot, 1998; Sidossis et al. 1998; Jensen et al. 2001).
Newsholme & Crabtree (1976) hypothesized that substrate cycling amplifies the response of substrate flux to a given change in regulator. Large changes in the demand for fat as a substrate occur in response to exercise and in the recovery period following exercise. Indeed, substantial changes have been reported in FA intra- and extracellular reesterification rates when going from rest to exercise and from exercise to recovery. The major changes were suggested to occur in extracellular FA reesterification (Wolfe et al. 1990). The accuracy of the estimations of intra- and extracellular FA reesterification depends on the assumption that 3 × glycerol appearance in the circulation is representative of the total FA appearance from lipolysis in the body (Wolfe et al. 1990; Campbell et al. 1992). This implies that no de novo glycerol synthesis takes place, and that glycerol cannot be directly reincorporated into TAG. However, lately evidence has been presented of a substantial glycerol metabolism in skeletal muscle (Landau et al. 1996; Coppack et al. 1999; Jensen, 1999). So far no data are available comparing systemic FA reesterification rates with direct measurements across the splanchnic area and adipose tissue. Therefore, the first aim of the present study was to determine TAG-FA cycling via whole body isotopic methods in healthy volunteers in the post-absorptive state during exercise and recovery when fat utilization is substantially changed. The second aim was to quantify the role of hepatic and adipose tissue in FA homeostasis by combining the arterial-venous difference technique and tracer methodology across the splanchnic area and subcutaneous adipose tissue of the anterior abdominal wall.
METHODS
Subjects
Six young, healthy male volunteers participated in the study. Their mean age (range) was 24.3 years (22–28), weight 79.8 kg (73–86), and height 186 cm (180–191). The average body mass index was 23.1 kg m−2 (20.9–25.0). The lean body mass was 66.8 kg (63–73) and the fat mass was 11.7 kg (6.9–15.3). The abdominal fat mass in the region from the diaphragm to the femoral heads was 2.7 kg (1.6–3.4). Peak oxygen uptake was 3716 ml min−1 (3307–4161). The subjects were given a written and an oral description of the study and the possible risks and discomfort involved before giving their voluntary consent to participate. The study was performed according to the Declaration of Helsinki II and was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark.
Protocol
About 2 weeks prior to the experiment the subjects had their maximal oxygen uptake determined. They exercised in a semi-recumbent position on an electrically braked cycle ergometer (Ergometrics er900L, Ergoline, Bitz, Germany) initially at 50 W increasing by 50 W every 2 min until exhaustion. Oxygen uptake and carbon dioxide output were measured continuously during the test by an Oxycon champion system (Jaeger, Wuerzburg, Germany) using a facemask and the breath-by-breath technique. Whole body composition was determined by DEXA-scanning (Lunar DPX-IQ, software version 4.6c, Lunar Corporation, Madison, WI, USA) using the medium scan mode and extended research analysis. Abdominal fat mass was determined in a region of interest as described in Jensen et al. (1995). On the days prior to the experiments the subjects consumed their habitual diet but refrained from food items containing carbohydrates from source with a high 13C-background. Twenty-four hours prior to the experiment they refrained from vigorous physical activity. The experiment started at 8 a.m. when the subjects arrived in the laboratory after an overnight fast from 10 p.m. The experiment consisted in general of three periods, a pre-exercise resting period (baseline), an exercise period in which the subjects exercised at 60 % of their maximal power output, and a post-exercise resting period. The baseline, resting period was of 30 min duration, and it was initiated 90 min after the start of the infusions of indocyaninegreen (ICG), and a prime of NaH13CO3 (1.5 μmol kg−1), a primed (1.5 μmol kg−1) constant infusion of [1,1,2,3,3-2H5] glycerol (0.142 μmol kg−1 min−1) and a constant infusion of [U-13C] palmitate (0.0135 μmol kg−1 min−1). After the baseline period the subjects exercised for 60 min at 60 % of maximal power output. The exercise was performed in the semi-recumbent position. At the onset of exercise the infusion rate of [U-13C]palmitate and [1,1,2,3,3-2H5]glycerol was doubled and immediately on termination of exercise the infusion rate was decreased to the pre-exercise resting infusion rate. After exercise the subjects were studied for another 3 h during rest. During both the pre- and post-exercise periods subjects were in the recumbent position.
Acetate recovery was assessed in three of the volunteers at least 2 weeks after the above-described study was performed to correct for underestimation of FA oxidation rates. In addition, acetate recovery was assessed in three additional volunteers. The protocol was identical to that described above, but recovery was extended to 6 h. In addition, three of the volunteers were studied during 9 h at rest. A catheter was inserted in a forearm vein for the continuous infusion of [1,2-13C]acetate (0.104 μmol kg−1 min−1) with a NaH13CO3 prime (1.5 μmol kg−1). The acetate infusion rate during exercise was doubled at the onset of exercise and decreased to the resting infusion rate at termination of exercise. Whole body acetate recovery was determined by measuring 13CO2 enrichment in the breath and used to correct whole body and splanchnic palmitate oxidation, as it has been shown that systemic acetate recovery is similar to splanchnic acetate recovery (Mittendorfer et al. 1998).
Catheterizations
The subjects were catheterized in a subcutaneous vein on the anterior abdominal wall, in a hepatic vein and in a radial artery. The subcutaneous, abdominal vein was catheterized as previously described (Simonsen et al. 1994) using a polyurethane catheter (o.d. 0.9 mm; Ohmeda, Swindon, UK). The catheterization was done by ultrasound Colour-Doppler guidance, because this enables catheterization of a vein in the subcutaneous tissue not visible through the skin. After catheterization, the catheter was kept patent throughout the study by continuous infusion of isotonic sodium chloride at a rate of 40 ml h−1. The right femoral vein was catheterized during local analgesia (lignocaine (lidocaine) 1 %, 5–10 ml) and a polyethylene catheter (o.d. 2.0 mm) was advanced to a right-sided hepatic vein and left in situ with the tip positioned 3–4 cm from wedge position during the rest of the experiment. The catheterization was done during fluoroscopic control. This catheter was kept patent during the experiment by regular flushing with isotonic sodium chloride containing 1 i.u. ml−1 heparin. Another catheter was inserted into the radial artery of the non-dominant arm during local analgesia (1 ml 1 % lignocaine). The catheterization was performed with an Artflon catheter (Ohmeda). The catheter was kept patent during the experiment by regular flushing with isotonic sodium chloride. A forearm vein was catheterized for infusion of ICG and the stable isotope tracers. After catheterization procedures, primed or constant infusions of ICG and metabolic tracers were started.
Measurements
Adipose tissue blood flow
Adipose tissue blood flow was measured by the 133Xe washout technique as described in Bülow (2001). About 1 MBq of 133Xe dissolved in 0.1 ml isotonic sodium chloride was injected into the subcutaneous, abdominal adipose tissue on the contralateral side of the catheter position. The washout of 133Xe was registered by a scintillation counter system (Mediscint Oakfield Instruments, Oxford, UK) strapped to the skin above the 133Xe depot. The calculation of adipose tissue blood flow was performed using an average tissue/blood partition coefficient of 8 for the subjects (Bülow et al. 1987).
Splanchnic blood flow
Splanchnic plasma flow was measured by continuous infusion of ICG (Henriksen & Winkler, 1987). Immediately after the catheterization a primed (1 mg) continuous infusion (170 μg min−1) of ICG was given for the rest of the experiment. Blood samples for determination of splachnic plasma flow were drawn in triplicate (5 min intervals) at the same time as the metabolites were measured. The ICG concentration was determined in plasma by spectrophotometry at 804 and 905 nm. Splanchnic blood flow was calculated from splanchnic plasma flow by correction with simultaneously measured haematocrit values.
Blood sampling
Blood samples were preferably drawn simultaneously from the three catheters. However, sometimes the blood flow level in the adipose tissue limited the rate at which blood could be drawn from the subcutaneous catheter, and in such situations a time delay developed between the samples drawn from this site and the samples drawn from the artery and hepatic vein. Generally, for measurements of glycerol, FA, TAG and 3-OH-butyrate, three sample-sets were drawn during the baseline rest period, three samples during exercise every 20 min, and finally every 30 min in the post-exercise period. The post-exercise period lasted 3 h. The blood was collected in vials at 4 °C, and whole blood was immediately deproteinized or the blood was collected in 4 °C EDTA-containing tubes and immediately centrifuged at 4 °C. The samples were stored at -20 °C until analysis.
Blood analysis
The analyses were performed in duplicate. 3-OH-butyrate levels were measured in neutralized, deproteinized extracts of whole blood, and glycerol, FA and TAG were measured in plasma as previously described (Bülow et al. 1999).
Tracer analysis
Glycerol enrichment was measured by gas chromatography-mass spectrometry (GC-MS, Automass II, Finnigan, France). In preparation for GC-MS analysis, plasma samples were processed to make a trifluorobutyrate derivative of glycerol. For the preparation of the fluorobutyrate derivative of glycerol, 3 ml of ethanol: choloroform (2.3: 1) was added to 200 μl of plasma, mixed and centrifuged. The top layer was extracted again with 2 ml choroform and 1 ml of H2O (adjusted to pH = 2, with HCl), mixed and centrifuged. The top layer was evaporated under a stream of N2. Two hundred microlitres of heptafluorobutyric acid anhydride in ethylacetate (1:3 v/v) was added to the residue and heated for 10 min at 70 °C. The solution was evaporated under a stream of N2 and the residue re-dissolved in 1 ml ethyl acetate. The glycerol enrichment was determined by splitless injection of 1 μl onto a 30 m capillary fused-silica column (CP-SIL 8CB, Chrompack, The Netherlands), injector temperature was set at 265 °C. Helium carrier gas was used at a flow rate of 1.8 ml min−1. Typically, the GC oven was programmed from an initial 50 °C to 300 °C (50 °C held 1 min; 50–85 °C, ramp 20 °C min−1, 85–88 °C, ramp 0.5 °C min−1; 88–300 °C, ramp 50 °C min−1, held for 10 min). The instrument was controlled by Finnigan Lucy 4.0 software. The isotopic enrichment of glycerol was determined using electron impact ionization; selectively monitoring ions at mass-to-charge ratio (m/z) of 252–256, representing the molecular ions of unlabelled (252) and labelled derivatives (256), respectively. The isotopic ratios corresponding to the ratio of isotopomer peak areas were automatically calculated at the end of each analysis by MassRatio V2.0 software (CMRC, Denmark).
Plasma palmitate concentration was determined by GC (Autosystem XL, Perkin Elmer, USA) using heptadecanoic acid as internal standard, and 13C-enrichment of plasma palmitate was determined by gas chromatograph-combustion-isotope ratio mass spectrometry (GC-C-IRMS, Hewlett Packard 5890- Finnigan GC combustion III-Finnigan Deltaplus, Finnigan MAT, Germany). In preparation of GC and GC-C-IRMS analysis, plasma samples were processed to make a methyl derivative of palmitate. To 200 μl of plasma 100 μl of the internal standard heptadecanoic acid (110 mg dl−1) was added, and lipids extracted according the Folch method (Folch et al. 1957). The plasma tri-, di- and mono-acylglycerols, phospholipids, cholesterol and FAs were isolated by thin-layer chromatography (petroleum ether, diethyl ether, acetic acid, 120:25:1.5 v/v/v) on silica gel 60 plates (Merck, Germany). The plates were left to dry under nitrogen and sprayed with rhodamine 6G solution (0.01 % in methanol). The different lipid fractions were visualized under long-wave ultraviolet light and the FAs, identified by means of FA standards run on separate lanes of the same plate, scraped off into glass tubes. The FAs were extracted twice with hexane, which was then evaporated under a stream of nitrogen. To the isolated FA band, 2 ml of methanol and iso-octane (4:1 v/v), and 0.2 ml of acetyl chloride were added and heated for 1 h at 100 °C. Thereafter, 5 ml of 6 % potassium carbonate was added and mixed. After centrifugation, the upper layer was evaporated under N2 and re-dissolved in iso-octane. The FA concentrations were determined by injecting 2 μl in the split mode (1/5), onto a 30 m capillary fused-silica column (Rtx-2330, Restex, USA), injector temperature at 300 °C. Helium carrier gas was used at a flow rate of 1.8 ml min−1. Typically, the GC oven was programmed from an initial 140 °C to 255 °C (140 °C held 1 min; 140–194 °C, ramp 2 °C min−1, 194–215 °C, ramp 4 °C min−1; 215–255 °C, ramp 20 °C min−1, held for 10 min). The 13C-palmitate enrichment was determined by injecting 2 μl onto a 30 m capillary fused-silica column (Rtx-2330, Restex, USA), via a HP-PTV injector. The injectors vent-flow was set at 5 p.s.i., and the initial temperature was 85 °C for 1 min followed by a ramp of 50 °C min−1 to 255 °C. Typically the GC oven was programmed from an initial 85 °C to 255 °C (85 °C held 2 min; 85–135 °C, ramp 10 °C min−1, 135–145 °C, ramp 1 °C min−1; 145–255 °C, ramp 20 °C min−1, held for 10 min). The C-IRMS was controlled by Isodat ver6 software. The isotopic enrichment of palmitate was expressed as the Δ difference between the 13C/12C ratio of the sample and a known laboratory reference standard related to Pee Dee Belemnitella (PDB) limestone. The methyl derivative of palmitate contains 17 carbons of which 16 are from the palmitate, thus tracer/tracee ratio (TTR) of palmitate was corrected by a factor 17/16.
Samples of arterial, adipose and hepatic venous blood, and expired breath for measurement of 13CO2 enrichment were determined by gas chromatograph-isotope ratio mass spectrometry (GC-IRMS, Deltaplus, Finnigan MAT, Germany). Ten ml of expired air was collected in a vacutainer. The 13C/12C ratio was determined by split injection (ratio 1: 4) of 20 μl of the expired air onto a poraplot Q column (Chrompack, The Netherlands), injector and column at 30 °C). For the determination of blood CO2 enrichment, CO2 was liberated by adding 0.5 ml of 2.5 m phosphoric acid to 0.5 ml of blood in a 10 ml vacutainer. The tubes were brought to pressure with pure helium. The 13C- to 12C ratio was determined by split injection (ratio 1:10) of 20 μl of the headspace on the GC-IRMS. The isotopic enrichment of breath or blood CO2 was expressed as the Δ per mil difference between 13C/12C of the sample and that of a known laboratory reference standard related to PDB.
Calculations
Whole body measurements
Whole body measurements of the rate of appearance (Ra) and disappearance (Rd) of palmitate and glycerol were calculated using the non-steady-state equations of Steele (Steele, 1959) adapted for stable isotopes (Wolfe, 1992):
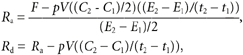 |
where F is the isotopic infusion rate (μmol min−1). E1 and E2 are the arterial isotope enrichments of palmitate or glycerol (TTR) at time 1 (t1) and 2 (t1) (min), respectively. C1 and C2 are the arterial concentrations at t1 and t2 (μmol l−1), respectively. pV is the volume of distribution, 0.04 and 0.23 l (kg body weight)−1 for palmitate and glycerol, respectively (Romijn et al. 1993). The assumption that this value is appropriate for resting and exercise conditions is obviated by the presence of near steady-state conditions. Palmitate oxidation rates were calculated:
 |
where ECO2 is the breath 13C/12C ratio, VCO2 is the carbon dioxide output (μmol min−1) and ar is the mean acetate correction factor as measured in an identical experiment with [1,2-13C]acetate infusion (Fig. 1).
Figure 1. Acetate recovery during 9 h of rest, and 2 h rest followed by 1 h of exercise and 6 h of recovery.
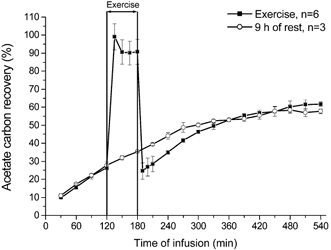
Values are means ± s.e.m. Six subjects were studied using the same protocol as the actual study, but with an extended recovery period of 6 h. Acetate recovery was studied in three subjects during 9 h of rest.
Regional measurements
Regional measurements were calculated using the following equations:
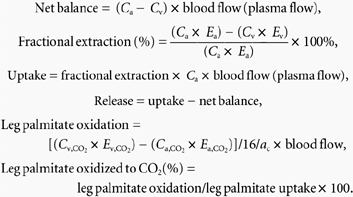 |
Cv,CO2, Ca,CO2, and Ev,CO2, Ea,CO2, are the blood CO2 concentration and enrichment in TTR in the hepatic vein and artery, respectively. Ca, Cv and Ea, Ev are the concentration and enrichment in TTR in the radial artery and hepatic or adipose vein, respectively. The parameter ac is the acetate correction factor measured at whole body level (Table 1) on the assumption that labelled carbon recovery from acetate across the splanchnic area is similar to that of the whole body (Mittendorfer et al. 1998). All palmitate data were converted to total FA by dividing the palmitate data by the fractional contribution of palmitate total FA concentration of each subject (0.22 ± 0.1).
Table 1.
Whole body and splanchnic FA oxidation, ratio of FA and glycerol Ra, and relative contribution of the regional uptake and release to systemic Rd and Ra of FA and glycerol
| RER | Fat oxidation (g min−1) | FA oxidation (%) | Ra,FA/Ra,glyc | FArel/Glycrel | FA upt/Rd,FA(%) | FArel/Ra,FA(%) | Glyc upt/Rdglyc(%) | Glycrel/Ra,glyc(%) | |
|---|---|---|---|---|---|---|---|---|---|
| Whole body | |||||||||
| Rest | 0.80 ± 0.02 | 0.08 ± 0.01 | 45 ± 4 | 2.4 ± 0.3 | – | – | – | – | – |
| Exercise | 0.85 ± 0.02* | 0.52 ± 0.10* | 71 ± 6* | 2.0 ± 0.3 | – | – | – | – | – |
| 3 h recovery | 0.77 ± 0.03* | 0.10 ± 0.02* | 41 ± 4 | 2.5 ± 0.2 | – | – | – | – | – |
| Splanchnic area | |||||||||
| Rest | – | – | 12 ± 4 | – | 4.8 ± 0.8 | 45 ± 7 | 17 ± 3 | 43 ± 10 | 7 ± 1 |
| Exercise | – | – | 15 ± 4 | – | 5.3 ± 1.3 | 24 ± 4* | 11 ± 3 | 37 ± 7 | 5 ± 1 |
| 3 h recovery | – | – | 13 ± 4 | – | 6.2 ± 1.2 | 50 ± 8* | 17 ± 4 | 38 ± 4 | 7 ± 1 |
| Abdominal fat tissue‡ | |||||||||
| Rest | – | – | n.d. | – | 2.8 ± 0.5 | 0.1 ± 1.0† | 35 ± 7 | 2.7 ± 1.6 | 27 ± 2 |
| Exercise | – | – | n.d. | – | 2.8 ± 0.4 | −1.3 ± 1.3† | 24 ± 4 | 2.2 ± 1.0 | 19 ± 3 |
| 3 h recovery | – | – | n.d. | – | 2.7 ± 0.4 | 2.0 ± 1.8† | 38 ± 5 | 2.0 ± 0.5 | 41 ± 4* |
RER is the respiratory exchange ratio; fat oxidation is the total fat oxidation measured via indirect calorimetry; FA is fatty acid; Ra is rate of appearance; Rd is rate of disappearance; n.d., not detectable
significantly different from rest
not different from zero.
Fat tissue was 11.7 ± 1.1 kg for the 6 subjects from DEXA measurements, and assuming that abdominal fat is representative for all fat depots. The recovery data presented are the average of the measurements made after 2.5 and 3 h of recovery.
Whole body and splanchnic FA reesterification
The total rate of lipolysis expressed as FA units was calculated as 3 × glycerol Ra, assuming that glycerol appears in the blood only as a product of lipolysis, cannot be directly incorporated into triglycerides, and that TAG undergoes complete hydrolysis. Intracellular FA reesterification, in which FA never leaves the cell, was determined from the difference between lipolysis and FA Ra. Extracellular FA reesterification, that is FA reesterification after release into the circulation before being reesterified, was calculated from the difference between FA Rd and FA oxidation:
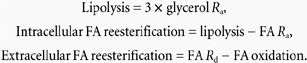 |
The fate of FA taken up by splanchnic tissue was estimated from the relative contributions of (1) oxidation, percentage palmitate taken up oxidized, (2) ketone body formation, estimated from the splanchnic 3-OH-butyrate output assuming that 1 FA is needed for the formation of 8 ketone bodies and that the 3-OH-butyrate and acetoacetate are formed in a 1:1 relation, (3) reesterification into TAG, estimated from the splanchnic net TAG release multiplied by 3.
Statistics
All the data are presented as mean ± s.e.m. The results were analysed by repeated-measures ANOVA with time as a within-subject factor. The significance of substrate net exchange and tracer exchange was analysed by comparing mean values with zero using a paired t test.
RESULTS
Whole body acetate recovery (Fig. 1)
Under resting conditions, acetate recovery increased steadily with infusion time and reached an apparent plateau after ≈8 h of infusion. In the test with exercise, acetate recovery during exercise was ≈90 % and acetate recovery was lower 10 min after the termination of exercise compared to rest just before the start of the exercise bout. After 2.5–3 h of recovery, acetate recovery was similar between the two tests. If the rest and exercise tests of the three volunteers that participated in both tests were compared then acetate recovery was virtually identical after 3 h of recovery and was similar for the remaining 3 h of recovery. The mean acetate recovery was used to correct whole body and splanchnic palmitate oxidation rates.
Regional blood flow (Fig. 2)
Figure 2. Splanchnic and abdominal adipose tissue blood flow at rest, during exercise and recovery.
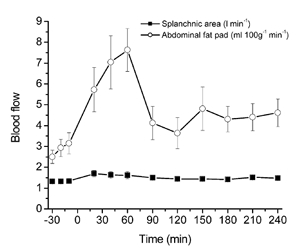
Values are means ± s.e.m. (n = 6).
The abdominal adipose tissue blood flow increased significantly during exercise. Initially post exercise the abdominal adipose tissue blood flow decreased but began to increase after 1 h of recovery, which continued until the end of the study, 3 h post exercise. The splanchnic blood flow varied around 1.3–1.6 l min−1 during the entire study period.
Whole body and regional FA metabolism (Figs 3 and 4)
Figure 3. Arterial and venous FA and glycerol concentrations and enrichments at rest, during exercise and recovery.
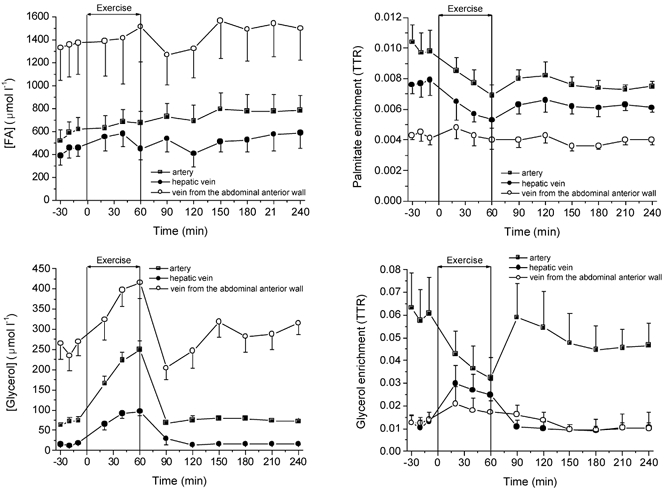
Values are means ± s.e.m. (n = 6).
Figure 4. Rate of FA appearance and regional FA exchange at rest, during exercise and recovery.
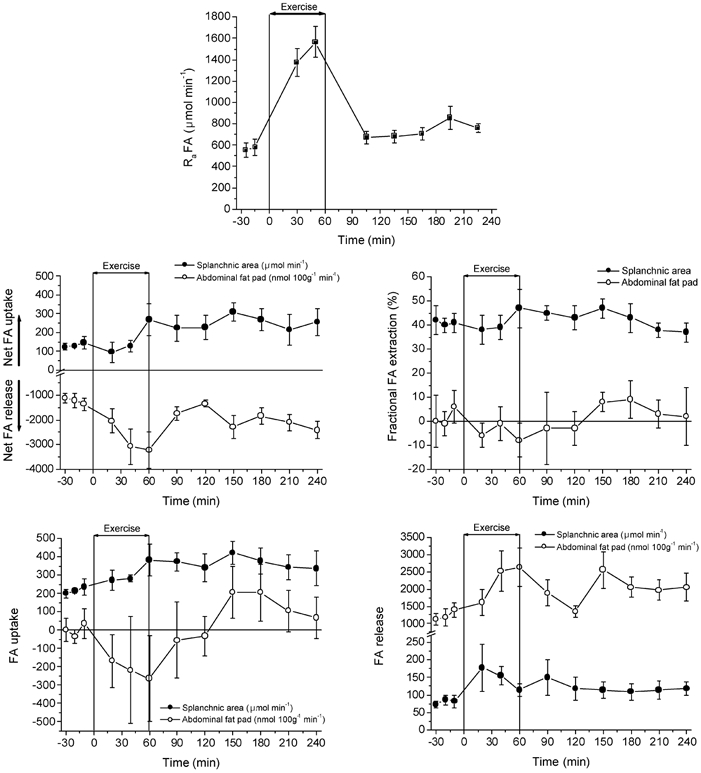
Values are means ± s.e.m. (n = 6).
The arterial FA concentration was lower at rest (≈600 μmol l−1) than in recovery, and reached a plateau at ≈780 μmol l−1 after 1.5 h of recovery. During the hour of exercise, FA concentrations were not higher than at rest. The rates of FA appearance (Ra) and disappearance (Rd) (not shown) in the circulation were lower at rest than in at any time in recovery. Post exercise there was a tendency for an increase of Ra and Rd with time. With 1 h of bicycle exercise the FA Ra increased ≈2.5-fold compared to rest. Whole body FA oxidation rate in recovery was significantly higher than at rest, but the percentage of FAs disappearing from the circulation that were oxidized was unchanged (Table 1). During exercise 71 % of FA Rd was oxidized (Table 1).
Net FA release from adipose tissue increased ≈2.5-fold with exercise. During the first hour of recovery, net FA release from adipose tissue decreased to near pre-exercise values but then increased, reaching a rate of net FA release nearly 2-fold higher than at rest. This steady increase in net FA release from adipose tissue during recovery coincided with the continuous rise in systemic FA Ra. No fractional extraction of FA across abdominal adipose tissue could be observed at any period of the study, implying that no FAs were taken up by abdominal subcutaneous adipose tissue.
The splanchnic net FA uptake at rest was 2-fold lower than in recovery. Fractional extraction of FA by the splanchnic area was constant at ≈40 % of FA delivery during the entire period. Thus, as a result splanchnic FA uptake was roughly 2-fold lower at rest than at the end of exercise and in recovery.
Whole body and regional glycerol exchange (Figs 3 and 5)
Figure 5. Rate of glycerol appearance and regional glycerol exchange at rest, during exercise and recovery.
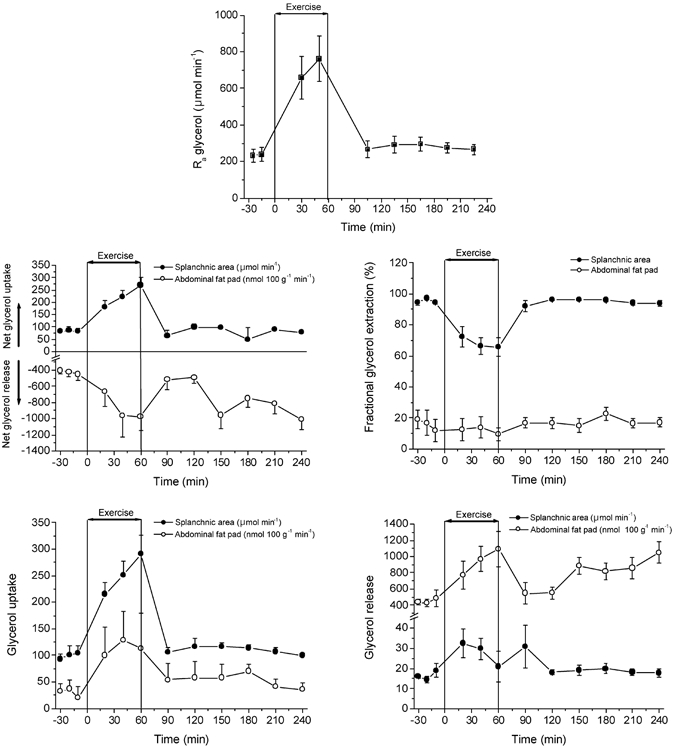
Values are means ± s.e.m. (n = 6).
The arterial glycerol concentration at rest (≈70 μmol l−1) increased rapidly with exercise, being ≈3.5-fold higher after 1 h of exercise (≈250 μmol l−1). In recovery, the arterial glycerol concentration dropped within 30 min to resting values and remained nearly unchanged over the 3 h of recovery. The glycerol Ra was similar at rest and recovery but increased 3- to 4-fold with exercise.
Net glycerol release from adipose tissue increased ≈2.5-fold with exercise. In the first hour of recovery the net glycerol release from adipose tissue decreased to near pre-exercise values but then increased continuously in recovery, reaching a value nearly 2- to 2.5-fold higher than at rest. This steady increase in net glycerol release from adipose tissue during recovery was not accompanied by a similarly enhanced glycerol Ra. A constant fractional extraction of glycerol was observed across the abdominal adipose tissue of 10–18 % at rest, exercise and recovery, implying that there was a glycerol uptake by abdominal tissue of ≈25 nmol (100 g)−1 min−1 at rest with a tendency for it to be higher during exercise and recovery.
Net glycerol uptake by the splanchnic area was similar at rest and in recovery (≈80 μmol min−1). During exercise net glycerol uptake was increased with exercise duration to ≈250 μmol min−1. The splanchnic fractional extraction of glycerol was ≈95 % of glycerol delivery at rest and recovery. However, during exercise, with substantially higher glycerol delivery, fractional extraction decreased to ≈65 %. Splanchnic glycerol uptake and release were similar at rest and in recovery. During exercise, glycerol uptake increased 3-fold whereas only a small increase in release was observed after 20 and 40 min of exercise after which it decreased to resting levels.
Regional contribution to whole body FA and glycerol Ra and Rd (Table 1)
Abdominal subcutaneous adipose tissue tracer-estimated FA and glycerol uptake and release rates were extrapolated to total body adipose tissue (11.7 kg) assuming that abdominal subcutaneous adipose tissue is representative for all adipose tissue depots. The contributions of total adipose tissue FA release to systemic FA Ra was between 35 and 38 % at rest and after 3 h of recovery. No significant FA uptake was observed, thus adipose tissue did not contribute to FA Rd. After 3 h of recovery from exercise the contribution of adipose tissue release to glycerol Ra was increased compared to rest and exercise.
The contribution of splanchnic FA release to Ra was about 11–17 % under all conditions. The contribution of splanchnic FA uptake to FA Rd was 45 % at rest and decreased with exercise to 24 %. After 3 h of recovery the relative contribution increased to 50 %, which was higher compared to rest and exercise.
Whole body and regional TAG and 3-OH-butyrate exchange (Fig. 6)
Figure 6. Arterial and venous concentration and net regional exchange of triacylglycerol and 3-OH-butyrate.
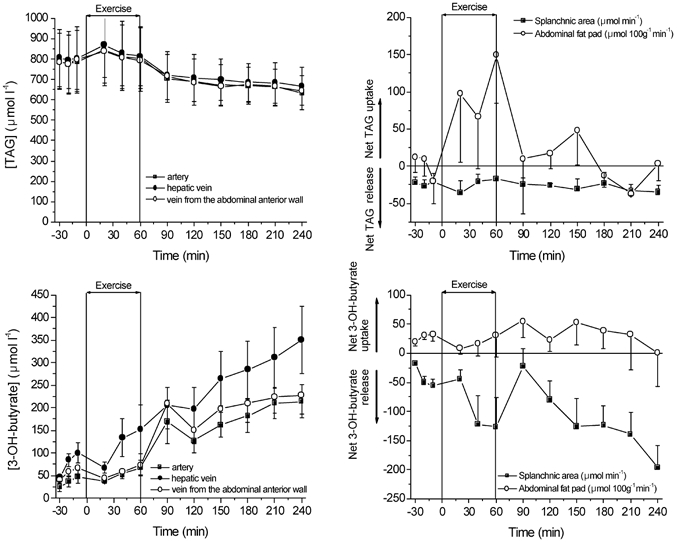
Values are means ± s.e.m. (n = 6).
The arterial TAG concentrations at rest and during exercise were similar but continuously decreased in recovery. In adipose tissue it was not possible to demonstrate a significantly different net TAG uptake during rest and in the recovery period. The net TAG uptake seemed to increase during exercise, although it only reached statistical significance after 1 h of exercise. The net splanchnic TAG release hardly changed, but a tendency existed for a higher splanchnic net TAG release during recovery (last hour of recovery 31 ± 10) compared to rest (23 ± 8). Clearly, the relatively small arterial-venous difference of TAG compared to the high plasma concentration induces considerable variability in the measurements. The arterial 3-OH-butyrate concentration increased during the entire study period reaching a 7-fold higher concentration after 3 h of recovery compared to rest. Adipose tissue showed a small net uptake of 3-OH-butyrate during the resting period. 3-OH-butyrate was released by the splanchnic area with a tendency to increase during exercise. During recovery the 3-OH-butyrate net release increased significantly to reach a level about 4-fold higher than at rest.
Whole body FA reesterification (Fig. 7)
Figure 7. Intracellular and extracellular FA reesterification at rest, exercise and recovery.
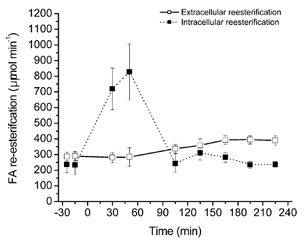
The intracellular FA reesterification rates are presented with a thin dotted line, as their values may be incorrect; see Discussion. Values are means ± s.e.m. (n = 6).
Whole body intracellular and extracellular FA reesterification rates were similar at rest (≈230–290 μmol min−1). During exercise, intracellular FA reesterification rate increased 3-fold but returned to pre-exercise values within 45 min of recovery. Extracellular FA reesterification rate did not change with exercise but increased continuously in recovery and reached an apparent steady state after 1.5 h of recovery of ≈390 μmol min−1.
Fate of FA taken up by the splanchnic area (Fig. 6)
The fate of carbon of FA taken up from the circulation by the splanchnic area was estimated at rest and in the last hour of recovery from the splanchnic net TAG, ketone body release, and FA oxidation. The splanchnic net TAG release at rest was 23 ± 8 and 31 ± 10, representing 69 and 93 μmol min−1 of FA at rest and in the third hour of recovery, respectively. The contribution of ketone formation was estimated from the splanchnic net 3-OH-butyrate release at rest (41 ± 11 μmol min−1) and the third hour of recovery (152 ± 32 μmol min−1), multiplied by 2 as acetoacetate and 3-OH-butyrate have been shown to be produced in the ratio 1:1 (Wahren et al. 1984), and divided by a factor 8 since 1 fatty acid gives rise to 8 ketone bodies. Thus, the splanchnic ketone body release represents 10 and 33 μmol min−1 of FA at rest and recovery, respectively. The proportion of the FA taken up by the splanchnic area that was oxidized was 12 ± 4 % at rest and 13 ± 4 % at the third hour of recovery (Table 1), accounting for 23 and 38 μmol min−1 of FA being oxidized, respectively. The splanchnic FA uptake was 186 μmol min−1 at rest and 299 μmol min−1 in recovery. In absolute terms, more of the FAs taken up by the splanchnic area were used for TAG, ketone synthesis, and FA oxidation in the third hour of recovery from exercise compared to rest. However, since the splanchnic FA uptake was increased in recovery, in relative terms the contribution remained similar. In total, about 45 % of the FAs taken up by the splanchnic area could not be accounted for by the three above-mentioned processes.
Discussion
The major findings of the present study are that in healthy post-absorptive individuals: (1) extracellular FA reesterification is unchanged by exercise but increased in recovery; (2) more then half of whole body extracellular FA reesterification occurs in the splanchnic area. Abdominal subcutaneous adipose tissue does not contribute, or contributes only in a very minor way, to extracellular FA reesterification; (3) glycerol is taken up by adipose tissue indicating that adipose tissue can metabolize glycerol.
An uptake of glycerol by abdominal adipose tissue was observed over the entire study period without differences between rest, exercise and recovery. A tracer-estimated glycerol uptake by adipose tissue has been demonstrated by some (Kurpad et al. 1994; Samra et al. 1999), but not by all (Coppack et al. 1999). Kurpad et al. (1994) argued that the glycerol uptake by adipose tissue originated from an inadequate equilibration of the free glycerol pool in adipose tissue since the tracer was infused for only one hour. This would result in the exchange of an enriched glycerol entering for an unenriched glycerol leaving the adipose tissue pool, resulting in a falsely calculated tracer glycerol uptake. However, it is unlikely that the 6 h of [2H5]glycerol infusion in the present study would not have caused an equilibration of the adipocyte free glycerol and venous glycerol pools; this is supported by the constant rate of glycerol uptake over the entire infusion period. In addition, Samra et al. (1999) reported similar glycerol uptake rates by adipose tissue between 8 and 14 h of [2H2]glycerol infusion under resting conditions. The uptake of glycerol by adipose tissue implies that glycerol has to be metabolized in adipocytes. The presence of glycerol kinase has been shown in human subcutaneous adipocytes of the anterior abdominal wall of non-obese subjects (Ryall & Goldrick, 1977). However, the glycerol kinase activity was relatively low compared to that of the liver and also compared with glycerol kinase activity of adipocytes of other species. Ryal & Goldrick (1977) estimated that adipocytes from anterior abdominal adipose tissue could account for glycerol re-utilization of 5–20 % of the produced glycerol. The calculated in vivo glycerol uptake by abdominal adipose tissue of the present study represents 5–8 % of glycerol release. Alternatively, adipose tissue accounts for approximately 80 % of adipocytes, thus other cell types within the abdominal adipose tissue may be responsible for the observed glycerol uptake.
In contrast with the consistent glycerol uptake, we did not observe an uptake of FA by adipose tissue. The absence of a FA uptake by adipose tissue has been reported previously in post-absorptive subjects (Coppack et al. 1999). However, they observed a substantial net TAG uptake by adipose tissue resulting in a LPL-mediated uptake of lipoprotein-FA of ≈400 nmol (100 g)−1 min−1 or ≈17 % of the FA release (Coppack et al. 1990, 1999). It has been suggested that FA trafficking in adipocytes is partitioned in uptake by lipoprotein lipase (LPL) and release by hormone-sensitive lipase (HSL) (Frayn et al. 1995, 1997). However, in the present study we did not observe a significant net uptake of TAG by adipose tissue. The adipose tissue net TAG uptake is difficult to measure due to the relative high TAG concentration and the small difference in TAG concentration between the artery and vein draining adipose tissue (Fig. 6). Nevertheless, the analysis would have been sensitive enough to detect the adipose tissue net TAG uptake in amounts as reported by Coppack et al. (1999). It seems that in the post-absorptive state the biological variability in adipose TAG utilization is large. A similar reflection can be made for the tracer-estimated adipose tissue FA uptake. However, the observed variability in the resting post-absorptive adipose FA release was minimal, suggesting a very low adipose tissue FA uptake. The very low or even absent FA and TAG uptake by abdominal subcutaneous adipose tissue in the present study shows that extracellular reesterification is low in adipose tissue in post-absorptive healthy normal weight volunteers.
The quantitative data of systemic FA and glycerol Ra, and the FA and glycerol arterial-abdominal adipose tissue venous differences were rather similar as shown previously (Coppack et al. 1999). However, the contribution of total adipose tissue to systemic Ra (24–38 %) was considerable lower than the ≈60 % estimated by Coppack et al. (1999). The difference can solely be explained by the ≈40 % lower adipose tissue blood flow in the present study. The difference in adipose tissue blood flow between the two studies may originate from experimental conditions. In the present study the room temperature was 22 °C and the subjects abdominal region was uncovered during the entire study. Higher room temperature and especially covering the abdominal region will cause higher blood flow in adipose tissue of the anterior abdominal wall. The extrapolation from local release or uptake to whole body fat mass must be interpreted conservatively because of heterogeneity of adipose tissues between different regions. Nevertheless, the extrapolation from adipose tissue to total fat mass seems to suggest that a large fraction of FA and glycerol Ra does not originate from adipose tissue, and/or that adipose tissue from the anterior abdominal wall has a lower lipolytic rate compared to other adipose tissue depots. The reported contribution of the splanchnic area FA and glycerol release to systemic Ra is generally attributed to visceral adipose tissue lipolysis. The net splanchnic FA release found in the present experiments corresponds to what was found by Boberg et al. (1972; 76 - 120 μmol min−1) and Jensen et al. (2001; 102 ± 12 μmol min−1), who applied comparable experimental techniques and calculations. The fractional extraction of FA in the splanchnic region was about 40 % in both rest periods as well as during exercise. Assuming that this is representative for the first pass extraction of FA derived from the visceral adipose tissue and all FA released originates from visceral adipose tissue, the visceral FA mobilization rate post exercise was about 160 μmol min−1. Twenty-five per cent of the total splanchnic blood flow comes from the hepatic artery and the remaining 75 % from the portal vein. Based on this blood flow distribution, the FA concentration in the portal vein can be estimated. Such an estimate shows that the portal vein concentration is about 250 μmol l−1 higher than the arterial concentration in these normal weight subjects. It is also possible to estimate the fraction of the total FA delivery to the liver being derived from the visceral adipose tissue depots. This shows that the visceral depots contribute about 25 % of the total FA supply to the liver. Fifty per cent of the abdominal adipose tissue mass is visceral adipose tissue in normal weight subjects (Thomas et al. 1998). In the subjects of the present study this corresponds to about 1.4 kg of visceral adipose tissue. It can be estimated that the mobilization rate of FA from these depots is about 5-fold higher than the mobilization rate from subcutaneous adipose tissue.
The fractional extraction of FA by the splanchnic area was unchanged during the entire study period, ranging from 38–46 %. In general FA fractional extraction seems to be independent of FA delivery, as shown previously (Havel et al. 1970; Ahlborg et al. 1974; Sidossis et al. 1999; Jensen et al. 2001). Moreover, Ahlborg et al. (1974) observed only marginal changes in splanchnic FA fractional extraction during 4 h of exercise causing a 3-fold change in splanchnic FA delivery. It has been shown that splanchnic FA delivery is closely correlated to liver TAG output (Havel et al. 1970), implying that over a substantial range of plasma FA concentration/delivery the liver is able to clear FA and increase TAG output. In the present study, splanchnic FA delivery was lower at rest compared to recovery resulting in a nearly 2-fold increase in splanchnic FA uptake during recovery. However, net splanchnic TAG output was increased by only ≈30 % at rest compared to the third hour of recovery. With respect to TAG formation it is obvious that the net splanchnic release is smaller than the TAG clearance in various tissues, since arterial TAG concentration significantly decreased post exercise. It was not possible either in the present or in a previous experiment (Mulla et al. 2000) to demonstrate an increased TAG clearance by adipose tissue as mentioned above. This may be due to the TAG analysis not being sensitive enough to detect small arterial-hepatic venous differences. However, another explanation may be that other tissues are responsible for the increased TAG clearance post exercise. Potentially, visceral adipose tissue could be one of the tissues that enhance TAG utilization in recovery from exercise. In that case, another implication would be that the hepatic TAG synthesis, estimated from splanchnic net TAG release, would be underestimated. Alternatively, hepatic TAG storage could be enhanced in recovery from exercise. The splanchnic glycerol fractional extraction was ≈95 % at rest and recovery. However, during exercise with a ≈2.5-fold increase in arterial glycerol delivery the fractional extraction decreased to 72 % after 20 min of exercise, and after 1 h of exercise with glycerol delivery 3.5-fold increased fractional extraction had decreased to 65 %. Thus, hepatic glycerol uptake or utilization can be limited.
The FA carbon balance across the splanchnic area indicated that between 37 % and 31 % of the FA taken up was incorporated into TAG, 12–13 % oxidized, and 5–11 % converted to ketone bodies, thus leaving ≈45 % unaccounted for at rest and recovery, respectively. The incorporation of FA into TAG and FA oxidation rates are similar as reported previously (Sidossis et al. 1998). However, splanchnic conversion of FA to ketone bodies was reported ≈30 % in post-absorptive subjects (Havel et al. 1970; Sidossis et al. 1998). It is unclear why in the present studies ketone body concentration and splanchnic release were considerably lower than reported previously in tracer studies, but our values for ketone body release compare well with other studies (Wahren et al. 1984; Coppack et al. 1990). The ≈45 % of splanchnic FA uptake not accounted for by oxidation and ketone and TAG formation may originate from underestimation of hepatic TAG synthesis caused by underestimation of net TAG release, as discussed above and/or storage of TAG and FA in the splanchnic region.
Extracellular FA reesterifcation represents the FA from TAG hydrolysis released into the circulation before being reesterified in tissues. The systemically calculated extracellular FA reesterification was ≈290 μmol min−1 at rest, which remained similar during exercise and increased to ≈390 μmol min−1 after 3 h of recovery. The FA released into the circulation in excess of oxidation is thought to be cleared via hepatic reesterification and subsequently released as TAG. In the present study the hepatic net TAG release was 23 μmol min−1 at rest in post-absorptive subjects, similar values to those reported previously (Sidossis et al. 1998), and 31 μmol min−1 in recovery. Thus, the hepatic reesterification of circulatory FA to TAG released accounted for 69 and 93 μmol FA min−1, or only ≈24 % of total systemic extracellular FA reesterification at rest and in recovery. However, systemic extracellular FA reesterification is determined as FA disappearing from the circulation minus FA oxidized. In analogy, the contribution of the splanchnic area would be the splanchnic FA uptake minus oxidation and ketone formation. Therefore, the splanchic contribution to systemic extracellular FA reesterification was 51 % at rest and 58 % during the third hour of recovery. Clearly, a considerable FA esterification occurs in extrahepatic tissues as suggested previously (Jensen, 1995; Diraison & Beylot, 1998; Jensen et al. 2001). Skeletal muscle is capable of reesterifying a significant amount of FA in humans (Sacchetti et al. 2002). In a study, with a similar protocol to the present one, leg FA kinetics were investigated. The leg muscle FA uptake not oxidized, and extrapolated to total body muscle mass was 20 % of FA Rd under resting condition. Thus skeletal muscle is an important site of extracellular free FA (FFA) reesterification. Wolfe et al. (1990) suggested that extracellular TAG-FA cycling played an important role in enabling a rapid response of fatty acid metabolism to major changes in energy demand. Furthermore, it was suggested that the increase in extracellular FA esterification immediately at the cessation of exercise was a major reason for the rapid fall in FA concentration. In the present study we cannot find any evidence to support these findings. Our data suggest that a rapid decrease in lipolysis keeps the FA concentration within reasonable limits despite large changes in FA utilization going from exercise to rest. However, in the present study only a 1.5-fold increase in FA concentration was caused by the 1 h of bicycle exercise compared to a 4-fold increase in FA concentration in the 4 h of treadmill running in the study of Wolfe et al. (1990). The extracellular reesterification rates at rest of both studies were similar. However, it is unlikely that the exercise duration and the consequent difference in FA metabolism can explain the 6-fold increase in recovery versus rest in extracellular FA reesterification compared to ≈30 % in the present study. In the present study extracellular FA reesterification was calculated as FFA Rd - FFA oxidation, compared to total FFA reesterification - intracellular reesterification in the study of Wolfe et al. (1990). However, estimating the extracellular recycling rate in the present study according to the formula of Wolfe et al. (1990), then extracellular FA reesterification rates gave values of 200 ± 45, -10 ± 170, and 320 ± 60 μmol min−1 at rest, during exercise and recovery, respectively. Thus the extracellular reesterifcation rates during rest and recovery are similar using the two different calculations, and thus do not explain the difference between the studies.
Intracellular FA reesterification represents the FA originating from TAG hydrolysis being reesterified in situ or in the same tissue, thus not appearing in the circulation. We have used the calculation of intracellular FA reesterifcation rates according to Wolfe et al. (1990). Intra- and extracellular recycling are presented in Fig. 7. The intracellular FA reesterification is thought to occur mainly in adipose tissue. However, if this is the case then the estimated intracellular FA reesterification in the present study would imply a 2-fold higher lipolytic rate in the subcutaneous adipose tissue than was actually measured. In the case of glycerol not being reincorporated in TAG that would mean that the FA release/glycerol release ratio is far lower than 3 for adipose tissue, even taking heterogeneity and underestimation of total adipose tissue mass and FA uptake into account. However, it has clearly been shown that the adipose tissue FA/glycerol release ratio of approximately 3 is well kept in the post-absorptive state (present study; Frayn et al. 1995; Mulla et al. 2000). This means either that glycerol is phosphorylated and reesterified to the same extent as FA in adipocytes, or that adipose tissue is not a major site of intracellular FA reesterification. An alternative explanation is that the assumptions in the calculation of intracellular FA reesterification are incorrect (3 × glycerol Ra - FFA Ra). The main assumptions are that glycerol appears in the blood solely as a product of lipolysis, meaning that there is no de novo glycerol synthesis, and glycerol cannot be directly reincorporated into TAG. Potentially, glycerol can be phosphorylated in adipose tissue and thus reincorporated into TAG as discussed above but this does not seem to affect glycerol Ra much and thus the rate of lipolysis is unaffected (Table 1). However, skeletal muscle takes up glycerol in substantial amounts and glycerol kinase has been shown to be present (Seltzer et al. 1989). Thus it is quite likely that glycerol can directly be incorporated into TAG (Guo & Jensen, 1999). Moreover, in a study with a similar protocol the tracer glycerol release from skeletal muscle could account for ≈24 % of total glycerol Ra at rest, with a substantial increase in muscle contribution during the first hour of recovery without any net glycerol release (van Hall et al. 2002). Similar values are reported in other studies (Landau et al. 1996; Coppack et al. 1999; Jensen et al. 2001). Most likely, the labelled glycerol taken up by skeletal muscle is phosphorylated and incorporated in TAG (Guo & Jensen, 1999). Simultanously TAG is broken down at the same rate as it is synthesized and an unenriched glycerol is released. Alternatively, the glycerol taken up by skeletal muscle is oxidized and glycerol is synthesized at the same rate and released. Moreover, glycerol de novo synthesis is likely to occur in skeletal muscle (Kanno & Maekawa, 1995; Miyajima et al. 1995). Therefore, glycerol Ra is not representative of adipose tissue lipolytic rate.
The whole body and splanchnic FA oxidation rates were determined by an intravenous continuous infusion of [U-13C]palmitate and its label appearance in breath and blood CO2. The carbon recovery of [1,2-13C]acetate was used to correct for label not appearing as CO2 but fixed in other metabolites mainly via tricarboxylic acid cycle exchange reactions (Sidossis et al. 1995). Acetate carbon recovery changes over time, and thus, for proper correction of FA oxidation rates, acetate carbon recovery needs to be determined over time. In the present study whole body acetate carbon recovery was used to correct for whole body FA oxidation as well as splanchnic FA oxidation since it has been shown that whole body and splanchnic acetate carbon recovery are similar (Mittendorfer et al. 1998). Under resting conditions the acetate carbon recovery had little variability over 9 h of acetate infusion in healthy post-absorptive subjects. A situation close to steady state in acetate carbon recovery was reached after 8 h, which confirms a previous finding that after 12 h a steady state is reached in acetate carbon recovery (Mittendorfer et al. 1998). During exercise an acetate carbon recovery of 91 % was observed after an initial 100 % recovery. In recovery, acetate carbon recovery was substantially lower than during the trial under resting condition. The difference in acetate correction in recovery lasted for about 2 h. The higher acetate carbon recovery at onset of exercise as well as the lower acetate carbon recovery during the first 2 h of recovery are caused by changes in the bicarbonate pool sizes and turnover rates and probably not by changes in the rate of exchange reactions involved in acetate carbon fixation (van Hall et al. 1999). The early changes in acetate carbon recovery going from rest to exercise and from exercise highlight the non-steady-state situation caused by major changes in metabolic rate. The changes in metabolic rate affect tracer kinetics and also affect measures of carbohydrate and fat oxidation made with indirect calorimetry. Therefore, accurate measurements cannot be made during the initial phase of exercise and recovery.
Acknowledgments
The Copenhagen Muscle Research Centre is funded by a grant from the Danish National Research Foundation (grant no. 504-14). The study was also supported by grants received from the Danish Medical Research Council (12-1610-1), The John and Birthe Meyer Foundation, the Danish Heart Foundation (97-1-3-48-22465), and the Novo Nordic Foundation.
References
- Ahlborg G, Gelig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man-splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. Journal of Clinical Investigations. 1974;53:1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J, Carlson LA, Freyschuss U. Determination of splanchnic secretion rate of plasma triglycerides and of total and splanchnic turnover of plasma fatty acids in man. European Journal of Clinical Investigations. 1972;2:123–132. doi: 10.1111/j.1365-2362.1972.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Bülow J. Measurement of adipose tissue blood flow. Methods in Molecular Biology. 2001;155:281–293. doi: 10.1385/1-59259-231-7:281. [DOI] [PubMed] [Google Scholar]
- Bülow J, Jelnes R, Atrup A, Madsen JVP. Tissue/blood partition coefficients for xenon in various adipose tissue in man. Scandinavian Journal of Clinical and Laboratory Investigations. 1987;47:1–3. doi: 10.1080/00365518709168861. [DOI] [PubMed] [Google Scholar]
- Bülow J, Simonsen L, Wiggins D, Humphreys SM, Frayn KN, Powell D, Gibbons GF. Co-ordination of hepatic and adipose tissue lipid metabolism after oral glucose. Journal of Lipid Research. 1999;40:2034–2043. [PubMed] [Google Scholar]
- Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. American Journal of Physiology. 1992;263:E1063–1069. doi: 10.1152/ajpendo.2006.263.6.E1063. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Frayn KN, Humphreys SM, Whyte PL, Hockaday DR. Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism. 1990;39:384–390. doi: 10.1016/0026-0495(90)90253-9. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. American Journal of Physiology. 1999;276:E233–240. doi: 10.1152/ajpendo.1999.276.2.E233. [DOI] [PubMed] [Google Scholar]
- Diraison F, Beylot M. Role of human liver lipogenesis and reesterification in triglycerides secretion and FFA reesterification. American Journal of Physiology. 1998;274:E321–327. doi: 10.1152/ajpendo.1998.274.2.E321. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Coppack SW, Fielding BA, Humpreys SM. Coordinated regulation of hormone-sensitive lipase and lipoproteinlipase in human adipose tissue in vivo: implications for the control of fat storage and fat mobilization. Advances in Enzyme Regulation. 1995;35:163–178. doi: 10.1016/0065-2571(94)00011-q. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry. 1957;226:497–510. [PubMed] [Google Scholar]
- Frayn KN, Fielding BA, Samra JS, Summers LKM, editors. Extracellular Metabolic Regulation in Adipose Tissue. Physiology, Stress and Malnutrition. Functional Correlates, Nutritional Intervention. Philadelphia: Lippincott-Raven Publishers; 1997. [Google Scholar]
- Guo Z, Jensen MD. Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. Journal of Biological Chemistry. 1999;274:23702–23706. doi: 10.1074/jbc.274.34.23702. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Balasse EO, Segel N, Basso LV. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normoxtriglyceridemic and hypotriglyceridemic humans. Journal of Clinical Investigations. 1970;49:2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen JH, Winkler K. Hepatic blood flow determination. A comparison of 99-Tc-diethyl-IDA and indocyanine green as hepatic blood flow indicator in man. Journal of Hepatology. 1987;4:66–70. doi: 10.1016/s0168-8278(87)80011-9. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Gender difference in regional fatty acid metabolism before and after meal ingestion. Journal of Clinical Investigations. 1995;96:2297–2303. doi: 10.1172/JCI118285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD. Regional and free fatty acid metabolism before and after meal ingestion. American Journal of Physiology. 1999;276:E863–869. doi: 10.1152/ajpendo.1999.276.5.E863. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ekberg K, Landau BR. Lipid metabolism during fasting. American Journal of Physiology Endocrinology and Metabolism. 2001;281:E789–793. doi: 10.1152/ajpendo.2001.281.4.E789. [DOI] [PubMed] [Google Scholar]
- Kanno T, Maekawa M. Lactate dehydrogenase M-subunit deficiencies: clinical features, metabolic background, and genetic heterogeneities. Muscle and Nerve. 1995;(suppl. 3):54S–60S. doi: 10.1002/mus.880181413. [DOI] [PubMed] [Google Scholar]
- Kurpad A, Khan K, Calder AG, Coppack S, MacDonald I, Elia M. Effect of noradrenaline on glycerol turnover and lipolysis in the whole body and subcutaneous adipose tissue in humans in vivo. Clinical Science. 1994;86:177–184. doi: 10.1042/cs0860177. [DOI] [PubMed] [Google Scholar]
- Landau BR, Wahren J, Previs SF, Ekberg K, Chandramouli V, Brunengraber H. Glycerol production and utilization in humans: sites and quatitation. American Journal of Physiology. 1996;271:E1110–1117. doi: 10.1152/ajpendo.1996.271.6.E1110. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. American Journal of Physiology. 1998;274:E978–983. doi: 10.1152/ajpendo.1998.274.6.E978. [DOI] [PubMed] [Google Scholar]
- Miyajima H, Takahashi Y, Kaneko E. Characterization of the glycolysis in lactate dehydrogenase-A deficiency. Muscle and Nerve. 1995;18:874–878. doi: 10.1002/mus.880180812. [DOI] [PubMed] [Google Scholar]
- Mulla NA, Simonsen L, Bülow J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: effect of exercise intensities. Journal of Physiology. 2000;524:919–928. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme EA, Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochemistry Society Symposium. 1976;41:61–109. [PubMed] [Google Scholar]
- Romijn JA, Klein S, Coyle EF, Sidossis LS, Wolfe RR. Strenuous endurance training increases lipolysis and triglycride-fatty acid cycling at rest. Journal of Applied Physiology. 1993;75:108–113. doi: 10.1152/jappl.1993.75.1.108. [DOI] [PubMed] [Google Scholar]
- Ryall RL, Goldrick RB. Glycerol kinase in human adipose tissue. Lipids. 1977;12:272–277. doi: 10.1007/BF02533346. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. Journal of Physiology. 2002;540:387–395. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samra JS, Clark ML, Humpreys SM, Bannister PA, Summers LKM, MacDonald IA, Frayn KN. Evidence for glycerol uptake by subcutaneous adipose tissue. Proceedings of the Nutritional Society. 1999;58:164A. [Google Scholar]
- Seltzer WK, Angelini C, Dhariwal G, Ringel SP, McCabe ERB. Muscle glycerol kinase in Duchenne dystrophy and glycerol kinase deficiency. Muscle and Nerve. 1989;12:307–313. doi: 10.1002/mus.880120409. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Coggan AR, Gastadelli A, Wolfe RR. A new correction factor for use in a tracer estimations of plasma fatty acid oxidation. American Journal of Physiology. 1995;269:E649–656. doi: 10.1152/ajpendo.1995.269.4.E649. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR. Effect of hyperglycemia-hyperinsulimia on whole body and regional fatty acid metabolism. American Journal of Physiology. 1999;276:E427–434. doi: 10.1152/ajpendo.1999.276.3.E427. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Mittendorfer B, Walser E, Chinkes D, Wolfe RR. Hyperglycemia-induced inhibition of splanchnic fatty acid oxidation increases hepatic triacylglycerol secretion. American Journal of Physiology. 1998;275:E798–805. doi: 10.1152/ajpendo.1998.275.5.E798. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Bülow J, Madsen J. Adipose tissue metabolism in humans determined by vein catherization and microdialysis techniques. American Journal of Physiology. 1994;266:E357–365. doi: 10.1152/ajpendo.1994.266.3.E357. [DOI] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Medical Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, Bell JD. Magnetic resonance imaging of total body fat. Journal of Applied Physiology. 1998;85:1778–1785. doi: 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- van Hall G. Correction factors for 13C-labelled substrate oxidation at whole-body and muscle level. Proceedings of the Nutritional Society. 1999;58:979–986. doi: 10.1017/s0029665199001299. [DOI] [PubMed] [Google Scholar]
- van Hall G, Sacchetti M, Rådegran G, Saltin B. Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. Journal of Physiology. 2002;543:1047–1058. doi: 10.1113/jphysiol.2002.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J, Sato Y, Östman J, Hagenfeldt L, Felig P. Turnover and splanchnic metabolism of free fatty acids and ketones in insulin-dependent diabetics at rest and in response to exercise. Journal of Clinical Investigations. 1984;73:1367–1376. doi: 10.1172/JCI111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotopes Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, Inc.; 1992. [Google Scholar]
- Wolfe RR, Klein S, Carraro F, Weber J-M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. American Journal of Physiology. 1990;258:E382–389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]


