Abstract
We have assessed the functional role of type 2 purinergic (P2) receptors within the caudal aspect of the commissural nucleus tractus solitarii (NTS) in mediating the peripheral chemoreceptor reflex cardiorespiratory response in the arterially perfused in situ working heart-brainstem preparation of rats. Microinjection in NTS of either suramin (100 pmol) or pyrinoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt (PPADS; 10 pmol) depressed the reflex bradycardia (by ≈50 %), but not the tachypnoea, following peripheral chemoreceptor stimulation. In contrast, the reflex bradycardia produced by stimulation of pharyngo-oesophageal receptors was unaffected. Furthermore, microinjections in NTS of the P2X receptor agonist α,β-methyleneadenosine 5′-triphosphate (10 pmol) evoked a bradycardia which was antagonized by suramin (100 pmol). This P2X agonist reversibly potentiated the peripheral chemoreceptor-evoked bradycardia. The effect of suramin was selective to purinergic receptors because the bradycardia evoked by microinjection of α,β-methyleneadenosine 5′-triphosphate was blocked while the bradycardic responses to microinjections of NMDA or non-NMDA receptor agonists were not affected. From whole-cell recordings, some NTS neurones received convergent excitatory synaptic inputs from both peripheral chemoreceptors and receptors at the pharyngo-oesophageal junction. The excitatory postsynaptic response evoked by chemoreceptor stimulation was depressed by suramin, but convergent excitatory inputs from pharyngo-oesophageal receptors were unperturbed. Our findings support the hypothesis that caudal commissural NTS P2 purinergic receptors play a role in the neurotransmission of the parasympathetic (bradycardic) component of the chemoreceptor reflex. This effect is highly selective in that the chemoreceptor afferent-evoked tachypnoea, as well as other visceral receptor-mediated reflex bradycardia, remain unaffected.
Activation of peripheral chemoreceptors by intravenous injection of cyanide salts produces bradycardia, hypertension and tachypnoea in conscious rats (Franchini & Krieger, 1992, 1993). There is evidence that the processing of chemoreceptor afferents at the level of the nucleus tractus solitarii (NTS) occurs mainly at the caudal aspect of the commissural NTS (Mifflin, 1992; Chitravanshi et al. 1994; Chitravanshi & Sapru, 1995). Haibara et al. (1995) documented that bilateral microinjection of dl-2-amino-5-phosphonopentanoic acid (AP5; a selective NMDA receptor antagonist) into the lateral aspect of the rostral commissural NTS (0.5 mm rostral to calamus scriptorius and 0.5 mm lateral to the midline) produced a dose-dependent suppression of the evoked bradycardia but no change in the pressor response to chemoreceptor reflex activation. In a subsequent study, Haibara et al. (1999) showed that the pressor response to chemoreflex activation was only partly reduced by the microinjection of 6,7-dinitroquinoxaline-2,3-(1H,4H)-dione (DNQX; a selective non-NMDA receptor antagonist) into the rostral and caudal aspects of the commissural NTS. An important observation by Haibara et al. (1995) was related to the fact that microinjection of kynurenic acid (a non-selective antagonist of excitatory amino acid (EAA) receptors) into the medial aspect of the caudal commissural NTS (at the level of calamus scriptorius and at the midline) produced no effect on the bradycardic component of the chemoreceptor reflex. These data of Haibara et al. (1995, 1999) suggested that neurotransmission of the parasympathethetic component of the chemoreceptor reflex may involve glutamatergic synapses in the lateral aspect of the rostral commissural NTS and postsynaptic receptors other than EAA in the caudal aspect of the commissural NTS, a subregion that appears critical for the processing of the autonomic responses to chemoreflex activation. In this study we have sought to determine which other receptors within the NTS play a role in mediating the peripheral chemoreceptor reflex bradycardia.
Immunohistochemical studies have documented the presence of type 2 ionotropic purinergic receptors: P2X2 and P2X3 receptors (Vulchanova et al. 1997), P2X3 receptors (Llewellyn-Smith & Burnstock, 1998) and all six P2X receptors (Yao et al. 2000) in the NTS of rats. Recently the P2X7 receptor was also found presynaptically on vagal afferents in the NTS (Deuchars et al. 2001). Functional studies performed in anaesthetized rats have shown that microinjection of α,β-methyleneadenosine 5′-triphosphate (α,β-methylene ATP) into the NTS produced hypotension and bradycardia which could be antagonized by suramin (Scislo et al. 1998; Scislo & O'Leary, 1998, 2000). Taking into consideration the fact that anaesthesia may affect cardiovascular responses evoked by microinjection of different neurotransmitters in the NTS, such as l-glutamate (see Machado & Bonagamba, 1992), our preliminary data indicated that microinjection of ATP into the caudal commissural NTS produced a dose-dependent increase in mean arterial blood pressure (MAP) and an intense bradycardia in conscious rats (de Paula et al. 2000), a pattern similar to that evoked by peripheral chemoreceptor activation. Since ionotropic glutamate receptor antagonism in the caudal commissural NTS failed to block the chemoreceptor reflex-evoked bradycardia and ATP microinjected into the same subregion of the NTS produced a pattern of response resembling the cardiovascular response observed during a chemoreceptor reflex, we evaluate in this study a possible role of purinergic P2 receptors in processing chemoreceptor reflex afferents in the NTS. Studies were performed in an unanaesthetized decerebrate working heart-brainstem preparation (WHBP) to avoid complexities of anaesthesia. Although this in situ preparation did not allow an analysis of the vascular response to chemoreceptor reflex activation, it did permit a subsequent intracellular analysis of the effects of P2 receptor blockade on afferent inputs to physiologically characterized NTS neurones. We report that non-selective P2 purinergic receptor antagonists in the caudal aspect of the commissural NTS attenuate the chemoreceptor reflex response in the rat and that this appears to be highly reflex selective.
METHODS
The WHBP
Sprague-Dawley rats (75–150 g; male) were anaesthetized deeply with a saturated atmosphere of halothane and a WHBP prepared (see Paton, 1996). Before surgery the level of anaesthesia was assessed as deep, since there was an absence of withdrawal reflexes following a pinch to a paw or the tail. Rats were decerebrated precollicularly and all brain rostral to this level was removed by aspiration. At this time anaesthesia was discontinued since the decerebration procedure rendered the animals insentient. Rats were cerebellectomized and transected subdiaphragmatically. The upper half of the animal was made viable by arterial perfusion: the descending aorta was cannulated with a double-lumen catheter and perfused using a Ringer solution containing 1.25 % ficoll (Sigma), gassed with carbogen (95 % O2-5 % CO2) and warmed to 31 °C. The second lumen was connected to a transducer for measurement of perfusion pressure (PP). Neuromuscular blockade was established using vecuronium bromide added to the perfusate (0.04 μg ml−1; Organon Teknica, Cambridge, UK). This neuromuscular agent was chosen since it has minimal antagonistic action on cardiac vagal motor transmission.
NTS microinjection
All microinjections were made using three-barrelled glass microelectrodes containing saline (0.9 %) and Pontamine Sky Blue. Bilateral NTS microinjections were made 400–900 μm ventral to the dorsal surface, ±0.25 mm rostral and caudal relative to calamus scriptorius and between 100 and 300 μm from the midline, with the most medial injection sites being the most caudal. In all cases the volume injected (40 nl) was assessed by the distance moved by the meniscus as viewed via a binocular microscope fitted with a precalibrated eyepiece graticule. Silicone rubber tubing was connected to the blunt ends of each microelectrode and these were connected to a syringe (50 ml) and pressure generated manually. Reflexes were retested between 2 and 7 min postmicroinjection. The effect of microinjected drugs washed out after 15 min approximately. In all experiments, saline (40 nl) microinjected either before or after pharmacological agonists/antagonists was without effect on baseline cardiorespiratory variables or reflex responses.
Three groups of microinjection experiments were conducted in separate preparations, as follows.
(1) Glutamate (concentration 100 mm; dose 4 nmol), NMDA (concentration 100 mm; dose 4 nmol) or ±-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA; concentration 10 μM; dose 0.4 pmol) was microinjected into the NTS to evoke a bradycardia. This was repeated after a microinjection of either suramin (dose range 100–200 pmol) or a more specific P2 receptor antagonist, pyrinoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt (PPADS; dose range 10–20 pmol) to assess a dose at which the antagonists did not cause non-specific blockade of glutamate receptors. Glutamate, NMDA or AMPA was microinjected within 60 s of an injection of a P2 receptor antagonist.
(2) The role of P2 receptors in the NTS for mediating the reflex bradycardia evoked following activation of peripheral chemoreceptors and pharyngo-oesophageal receptors was tested with microinjection of either suramin or PPADS. The doses of suramin and PPADS were 100 and 10 pmol, respectively, since these doses were found to produce minimal disturbance to basal phrenic nerve activity (PNA) and heart rate (HR) but, importantly, failed to affect glutamate-, NMDA- and AMPA-evoked responses from the NTS.
(3) To establish evidence that P2X receptors in the NTS mediate the chemoreceptor reflex, a P2X agonist (α,β-methylene ATP; 4, 10 and 20 pmol; concentrations 100, 250 and 500 μM) was microinjected and its effect on the peripheral chemoreceptor reflex assessed. The effect of α,β-methylene ATP was then shown to be antagonized with suramin using a dose that did not affect glutamate transmission.
PNA and ECG recording
A glass suction electrode held in a three-dimensional micromanipulator was used to record PNA from the central end of a cut phrenic nerve within the thorax. The ECG was recorded either through this electrode or through a separate silver wire electrode placed intrathoracically. Signals were amplified and filtered (8 Hz to 3 kHz). Transistor-transistor logic pulses were derived from the R-wave of the ECG using a window discriminator and were used to compute heart rate. Rhythmic, ramping PNA persisted uninterrupted for up to 7 h. This motor pattern was indicative of eupnoea and gave a continuous physiological index of preparation viability.
Intracellular recording from NTS neurones
‘Blind’ whole-cell patch techniques were used to make current clamp recordings as described previously (e.g. Paton et al. 1999, 2001). Only neurones with a resting membrane potential more negative than -50 mV and an input resistance of >150 Ω were included. Patch pipettes were manufactured using a two-stage pulling programme (Narishige PP830) with a resistance of 4–6 MΩ. The dorsal surface of the NTS was exposed by peeling off the dorsal column nuclei using fine watchmaker's forceps. This reduced blockage of pipette tips on their descent into the NTS. Removal of the dorsal columns did not affect reflex changes in the cardiorespiratory variables measured. Before seal formation, pipettes were pressurized (10–45 mmHg) and advanced (1.4 μm steps) into the NTS using a stepping motor held in a 3-D micromanipulator. Patch pipettes were placed into the NTS and orientated relative to the calamus scriptorius and the exposed solitary tract under visual control. Recordings were made 1 mm rostral to 1 mm caudal relative to calamus scriptorius and up to 500 μm from the midline. After establishing the whole-cell configuration, recordings were made in bridge mode using an Axoclamp 2A (Axon Instruments; filtered at 3 kHz) and displayed on an oscilloscope (Gould DSO 400). Seal and access resistances were >1.5 GΩ and 20–30 MΩ, respectively. Junction potentials were measured (-6 ± 0.2 mV; n = 7) and corrected for at the time of recording. On gaining neuronal access, capacitance transients were compensated fully and the bridge circuit of the amplifier balanced. All recorded variables were digitized (CED 1401 plus, neurone sampling rate 6 kHz) and stored on the hard disk of a computer for off-line analysis using Spike2 software (CED). Suramin (100 μM) was applied to recorded neurones via a perfusable well (Pierrefiche et al. 1996; Paton, 1998). This device was placed on the dorsal surface of the exposed NTS and has both infusion and aspiration ports. Recording electrodes were driven down through the well. Both the absence of the pial membrane and the capillary action of the microelectrode shaft facilitated suramin delivery into the vicinity of the recorded neurone. All data from WHBP were relayed via a CED 1401 plus interface to a computer running Spike2 software (CED) with custom-written scripts for data acquisition and on- and off-line analysis.
Stimulation methods in the WHBP
Peripheral chemoreceptors were stimulated with sodium cyanide (CN) solution (0.03 %) injected directly into the descending aorta. A range of CN doses were given (7–30 μg bolus) and the dose used was suprathreshold but submaximal. Note that in the WHBP, the PNA and heart rate response can be measured, whereas the changes in vascular resistance are compromised owing to the limited number of vascular beds and low peripheral resistance of this preparation. In experiments where α,β-methylene ATP was microinjected, doses of cyanide were reduced to <15 μg. This reduced the control reflex bradycardia in an attempt to maximize the potentiating effect of α,β-methylene ATP. Once selected, the dose of CN was not changed throughout the course of the experiment. Our dose range of CN was relatively selective for stimulating arterial chemoreceptors (Franchini & Krieger, 1993; Daly, 1997). Furthermore, the chemoreceptor reflex response can be abolished completely by denervation of the vagi and carotid sinus nerves (Paton et al. 1999), indicating no direct effects on central cardiorespiratory activity. Repeated stimulation of peripheral chemoreceptors with CN gives consistent reflex responses in the WHBP and does not cause toxic side effects owing to the large circulating volume of 200 ml.
Pharyngo-oesophageal receptors were activated using an arterial embolectomy balloon-tipped catheter (3F; Baxter). This was introduced into the oesophageal lumen at the level of the diaphragm and advanced rostrally into the pharyngo-oesophageal junction by oral inspection (see Paton et al. 1999). Its exact position was confirmed after the experiment. Inflation of the balloon in this region evoked a swallow (before neuromuscular blockade) accompanied by bradycardia and a prolongation of expiratory time (Paton et al. 1999). The balloon was fluid filled (distilled water) and inflated with a volume of up to 50 μl for 2 ± 1 s. The exact volume used in each preparation was determined at the start of the experiment and was the minimal volume needed to evoke a reflex response. Once ascertained, all subsequent inflations were set to give the same volume for the duration of the experiment.
Histological analysis
At the end of the experimental procedure in the WHBP, the brainstem was removed and fixed in 4 % paraformaldehyde and then in 30 % sucrose for 24 h before cutting transverse sections (40–60 μm). Material was stained using Neutral Red. Only the rats in which the sites of microinjections were bilaterally located in the caudal aspects of the NTS were considered for data analysis. Microinjection sites were documented on predrawn transverse sections of the medulla at the level of the NTS (Paxinos & Watson, 1986). The location, somatic and dendritic morphology and axonal projections of the intracellularly recorded NTS neurones responding to chemoreceptor and pharyngo-esophageal inputs have been published recently (see Paton et al. 2001).
Data analysis
Baseline and peak reflex responses in arterial pressure, heart rate and phrenic nerve activity cycle length were measured. Phrenic nerve activity cycle length was measured (i.e. the time interval between the onset of 2 consecutive bursts) during control conditions (>3 control cycles) and the first cycle length during the evoked reflex response. Resting membrane potential and membrane input resistance were measured before and after drug application. Input resistance was calculated from the voltage deflection in response to hyperpolarizing pulses (-0.01 to -0.06 nA, 500 ms, 1 Hz) injected into neurones from their resting membrane potential. The peak firing response and the total number of spikes (above basal on-going activity) evoked following stimulation of chemoreceptors and pharyngo-oesophageal receptors were measured before, 3 min after suramin and >10 min following drug application. Data were normalized by expressing percentages. All values quoted are means ± s.e.m. and n is the number of preparations or, in neuronal recording studies, the number of neurones. Differences were taken as significant at the 95 % confidence limit using Student's two-tailed t test.
RESULTS
Assessing the specificity of suramin and PPADS in the NTS
Due to the reported non-selective action of suramin in blocking glutamatergic receptors (e.g. Peoples & Li, 1998), we assessed whether the bradycardia evoked by NTS microinjections of glutamate, NMDA or AMPA was affected by microinjection of P2 receptor antagonists. The bradycardia evoked by NTS microinjection of glutamate (-118 ± 15 b.p.m.), NMDA (Fig. 1B; -156 ± 19 b.p.m.) and AMPA (Fig. 1A; -168 ± 25 b.p.m.) were unaffected after suramin (100 pmol; Fig. 1A and B; n = 3; P = 0.97, 0.36 and 0.98, respectively). Likewise, PPADS (10 pmol) failed to alter glutamate-, NMDA- and AMPA-evoked bradycardia (n = 3; P = 0.84, 0.77 and 0.85, respectively). In contrast, doses of suramin and PPADS >100 and >10 pmol, respectively, blocked glutamatergic receptor-evoked bradycardia (data not shown). Thus, these results suggest that 100 pmol suramin and 10 pmol PPADS do not block glutamate-, NMDA- and AMPA receptor-mediated bradycardia from the NTS; hence these doses of the P2 receptor antagonists were used in all other microinjection studies.
Figure 1. Assessing specificity of suramin (100 pmol) in the NTS.
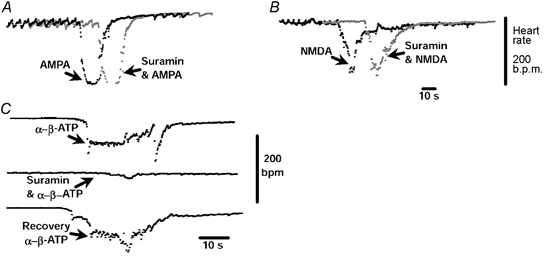
NTS microinjections of either AMPA (A; 0.4 pmol) or NMDA (B; 4 nmol) induced bradycardia which was similar in magnitude and duration after a microinjection of suramin (100 pmol). In C, the bradycardia evoked by the P2X receptor agonist (α,β-methylene ATP; 10 pmol) was almost completely antagonized by suramin (100 pmol). These findings suggest that 100 pmol suramin in the NTS does not antagonize glutamatergic synapses but does block P2X receptors in the WHBP.
Effect of suramin blockade of P2 receptors in the NTS on reflex-evoked bradycardia
In the WHBP, resting HR and PNA cycle length were 261 ± 6 b.p.m. and 5.5 ± 0.6 s, respectively. CN activation of peripheral chemoreceptors evoked a bradycardia of -157 ± 6 b.p.m. and a decrease in PNA cycle length of -3.45 ± 0.5 s (Fig. 2 and Fig. 3A; P < 0.01; n = 10). When 100 pmol suramin was microinjected into medial regions of the caudal NTS, the bradycardia evoked by chemoreceptor stimulation was attenuated to -66.8 ± 9 b.p.m. (Fig. 2 and Fig. 3; n = 10, P < 0.01) but the evoked decrease in PNA cycle length was unaltered (i.e. -3.45 ± 0.5 versus -3.1 ± 0.4 s; n = 7, P = 0.5). Ten to fifteen minutes following the microinjection of suramin, the chemoreceptor-evoked bradycardia had returned to control levels (i.e. -162 ± 8 b.p.m.; Fig. 2 and Fig. 3A). During an NTS microinjection of suramin, a transient central apnoea and variable changes in HR were evoked (n = 7); both variables had returned to control values after 20 s, the time at which the chemoreceptor reflex was retested.
Figure 2. Suramin blockade of P2 receptors in the NTS selectively attenuates the reflex bradycardia in response to peripheral chemoreceptor but not pharyngo-oesophageal receptor stimulation.
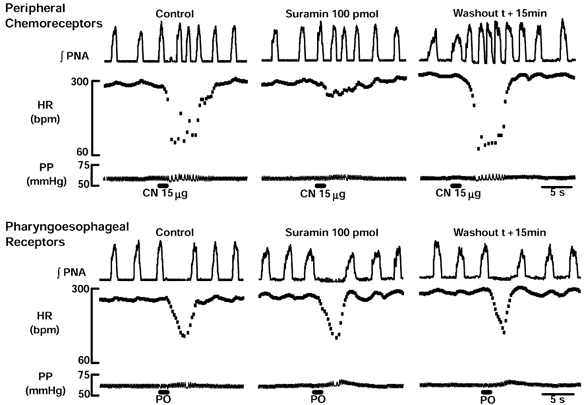
A representative WHBP in which CN stimulation of peripheral chemoreceptors (top) evoked a decrease in PNA cycle length and bradycardia. The cardiac, but not the respiratory component of this response was attenuated by suramin (100 pmol). The attenuating effect of suramin on the chemoreceptor reflex bradycardia was abolished after 15 min. Distension of pharyngo-oesophageal junction (PO; bottom) using a small balloon caused an increase in PNA cycle length and bradycardia that persisted after an NTS microinjection of suramin. Note that the persistence of the latter response occurred at a time when the chemoreceptor reflex-mediated bradycardia was attenuated. HR, heart rate; PP, perfusion pressure.
Figure 3. Suramin (A; 100 pmol) and PPADS (B;10 pmol) antagonism of P2 receptors in the NTS attenuate the peripheral chemoreceptor reflex bradycardia selectively.

The effect of blocking P2 receptors in the NTS was selective for the chemoreceptor reflex circuit mediating the bradycardia (n = 6) since responses following pharyngo-oesophageal afferent activation were unperturbed (n = 6). ▪, stimulation of peripheral chemoreceptors; □, pharyngo-oesophageal distension.
In the same preparations, pharyngo-oesophageal distension evoked a bradycardia and increase in PNA cycle length (-94.2 ± 11 b.p.m. and 3.1 ± 0.7 s, respectively; Fig. 2 and Fig. 3A; n = 10, P < 0.01) but both components of this reflex were unaffected following suramin microinjection (n = 10, P = 0.98 and 0.93, respectively). Notably, this lack of effect of suramin occurred at a time when the chemoreceptor reflex bradycardia was attenuated, indicating a reflex-selective effect.
Effect of PPADS blockade of P2 receptors in the NTS on reflex-evoked bradycardia
In six naive WHBP, resting HR and PNA cycle length were 265 ± 9.6 b.p.m. and 5.69 +0.4 s, respectively. CN evoked a bradycardia (-130.8 ± 6 b.p.m.) and decrease in PNA cycle length (-3.3 ± 0.3 s; Fig. 3B; P < 0.01). After PPADS (10 pmol) was microinjected into NTS, the chemoreceptor-induced bradycardia was attenuated to -55.2 ± 7.2 b.p.m. (Fig. 3B; n = 6, P < 0.01), while the PNA response was unchanged (P = 0.7). The attenuation of the CN-evoked bradycardia was abolished after 10–15 min, when the response was -144 ± 7.8 b.p.m., which was not different from control values (Fig. 3B; P = 0.2). PPADS failed to affect baseline HR but caused a transient central apnoea lasting ≈10 s. All tests were performed after this variable had returned to control levels.
As with suramin, PPADS in these WHBP did not have any effect on the cardiorespiratory reflex response to pharyngo-oesophageal distension. Pharyngo-oesophageal distension evoked a bradycardia and increase in PNA cycle length of -103.8 ± 7 b.p.m. and 2.1 ± 0.2 s, respectively. When PPADS was microinjected into NTS the reflex-evoked bradycardia (-114 ± 6 b.p.m.) and increase in cycle length (1.7 ± 0.3 s) were not different from control values (Fig. 3B; n = 6, P = 0.14 and 0.19, respectively). This lack of effect of PPADS occurred at the time when the chemoreceptor reflex bradycardia was attenuated.
Microinjection sites
Figure 4 depicts a representative NTS microinjection site at which suramin reduced the chemoreceptor reflex bradycardia but was without effect on the pharyngo-oesophageal reflex response. All microinjection sites that were recovered were located within the commissural NTS.
Figure 4. Photomicrograph of a representative transverse section of the medulla oblongata showing the sites of a bilateral microinjection of suramin into the NTS of a WHBP.
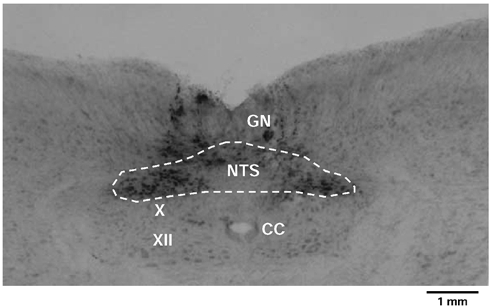
All effective sites were confined to the commissural NTS and marked with Pontamine Sky Blue. The dark staining of neuronal somata by this dye is mainly confined to the NTS with some spread into the gracile nuclei (GN). Other abbreviations: CC, central canal; X, dorsal vagal motor nucleus; XII, hypoglossal motor nucleus.
Effect of P2X receptor stimulation in the NTS
In five WHBP, we used a P2X receptor agonist (α,β-methylene ATP) in doses of 4, 10 and 20 pmol, which evoked bradycardia of -67.8 ± 19, -110.4 ± 16 and -98.4 ± 4 b.p.m., respectively. This effect was repeatable but only after a long washout period of 30–35 min, consistent with a desensitization of the receptor. The bradycardia evoked by α,β-methylene ATP recovered after 20–30 s to a level not different from control values. When microinjected after suramin (100 pmol), α,β-methylene ATP (20 pmol) failed to evoke a significant change in heart rate (-9.0 ± 3 b.p.m.; Fig. 1C; n = 5). This effect was reversible in all cases. Based on these findings, we assessed the effect of 10 pmol α,β-methylene ATP on the reflex bradycardia following stimulation of peripheral chemoreceptors.
Effect of P2X receptor stimulation in the NTS on the chemoreceptor reflex bradycardia
Since blockade of P2 receptors in the NTS depressed the reflex bradycardia in response to chemoreceptor stimulation, we sought to determine whether α,β-methylene ATP would facilitate this component of the response. In six WHBP, microinjection of α,β-methylene ATP (10 pmol) into the NTS evoked a transient bradycardia of -98.3 ± 3.5 b.p.m. from a resting level of 326 ± 5 b.p.m. (Fig. 5; n = 6, P < 0.05). This lasted 20–30 s and once HR had returned to control levels, the chemoreceptor reflex was challenged again with a dose of CN just above the bradycardia threshold. From a control chemoreceptor reflex bradycardia of -69.7 ± 12 b.p.m., α,β-methylene ATP facilitated this response significantly to -140 ± 12 b.p.m. (Fig. 5; n = 6, P < 0.05). This potentiation declined to -60.2 ± 11 b.p.m. after 10–15 min, a level not different from control values. There were no consistent changes in the PNA component of the chemoreceptor reflex response.
Figure 5. Facilitation of the chemoreceptor reflex bradycardia by stimulation of P2 receptors in the NTS.
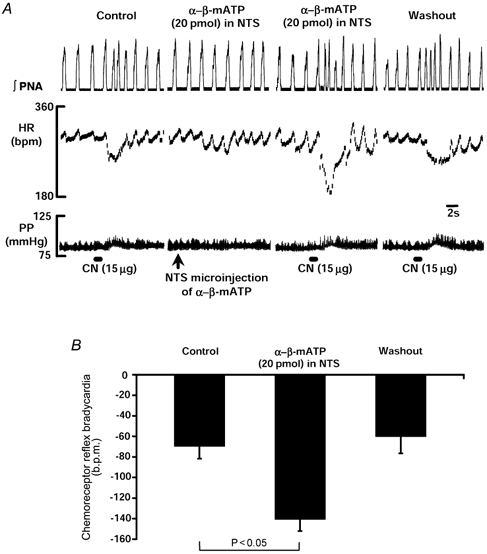
A, NTS microinjection of α,β-methylene ATP, a P2X receptor agonist, produced a transient bradycardia. Once HR returned to baseline, the bradycardia evoked reflexly by CN stimulation of the chemoreceptors was significantly potentiated. This effect returned to control values after 10–15 min. The group data for 6 WHBP are shown in B.
Effect of P2 receptor blockade on synaptic responses evoked from physiologically characterized NTS neurones
Seven neurones were excited following CN stimulation of peripheral chemoreceptors and termed chemoreceptive (Fig. 6). These neurones were recorded from separate preparations. A total of 15 preparations were used to obtain these recordings. Collectively, the chemoreceptive neurones had a resting membrane potential and input resistance of -53.6 + 0.5 mV and 239 ± 9 MΩ, respectively. In response to chemoreceptor stimulation, these neurones exhibited a peak firing frequency of 67.6 ± 5 Hz with a total of 107.7 ± 10 spikes above baseline firing (1.7 ± 0.3 Hz). Following 3 min of exposure to topically applied suramin, there was a significant reduction in both the peak firing rate (28.3 ± 3.1 Hz; P < 0.05) and the total number of spikes (49.6 ± 7 spikes; Fig. 6 and Fig. 7; P < 0.05) evoked by CN injection. Suramin was without effect on resting membrane potential, on-going firing or membrane input resistance in five neurones. In the remaining two neurones, suramin produced a hyperpolarization of 4–5 mV and a 15–18 % increase in membrane input resistance. The effect of suramin on the synaptic response was partly reversible (Fig. 6 and Fig. 7). The peak firing frequency response and total number of spikes evoked recovered towards control levels (i.e. 44.5 ± 3.2 Hz and 71.5 ± 4.3 spikes) after a 15 min washout period. These firing responses were significantly larger than those following suramin administration (n = 7; P < 0.05).
Figure 6. Suramin preferentially blocks the excitatory synaptic response evoked by chemoreceptor stimulation.
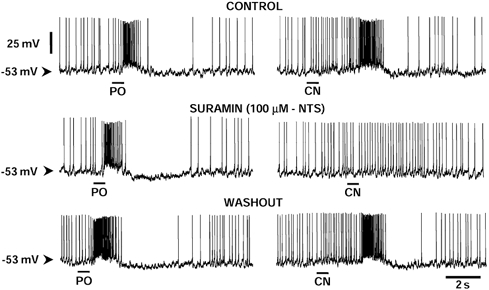
Whole-cell recording of an NTS neurone responding to activation of peripheral chemoreceptors and receiving a convergent input from pharyngo-oesophageal receptors (PO). Note that the convergent PO-evoked input was unaffected. This cell had an input resistance of 220 MΩ and showed no change in this variable, resting membrane potential or on-going firing rate after suramin. Suramin (100 μM) was applied topically to recorded neurones via a perfusable well (see Paton, 1998); the absolute concentration at the neurone is unknown but <100 μM.
Figure 7. Selective modulation by suramin of the peripheral chemoreceptor input but not the excitatory synaptic response from pharyngo-oesophageal receptors (PO) to NTS neurones.
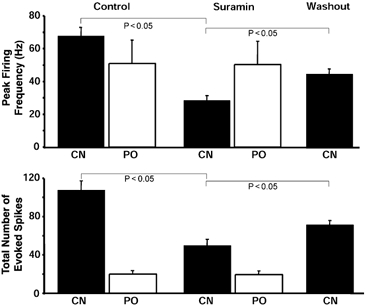
Seven neurones responded to CN stimulation of chemoreceptors, of which five received synaptic input from PO receptors also. The former but not the latter inputs were attenuated by suramin as measured from the peak frequency of the evoked firing response and the total number of action potentials elicited above the basal discharge rate. Effects had partly reversed after a 10–15 min washout period. ▪, stimulation of peripheral chemoreceptors; □, stimulation of receptors at the pharyngo-oesophageal junction.
Five of the seven neurones activated by chemoreceptor stimulation received a convergent excitatory input from pharyngo-oesophageal receptors as described previously (Paton et al. 1999; see Fig. 6). Activation of these receptors produced a peak firing response and total spike count of 51.0 ± 14.3 Hz and 20.0 ± 3.6 spikes, respectively. In contrast to the depressant effect seen with the chemoreceptor input, there was no change in either the peak firing frequency or the total spikes evoked by pharyngo-oesophageal receptor stimulation after exposure to suramin (i.e. 50.3 ± 14 Hz and 19.5 ± 3.7 spikes; Fig. 6 and Fig. 7).
Discussion
Activation of arterial chemoreceptors with CN salts in conscious rats produced an intense bradycardia and hypertension (Franchini & Krieger, 1992, 1993; Haibara et al. 1995, 1999). The bradycardia was also seen in the WHBP, but owing to the low peripheral resistance and limited vasculature we were unable to measure vasomotor responses in this preparation. In the conscious rat, Haibara et al. (1995) have shown that the bradycardia in response to chemoreceptor reflex activation was blocked in a dose-dependent manner by NTS microinjections of AP5, an NMDA receptor antagonist, into the lateral aspect of the rostral commissural NTS. In contrast, the pressor response was unaffected. Subsequently, Haibara et al. (1999) showed that either DNQX (a non-NMDA antagonist) or metabotropic (α-methyl-4-carboxyphenylglycine; MCPG) receptor antagonists, in a selective range of doses, were unable to block the pressor and bradycardic components of the chemoreceptor reflex. In addition, microinjection of kynurenic acid, a non-selective antagonist of the EAA receptors, into the caudal commissural NTS failed to block the bradycardic response to chemoreflex activation (Haibara et al. 1999). Thus, receptors other than glutamatergic types may also be involved in mediating the cardiovascular response at the level of the NTS. It was on the basis of these findings by Haibara et al. (1995, 1999), together with studies indicating the presence and functional role for purinergic receptors in the NTS (Phillis et al. 1997; Scislo et al. 1998; Scislo & O'Leary, 1998, 2000), that we sought to determine whether P2 receptors play any part in mediating the chemoreceptor reflex in the in situ WHBP.
Different studies have shown that the first synapse of the carotid chemoreceptor afferents occurs in the lateral (rat: Finley & Katz, 1992; cat: Mifflin, 1992) and caudal commissural NTS (rat: Chitravanshi et al. 1994; Chitravanshi & Sapru, 1995). This was supported by our recent morphological data revealing cell bodies of some ‘chemoreceptive’ NTS neurones within this region (Paton et al. 2001). Therefore, in order to evaluate the possible role of purinergic receptors in the neurotransmission/neuromodulation of the chemoreceptor reflex bradycardia, we microinjected either suramin or PPADS into the lateral and caudal aspects of the commissural NTS in the WHBP. The data show that bilateral microinjection of either suramin or PPADS into the commissural NTS at a level caudal to calamus scriptorious produced a significant reduction in the bradycardic responses to chemoreceptor reflex activation. These findings fully support the earlier work of Chitravanshi et al. (1994) and Chitravanshi & Sapru (1995) in demonstrating that the cardiovascular response to peripheral chemoreceptor stimulation is mediated via the caudal commissural NTS.
Microinjections into the NTS are likely to affect multiple sensory circuits integrating specific sensory channels of visceral information (see Paton, 1999, for review). Indeed, peripheral chemoreceptor-activated NTS neurones that are intermingled with neurones mediating other cardiovascular and non-cardiovascular inputs (see Paton & Kasparov, 2000, for review). With this in mind, effects on non-chemoreceptor circuitry might explain the increase in baseline MAP after P2 receptor blockade in the commissural NTS of conscious rats (de Paula et al. 2000). This could indicate an endogenous release of ATP within NTS producing sympathoinhibition. This would be analogous to the effects of kynurenic acid at the same NTS sites where increases in MAP are elicited (Haibara et al. 1999).
The present results substantiate a physiological role for P2 receptors within the NTS for mediating the peripheral chemoreceptor reflex bradycardia. We showed that both suramin and PPADS attenuated the reflex bradycardia by about 50 %, while the respiratory component of the reflex was unaffected. Two possibilities may account for such a difference, as follows. First, NTS sites mediating the cardiovascular and respiratory reflex responses are anatomically distinct within the NTS. Hence, the sites studied in the present paper did not encompass regions mediating the reflex tachypnoea following chemoreceptor reflex activation. Second, the P2 receptors in the NTS do not mediate the respiratory component of the reflex response. Certainly one would predict that if ATP was the transmitter for mediating the tachypnoea of the chemoreceptor reflex then a microinjection of ATP into the NTS should mimic this effect. This does not appear to be the case. Recent findings indicate that ATP in the caudal NTS produced apnoea in conscious rats (V. R. Antunes and B. H. Machado, unpublished observations). Moreover, in the WHBP, blockade of P2 receptors in the NTS appeared to be reflex specific. For example, both P2 receptor antagonists failed to affect the reflex bradycardia in response to pharyngo-oesophageal stimulation (see above). Our preliminary data suggest that other reflex-evoked bradycardia, such as that evoked following corneal receptor stimulation, is also unaffected (P. Boscan and J. F. R. Paton, unpublished observations), whereas in anaesthetized rats P2 receptors in the NTS were shown to modulate the baroreflex-evoked bradycardia (Scislo et al. 1998). Nevertheless, our data reveal a relatively high level of organization within NTS circuitry mediating the peripheral chemoreceptor reflex such that P2 receptors are important for some of its components.
We fully acknowledge the limitations of the specificity of the antagonists we have used. Currently, these are the only pharmacological tools available commercially and have been used in other studies on central nervous mechanisms relating to cardiorespiratory control (Scislo et al. 1998; Scislo & O'Leary, 1998, 2000). Despite these limitations, the data presented argue for the involvement of P2 receptors. First, if the effects of suramin and PPADS were non-specific it would be expected that all components of the chemoreceptor reflex would have been attenuated. This was not found to be the case. Second, we showed that at the doses used to attenuate the chemoreceptor reflex bradycardia, suramin and PPADS were ineffective in altering glutamate-, NMDA- and AMPA-induced bradycardia, suggesting that at the dose used these P2 receptor antagonists were not affecting glutamatergic excitatory neurotransmission. Indeed, we consider that glutamatergic transmission is important for mediating the chemoreceptor reflex-induced bradycardia that remains following suramin or PPADS blockade of P2 receptors in the NTS. Third, both suramin and PPADS also failed to attenuate a reflex bradycardia produced by stimulating the pharyngo-oesophageal reflex. Moreover, suramin preferentially attenuated the chemoreceptor- but not the pharyngo-oesophageal reflex-evoked excitatory synaptic response in intracellularly recorded NTS neurones. Fourth, we used a selective P2X receptor agonist and found that this produced a bradycardia that was fully antagonized by suramin. With the finding that α,β-methylene ATP also potentiated the chemoreceptor reflex bradycardia, we suggest a role for P2X receptors in the NTS for mediating the chemoreceptor reflex bradycardia.
Our findings raise the question of how P2 receptor blockade can affect one input but not another that both converge onto the same neurone. Although our data are clear in showing a difference in terms of P2 receptor involvement in chemoreceptor- versus pharyngo-oesophageal receptor-evoked bradycardia in the NTS, the organization remains unclear. A possibility is that P2 receptors are located presynaptically on chemoreceptor afferents but not on sensory fibres from pharyngo-oesophageal receptors. There is evidence to support the functional presence of P2X receptors on afferent endings of type I chemoreceptors (Zhang et al. 2000). Furthermore, our results would support this, since in the majority of NTS neurones tested with suramin, there was no significant change in either resting membrane potential or input resistance. Recent evidence supports the suggestion that P2X7 receptors, known to be on vagal sensory endings in the NTS, promote release of excitatory neurotransmitter (Deuchars et al. 2001). The presence of this subtype of P2X receptor on some chemoreceptor afferents would explain the present data. A more difficult result of the present study to explain is the absence of effect of P2 receptor blockade on the reflex tachypnoea following chemoreceptor activation. This remains unclear but provokes the hypothesis that NTS neurones mediating the respiratory component of the chemoreceptor reflex are distinct from those outputting to the cardiovascular system. Either P2 receptors are distributed exclusively on those chemoreceptor afferents impinging on NTS neurones affecting cardiovascular outflows or the neurones mediating the respiratory component of the chemoreceptor reflex are located in a different region of the NTS or brainstem.
We conclude that P2 receptors in the commissural NTS may play a key role in the neurotransmission of the parasympathetic, but not the respiratory component, of the peripheral chemoreceptor reflex.
Acknowledgments
In the UK, these studies were funded by the British Heart Foundation (BS/93003), the BBSRC (24/S11296) and Merck Sharp & Dohme. P. B. was funded by a University of Bristol scholarship. In Brazil, funding sources were FAPESP (95/4685-8 and 97/01814-7), CNPQ (522150/95-0) and PRONEX (357/96).
References
- Chitravanshi VC, Kachroo A, Sapru HN. A midline area in the nucleus commissuralis of NTS mediates the phrenic nerve responses to carotid chemoreceptor stimulation. Brain Research. 1994;662:127–133. doi: 10.1016/0006-8993(94)90804-4. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. American Journal of Physiology. 1995;268:R851–858. doi: 10.1152/ajpregu.1995.268.4.R851. [DOI] [PubMed] [Google Scholar]
- Daly MdeB. Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. Oxford: Monograph of the Physiological Society. Clarendon Press; 1997. [Google Scholar]
- de Paula PM, Bonagamba LGH, Machado BH. Involvement of purinergic mechanisms in the neurotransmission of the chemoreflex in the nucleus tractus solitarius of awake rats. Autonomic Neuroscience: Basic and Clinical. 2000;82:55. [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TFC, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. Journal of Neuroscience. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Research. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Krieger EM. Carotid chemoreceptors influence arterial pressure in intact and aortic denervated rat. American Journal of Physiology. 1992;262:R677–683. doi: 10.1152/ajpregu.1992.262.4.R677. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. Journal of the Autonomic Nervous System. 1993;42:63–70. doi: 10.1016/0165-1838(93)90342-r. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Bonagamba LGH, Machado BH. Sympathoexcitatory neurotransmission of the chemoreflex in the NTS of awake rats. American Journal of Physiology. 1999;276:R69–80. doi: 10.1152/ajpregu.1999.276.1.R69. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Colombari E, Chianca DA, Jr, Bonagamba LGH, Machado BH. NMDA receptors in NTS are involved in bradycardic but not in pressor response of chemoreflex. American Journal of Physiology. 1995;269:H1421–1427. doi: 10.1152/ajpheart.1995.269.4.H1421. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Burnstock G. Ultrastructural localization of P2X3 receptors in rat sensory neurons. NeuroReport. 1998;9:2545–2550. doi: 10.1097/00001756-199808030-00022. [DOI] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LGH. Microinjection of l-glutamate into the nucleus tractus solitarii increases arterial pressure in conscious rats. Brain Research. 1992;576:131–138. doi: 10.1016/0006-8993(92)90618-j. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. American Journal of Physiology. 1992;263:R368–375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. Journal of Neuroscience Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Importance of neurokinin-1 receptors in the nucleus tractus solitarii of mice for the integration of cardiac vagal inputs. European Journal of Neuroscience. 1998;10:2261–2275. doi: 10.1046/j.1460-9568.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Paton JFR. The Sharpey-Schaffer Prize Lecture. Nucleus tractus solitarii: integrating structures. Experimental Physiology. 1999;84:815–833. [PubMed] [Google Scholar]
- Paton JFR, Deuchars J, Li Y-W, Kasparov S. Morphological and electrophysiological comparison of solitary tract neurones responding to peripheral chemoreceptor stimulation. Neuroscience. 2001;105:231–248. doi: 10.1016/s0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Sensory channel specific modulation in the nucleus of the solitary tract. Journal of the Autonomic Nervous System. 2000;80:117–129. doi: 10.1016/s0165-1838(00)00077-1. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Li Y-W, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemo- receptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Brain in Stereotaxic Coordinates. 2. Sydney, Australia: Academic Press; 1986. [Google Scholar]
- Peoples RW, Li C. Inhibition of NMDA-gated ion channels by the P2 purinoceptor antagonists suramin and reactive blue 2 in mouse hippocampal neurones. British Journal of Pharmacology. 1998;124:400–408. doi: 10.1038/sj.bjp.0701842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Scislo TJ, O'Leary DS. Purines and the nucleus tractus solitarius: effects on cardiovascular and respiratory function. Clinical and Experimental Pharmacology and Physiology. 1997;24:738–742. doi: 10.1111/j.1440-1681.1997.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. Journal of Physiology. 1996;494:399–409. doi: 10.1113/jphysiol.1996.sp021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scislo TJ, Ergene E, O'Leary DS. Impaired arterial baroreflex regulation of heart rate after blockade of P2-purinoceptors in the nucleus tractus solitarius. Brain Research Bulletin. 1998;47:67. doi: 10.1016/s0361-9230(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A2a and P2X purinoceptors. American Journal of Physiology. 1998;275:H2130–2139. doi: 10.1152/ajpheart.1998.275.6.H2130. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Differential role of ionotropic glutamatergic mechanisms in responses to NTS P2X and A2a receptor stimulation. American Journal of Physiology. 2000;278:H2057–2068. doi: 10.1152/ajpheart.2000.278.6.H2057. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. Comparative study on the distribution patterns of P2X(1)-P2X(6) receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with cathecholamine cell groups. Journal of Comparative Neurology. 2000;427:485–507. [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. Journal of Physiology. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


