Abstract
Information on the effects of thyroid hormone on smooth muscle contractile protein expression and mechanical properties is sparse. We have addressed the following questions. (1) Can thyroxine hormone alter myosin isoform composition in smooth muscle? (2) Can a change in myosin isoform composition lead to altered mechanical properties in smooth muscle? (3) Are alterations, if occurring, equal in fast and slow smooth muscle types? Guinea-pigs were treated with thyroxine (T4) for 12 days. Control animals were given physiological saline solution. Maximal unloaded shortening velocity (Vmax) was measured in chemically skinned, maximally activated muscle preparations from the aorta and the taenia coli. Vmax increased following thyroxine treatment, by approximately 20 % in the taenia coli. In the aorta, no significant increase in Vmax could be detected. The sensitivity of isometric force to inorganic phosphate (Pi) was increased in the taenia coli following thyroxine treatment. The expression of mRNA (determined with RT-PCR) for the myosin heavy chain with the seven amino acid insert increased by approximately 70 % in the aorta and about 25 % in the taenia coli following thyroxine treatment. Western blot analysis showed an increase in the inserted myosin heavy chain form in the taenia coli. Expression of mRNA for the myosin essential light chains and the corresponding proteins did not change significantly in either muscle type. No alterations in non-muscle myosin heavy chain isoforms could be detected after thyroxine treatment. In conclusion, thyroxine treatment alters the isoform composition of myosin in fast and slow smooth muscles in vivo. This change is sufficient to increase shortening velocity and sensitivity of isometric force to Pi in the fast, but not in the slow, smooth muscle type.
Smooth muscle performs a large variety of physiological tasks, such as keeping constant tension in the wall of large arteries or performing propulsive movement in the intestines. This is reflected in a large variability in contractile properties between smooth muscle types (Somlyo & Somlyo, 1968). The functional differences are present at the level of the contractile protein interaction, large differences in the maximal shortening velocity, rate of tension development and nucleotide dependencies exist between slow and fast smooth muscle types (Malmqvist & Arner, 1991; Fuglsang et al. 1993; Löfgren et al. 2001). Smooth muscle organs can also undergo dramatic changes in their structure and function in several physiological and pathophysiological situations. This can be accompanied by adaptive changes in the smooth muscle contractile system, towards a slower or faster contractile phenotype. The reported magnitude of these changes appears to be comparatively small, e.g. hypertrophy of the urinary bladder is associated with an ≈20 % decrease in maximal shortening velocity (Sjuve et al. 1996).
The myosin molecule is the major determinant of the actin-myosin kinetics in muscle, and altered expression of its isoforms is an important mechanism for long-term modulation of contractile kinetics. In skeletal and cardiac muscle several myosin heavy chain genes are differentially expressed depending on developmental stage and tissue (see Sellers et al. 1996). Control of these genes and the resulting tissue content of the respective myosin isoforms is an important mode of modulating the contractile properties in the striated muscle, although other contractile and regulatory proteins can also be involved. In contrast, the smooth muscle myosin heavy chains are formed from a single gene and isoforms are produced by alternative splicing. The two main smooth muscle myosin heavy chain variants, SM-1 and SM-2, differ in the C-terminal part and are produced by alternative splicing in the 3′ region (Rovner et al. 1986; Babij & Periasamy, 1989). Expression of these isoforms has not been found to correlate with muscle shortening velocity or actin filament displacement velocity in vitro (Malmqvist & Arner, 1991; Kelley et al. 1992). Additional isoforms are formed by alternative splicing in the myosin heavy chain head region (denoted SM-A and SM-B; White et al. 1993). This domain, near the ATP binding site, appears to have dramatic effects on myosin kinetics (Sweeney et al. 1998). SM-B contains an additional seven amino acid insert compared to SM-A (White et al. 1993) and has a higher velocity of actin displacement in vitro (Kelley et al. 1993; Rovner et al. 1997; Lauzon et al. 1998). Consistent with these data, smooth muscle from mice lacking the SM-B isoform appear to have a slower maximal shortening velocity (Babu et al. 2001). The myosin essential light chain exists in two isoforms in smooth muscle, LC17a and LC17b (Lash et al. 1990). Several studies have found a correlation between high relative tissue expression of LC17b and lower shortening velocity (Malmqvist & Arner, 1991; Fuglsang et al. 1993; Sjuve et al. 1996; Matthew et al. 1998). In contrast, no correlation between essential light chain isoform and actin displacement velocity has been found in in vitro motility studies (Kelley et al. 1993; Rovner et al. 1997). Thus, there are indications that both myosin heavy chain and essential light chain isoforms are important for determining the kinetic phenotype of smooth muscle, although the molecular mechanism, their relative roles and the regulation of their tissue expression in the intact smooth muscle cell are unknown.
Thyroid hormone is a potent factor influencing myosin isoform expression and mechanical properties of striated muscle. In both cardiac and skeletal muscle, increased levels of thyroid hormone in vivo are associated with a change towards faster muscle phenotypes and a higher expression of fast myosin isoforms (Buccino et al. 1967; Hoh et al. 1978; Hoh & Egerton, 1979; Izumo et al. 1986; Caiozzo et al. 1991). The active form of the thyroid hormone, triiodothyronine (T3) is formed by deiodination of thyroxine (T4), which is the main hormone released from the thyroid gland. T3 is considered to exert its effects on gene transcription by binding to an intracellular thyroid hormone receptor. At the transcriptional level, thyroid hormone exerts its effects through influence of T3 (in complex with a thyroid hormone receptor) on T3 responsive elements (TREs) located in the promoter regions of thyroid hormone responsive genes (Brent, 1994). In the cardiac α- and β-myosin heavy chain genes, TREs have been found in the promoter regions (see Morkin, 2000). TREs have also been identified in the MyoD and myogenin genes, believed to be involved in the differentiation of skeletal muscle (Downes et al. 1993; Muscat et al. 1994).
Information on the effects of thyroid hormone on the contractile system in smooth muscle is sparse. Thyroid hormone has acute relaxant effects on isolated smooth muscle cells and vascular preparations in vitro (Ishikawa et al. 1989; Ojamaa et al. 1996; Park et al. 1997; Zwaveling et al. 1997), suggesting non-genomic direct effects. A few studies have examined the effects of long-term treatment with thyroid hormone on smooth muscle structure and function. In a study on rabbits treated with thyroxine, Giuriato et al. (1991) reported intimal thickening and upregulation of non-muscle myosin in the aorta. It has been found that the thyroid state influences the density of α- and β-adrenoreceptors in smooth muscle, correlated with changed responses to catecholamines (Gunasekera & Kuriyama, 1990). Also, hyperthyroidism leads to increased acetylcholine- and potassium chloride-induced contractions of urinary bladder strips (Adeniyi et al. 1994) and increased acetylcholine-mediated relaxation of blood vessels (McAllister et al. 1998). In smooth muscle both heavy and light chain myosin isoforms are formed by alternative splicing from single genes. The regulation of this splicing process, the control of the non-muscle myosin expression and the molecular mechanism for thyroxine effects on smooth muscle myosin isoform composition are not known at present.
In an early study by Peiper (1977), performed on the intact portal vein from the rat, it was shown that shortening velocity was increased following long-term thyroxine (T4) or 1-triiodothyronine-hydrochloride (T3) treatment. At that time, knowledge of the different myosin isoforms and their correlation to muscle mechanics was lacking. In view of these previously published results, we have in this study addressed the following questions. (1) Can an alteration in myosin isoform composition of smooth muscle occur in vivo as a result of increased levels of thyroid hormone? (2) Can a change in the myosin composition of the smooth muscle contractile system lead to altered mechanical properties (shortening velocity and properties of the cross-bridge cycle)? (3) Are alterations, if occurring, equal in fast and slow smooth muscle types? We treated guinea-pigs with thyroxine (T4) for 12 days and examined the expression of smooth muscle myosin heavy and essential light chain isoforms in a fast (taenia coli) and a slow (aorta) smooth muscle type. We also examined expression of non-muscle isoforms. These biochemical data were correlated with determinations of force-velocity relationships in maximally activated preparations of these muscles.
A preliminary report of these results has been presented at the XXIX European Muscle Conference 2000, Berlin.
METHODS
Animals
Twenty-one female guinea-pigs (M & B A/S, Ry, Denmark) weighing about 400 g were used in this study, 11 receiving thyroxine treatment and 10 controls. Hyperthyroidism was induced by daily i.p. injections with 0.4 mg (kg body weight)−1 thyroxine (Nycomed, Lidingö, Sweden) for 12 days. Controls were given the same volume of saline solution. During the treatment the animals were weighed daily. On the 13th day, the animals were killed by cervical dislocation and the muscle preparations were cut out. The experiments were approved by the local ethics committee and carried out according to the Swedish national ethical guidelines for animal research.
Muscle preparations
The taenia coli and thoracic aorta were dissected and either frozen in liquid N2 for biochemistry and RT-PCR or skinned with Triton X-100 for mechanical experiments, as described previously (Arner & Hellstrand, 1985). The skinned muscle preparations were kept at −20 °C for a maximum of 3 weeks. The taenia coli preparations for the mechanical experiments had a diameter of about 0.15 mm and a length of 2–3 mm. For experiments on the aorta, a 1.2–1.8 mm long segment was cut open and mounted with the circular muscle layer in the long axis of the preparation, which gave preparations of 2–3 mm length with the full thickness of the media layer (approximately 0.05 mm).
Solutions for the mechanical experiments
All the experiments were performed at 22 °C. The solutions contained 30 mm Tes, 4 mm EGTA and 2 mm free Mg2+. All solutions were adjusted to pH 6.9 with KOH and to an ionic strength of 150 mm using KCl. The standard relaxation and activation solutions contained 3.2 mm MgATP, 12 mm phosphocreatine (PCr) and 0.5 mg ml−1 creatine kinase (CK). Rigor solutions did not contain ATP, PCr or CK. Thiophosphorylation of the regulatory myosin light chains was performed in a rigor solution with calcium (pCa 4.5), 0.5 μM calmodulin and 2 mm ATP-γ-S. The composition of all solutions was calculated using a computer program and stability constants essentially as described by Fabiato & Fabiato (1979) and Fabiato (1981). All chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA) and Boehringer Mannheim (Mannheim, Germany). Calmodulin was a generous gift from Dr E. Thulin, Department of Physical Chemistry II, Lund University.
Quick-release experiments
Small clips of aluminium foil were wrapped around each end of the muscle preparations which then were mounted between a stainless-steel pin attached to an AE 801 force transducer (SensoNor a.s., Horten, Norway) and an isotonic lever as described by Arner & Hellstrand (1985). Electromagnetic relays could release the lever arm. The afterload was determined by a spring load on the lever and reached stable values within 5 ms after release. An RTI-800 Analog Devices A/D board, with a sampling rate of 1 kHz, in a personal computer was used to record force and muscle length. Velocities were determined 100 ms after release. To calculate the maximal shortening velocity at each contraction, the Hill (1938) equation:
was fitted to the force and velocity (V) data. The values a and b in the equation are constants, P is the afterload and Po is the isometric force of the contraction. The curve was extrapolated to P/Po = 0, which gave the maximal shortening velocity (Vmax).
At the beginning of the experiment, the preparations were stretched to a passive tension of approximately 0.1 mN and placed in relaxing (pCa 9) solution. During the experiment the muscles were kept maximally activated by thiophosphorylation of the regulatory light chains, as described previously (Arheden et al. 1988). This results in near-maximal thiophosphorylation of the regulatory light chains (Hellstrand & Arner, 1985). After the thiophosphorylation, the preparations were rinsed in calcium-free rigor solution and thereafter contracted by transfer into an ATP-containing solution. At the plateau of the contraction the preparations were transferred to fresh solution and after 2 min a series of approximately 20 releases to different afterloads was executed. At the end of the experiment, the length of the muscle preparation was measured using a microscope with an ocular scale.
Isometric force
The same type of preparations as described for the quick-release experiments were used in the experiments measuring the active force at different concentrations of inorganic phosphate (Pi). The preparations were attached to a force transducer (AE 801, SensoNor) and a stainless-steel pin. Maximal activation was achieved using thiophosphorylation as described above and contraction was elicited with addition of solution containing 3.2 mm MgATP and different concentrations of Pi. A maximum of six contractions was performed on each muscle preparation.
SDS-gel electrophoresis
The relative amounts of LC17a and LC17b were determined using 2-D-gel electrophoresis with ampholytes in the pH range 4.5–6, as described previously (Malmqvist & Arner, 1991). The first isoelectric focusing dimension separates the proteins according to isoelectric point. A 15 % polyacrylamide gel separates the proteins according to molecular weight in the second dimension. Coomassie blue was used to stain the gels before quantification with densitometric scanning.
To determine the actin/myosin ratio and the relative content of non-muscle myosin isoforms and myosin heavy chain with the seven amino acid insert, samples were homogenised in a SDS-containing buffer and separated on 8 % polyacrylamide gels. Four different amounts of extracts from each preparation together with a standard from newborn mouse urinary bladders (containing both non-muscle A and B isoforms; produced in-house from newborn mice that were humanely killed by decapitation) were separated in parallel on each gel. Two gels were run in parallel, one for Coomassie blue staining and evaluation of actin and myosin bands, and the other for Western blotting with non-muscle A antibody, a gift from Dr Adelstein (Kelley et al. 1996), non-muscle B antibody, a gift from Drs Haase and Morano (Sjuve et al. 2001) or inserted myosin heavy chain antibody, also a gift from Drs Haase and Morano (Haase & Morano, 1996). These antibodies were derived from specific sequences in human non-muscle myosin heavy chain A, human non-muscle myosin heavy chain B and the myosin insert sequence. The enhanced chemiluminescence (ECL) reactions on the Western blot of non-muscle myosins and inserted myosin were expressed relative to the intensity of the myosin band on the Coomassie blue-stained gel. The urinary bladder extract was used as a standard to enable comparisons between gels.
RT-PCR
Relative amounts of the splice variants of mRNA for the myosin heavy (HC) and essential light (LC) chains were determined in the tissues using the RT-PCR technique. Oligonucleotide primers of the following sequences (listed from the 5′ to 3′ end) were used to determine HC composition: ATGTACAAGGGCAAGAAGAGGC and GAGGAGTTGTCGTTCTTGAC. For gizzard myosin, the corresponding primers would generate two fragments of 351 and 330 bp (Fisher et al. 1997), corresponding to the content of mRNA for HC with and without the seven amino acid insert, respectively. We used the corresponding primers from the mammalian sequence (mouse; NCBI GI-number: 7305294). For the LC, oligonucleotide primers (Fisher et al. 1997) of the following sequences (listed from the 5′ to 3′ end, using the mouse sequence; A. Arner, unpublished observations) were used: ATGTGTGACTTCACCGAGGAC and CATTCAGCACCATCCGGAC, generating two fragments of 451 and 496 bp, corresponding to the content of mRNA for LC17a and LC17b, respectively. The frozen tissue was homogenised and the minimessage maker kit (R&D Systems, Abingdon, UK) or Microfast track kit (Invitrogen, Carlsbad, USA) was used to extract mRNA from the tissue. cDNA was generated using the Superscript first strand synthesis system for RT-PCR (Life Technologies, Carlsbad, USA). The PCR reactions were performed with denaturation at 94 °C for 1 min, annealing at 51.7 °C (LC) or 53.1 °C (HC) and extension at 72 °C for 2 min for 35 cycles using a Progene thermocycler (Techne, Cambridge, UK) and Taq polymerase (Qiagen, Washington, USA). Agarose gels (2 %) with ethidium bromide were used to visualise the PCR products. The gels were evaluated using Gel Doc 2000 and Quantity One software (Bio-Rad, Richmond, CA, USA). Alternatively, the gels were photographed and the photographs were scanned using a Fuji Film Las 1000 Plus and a PC with Image Gauge V3.41 software. The splice-in and splice-out variants are expressed as a percentage of the total PCR products.
Statistics
All values are given as means ± s.e.m. with n being the number of observations. Student's t test or Bonferroni test was used to test statistical significance. P < 0.05 was considered significant.
RESULTS
Characteristics of the animals
The two groups of animals, control (receiving saline injections) and thyroxine (T4) treated, had similar initial mean weights (control: 346 ± 9.6 g, n = 10; T4 treated: 357 ± 8.1 g, n = 11). After 12 days of treatment, the mean weights of the animals were 406 ± 13.2 g (control, n = 10) and 330 ± 10.4 g (T4 group, n = 11), showing a weight loss in the thyroxine-treated animals. No apparent signs of discomfort were observed in either group of animals.
Tissue expression of myosin isoforms
Using the RT-PCR technique we identified PCR products corresponding to two isoforms of myosin heavy chain (MHC), with and without the seven amino acid insert in the head region, and two forms of essential 17 kDa light chain, LC17a and LC17b (Fig. 1 and Fig. 2). The summarised data from control animals (open bars in Fig. 1) show that the expression of mRNA for the inserted heavy chain form was higher in the taenia coli than in the aorta. Following thyroxine treatment (filled bars), the expression of mRNA for the inserted myosin heavy chain form relative to the total smooth muscle myosin heavy chain increased significantly in both tissues (taenia coli: MHC + insert/ total SM-MHC controls, 69.8 ± 2.7 %, n = 9; MHC + insert/total SM-MHC thyroxine treated, 87.5 ± 1.8 %, n = 7; aorta: MHC + insert/total SM-MHC controls, 28.9 ± 3.6 %, n = 6; MHC + insert/total SM-MHC thyroxine treated, 49.6 ± 3.4 %, n = 7). To investigate whether the increase in expression of mRNA encoding the myosin heavy chain with insert was also associated with an increase in the expression of the corresponding protein, we performed quantitative Western blot analysis. Western blot analysis does not provide absolute values of the amount of the inserted myosin, but in relative units (ECL reaction of MHC + insert on the Western blots/intensity of SM-MHC on the Coomassie blue-stained gels), the expression increased from 8.3 ± 1.9 to 28.5 ± 8.3 (P < 0.05, n = 5) in the taenia coli, confirming the results from the RT-PCR. In the aorta preparation the expression of the myosin heavy chain insert was very low and we could therefore not detect the inserted myosin at levels high enough to enable comparisons between control and T4-treated vessels.
Figure 1. Expression of mRNA for myosin heavy chain.
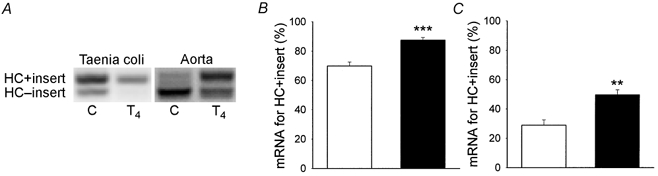
A, an original photograph of an agarose-ethidium bromide gel of RT-PCR products showing the myosin heavy chains (HC). The left lanes show data from a control animal (C) and the right lanes show data from a thyroxine-treated animal (T4). PCR products for the inserted and non-inserted myosin heavy chains had gel mobilities with a predicted difference in size of 21 bp. B (taenia coli) and C (aorta) show the mean values of the relative expression of mRNA for the myosin heavy chain with the seven amino acid insert. □, control experiments; ▪, experiments on muscles from thyroxine-treated animals. **P < 0.01 and ***P < 0.001 (n = 6–9).
Figure 2. Expression of mRNA for LC17.
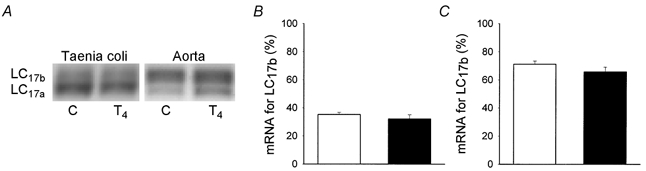
A, an original photograph of an agarose-ethidium bromide gel of RT-PCR products showing the myosin essential light chains (LC). The left lanes show data from a control animal (C) and the right lanes show data from a thyroxine-treated animal (T4). The PCR products for the different isoforms of myosin essential light chains (LC17a and LC17b) had mobilities corresponding to 451 and 496 bp, respectively. B (taenia coli) and C (aorta) show the mean values of the relative expression of mRNA for the myosin essential light chain 17b (LC17b). □, results from control experiments; ▪, results from experiments on muscles from thyroxine-treated animals (n = 8–9).
Figure 2 shows the tissue expression of mRNA for the myosin essential light chain isoforms. In control animals (open bars), the expression of the LC17b form was higher in the aorta than in the taenia coli. Treatment with thyroxine did not significantly alter the expression in either tissue, although the expression of mRNA for LC17b tended to decrease. To examine whether the expression of LC17 protein was altered following thyroxine treatment, we performed 2-D-gel electrophoresis. The relative amount of LC17b was higher in the aorta (58.9 ± 2.7 %, n = 4) than in the taenia coli (15.7 ± 0.5 %, n = 4), but was not significantly altered following thyroxine treatment in either tissue (58.4 ± 4.9 %, n = 4, in the aorta and 11.9 ± 1.5 %, n = 5, in the taenia coli).
We evaluated changes in tissue expression of non-muscle myosin isoforms A and B using semi-quantitative Western blots. No significant changes in the expression of non-muscle myosin A or B isoforms could be detected following T4 treatment (Table 1). Although no absolute measurements of tissue content of non-muscle myosins was performed, it should be noted that the aorta had a significantly higher content of non-muscle myosins than the taenia coli; the content of non-muscle myosin-B relative to the myosin band was more than 10 times higher in the aorta than in the taenia coli. A slight increase in the myosin/actin ratio was observed following T4 treatment in the aorta.
Table 1.
Contractile protein content and active force in control and thyroxine-treated animals
| Taenia coli | Aorta | |||
|---|---|---|---|---|
| Control | T4 treated | Control | T4 treated | |
| Myosin/actin ratio | 0.43 ± 0.03 | 0.44 ± 0.07 | 0.37 ± 0.02 | 0.45 ± 0.02* |
| NM-MHC-A (relative units) | 0.27 ± 0.08 | 0.21 ± 0.08 | 0.71 ± 0.10 | 1.08 ± 0.22 |
| NM-MHC-B (relative units) | 0.15 ± 0.04 | 0.22 ± 0.09 | 2.57 ± 0.62 | 2.18 ± 0.52 |
| Force (mN) | 4.83 ± 0.42 | 4.23 ± 0.25 | 2.16 ± 0.14 | 1.60 ± 0.24 |
The myosin/actin ratio was calculated from Coomassie blue-stained SDS-PAGE gels. The content of non-muscle heavy chain A (NM-MHC-A)and B (NM-MHC-B)in the respective tissues is expressed as relative units of ECL intensity on Western blots compared to Coomassie blue intensity of the myosin band. Data were normalised to the ratio in a standard homogenate of urinary bladder tissue from newborn mice, containing both of these non-muscle isoforms. The force values are given in millinewtons, the sizes of the preparations for the force measurements were equal in control and thyroxine-treated animals for the taenia coli and aorta, respectively.
P < 0.05 vs. Control (n = 4–6).
Mechanical characteristics
The active force of the skinned fibre preparation appeared to be slightly decreased after T4 treatment, although no significant changes were detected (Table 1). Figure 3 shows force-velocity relationships and Vmax obtained in quick-release experiments on the taenia coli (Fig. 3A) and the aorta (Fig. 3B) from control animals (open circles and bars) and animals treated with thyroxine (filled circles and bars). The experiments were carried out on fully activated (thiophosphorylated) skinned muscle preparations. The Vmax of the taenia coli was approximately three times higher than that of the aorta. Vmax was significantly increased in the taenia coli preparations following thyroxine treatment (Vmax control: 0.162 ± 0.011 muscle lengths (ML) s−1, n = 10; Vmax thyroxine treated: 0.194 ± 0.008 ML s−1, n = 11). In the aorta the mean value was not significantly changed (Vmax control: 0.035 ± 0.005 ML s−1, n = 10; Vmax thyroxine treated: 0.047 ± 0.010 ML s−1, n = 9).
Figure 3. Force-velocity relationships and maximal unloaded shortening velocity (Vmax) from quick-release experiments performed on taenia coli (A) and aorta (B).

The left panels show original plots of afterload (P/Po) and velocity (in muscle lengths s−1) from a control animal (○) and a thyroxine-treated animal (•). The right panels show mean values of Vmax from control (□) and thyroxine-treated (▪) animals. *P < 0.05 (n = 9–11).
Figure 4 shows the effects of Pi on active force on the thiophosphorylated skinned muscle preparations. In both types of smooth muscle tissue, Pi reduced force in a dose-dependent manner. The effect was more pronounced in the taenia coli muscle where approximately 40 % inhibition was observed at 40 mm Pi. In contrast, the force of the aorta was less affected, with approximately 15 % inhibition at 40 mm Pi. In taenia coli preparations from thyroxine-treated animals a larger effect of Pi was observed at all Pi concentrations. In the aorta, no significant differences could be detected between preparations from control and thyroxine-treated animals regarding the effects of Pi.
Figure 4. Effects of Pi on isometric force.
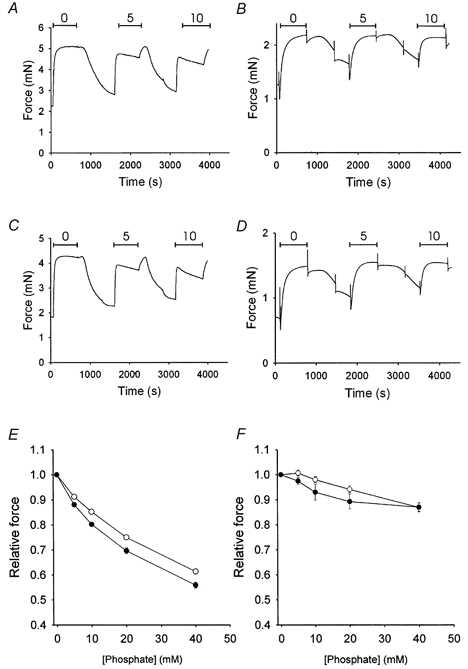
A-D, original recordings from experiments investigating the effects of Pi on active isometric force in taenia coli (A and C) and aorta (B and D) preparations from control (A and B) and thyroxine-treated (C and D) animals. In these panels, three contractions at different concentrations (0, 5 and 10 mm) of Pi are shown. Between the contractions there are periods of treatment with ATP-γ-S and subsequent rinsing periods in rigor solution. Mean values of force at different [Pi] are shown in E (taenia coli) and F (aorta) for control (○) and thyroxine-treated (•) animals. The force from the T4-treated preparations was significantly lower than that from the controls at all concentrations of added Pi (P < 0.05, Bonferroni test) in the taenia coli (E). (n = 5–6).
T4 has been suggested to influence myosin light chain kinase (MLCK) activity, possibly by a direct binding of the hormone to the enzyme (Hagiwara et al. 1988, 1989). This effect is likely to be minimal in the skinned preparations, but to exclude inhibition of MLCK in the T4-treated preparations, we performed additional control experiments. Taenia coli preparations were first thiophosphorylated for 3 min at pCa 4.5. This resulted in a submaximal contraction when ATP was added. The force of this contraction relative to maximal, which reflects the extent of MLCK-induced thiophosphorylation during the 3 min period, was not different in the T4-treated compared to control preparations (control: 0.65 ± 0.04, n = 6; T4 treated: 0.65 ± 0.08, n = 6). This suggests that MLCK activity was not altered by T4 treatment in these preparations. Subsequently, the preparations were treated with ATP-γ-S at pCa 4.5 in the presence of microcystine (30 nm) for 30 min. This did not increase the ATP-induced force above the level obtained using our standard protocol and the difference in Pi-induced relaxation was still present between T4-treated and control taenia coli preparations (control: 40.4 ± 1.7 %; T4 treated: 47.3 ± 1.0 % relaxation at 40 mm Pi; P < 0.05, n = 6). These results show that further treatment with ATP-γ-S and phosphatase inhibitors did not result in a higher force or a changed Pi sensitivity.
Discussion
We have examined two muscles, the aorta and the taenia coli, representing a slow and a fast smooth muscle type. Using RT-PCR and gel electrophoresis, we confirmed that the taenia coli with a comparatively fast Vmax had a higher expression of myosin heavy chain with the seven amino acid insert (MHC + insert; Kelley et al. 1993) and acidic essential light chain (LC17a; Malmqvist & Arner, 1991) compared to the slow aorta muscle. Using thyroid hormone treatment we showed that the velocity and Pi sensitivity of force can be modulated in vivo and that these mechanical changes can be correlated with altered expression of the myosin heavy chain isoforms.
In a study by Peiper (1977), it was reported that the shortening velocity of the smooth muscle in the rat portal vein increased following thyroid hormone treatment. However, since intact preparations were used in that study it is difficult to exclude that altered degree of activation was involved in the effects of thyroid hormone on shortening velocity. In the present study we therefore used permeabilised, maximally activated preparations and can conclude that the changes in shortening velocity induced by thyroid hormone occur at the level of contractile proteins.
The composition of myosin heavy chains and essential light chains are important for determining the kinetic phenotype of a smooth muscle tissue. Data from in vitro motility experiments strongly favour the myosin heavy chain insert as being the important modulator of velocity in smooth muscle (Kelley et al. 1993). Data from organised contractile systems in muscle preparations are less conclusive since light and heavy chain composition appears to be modulated in a co-ordinated manner in smooth muscles altering their contractile phenotype during hypertrophy (Morano et al. 1993; Sjuve et al. 1996). Studies on intact smooth muscles from mice where the gene sequence for the myosin heavy chain insert is ablated reveal a slowing of the shortening velocity (Babu et al. 2001), although the magnitude of the change is not as large as the span between the slowest and the fastest smooth muscles. In this study, we observe that thyroxine treatment results in a dramatic change in the myosin heavy chain composition towards more of the inserted type and that this change is correlated with a faster phenotype in the taenia coli. This suggests that the myosin heavy chain composition of the taenia coli has functional effects on shortening velocity and that a change towards more of the inserted form is sufficient to shift the taenia coli towards a faster phenotype.
In addition to the smooth muscle myosin heavy chain, smooth muscle cells of large elastic arteries can also contain non-muscle myosin heavy chains (Larson et al. 1984). We have confirmed these results and show that the aorta has a significantly higher relative content of both NM-MHC-A and NM-MHC-B than the taenia coli. At present we do not know the absolute content or cellular distribution of these isoforms in the smooth muscle cells. The preparations from the aorta were not denuded of endothelial cells which could also contribute somewhat to the amount of NM-MHC found in this tissue. Both non-muscle myosin isoforms can be expressed in smooth muscle and exhibit a low shortening velocity in muscle fibres (Morano et al. 2000). Non-muscle myosin isoforms might thus contribute to the low velocity of the aorta. In a study by Giuriato et al. (1991), it was found that the aortic media of rabbits treated with thyroid hormone had an increased number of smooth muscle cells expressing non-muscle myosin heavy chain (NM-MHC). In the present investigation, however, we did not observe any change in non-muscle myosin expression after thyroxine treatment, suggesting that these isoforms are not responsible for the alterations in Vmax observed.
Interestingly, the largest change in myosin heavy chain isoform composition was observed in the aorta, where we could not find any statistically verified changes in Vmax. This is in contrast to the findings in skeletal muscle, where both the myosin isoform and the mechanical properties are more strongly affected in the slow muscle types (Caiozzo et al. 1991). The fact that we do not observe a change towards a faster contractile phenotype in the aorta might be due to its specific myosin isoform composition. One possibility for the small mechanical effects of T4 treatment in the aorta is that a high expression of LC17b, non-inserted heavy chain, non-muscle myosin or a different expression of cytoskeletal proteins introduces an intrinsic load or is associated with a structural state that makes the change in the heavy chain region have less influence on the filament velocity. It is possible that the amount of inserted myosin heavy chain has to exceed a certain threshold in order to have an effect on Vmax. Another possibility could be that the structure of the aorta somehow limits the influence of alterations in the kinetics of the contractile system on the shortening velocity.
An altered Vmax can reflect a difference in the kinetics of the actin-myosin interaction induced by the T4 treatment. However, it is difficult to exclude that alterations in an internal load, in the contractile unit length or in the mechanical coupling in the tissue contribute to the change in Vmax. It might be possible that T4 alters the cell/tissue structure or expression of cytoskeletal proteins that can be involved in the change in Vmax by a pure structural mechanism. In this respect, an independent measure of cross-bridge kinetics would be important in order to link the mechanical changes to alterations in enzymatic properties of myosin. Vmax is considered to be rate-limited by the ADP release reaction (Siemankowski et al. 1985; Geeves, 1989). In smooth muscle, addition of ADP slows shortening velocity (Löfgren et al. 2001) and this effect is significantly stronger in slow muscle types. Most probably the alterations in Vmax following T4 treatment are associated with alterations in the ADP release reaction. However, measurements of the apparent inhibition constant for ADP in cycling cross-bridges is complex, e.g. as ATP back-up systems cannot be used. We predicted that the change in Vmax following T4 treatment would be associated with a difference in ADP binding that would be too small to be accurately detected with the quick-release method used here. It has been shown that release of Pi from the actin-myosin-ADP-Pi complex is coupled to force generation of smooth and skeletal muscle (Hibberd et al. 1985; Österman & Arner, 1995). Active force is less influenced by Pi in smooth than in skeletal muscle, suggesting that the kinetics of the Pi release reaction differs between these muscle types. We have recently shown that active force is inhibited by Pi at lower concentrations in fast compared to slow smooth muscle (Löfgren et al. 2001), which seems to suggest that the myosin isoform in a co-ordinated manner influences both the kinetics of cross-bridge reactions associated with force generation and the reactions rate-limiting for filament sliding. In the present study, we used the Pi sensitivity of force for probing cross-bridge reactions and observed an increase in the sensitivity to Pi in the taenia coli following thyroxine treatment. The result from the taenia coli, consistent with the notion that faster smooth muscle phenotypes have higher sensitivity to Pi, suggests that mechanical effects of thyroxine treatment do not simply involve structural changes, but reflect altered kinetics of the cross-bridge cycle.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (04x-8268), the Medical Faculty Lund University, the Crafoord foundation and the Swedish Heart Lung foundation. We are very grateful for the gifts of non-muscle myosin heavy chain and inserted myosin heavy chain antibodies from Drs Haase and Morano, MDC, Berlin, Germany and Dr Adelstein, NIH, Washington, USA.
References
- Adeniyi KO, Ogunkeye OO, Senok SS, Udoh FV. Influence of the thyroid state on the intrinsic contractile properties of the bladder muscle. Acta Physiologica Hungarica. 1994;82:69–74. [PubMed] [Google Scholar]
- Arheden H, Arner A, Hellstrand P. Cross-bridge behaviour in skinned smooth muscle of the guinea-pig taenia coli at altered ionic strength. Journal of Physiology. 1988;403:539–558. doi: 10.1113/jphysiol.1988.sp017263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner A, Hellstrand P. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. Journal of Physiology. 1985;360:347–365. doi: 10.1113/jphysiol.1985.sp015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij P, Periasamy M. Myosin heavy chain isoform diversity in smooth muscle is produced by differential RNA processing. Journal of Molecular Biology. 1989;210:673–679. doi: 10.1016/0022-2836(89)90142-3. [DOI] [PubMed] [Google Scholar]
- Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nature Cell Biology. 2001;3:1025–1029. doi: 10.1038/ncb1101-1025. [DOI] [PubMed] [Google Scholar]
- Brent GA. The molecular basis of thyroid hormone action. New England Journal of Medicine. 1994;331:847–853. doi: 10.1056/NEJM199409293311306. [DOI] [PubMed] [Google Scholar]
- Buccino RA, Spann JF, Jr, Pool PE, Sonnenblick EH, Braunwald E. Influence of the thyroid state on the intrinsic contractile properties and energy stores of the myocardium. Journal of Clinical Investigation. 1967;46:1669–1682. doi: 10.1172/JCI105658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaioOZZ VJ, Herrick RE, Baldwin KM. Influence of hyperthyroidism on maximal shortening velocity and myosin isoform distribution in skeletal muscles. American Journal of Physiology. 1991;261:C285–295. doi: 10.1152/ajpcell.1991.261.2.C285. [DOI] [PubMed] [Google Scholar]
- Downes M, Griggs R, Atkins A, Olson EN, Muscat GE. Identification of a thyroid hormone response element in the mouse myogenin gene: characterization of the thyroid hormone and retinoid X receptor heterodimeric binding site. Cell Growth and Differentiation. 1993;4:901–909. [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. Journal of General Physiology. 1981;78:457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Fisher SA, Ikebe M, Brozovich F. Endothelin-1 alters the contractile phenotype of cultured embryonic smooth muscle cells. Circulation Research. 1997;80:885–893. doi: 10.1161/01.res.80.6.885. [DOI] [PubMed] [Google Scholar]
- Fuglsang A, Khromov A, Torok K, Somlyo AV, Somlyo AP. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. Journal of Muscle Research and Cell Motility. 1993;14:666–677. doi: 10.1007/BF00141563. [DOI] [PubMed] [Google Scholar]
- Geeves MA. Dynamic interaction between actin and myosin subfragment 1 in the presence of ADP. Biochemistry. 1989;28:5864–5871. doi: 10.1021/bi00440a024. [DOI] [PubMed] [Google Scholar]
- Giuriato L, Borrione AC, Zanellato AM, Tonello M, Scatena M, Scannapieco G, Pauletto P, Sartore S. Aortic intimal thickening and myosin isoform expression in hyperthyroid rabbits. Arteriosclerosis and Thrombosis. 1991;11:1376–1389. doi: 10.1161/01.atv.11.5.1376. [DOI] [PubMed] [Google Scholar]
- Gunasekera RD, Kuriyama H. The influence of thyroid states upon responses of the rat aorta to catecholamines. British Journal of Pharmacology. 1990;99:541–547. doi: 10.1111/j.1476-5381.1990.tb12965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Morano I. Alternative splicing of smooth muscle myosin heavy chains and its functional consequences. Journal of Cellular Biochemistry. 1996;60:521–528. doi: 10.1002/(SICI)1097-4644(19960315)60:4%3C521::AID-JCB8%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Mamiya S, Hidaka H. Selective binding of l-thyroxine by myosin light chain kinase. Journal of Biological Chemistry. 1989;264:40–44. [PubMed] [Google Scholar]
- Hagiwara M, Mamiya S, Ochiai M, Hidaka H. Thyroid hormones inhibit the Ca2+ calmodulin-induced activation of myosin light chain kinase. Biochemical and Biophysical Research Communications. 1988;152:270–276. doi: 10.1016/s0006-291x(88)80710-1. [DOI] [PubMed] [Google Scholar]
- Hellstrand P, Arner A. Myosin light chain phosphorylation and the cross-bridge cycle at low substrate concentration in chemically skinned guinea pig Taenia coli. Pflügers Archiv. 1985;405:323–328. doi: 10.1007/BF00595684. [DOI] [PubMed] [Google Scholar]
- Hibberd MG, Dantzig JA, Trentham DR, Goldman YE. Phosphate release and force generation in skeletal muscle fibers. Science. 1985;228:1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of shortening. Proceedings of the Royal Society. 1938;126:231–252. [Google Scholar]
- Hoh JF, Egerton LJ. Action of triiodothyronine on the synthesis of rat ventricular myosin isoenzymes. FEBS Letters. 1979;101:143–148. doi: 10.1016/0014-5793(79)81313-7. [DOI] [PubMed] [Google Scholar]
- Hoh JF, McGrath PA, Hale PT. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. Journal of Molecular and Cellular Cardiology. 1978;10:1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Chijiwa T, Hagiwara M, Mamiya S, Hidaka H. Thyroid hormones directly interact with vascular smooth muscle strips. Molecular Pharmacology. 1989;35:760–765. [PubMed] [Google Scholar]
- Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231:597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kelley CA, Sellers JR, Gard DL, Bui D, Adelstein RS, Baines IC. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. Journal of Cell Biology. 1996;134:675–687. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CA, Sellers JR, Goldsmith PK, Adelstein RS. Smooth muscle myosin is composed of homodimeric heavy chains. Journal of Biological Chemistry. 1992;267:2127–2130. [PubMed] [Google Scholar]
- Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. Journal of Biological Chemistry. 1993;268:12848–12854. [PubMed] [Google Scholar]
- Larson DM, Fujiwara K, Alexander RW, Gimbrone MA., Jr Heterogeneity of myosin antigenic expression in vascular smooth muscle in vivo. Laboratory Investigation. 1984;50:401–407. [PubMed] [Google Scholar]
- Lash JA, Helper DJ, Klug M, Nicolozakes AW, Hathaway DR. Nucleotide and deduced amino acid sequence of cDNAs encoding two isoforms for the 17,000 dalton myosin light chain in bovine aortic smooth muscle. Nucleic Acids Research. 1990;18:7176. doi: 10.1093/nar/18.23.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon AM, Tyska MJ, Rovner AS, Freyzon Y, Warshaw DM, Trybus KM. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. Journal of Muscle Research and Cell Motility. 1998;19:825–837. doi: 10.1023/a:1005489501357. [DOI] [PubMed] [Google Scholar]
- Löfgren M, Malmqvist U, Arner A. Substrate and product dependence of force and shortening in fast and slow smooth muscle. Journal of General Physiology. 2001;117:407–418. doi: 10.1085/jgp.117.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister RM, Grossenburg VD, Delp MD, Laughlin MH. Effects of hyperthyroidism on vascular contractile and relaxation responses. American Journal of Physiology. 1998;274:E946–953. doi: 10.1152/ajpendo.1998.274.5.E946. [DOI] [PubMed] [Google Scholar]
- Malmqvist U, Arner A. Correlation between isoform composition of the 17 kDa myosin light chain and maximal shortening velocity in smooth muscle. Pflügers Archiv. 1991;418:523–530. doi: 10.1007/BF00370566. [DOI] [PubMed] [Google Scholar]
- Matthew JD, Khromov AS, Trybus KM, Somlyo AP, Somlyo AV. Myosin essential light chain isoforms modulate the velocity of shortening propelled by nonphosphorylated cross-bridges. Journal of Biological Chemistry. 1998;273:31289–31296. doi: 10.1074/jbc.273.47.31289. [DOI] [PubMed] [Google Scholar]
- Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, Haase H, Bader M. Smooth-muscle contraction without smooth-muscle myosin. Nature Cell Biology. 2000;2:371–375. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- Morano I, Erb G, Sogl B. Expression of myosin heavy and light chains changes during pregnancy in the rat uterus. Pflügers Archiv. 1993;423:434–441. doi: 10.1007/BF00374938. [DOI] [PubMed] [Google Scholar]
- Morkin E. Control of cardiac myosin heavy chain gene expression. Microscopy Research and Technique. 2000;50:522–531. doi: 10.1002/1097-0029(20000915)50:6<522::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Muscat GE, Mynett-Johnson L, Dowhan D, Downes M, Griggs R. Activation of myoD gene transcription by 3,5,3′-triiodo-l-thyronine: a direct role for the thyroid hormone and retinoid X receptors. Nucleic Acids Research. 1994;22:583–591. doi: 10.1093/nar/22.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojamaa K, Klemperer JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid. 1996;6:505–512. doi: 10.1089/thy.1996.6.505. [DOI] [PubMed] [Google Scholar]
- Österman A, Arner A. Effects of inorganic phosphate on cross-bridge kinetics at different activation levels in skinned guinea-pig smooth muscle. Journal of Physiology. 1995;484:369–383. doi: 10.1113/jphysiol.1995.sp020671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Dai HB, Ojamaa K, Lowenstein E, Klein I, Sellke FW. The direct vasomotor effect of thyroid hormones on rat skeletal muscle resistance arteries. Anesthesia and Analgesia. 1997;85:734–738. doi: 10.1097/00000539-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Peiper U. Force-velocity relations of the portal vein of hyperthyroid rats. Pflügers Archiv. 1977;372:23–27. doi: 10.1007/BF00582202. [DOI] [PubMed] [Google Scholar]
- Rovner AS, Freyzon Y, Trybus KM. An insert in the motor domain determines the functional properties of expressed smooth muscle myosin isoforms. Journal of Muscle Research and Cell Motility. 1997;18:103–110. doi: 10.1023/a:1018689102122. [DOI] [PubMed] [Google Scholar]
- Rovner AS, Thompson MM, Murphy RA. Two different heavy chains are found in smooth muscle myosin. American Journal of Physiology. 1986;250:C861–870. doi: 10.1152/ajpcell.1986.250.6.C861. [DOI] [PubMed] [Google Scholar]
- Sellers JR, Goodson HV, Wang F. A myosin family reunion. Journal of Muscle Research and Cell Motility. 1996;17:7–22. doi: 10.1007/BF00140320. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proceedings of the National Academy of Sciences of the USA. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjuve R, Haase H, Ekblad E, Malmqvist U, Morano I, Arner A. Increased expression of non-muscle myosin heavy chain-B in connective tissue cells of hypertrophic rat urinary bladder. Cell and Tissue Research. 2001;304:271–278. doi: 10.1007/s004410000262. [DOI] [PubMed] [Google Scholar]
- Sjuve R, Haase H, Morano I, Uvelius B, Arner A. Contraction kinetics and myosin isoform composition in smooth muscle from hypertrophied rat urinary bladder. Journal of Cellular Biochemistry. 1996;63:86–93. doi: 10.1002/(SICI)1097-4644(199610)63:1%3C86::AID-JCB7%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacological Reviews. 1968;20:197–272. [PubMed] [Google Scholar]
- Sweeney HL, Rosenfeld SS, Brown F, Faust L, Smith J, Xing J, Stein LA, Sellers JR. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. Journal of Biological Chemistry. 1998;273:6262–6270. doi: 10.1074/jbc.273.11.6262. [DOI] [PubMed] [Google Scholar]
- White S, Martin AF, Periasamy M. Identification of a novel smooth muscle myosin heavy chain cDNA: isoform diversity in the S1 head region. American Journal of Physiology. 1993;264:C1252–1258. doi: 10.1152/ajpcell.1993.264.5.C1252. [DOI] [PubMed] [Google Scholar]
- Zwaveling J, Pfaffendorf M, van Zwieten PA. The direct effects of thyroid hormones on rat mesenteric resistance arteries. Fundamental and Clinical Pharmacology. 1997;11:41–46. doi: 10.1111/j.1472-8206.1997.tb00167.x. [DOI] [PubMed] [Google Scholar]


