Abstract
We investigated the thermoregulatory responses of sympathetic fibres supplying the tail in urethane-anaesthetised rats. When skin and rectal temperatures were kept above 39 °C, tail sympathetic fibre activity was low or absent. When the trunk skin was cooled episodically by 2–7 °C by a water jacket, tail sympathetic activity increased in a graded fashion below a threshold skin temperature of 37.8 ± 0.6 °C, whether or not core (rectal) temperature changed. Repeated cooling episodes lowered body core temperature by 1.3–3.1 °C, and this independently activated tail sympathetic fibre activity, in a graded fashion, below a threshold rectal temperature of 38.4 ± 0.2 °C. Tail blood flow showed corresponding graded vasoconstrictor responses to skin and core cooling, albeit over a limited range. Tail sympathetic activity was more sensitive to core than to trunk skin cooling by a factor that varied widely (24-fold) between animals. Combined skin and core cooling gave additive or facilitatory responses near threshold but occlusive interactions with stronger stimuli. Unilateral warming of the preoptic area reversibly inhibited tail sympathetic activity. This was true for activity generated by either skin or core cooling. Single tail sympathetic units behaved homogeneously. Their sensitivity to trunk skin cooling was 0.3 ± 0.08 spikes s−1 °C−1 and to core cooling was 2.2 ± 0.5 spikes s−1° C−1. Their maximum sustained firing rate in the cold was 1.82 ± 0.35 spikes s−1.
The rat's tail serves as a variable heat exchanger. This function is regulated by its blood flow, which is under the control of sympathetic vasoconstrictor nerves (O'Leary et al. 1985). The vasoconstrictor supply to the tail thereby plays an important role in thermoregulation (Rand et al. 1965; O'Leary et al. 1985). Despite considerable recent interest in the activity patterns of the sympathetic supply to the tail (e.g. Johnson & Gilbey, 1994; Chang et al. 1999; Häbler et al. 1999; Smith & Gilbey, 2000), little direct information exists about its regulation by thermal stimuli. Work from two laboratories has shown that whole-body heating inhibits the activity of rat tail sympathetic fibres (Johnson & Gilbey, 1994; Johnson & Gilbey, 1996; Johnson & Gilbey, 1998; Häbler et al. 2000), but no study has yet examined their response to cooling; nor are the relative roles of cutaneous and central thermoreceptors in the control of this thermoregulatory outflow yet understood.
Such information is needed for two reasons. Firstly, tail vasoconstrictor neuron activity represents the CNS output to a defined thermoregulatory effector organ. It can therefore give us a precise, direct measure of the CNS response during thermoregulatory reflexes. Measuring tail blood flow, by contrast, gives an indirect measure of the CNS response, whose magnitude and sensitivity may be strongly affected by extrinsic factors such as local temperature (Cassell et al. 1988; cf. Yanagiya et al. 1999; Minson et al. 2001). Further advantages of neural recording are that it has a superior time resolution and, as became apparent during the present study, a wider dynamic response range.
A second, related, reason is that precise information is needed about the behaviour of this neural outflow if we are to identify the central neurons and pathways that control it. Recent studies on rats indicate that different thermoregulatory effectors are driven by quite distinct temperature-sensitive neural pathways (Kanosue et al. 1998; Kanosue et al. 2000). In response to direct heating of the preoptic area, for example, the efferent pathways mediating thermal salivation, saliva spreading and skin vasodilatation have been distinguished from each other by differences in decussation, and dissociated by either discrete knife cuts or small variations in the site being heated (Kanosue et al. 2000; Kanosue et al. 1998). In this context, we recently identified a candidate population of raphé-spinal neurons whose activity was increased by mild cooling (Rathner et al. 2001). However, before these or any other central neurons can be identified as those which control a specific thermoregulatory response such as tail vasoconstriction, we need detailed knowledge of how that neural outflow behaves in response to controlled stimuli relevant to thermoregulation. Detailed information is lacking for cutaneous vasoconstrictor outflows such as that to the tail.
The present study was therefore undertaken to define the responses of this primarily thermoregulatory outflow to thermal stimuli. Some of these findings have been reported in abstract form (Owens & McAllen, 2000; Owens et al. 2000, 2001; McAllen et al. 2001).
METHODS
Preparation
All experiments were performed in accordance with the Australian National Health and Medical Research Council code of practice for the care and use of animals for scientific purposes and were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute. Male Sprague-Dawley rats were first anaesthetised with Brietal Sodium (80 mg kg−1i.p.) and the hair shaved from the trunk. After cannulation of the trachea, animals were ventilated artificially at 55–65 breaths min−1 and a tidal volume of 0.8 ml kg−1 with pure oxygen, and held under anaesthesia with isofluorane (Forthane; Abbott Australasia Pty Ltd, Kurnel, Australia). The concentration of isofluorane (2.0 to 2.2 %) was set to ensure a deep surgical level of anaesthesia. The right carotid artery and jugular vein or femoral artery and vein were cannulated for recording arterial blood pressure (BP) and for intravenous administration of drugs. The bladder was cannulated suprapubically and allowed to drain freely.
At the conclusion of all surgical manipulations anaesthesia was switched over approximately 30 min to urethane (1 to 1.5 g kg−1i.v.). The depth of anaesthesia was frequently tested during the entire course of the experiment by the absence of response to firm hind paw pinch and corneal probing. Supplementary doses of urethane (40 mg) were given when appropriate to ensure adequate anaesthesia.
Body temperature was maintained via a custom-made water jacket placed around the animal's trunk (flow rate 60–120 ml min−1), which did not cover the tail and only partly contacted the limbs and scrotum. The rat's skin temperature was taken as the mean value of three thermocouples placed across the trunk and attached with cyanoacrylate glue to the skin under the water jacket. Core temperature was monitored by a single thermocouple inserted approximately 3 cm into the rectum. Ventilation rate was measured via a pressure transducer connected to the expiratory air line.
Tail sympathetic nerve fibre recording
In 12 rats, fascicles of a ventral collector nerve in the tail were exposed by dissection of the tissue overlying the ventral artery. A paraffin pool was made around the tail, using a section cut from a syringe case as a base, and cotton wool soaked in warm 4 % agar to seal the ends. The nerve trunk was cleaned and desheathed by fine dissection. Nerve filaments were attached to a platinum wire electrode, and their activity recorded differentially with respect to a nearby thread of connective tissue. The signal was amplified × 10 000–20 000, filtered (bandpass 60–800 Hz), displayed on an oscilloscope, played through a loudspeaker and recorded onto magnetic tape along with blood pressure, skin and rectal temperatures, airway pressure and a voice/event channel. The signal was also led through a time-window discriminator, whose performance was monitored continuously by displaying spike waveforms and discrimination limits on a variable persistence storage oscilloscope. Either on- or off-line (from tape) the nerve signal (digitized at 20 kHz), blood and airway pressures (100 Hz) skin and rectal temperatures (10 Hz) and discriminated spikes were recorded on a computer-based analysis system (CED 1401 Plus interface and SPIKE2 software; Cambridge Electronic Design, Cambridge, UK). Single unit activity was later extracted off-line from some of these recordings, using spike shapes to separate units (using the spike recognition routine in the SPIKE2 program). Single unit discrimination was verified by a consistent spike shape (within the limits imposed by baseline noise and the slow drift of fibre recording conditions) and the presence of an appropriate refractory period (at least 5 ms, usually much longer) in its interspike interval histogram.
During the experiment, all spikes recorded from the tail that were above a chosen threshold voltage were discriminated and counted, in 15 s epochs unless otherwise stated, as few-fibre activity. Selected nerve filaments were first tested for thermosensitivity by an excitatory response of the few-fibre preparation to a 2 min cooling episode. Occasionally, we recorded irregular, intermittent spike activity that was unresponsive to cooling; this was attributed to damage, and discarded. Only filament preparations that were reflexly activated by lowering skin temperature were included in the present study. Hexamethonium (4–8 μg i.v.) given at the end of the experiment always abolished that activity, showing it to be postganglionic sympathetic activity. Other tests applied during experiments included raising blood pressure by > 30 mmHg with phenylephrine (0.5–1 μg, i.v. bolus) and pinching the tail tip firmly with a haemostat.
Preoptic warming and histology
In four rats where tail sympathetic nerve activity was recorded, the preoptic region was transiently warmed by passing radio frequency current through a thermode while tail sympathetic nerve activity was monitored. A small hole was drilled in the skull and a 1 mm diameter thermode (Unique Medical, Osaka, Japan) was inserted stereotaxically into the right preoptic region (0 mm rostral and 0.8 mm lateral to bregma, 9.5 mm below the brain surface). The thermode was electrically insulated up to its tip and its tip temperature was monitored continuously by an inbuilt thermocouple. The tip temperature was warmed to 45 °C by passing 500 kHz alternating current between the thermode and an indifferent silver wire electrode placed in the neck muscles.
At the conclusion of each experiment animals were killed by i.v. administration of pentobarbitone (120 mg kg−1). The brain was then excised and placed in 10 % formalin in normal saline for at least two days. After cryoprotection (immersing the brain in 20 % sucrose in phosphate buffered saline, pH 7.4, until the tissue sank), 40 μm coronal sections were cut serially on a freezing microtome and mounted onto gelatine-subbed slides. Section images were captured using a digital camera (SPOT camera Real time, Diagnostic Instruments Inc., MI, USA) connected to a light microscope (Leitz DMV, Nussloch, Germany), and the location of the thermode tip was reconstructed from its tract.
Blood flow
In a separate set of experiments on three anaesthetised rats, blood flow to the tail was measured by surgically implanting a transit time flow probe (1 mm; Transonic Instrument Co., Ithaca, NY, USA) around the caudal artery at its origin next to the aortic bifurcation. Nerve activity was not recorded. The presence of a good pulsatile flow signal was checked at intervals throughout the experiment, but the average output signal (0.1 Hz low-pass filter) was used for measurements during data collection. Minimum tail blood flow was determined by briefly tightening a tourniquet around the base of the tail. Maximum flow was assessed by the maximum flow rate in the warm and by the effect of sodium nitroprusside (0.2 μg kg−1i.v. bolus) at the end of the experiment. Blood flow measurements were converted to conductance by dividing flow by mean blood pressure.
Experimental procedures and analysis
Skin and core temperatures were manipulated by passage of warm water or cold water through the water jacket. Skin temperature was manipulated by passage of cold instead of warm water through the water jacket for brief periods (0.5–5 min). This reversibly lowered skin temperature by 2–7 °C. Several repeated periods of skin cooling were then applied, and this lowered core temperature by 1.3–3.1 °C. Cooling was then discontinued, allowing skin and core temperatures to rewarm and tail sympathetic activity to return to low levels.
Thresholds and thermal sensitivities of both few- and single fibre records were estimated by plotting 15 s spike counts against mean skin temperature or mean rectal temperature. Linear regression lines were calculated from selected sections of the data (see below). Thermal sensitivity for skin cooling (Kskin) and core cooling (Kcore) were measured by the slopes of the regression lines in the respective plots against skin and rectal temperatures. To compare these two values, the K ratio was defined as Kcore/Kskin. Thresholds were taken from the intercept of the respective regression line with the temperature axis.
Judgement was used to select appropriate sections of data for the calculation of thermal sensitivities and thresholds. The basic procedure is described in Results and shown in Fig. 1A-C. Data were taken from periods in the record where the one variable (skin or core temperature) was exerting a strong action while the other was likely to be having minimal, or no effect. Linear regression analysis was used to measure the relation between tail fibre activity and either skin or core temperature, in each case over the region of maximum slope close to threshold. Data points above and below that region were excluded from the calculation.
Figure 1. Few-fibre tail sympathetic nerve responses to mild cooling.
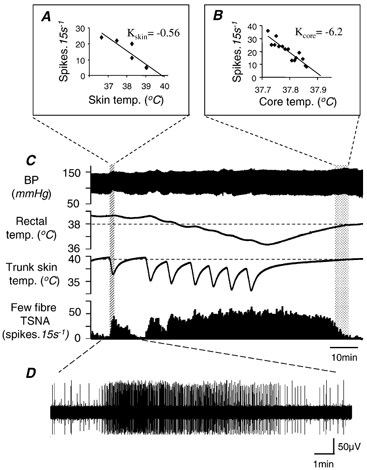
A and B, plots of few-fibre tail sympathetic nerve activity (TSNA) against trunk skin temperature and rectal temperature, respectively. Data were taken from the stippled regions, as indicated. Kskin and Kcore are estimates of the thermal sensitivity of TSNA to skin and core cooling, respectively, taken from their respective regression lines. C, chart record from a representative cooling experiment showing (from above) blood pressure, rectal temperature, trunk skin temperature and 15 s spike counts of TSNA. Starting from warm conditions, the record shows how cooling the trunk skin below a threshold (dashed line) caused a graded, reversible increase in TSNA. Repeated cooling episodes caused rectal temperature to fall. When it fell below approximately 38 °C (dotted line), this independently activated TSNA. Further explanation is given in the text. D, excerpt from the few-fibre tail sympathetic recording.
In one single fibre, the activity was low and variable. In this case, the data were smoothed by taking 30 s spike counts and subjecting them to a 3-point moving average before analysis. In one further experiment, tail sympathetic nerve activity increased with lowered skin and core temperature in a manner qualitatively the same as in other animals, but their responses were delayed by 60–90 s. The reasons for this lag were not clear, and the data were excluded from the group analysis.
Because methodological factors such as body size and contact with the water jacket might influence these measurements, the efficacy of thermal conduction between water jacket and rat in any one experiment was estimated by calculating a ‘heat transfer factor’. This was taken from a complete cooling and rewarming cycle, as the cooling-time integral (‘area over curve’) of rectal temperature expressed as a proportion of the cooling-time integral of trunk skin temperature.
Associations between experimental variables were assessed (using SigmaStat 2.03, SPSS Inc., Chicago, IL, USA) by Pearson product moment correlation, taking P < 0.05 to indicate a significant association. Unless otherwise noted data are presented as means ± s.e.m.
RESULTS
Tail sympathetic responses to cooling
Twelve rats were subjected to cooling protocols similar to that illustrated in Fig. 1 while sympathetic few-fibre activity was recorded from the tail. Activity was initially kept low by keeping both skin and rectal temperatures warm (≈39 °C). Against this background, a single 2 min cooling episode lowered trunk skin temperature by 2–7 °C, but had little effect on rectal temperature (e.g. Fig. 1C). This was accompanied by a robust increase in tail fibre activity that returned to baseline levels once the skin had rewarmed (Fig. 1C, hatched area). From episodes such as this, when the stimulus appeared to come entirely from the cooled skin, it was possible to estimate a threshold (Tskin; lower dotted line in Fig. 1C) and a thermal sensitivity coefficient for skin cooling (Kskin) for the few-fibre activity in each animal (e.g. Fig. 1A). Skin temperature was always kept within the non-noxious range (41–28 °C; Defrin et al. 2002).
Following repeated cooling episodes, rectal temperature fell and tail sympathetic activity no longer returned to low levels when the skin was rewarmed (Fig. 1C, middle area). After the skin cooling episodes were stopped, the animal gradually rewarmed. It was then possible to estimate the effect of body core temperature, measured here by rectal temperature, on tail sympathetic activity. The corresponding threshold (Tcore; upper dotted line in Fig. 1C) and thermal sensitivity coefficient (Kcore) were estimated from a section of the record where skin temperature had risen beyond its threshold or its effect was minimal (Fig. 1C, stippled section and Fig. 1B). Blood pressure changed little during the cooling protocol (Fig. 1C).
Grouped data
The mean estimated threshold skin temperature for a tail sympathetic fibre response in 11 rats was 37.8 ± 0.6 °C (range 34.8–40.8), while the mean estimated threshold rectal temperature was 38.4 ± 0.2 °C (range 37.4–39.5). The absolute values of Kskin and Kcore measured from few-fibre activity are not meaningful unless the number of fibres counted is known. Within-animal comparisons of Kcore to Kskin (K ratio) are meaningful, however, because the same number of fibres should have been counted in each case. Kcore always exceeded Kskin, but the K ratio varied between animals over a 24-fold range (1.34–32.73; see Table 1). The relative influence of skin and core cooling on each animal could be seen clearly in the shape of its response to the standard cooling protocol. In Fig. 1 and Fig. 3A, for example, the responses to skin cooling were prominent; by contrast, Fig. 3C shows that skin cooling was a relatively weak stimulus compared with core cooling in this animal.
Table 1.
Few-fibre data:thresholds and sensitivities
| Experiment number | K ratio (Kskin/Kcore) | Skin Th (°C) | Core Th (°C) |
|---|---|---|---|
| 1 | 1.34 | 38.9 | 39.5 |
| 2 | 1.91 | 40.6 | 39.1 |
| 3 | 3.10 | 37.8 | 38.5 |
| 4 | 4.37 | 34.8 | 37.7 |
| 5 | 4.77 | 37.1 | 38.4 |
| 6 | 4.94 | 36.5 | 38.6 |
| 7 | 5.11 | 35.6 | 37.4 |
| 8 | 6.51 | 38.4 | 38.1 |
| 9 | 7.80 | 39.1 | 39.1 |
| 10 | 8.03 | 40.8 | 38.0 |
| 11 | 32.73 | 36.6 | 38.4 |
| Median | 4.94 | 37.8 | 38.4 |
The few-fibre data included in the study displayed in order of their relative sensitivity of skin to core (rectal)temperature (K ratio). Corresponding threshold values to skin (Skin Th) or core (Core Th)cooling are also presented, as are the median values of the columns along the bottom row. Experiment numbers correspond with those in Table 2.
Figure 3. Skin and core temperature interactions.
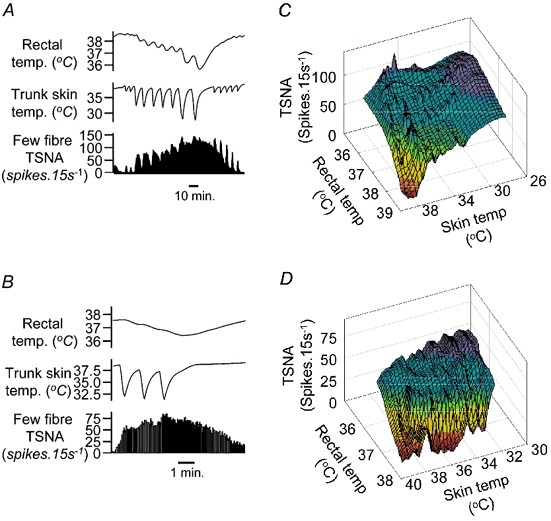
Examples of stimulus interactions between skin and core cooling as determinants of few-fibre TSNA in two experiments. A and C, chart records of responses to skin and core cooling (traces as in Fig. 1C, omitting blood pressure). In A, the response to skin cooling was strong; in C it was relatively weak. B and D, the corresponding 3D surface plots of TSNA against skin and rectal temperatures (note descending scales on temperature axes). Colour coding indicates the normalised ordinate value: red = 0 %, green =50 % and violet = 100 % of maximum TSNA. Concave surfaces indicate facilitatory interactions while convex surfaces (e.g. near summit) indicate occlusive interactions.
The surprisingly wide range of K ratios could not be explained on the basis of methodological factors. As far as could be ascertained, anaesthetic levels were equivalent between animals. To check whether such differences in response could be explained by differences in skin contact with the water jacket, we estimated a skin-to-body ‘heat transfer factor’ for each experiment (see Methods). This varied from 0.25 to 0.55 - a 2.2-fold range. No significant relationship between an animal’ s ‘heat transfer factor’ and its K ratio was found (r2 = 0.025; P > 0.6). Correlation analysis also showed no significant relation between an animal's K ratio and its weight (r2 = 0.035; P > 0.6) or between its K ratio and the rate of skin cooling in that experiment (r2 = 0.272; P > 0.05).
Effects of preoptic warming
The activation of tail sympathetic activity in parallel with reduced rectal temperature suggested that central thermoreceptors were responsible. Accordingly, we sought direct evidence that central thermoreceptors of the preoptic region influenced tail sympathetic nerve activity. A thermode was inserted stereotaxically into this region on the right side of four rats and briefly warmed during periods of cold-induced tail sympathetic activity. During preoptic warming, tail sympathetic activity declined to a minimum over a 0.5–2 min period (Fig. 2). In all four animals, unilateral preoptic warming to 45 °C inhibited ongoing tail sympathetic activity by 70–100 %. The effect was repeatable and reversible. Interestingly, this was the case irrespective of whether the activity was generated primarily by lowered skin temperature (Fig. 2, first warming period) or by lowered core temperature (Fig. 2, second warming period).
Figure 2. Effect of preoptic warming.
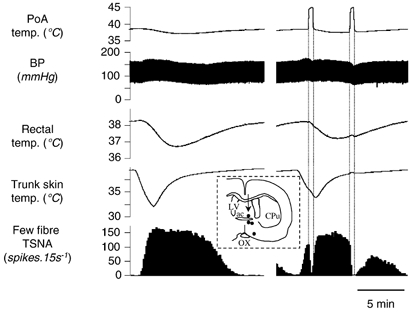
Chart record from an experiment where the preoptic area (PoA) was heated unilaterally with a thermode to test for the involvement of central thermoreceptors. The top trace shows thermode surface temperature; other traces are as in Fig. 1C. The left record shows a control response to cooling via the water jacket. The right record shows the response to a similar cooling episode, whose effects on TSNA were interrupted by two periods of preoptic warming to 45 °C. The first period was timed to occur when TSNA was driven predominantly by skin cooling, the second while it was driven by the fall in core (rectal) temperature. The inset shows thermode tip locations in four experiments (black dots) on a coronal section through the preoptic area. An arrow marks the site warmed in the record above. Abbreviations: ac, anterior commissure nucleus; CPu, caudate-putamen; LV, lateral ventricle; OX, optic chiasm.
Stimulus interactions between skin and rectal temperatures
Tail sympathetic nerve responses to combined skin and core cooling were often not the simple sum of the two components. Close to firing threshold, the effects of combined skin and core cooling were usually additive or facilitatory (Figs 3A and C). On three-dimensional graphs of firing rate against skin and rectal temperatures, these interactions may be seen as a flat or concave surface near the base (Fig. 3B). As firing rates approached their maxima, interactions were occlusive, giving a convex peak to the three-dimensional plot (Fig. 3D).
Tail blood flow changes reflect sympathetic nerve activity
Because tail sympathetic nerve activity responded to lowered skin and rectal temperatures in a graded fashion, whereas tail blood flow has been reported to respond to ambient temperature in an ‘on-off’ manner (Young & Dawson, 1982), we tested the effect of our cooling protocol on tail blood flow in three rats. Careful manipulation of the animal's core temperature kept tail blood flow at a level between its maximum and minimum values and able to respond to external stimuli with a measurable constriction or dilatation. Figure 4 shows how repeated episodes of cooling the trunk skin could produce graded vasoconstrictions of the tail circulation, but only in the middle section of the record when blood flow was in its responsive range. At the start of the record the tail was maximally vasodilated, and unresponsive to skin cooling; at the end of the record it was fully constricted, and scarcely dilated when the skin was rewarmed. This change in background state was brought about by the progressive fall in rectal temperature over the course of the record. The fall in caudal artery conductance thus followed the fall in rectal temperature in a graded fashion, as shown by the declining peak flow levels over successive periods when the trunk skin was warm. Similar results were obtained in three rats.
Figure 4. Tail blood flow responses to cooling.
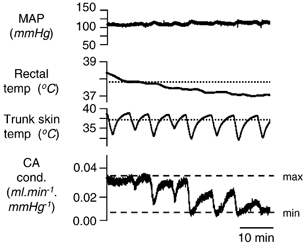
Traces from above show mean arterial pressure (MAP), rectal and trunk skin temperatures (dotted lines show estimated thresholds), and caudal artery conductance (CA cond.). The dotted lines labelled ‘max’ and ‘min’ give the maximum and minimum values of caudal artery conductance, measured when the rat was hot at the beginning of the trace and when the base of the tail was occluded with a tourniquet (not shown), respectively. Note how graded constrictor responses to both skin and core cooling could be obtained while the conductance was in its responsive range between ‘max’ and ‘min’. At the beginning and end of the record, where the tail was maximally dilated or constricted, it became unresponsive.
Single fibre responses reflect those of the population
The activity of nine single units was discriminated from the tail fibre recordings of seven rats. Single unit discrimination was verified by a constant spike shape (Fig. 5B) and the presence of an appropriate refractory period in the fibre's interspike interval histogram (Fig. 5C). When tested for their responses to the standard cooling protocol, all single units increased their activity in parallel with the remaining units in the few-fibre record, responding to both skin and core cooling in a similar manner (Fig. 5D). When the activity of the single fibre was correlated with that of the remaining spikes of the few-fibre preparation over 15–45 s epochs, the resulting correlations were usually strong (r2 = 0.71 ± 0.06, range 0.51–0.92; e.g. Fig. 5E).
Figure 5. Single fibre properties.
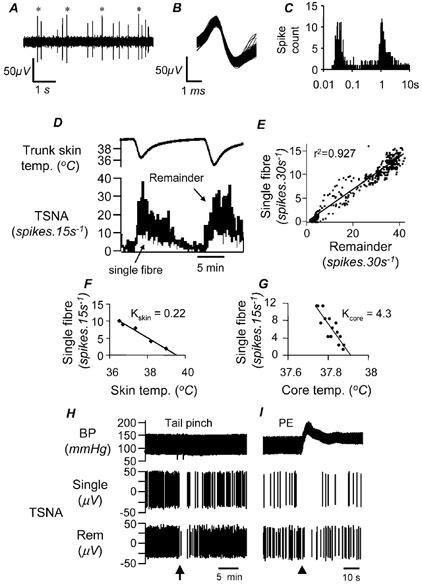
Properties are shown of a representative single fibre, discriminated from the same record as shown in Fig. 1C. A, an expanded section of the few-fibre record, with discriminated single unit spikes marked by asterisks. The discriminated single fibres showed a constant spike shape (see overlay in B) and an appropriate refractory period in the interval histogram (C; 1 ms bins, 3333 counts, note the semi-log plot). Single fibre activity was strongly correlated to the remainder of the activity (few-fibre activity with the single fibre spikes subtracted), as demonstrated by the response to two episodes of skin cooling (D) and the scatter plot of their 30 s spike counts from the whole 96 min record (E). Single unit values for Kskin and Kcore and thresholds were calculated by regression analysis in the same way as for few-fibre activity (F, G). H and I, sections of chart records showing similar responses of discriminated single fibre (Single) and remainder (Rem) spikes to tail pinch (H) and raising blood pressure with phenylephrine (PE; I). Only the waveforms of discriminated spikes, not baseline noise, are shown in this record.
Using the approach described above for few-fibre recordings, it was possible to estimate true values of Kskin and Kcore for single fibres (Fig. 5F and G). The mean values for nine single fibres were 0.31 ± 0.08 spikes s−1° C−1 and 2.20 ± 0.51 spikes s−1° C−1 for Kskin and Kcore, respectively. The K ratios obtained for single fibres were 10.09 ± 2.92 (n = 9, range 1.82–27.12). In each case the K ratio was similar to the value calculated from few-fibre activity in the same animal. The maximal firing rate of single fibres during strong cooling was 1.82 ± 0.35 spikes s−1 (range 0.79–4.17 spikes s−1). These data are summarised in Table 2.
Table 2.
Properties of tail sympat sympathetic nerve single fibres
| General | Skin | Core | ||||
|---|---|---|---|---|---|---|
| Experiment number | K ratio (Kskin/Kcore) | max.rate (sp s−1) | Th (°C) | K (sp s−1 °C−1) | Th (°C) | K (sp s−°C−1) |
| 1 | 1.82 | 2.27 | 39.1 | 0.79 | 38.5 | 1.44 |
| 6 | 2.46 | 0.79 | 34.5 | 0.14 | 37.6 | 0.35 |
| 8 | 3.85 | 1.27 | 39.6 | 0.25 | 37.4 | 0.96 |
| 7 | 4.99 | 4.17 | 39.1 | 0.25 | 38.7 | 1.26 |
| 6 | 5.28 | 1.88 | 35.1 | 0.58 | 37.8 | 3.08 |
| 8 | 12.64 | 1.90 | 40.1 | 0.37 | 37.4 | 4.64 |
| 5 | 12.66 | 2.11 | 37.1 | 0.17 | 38.3 | 2.17 |
| 9 | 19.96 | 1.20 | 39.6 | 0.22 | 37.9 | 4.34 |
| 7 | 27.12 | 0.81 | 36.1 | 0.05 | 38.3 | 1.48 |
| Median | 5.28 | 1.88 | 39.1 | 0.25 | 38.3 | 1.48 |
The table details the properties of all the single fibres included in the study displayed in order of their relative sensitivity of skin to core (rectal) temperature (K ratio).The experiment numbers match those shown in Table 1. Maximum firing rates over a 10 s period are given as well as threshold values and thermal sensitivities for skin and core cooling. Median values of the columns are presented on the bottom row.
When single units were tested for their responses to other stimuli that affected few-fibre recordings, they responded in the same direction as the parent few-fibre recording. For example, they were inhibited by phenylephrine-induced rises in blood pressure (5/5) and by noxious tail pinch (8/8) (Fig. 5H; Owens et al. 2000; Häbler et al. 1999). Each single unit thus showed the principal features of the few-fibre response.
Discussion
This study has characterised, for the first time, the responses of rat tail sympathetic fibres to mild cooling of skin and core temperatures. As expected from previous studies on cutaneous blood flow (Young & Dawson, 1982; Sakurada et al. 1993; Sawasaki et al. 2001), both skin and core temperatures were important determinants of tail sympathetic fibre activity. The effects of these two stimuli have now been measured directly, and this has uncovered a surprisingly wide variation between individual animals in their relative responsiveness to the two stimuli. Against predictions based on blood flow, tail sympathetic fibre responses to skin and core cooling were found to be graded. Single unit analysis has provided new, quantitative data on this vasomotor outflow's sensitivity to skin and core cooling. It also showed that fibres behaved homogeneously, providing no evidence for functional subsets with distinct functions.
The present investigation focused not on the detailed temporal firing patterns of tail sympathetic fibres, which have been well studied elsewhere (Johnson & Gilbey, 1994; Chang et al. 1999; Häbler et al. 1999), but on their responses to cooling. Importantly, the levels of skin and core cooling used in the present study were mild and would not have activated cold-responsive noxious sensory pathways (Craig et al. 2001; Defrin et al. 2002). Mild skin cooling was a very effective stimulus in the present experiments, presumably because it was applied to a large skin area, from which afferent information would have been summed centrally (Hellon & Mitchell, 1975).
The major inevitable limitation of this study is that it was performed on animals under general anaesthesia. It is to be expected that this would have blunted their thermoregulatory control (Sessler et al. 1988) and perhaps restricted the range of responses to cooling. Nevertheless, basic thermoregulatory reflexes such as cold-induced vasoconstriction evidently function reasonably effectively in urethane-anaesthetised rats (Hellon & Taylor, 1982). We cannot comment on whether it affected sensitivity to skin cooling more or less than the response to core cooling. Anaesthetic levels were maintained as far as possible at a steady, fairly deep level. The present findings thus relate directly to the extensive literature on this preparation and should be at least qualitatively applicable to conscious animals, although quantitative aspects should be extrapolated with caution.
A second limitation is that we made no attempt to compensate for the effect of cooling on metabolic rate and its consequences for central respiratory drive (which was not measured). Artificial ventilation was kept constant throughout cooling tests; respiratory drive would therefore be expected to fall when core temperature was low. This could have affected our estimates of Kcore and maximum firing rates in the cold. These errors were probably small, however. The available evidence suggests that the effects of hyperventilation on mean tail fibre activity, as opposed to its rhythmic pattern, are minor. Ventral collector nerve responses to hyperventilation were found to be equivalent, whether or not CO2 was added to prevent respiratory alkalosis (Chang et al. 2000).
A third potential limitation is that we measured Kskin from the leading edge of the cooling protocol and Kcore from its trailing edge. It is possible that the direction of temperature change could have influenced these values and threshold measurements. No major asymmetries in threshold to rising and falling temperatures were apparent, but direct experiments would be needed to confirm this. It is also possible that the tendency of spike amplitudes in few-fibre recordings to increase over time could have resulted in small spikes being subthreshold for counting at the start of the record but suprathreshold at the end. If so, this would have caused a small increase in the estimate of the K ratio in few-fibre recordings. It would have had no influence on data from single fibre recordings.
The method of cooling the rat via the water jacket enabled us to separate the influences of skin and core temperatures on tail sympathetic activity. The early effect of cooling skin against a warm background body temperature was a ‘clean’ stimulus, affecting only cutaneous receptors. The effects obtained later in the cooling protocol, when the trunk skin had rewarmed but rectal temperature was still depressed, were attributed to body core cooling. These, however, could have been mediated by receptors in more than one location, including skin outside the blanket. For this reason, we demonstrated formally that tail sympathetic activity generated in response to that stimulus could be abolished by directly warming the preoptic region. We can make no comment about the involvement of other areas that may sense deep body temperature (Simon, 1974, 2000). Interestingly, preoptic warming also inhibited tail sympathetic activity generated by skin cooling, suggesting a complex, tonic interaction between central and peripheral thermoreceptor signals (e.g. Boulant, 1974; Simon, 2000). Further hints for such interactions are provided by the finding that skin and whole-body cooling stimuli summed in a non-linear fashion that varied between temperature ranges and between rats. Future experiments will be needed to determine the factors and pathways involved.
The sympathetic fibres recorded here were all dissected from fascicles of a ventral collector nerve, which supplies both nutrient cutaneous vessels and arteriovenous anastomoses (Dawson & Keber, 1979; Sittiracha et al. 1987). The sympathetic supply to these vessels is known to be thermosensitive: it is doubtful that we sampled from any sympathetic fibres that might supply any other tissues in the tail. We found no obvious functional divisions between individual fibres in the same animal, suggesting that even if different fibres supply the different vessel types (Morris & Gibbins, 1997), their patterns of sympathetic drive are similar. Local temperature affects flow distribution between the nutrient vessels and arteriovenous anastomoses (Brown & Baust, 1980), and it may be that this is enough to explain any functional differences. The main distinctions found here - differences in relative sensitivity to skin cooling compared with core cooling - were between, not within, animals. However, we cannot exclude the possibility that fibres recorded in the same animal behaved so similarly because we sampled from a single subtype whose fibres were grouped together within one nerve fascicle.
Previous investigations of this type, in which the effects of skin and core temperatures have been investigated, measured blood flow rather than neural activity (Hellstrom, 1975; Young & Dawson, 1982; Raman et al. 1983; Nakajima et al. 1999). Studying end organ effects has the advantage that the final output is not in doubt, but it has the disadvantage that inferences about sympathetic neural drive are complicated by the contributions of other factors, such as local temperature, which directly affect vessels and neurovascular transmission (Sittiracha et al. 1987; Cassell et al. 1988). One relevant issue is the view that rat tail vessels respond to ambient temperature changes in an ‘on-off’ manner (Dawson & Keber, 1979). In the present experiments we showed clearly that the central vasomotor drive to the tail responds in a graded manner to thermal stimuli and that the same graded responses may be demonstrated in tail blood flow, provided care is taken to keep the preparation within its limited responsive range. If such precautions are not specifically taken, and thermal stimuli to the animal's skin and core temperatures are moving in the same direction at the same time (e.g. Dawson & Keber, 1979), it is easy to see how such graded vasoconstrictor behaviour could be missed. For overall thermoregulatory function, indeed, the ability to switch rapidly between a vasoconstricted and a dilated tail might be an advantage. But the issue is important if one wishes to investigate the nature of the control of neural drive from the central nervous system and the central pathways responsible (e.g. Blessing & Nalivaiko, 2001; Rathner et al. 2001; Tanaka et al. 2002).
In this context, it appears that neurons of the medullary raphé may mediate the tail vasoconstrictor responses to cold (Ootsuka et al. 2000; McAllen et al. 2001; Rathner et al. 2001) and perhaps other stimuli (Blessing & Nalivaiko, 2000). Interestingly, at least two other autonomic responses that are activated by cooling appear to relay in the same brainstem region: activation of the sympathetic supply to brown adipose tissue (Morrison, 1999), and the parasympathetic secretomotor supply to the stomach (Yang et al. 1993, 1994). To what extent these different responses are co-ordinated and perhaps driven by the same central pathways remains to be determined. However, the limited facts known about the organisation of thermoregulatory efferent pathways (Zhang et al. 1997) suggest that despite their overlapping anatomy, different neurons and neural pathways are likely to drive the different effector responses. Definitive tests to identify which central neurons control which thermoregulatory outflow await further study, for which the present findings help provide a basis.
Acknowledgments
We are grateful to David Trevaks for his expert help with technical and computing aspects of this study. We thank the National Heart Foundation of Australia, the National Health and Medical Research Council of Australia (block grant 983001) and the Ronald Geoffrey Arnott Foundation for supporting this work. Dr Ootsuka is ISH Postdoctoral Fellow of the Foundation for High Blood Pressure Research.
References
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. Journal of Physiology. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Raphe magnus/pallidus neurons regulate tail but not mesenteric arterial blood flow in rats. Neuroscience. 2001;105:923–929. doi: 10.1016/s0306-4522(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Boulant JA. The effect of firing rate on preoptic neuronal thermosensitivity. Journal of Physiology. 1974;240:661–669. doi: 10.1113/jphysiol.1974.sp010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Baust JG. Time course of peripheral heterothermy in a homeotherm. American Journal of Physiology. 1980;239:R126–129. doi: 10.1152/ajpregu.1980.239.1.R126. [DOI] [PubMed] [Google Scholar]
- Cassell JF, McLachlan EM, Sittiracha T. The effect of temperature on neuromuscular transmission in the main caudal artery of the rat. Journal of Physiology. 1988;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HS, Staras K, Gilbey MP. Multiple oscillators provide metastability in rhythm generation. Journal of Neuroscience. 2000;20:5135–5143. doi: 10.1523/JNEUROSCI.20-13-05135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HS, Staras K, Smith JE, Gilbey MP. Sympathetic neuronal oscillators are capable of dynamic synchronization. Journal of Neuroscience. 1999;19:3183–3197. doi: 10.1523/JNEUROSCI.19-08-03183.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. Journal of Neurophysiology. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- Dawson NJ, Keber AW. Physiology of heat loss from an extremity: the tail of the rat. Clinical and Experimental Pharmacology and Physiology. 1979;6:69–80. doi: 10.1111/j.1440-1681.1979.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- Häbler H, Bartsch T, Jänig W. Rhythmicity in single fiber postganglionic activity supplying the rat tail. Journal of Neurophysiology. 1999;81:2026–2036. doi: 10.1152/jn.1999.81.5.2026. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Bartsch T, Jänig W. Respiratory rhythmicity in the activity of postganglionic neurones supplying the rat tail during hyperthermia. Autonomic Neuroscience. 2000;83:75–80. doi: 10.1016/S0165-1838(00)00156-9. [DOI] [PubMed] [Google Scholar]
- Hellon RF, Mitchell D. Convergence in a thermal afferent pathway in the rat. Journal of Physiology. 1975;248:359–376. doi: 10.1113/jphysiol.1975.sp010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon RF, Taylor DCM. An analysis of a thermal afferent pathway in the rat. Journal of Physiology. 1982;326:319–328. doi: 10.1113/jphysiol.1982.sp014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom B. Cold vasodilatation of the rat tail. Canadian Journal of Physiology and Pharmacology. 1975;53:207–210. doi: 10.1139/y75-030. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. Sympathetic activity recorded from the rat caudal ventral artery in vivo. Journal of Physiology. 1994;476:437–442. doi: 10.1113/jphysiol.1994.sp020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. On the dominant rhythm in the discharges of single postganglionic sympathetic neurones innervating the rat tail artery. Journal of Physiology. 1996;497:241–259. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. Focally recorded single sympathetic postganglionic neuronal activity supplying rat lateral tail vein. Journal of Physiology. 1998;508:575–585. doi: 10.1111/j.1469-7793.1998.575bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanosue K, Hosono T, Zhang Y, Chen X. Neuronal networks controlling thermoregulatory effectors. Progress in Brain Research. 1998;115:49–62. doi: 10.1016/s0079-6123(08)62029-4. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Yoshida K, Maruyama M, Nagashima K. The Central Organisation of the Thermoregulatory System. In: Kosaka M, Simon E, editors. Tokyo: Springer; 2000. pp. 2–11. [Google Scholar]
- McAllen RM, Owens NC, Rathner JA, Ootsuka Y, Trevaks D. Comparison of activity patterns in rat tail vasomotor fibres and their putative premotor neurons. Proceedings of the Australian Neuroscience Society. 2001;12:111. [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. Journal of Applied Physiology. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Morris JL, Gibbins IL. Autonomic Innervation of the Skin. Chur, Switzerland: Harwood Academic Publishers; 1997. [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. American Journal of Physiology. 1999;276:R962–973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Nose H, Takamata A. Comparison between tail skin blood flow measurements by ultrasonic Doppler flowmetry and plethysmography during heating in anesthetized rats. Japanese Journal of Physiology. 1999;49:121–124. doi: 10.2170/jjphysiol.49.121. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Johnson JM, Taylor WF. Mode of neural control mediating rat tail vasodilation during heating. Journal of Applied Physiology. 1985;59:1533–1538. doi: 10.1152/jappl.1985.59.5.1533. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Owens NC, McAllen RM. Role of brainstem raphé neurons in cutaneous vasomotor control. High Blood Pressure Research Council of Australia. 2000;22:112. [Google Scholar]
- Owens NC, Kanosue K, McAllen RM. Skin and core temperature influence sympathetic drive to the rat's tail. Society for Neuroscience Abstracts. 2000;26:1739. [Google Scholar]
- Owens NC, McAllen RM. Factors controlling sympathetic drive to the rat's tail. Proceedings of the Australian Neuroscience Society. 2000;11:43. [Google Scholar]
- Owens NC, Ootsuka Y, McAllen RM. Neural control of blood flow to the rat's tail. Proceedings of the Australian Neuroscience Society. 2001;12:112. [Google Scholar]
- Raman ER, Roberts MF, Vanhuyse VJ. Body temperature control of rat tail blood flow. American Journal of Physiology. 1983;245:R426–432. doi: 10.1152/ajpregu.1983.245.3.R426. [DOI] [PubMed] [Google Scholar]
- Rand RP, Burton AC, Ing T. The tail of the rat, in temperature regulation and acclimation. Canadian Journal of Physiology and Pharmacology. 1965;43:257–267. doi: 10.1139/y65-025. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphé-spinal neurons in rats. Journal of Physiology. 2001;535:841–854. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada S, Shido O, Fujikake K, Nagasaka T. Relationship between body core and peripheral temperatures at the onset of thermoregulatory responses in rats. Japanese Journal of Physiology. 1993;43:659–667. doi: 10.2170/jjphysiol.43.659. [DOI] [PubMed] [Google Scholar]
- Sawasaki N, Iwase S, Mano T. Effect of skin sympathetic responses to local or systemic cold exposure on thermoregulatory functions in humans. Autonomic Neuroscience: Basic and Clinical. 2001;87:274–281. doi: 10.1016/S1566-0702(00)00253-8. [DOI] [PubMed] [Google Scholar]
- Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Review of Physiology and Biochemical Pharmacology. 1974;71:1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]
- Simon E. The enigma of deep-body thermosensory specificity. International Journal of Biometeorology. 2000;44:105–120. doi: 10.1007/s004840000060. [DOI] [PubMed] [Google Scholar]
- Sittiracha T, McLachlan EM, Bell C. The innervation of the caudal artery of the rat. Neuroscience. 1987;21:647–659. doi: 10.1016/0306-4522(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Smith JE, Gilbey MP. Coherent rhythmic discharges in sympathetic nerves supplying thermoregulatory circulations in the rat. Journal of Physiology. 2000;523:449–457. doi: 10.1111/j.1469-7793.2000.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphe in thermoregulatory vasomotor control in rats. Journal of Physiology. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagiya Y, Yoshimura R, Hori M, Kuwahara M, Tsubone H, Sugano S. The influence of chronic sympathectomy on cutaneous blood flow in the rat tail. Journal of Veterinary Medical Science. 1999;61:795–801. doi: 10.1292/jvms.61.795. [DOI] [PubMed] [Google Scholar]
- Yang H, Ohning G, Taché Y. TRH in dorsal vagal complex mediates acid response to excitation of raphe pallidus neurons in rats. American Journal of Physiology. 1993;265:G880–886. doi: 10.1152/ajpgi.1993.265.5.G880. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu SV, Ishikawa T, Taché Y. Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience. 1994;61:655–663. doi: 10.1016/0306-4522(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Young AA, Dawson NJ. Evidence for on-off control of heat dissipation from the tail of the rat. Canadian Journal of Physiology and Pharmacology. 1982;60:392–398. doi: 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Yamada K, Hosono T, Chen XM, Shiosaka S, Kanosue K. Efferent neuronal organization of thermoregulatory vasomotor control. Annals of the New York Academy of Science. 1997;813:117–122. doi: 10.1111/j.1749-6632.1997.tb51681.x. [DOI] [PubMed] [Google Scholar]


