Abstract
This study was conducted to investigate skeletal muscle fatty acid (FA) and glycerol kinetics and to determine the contribution of skeletal muscle to whole body FA and glycerol turnover during rest, 2 h of one-leg knee-extensor exercise at 65 % of maximal leg power output, and 3 h of recovery. To this aim, the leg femoral arterial-venous difference technique was used in combination with a continuous infusion of [U-13C]palmitate and [2H5]glycerol in five post-absorptive healthy volunteers (22 ± 3 years). The influence of contamination from non-skeletal muscle tissues, skin and subcutaneous adipose tissue, on FA and glycerol kinetics was studied by catheterization of the femoral vein in antegrade and retrograde directions. Substantially higher net leg FA and glycerol uptakes were observed with a retrograde compared to an antegrade catheter position, as a result of a much lower tracer-calculated leg FA and glycerol release. The whole body FA rate of appearance (Ra) increased with exercise and decreased rapidly in recovery but stayed higher compared to pre-exercise. The leg net FA uptake decreased immediately on cessation of exercise to near pre-exercise level, but the tracer FA uptake and release decreased slowly and reached constant values after ≈1.5 h of recovery similar to pre-exercise. Whole body FA reesterification (FA Rd - FA oxidation; Rd, rate of disappearance) was ≈400 μmol min−1 at rest and during exercise, and increased during recovery to 495 μmol min−1. Leg FA reesterification was 17 μmol min−1 at rest and decreased to 9 μmol min−1 during recovery, due to a larger fraction of leg FA uptake being directed to oxidation. A net glycerol exchange across the leg could not be detected under all conditions, but a substantial leg glycerol uptake was observed, which was substantially higher during exercise. Total body skeletal muscle FA and glycerol uptake/release was estimated to account for 18–25 % of whole body Rd or Ra. In conclusion: (1) skeletal muscle FA and glycerol metabolism, using the leg arterial-venous difference method, can only be studied if contamination from skin and subcutaneous adipose tissue is prevented; (2) whole body FA reesterification is unchanged when going from rest to exercise, but is increased during recovery; (3) in post-absorptive man total body skeletal muscle contributes 17–24 % to whole body FA and glycerol turnover and FA reesterification at rest; (4) glycerol is taken up by skeletal muscle and the uptake increases many fold during exercise.
Fat represents a major fuel for skeletal muscle at rest and during exercise at low and moderate intensities. During prolonged exercise the respiratory exchange ratio (RER), as well as the respiratory quotient (RQ), decreases across an exercising limb, indicating a shift towards an increased reliance on fat as a fuel. The fat oxidized by skeletal muscle can originate from circulating fatty acids (FA), very low density lipoprotein triacylglycerol and intramuscular triacylglycerol (mTAG). In order to differentiate between these sources of fat, carbon tracers of FA have been used, with or without measurements across a limb. However, the validity of the tracer estimates of plasma FA oxidation has been questioned, based on the observation of a significant label fixation during infusion of FA tracers in normal subjects. The uptake of 14/13C from carbon-labelled FA by skeletal muscle was far higher than could be recovered as 14/13CO2. It was thought that the major reason for this low 14/13CO2 production, i.e. incomplete oxidation of FA, by skeletal muscle was a consequence of rapid reesterification into mTAG rather than direct oxidation (Havel et al. 1967; Dagenais et al. 1976). However, an alternative explanation for the label retention has been suggested. The plasma FA entering muscle is not incorporated into mTAG, but directly oxidized and the observed label retention mainly originates from label fixation after the entrance of acyl-CoA via the exchange reaction in the tricarboxylic acid cycle (TCA cycle) (Sidossis et al. 1995). In order to correct for this label fixation via the TCA cycle the acetate carbon correction factor was introduced (Sidossis et al. 1995; Mittendorfer et al. 1998). However, even when skeletal muscle FA oxidation rates were corrected for label loss with the acetate correction factor (Mittendorfer et al. 1998) only 50 % of the FA taken up by the leg was found to be oxidized (Sidossis et al. 1999). Indeed, plasma FA taken up by skeletal muscle has been shown to be incorporated into mTAG of rat (Guo et al. 1998) and man (Sacchetti et al. 2002). Newsholme & Crabtree (1976) hypothesized that substrate cycling amplifies the response of substrate flux to a given change in regulator. The triglyceride-fatty acid cycle (TAG-FA cycle) is such a cycle where fatty acids are released into plasma during the process of TAG hydrolysis and subsequently reesterified into TAG. Large changes in the demand for fat as a substrate occur in response to exercise, as well as on termination of exercise. Indeed, large changes have been reported in whole body FA reesterification going from rest to exercise and from exercise to recovery (Wolfe et al. 1990). The recently reported rapid skeletal muscle TAG turnover suggests that skeletal muscle also contributes to whole body TAG-FA cycling, the function of which may be to quickly modify the supply of FA in response to the large changes in FA demand that occur in skeletal muscle.
Many studies investigating whole body fat metabolism and FA reesterification have used whole body glycerol appearance as a reflection of whole body lipolysis, with the assumption that all glycerol released in the process of lipolysis, whether in adipose tissue or skeletal muscle, appears in plasma, and that glycerol cannot be produced in the body other than from lipolysis. Similarly, skeletal muscle net leg glycerol release has been used as a reflection of muscle lipolysis. However, suggestions have been made that glycerol is taken up by skeletal muscle and utilized, implying that the assumption underlying the use of whole body glycerol appearance and net leg glycerol release as measures of whole body and skeletal muscle lipolysis is incorrect (Guo & Jensen, 1999).
The aims of the present study were to obtain quantitative measures of skeletal muscle FA metabolism, and examine the role of whole body and skeletal muscle FA reesterification (TAG-FA cycle), and the contribution of skeletal muscle to whole body fat and FA turnover during rest, exercise and recovery. In addition, skeletal muscle glycerol kinetics were determined in order to establish whether glycerol can be used as a reflection of lipolysis. In order to obtain quantitative skeletal muscle data the leg model was used as it gives the possibility of avoiding contamination from non-skeletal muscle tissues, compared to the forearm model (van Hall et al. 1999). The critical issue of contamination from non-skeletal muscle tissue when using a limb model was investigated by femoral venous catheterization in the commonly used antegrade direction and in the retrograde position. The latter, bypassing the saphenous vein and small veins from the lower abdomen, thus avoided contamination from subcutaneous adipose tissue and skin. Furthermore, one-leg knee-extensor exercise instead of bicycle exercise was used, as this exercise model has the advantage that the skeletal muscle mass involved in exercise could be estimated reasonably accurately, and a cuff below the knee can be inflated during blood sampling, thereby prohibiting contamination from the lower leg and shunting via the foot (van Hall et al. 1999).
METHODS
Subjects
Five healthy, physically active male subjects, aged 22 ± 3 years participated in the study. The subjects were informed about the possible risks and discomfort involved before giving their voluntary consent to participate. The study was performed according to the Declaration of Helsinki II and was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark.
Protocol
Each subject underwent a preliminary exercise test on the one-leg knee-extensor to become familiarized with knee extensor exercise and to determine their leg peak power output (Wmax,leg). On the day of the experiment the subjects were instructed to eat two slices of bread with marmalade at 07.00 h and to report to the laboratory at 08.00 h. The subjects changed and remained supine for the next 3 h. After 10 min in supine position, the femoral artery and vein from the leg to be exercised were catheterized under local anaesthesia (lidocaine (lignocaine), 20 mg ml−1). The Seldinger technique was used to insert the catheters (20 gauge; Ohmeda, Swindon, UK). The femoral arterial catheter was inserted ≈2–5 cm below the inguinal ligament and advanced ≈5–10 cm in proximal direction. The femoral venous catheter was inserted ≈2 cm above the inguinal ligament and advanced ≈7 cm in the distal direction. The retrograde orientation of the femoral venous catheter is crucial since the blood is otherwise contaminated with blood drained from the lower abdomen including fat tissue, as discussed previously (van Hall et al. 1999). To evaluate the effect of retrograde and antegrade femoral venous catheterization on quantitative measurements of FA and glycerol kinetics, another femoral venous catheter was inserted ≈2 cm below the inguinal ligament and advanced ≈7 cm in the proximal direction (antegrade catheter) in three of the five subjects. Thirty minutes later breath and blood samples were obtained for tracer background enrichment of breath and blood CO2, and palmitate and glycerol. Immediately after taking the background samples a prime of sodium [13C]bicarbonate (1 μmol kg−1) was given, and a constant infusion of [2H5]glycerol (0.1 μmol min−1 kg−1, primer 1.5 μmol kg−1) and [U-13C]palmitate (0.015 μmol min−1 kg−1) was started. All isotopes were purchased from Cambridge Isotope Laboratories (Andover, MA, USA). For each subject the actual infusion rate was calculated from the infusate concentration multiplied by the infusion rate. Indirect calorimetry measurements (CPX, Medical Graphics Corporation, St Paul, MN, USA) and breath and blood samples were obtained at regular intervals throughout the study. Shortly before each measurement a cuff placed below the knee, around the calf muscles, was temporarily inflated to a suprasystolic (≈240 mmHg) blood pressure to eliminate any contribution by blood from the lower leg. Just before the blood sample was taken the femoral arterial blood flow was measured using ultrasound doppler (model CFM 800, Vingmed Sound, Horten, Norway) (Rådegran, 1997). Measurements were carried out at rest, 90, 105 and 120 min after the start of the tracer infusions, and then after 30, 60, 90, 105 and 120 min of one-leg knee-extensor exercise at 65 % of maximal single leg power output, and at 10, 20 and 30 min and then every 30 min during the 3 h of recovery. At each sample point breath for 13CO2 enrichment was collected in a 10 l bag (Hans Rudolph, USA) from which a 10 ml vacutainer was filled via a needle. Femoral and arterial and venous blood samples were taken anaerobically with a heparinized syringe for analysis of haematocrit, haemoglobin and oxygen saturation (OSM3 hemoxymeter, Radiometer, Denmark), and blood pH, O2 and CO2 tension (ABL5, Radiometer, Denmark). Further femoral arterial and venous blood samples were taken and immediately transferred to ice-cold tubes that contained 10 μl of 0.33 m EDTA (ml blood)−1. Blood samples were mixed and centrifuged at 4 °C for 10 min and the plasma immediately frozen in liquid nitrogen and stored at -80 °C until analysis.
Diet and activity prior to the test
Subjects were asked to maintain their normal activity schedule the week prior to the test. They were, however, not allowed to perform any substantial exercise the day before the experiment. Furthermore, during the week preceding the test the subjects were asked not to consume any food items with a high natural abundance of 13C such as carbohydrates derived from C4 plants, e.g. maize (corn) and cane sugar.
Analytical procedures
Plasma was analysed enzymatically for glycerol, and FA (FA-C kit, Wako Chemical, Germany) on an automatic analyser (Cobas Fara, Roche, Switzerland). Glycerol enrichment was measured by gas chromatography-mass spectrometry (GC-MS, Automass II, Finnigan, France). In preparation of GC-MS analysis, plasma samples were processed to make a trifluorobutyrate derivative of glycerol. For the preparation of the fluorobutyrate derivative of glycerol, 3 ml of ethanol:choloroform (2.3:1) was added to 200 μl of plasma, and the solution was mixed and centrifuged. The top layer was extracted once more with 2 ml choroform and 1 ml of water (pH 2, with HCl), mixed and centrifuged. The top layer was then evaporated under a stream of N2. Two hundred microlitres of heptafluorobutyric acid anhydride in ethyl acetate (1:3 v/v) were added to the residue and heated for 10 min at 70 °C. The solution was evaporated under a stream of N2 and the residue re-dissolved in 1 ml ethylacetate. The glycerol enrichment was determined by splitless injection of 1 μl onto a 30 m capillary fused-silica column (CP-SIL 8CB, Chrompack, The Netherlands). The isotopic enrichment of glycerol was determined using electron impact ionization, selectively monitoring ions at mass-to-charge ratio (m/z) of 252–256 were determined, representing the molecular ions of unlabelled (252) and labelled derivatives (256), respectively.
Plasma palmitate concentration was determined by GC (Autosystem XL, Perkin Elmer, USA) using heptadecanoic acid as internal standard, and plasma [U-13C]palmitate enrichment was determined by gas chromatograph-combustion-isotope ratio mass spectrometry (GC-C-IRMS, Hewlett Packard 5890-Finnigan GC combustion III-Finnigan Deltaplus, Finnigan MAT, Germany). In preparation of GC and GC-C-IRMS analysis, plasma samples were processed to make a methyl derivative of palmitate. Briefly, heptadecanoic acid (30 nmol) was added, as an internal standard, to 200 μl of plasma and the proteins were precipitated with ice-cold acetone. After centrifugation, lipids were extracted with hexane. The plasma tri-, di-, mono-acylglycerols, phospholipids, cholesterol and free fatty acids were isolated by thin-layer chromatography (petroleum ether, diethyl ether, acetic acid, 120:25:1.5 v/v/v) on silica gel 60 plates (Merck, Germany). After development of the plates the free fatty acid band was isolated and 2 ml of methanol and iso-octane (4:1 v/v), and 0.2 ml of acetyl chloride was added and heated 1 h at 100 °C. Thereafter, 5 ml of 6 % potassium carbonate were added and mixed. After centrifugation, the upper layer was evaporated under N2 and re-dissolved in iso-octane. The palmitate concentration was determined by injecting 2 μl in the split mode (1/5), onto a 30 m capillary fused-silica column (Rtx-2330, Restex, USA), injector temperature at 300 °C. Helium carrier gas was used at a flow rate of 1.8 ml min−1. The instrument was controlled and the palmitate concentration automatically calculated from a palmitic and heptadecanoic acid standard curve. The palmitate enrichment was determined by injecting 2 μl onto a 30 m capillary fused-silica column (Rtx-2330, Restex, USA), via an HP-PTV injector. The vent-flow of the injector was set at 5 p.s.i., and the initial temperature was set at 85 °C for 1 min then increased with a ramp of 500 °C min−1 to 255 °C. The isotopic enrichment of palmitate was expressed as the Δ difference between the 13C/12C ratio of the sample and a known laboratory reference standard related to Pee Dee Belemnitella (PDB) limestone. The methyl derivative of palmitate contains 17 carbons of which 16 are palmitate, and thus the tracer/tracee ratio (TTR)of palmitate was corrected by a factor 17/16.
Samples of femoral arterial and venous blood and expired breath for measurement of 13CO2 enrichment were determined by gas chromatograph-isotope ratio mass spectrometry (GC-IRMS, Deltaplus, Finnigan MAT, Germany). Ten millilitres of expired air was collected in a vacutainer. The 13C/12C ratio was determined by split injection (ratio 1:4) of 20 μl of the expired air onto a poraplot Q column (Chrompack, The Netherlands), with the injector and column at 30 °C. For the determination of blood CO2 enrichment, 0.5 ml of 2.5 m phosphoric acid was added to 0.5 ml of blood in a 10 ml vacutainer to release CO2. The tubes were brought to pressure with pure helium. The 13C/12C ratio was determined by split injection (ratio 1:10) of 20 μl of the headspace on the GC-IRMS. The isotopic enrichment of breath or blood CO2 was expressed as the Δ difference between 13C/12C of the sample and a known laboratory reference standard related to PDB.
Calculations
Whole body FA and glycerol kinetics
Fat oxidation was calculated using stoichiometric equations (Frayn 1983; Péronnet & Massicotte 1991). Total fat oxidation was determined by converting the rate of TAG oxidation to its molecular equivalent, with the assumption that the average molecular weight of TAG is 860 g mol−1 and multiplied by three to express fat oxidation in FA units, since for each TAG molecule hydrolysed three FA molecules are liberated.
Whole body measurements of the rate of appearance (Ra) and disappearance (Rd) of palmitate and glycerol were calculated using the non-steady-state equations of Steele (Steele, 1959) adapted for stable isotopes (Wolfe, 1982):
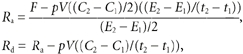 |
where F is the isotopic infusion rate (μmol min−1). E1 and E2 are the arterial isotope enrichments of palmitate or glycerol (TTR) at sample times 1 (t1) and 2 (t2) (min), respectively. C1 and C2 are the arterial concentrations at times 1 and 2 (μmol l−1), respectively. pV is the volume of distribution: 0.04 and 0.23 l (kg body weight)−1 for palmitate and glycerol, respectively (Romijn et al. 1993).
The expired 13CO2 in the breath from the infused palmitate tracer was calculated as:
ECO2 is the 13C/12C ratio in breath CO2 (TTR), VCO2 is the respiratory CO2 production (μmol min−1). ac is the acetate correction factor as determined by a continuous infusion of [1,2-13C]acetate in the same subjects at least 2 weeks after the present study using an identical protocol (van Hall et al. 2002). The percentage of plasma palmitate that disappears from the circulation and subsequently oxidized can then be calculated as:
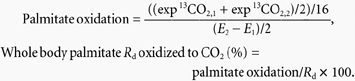 |
The factor of 16 is the number of 13C atoms in [U-13C]palmitate. The whole body FA Ra, Rd, FA oxidation and FA Rd oxidized to CO2 were calculated by dividing the palmitate data by the fractional contribution of palmitate to total FA concentration, on average palmitate was 0.22 ± 0.01 % of total FA.
Whole body FA reesterification (μmol min−1) was calculated as:
Leg FA and glycerol kinetics
For the FA and glycerol kinetics, plasma flow was used for palmitate calculations and blood flow for glycerol calculations.
 |
Leg palmitate oxidation and the percentage of palmitate taken up by the legend subsequently oxidation can then be calculated as:
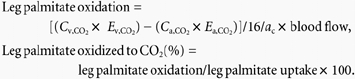 |
Cv,CO2, Ca,CO2 and Ev,CO2, Ea,CO2 are the blood CO2 concentration and 13C-enrichment in TTR in the femoral vein and artery, respectively. Ca, Cv and Ea, Ev are the plasma palmitate concentration and 13C-enrichment in TTR in the femoral artery and vein, respectively. The factor of 16 is the number of 13C atoms in [U-13C]palmitate. ac is the leg acetate correction factor as determined by a continuous infusion of [1,2-13C]acetate in the same subjects at least 2 weeks after the present study with an identical protocol (van Hall et al. 2002). The leg FA fractional extraction, uptake, release and oxidation were calculated by dividing the palmitate data by the fractional contribution of palmitate to total FA concentration, with palmitate being, on average, 0.22 ± 0.01 % of total FA. Leg FA reesterification (μmol min−1) was calculated as:
Statistics
All the date are presented as means ± s.e.m. The results were analysed by repeated-measures ANOVA with time as a within-subject factor. The significance of substrate net exchange and tracer exchange was analysed by comparing mean values with zero using Student's paired t test.
RESULTS
Whole body and leg FA kinetics (Figs 1 and 2)
Figure 1. Arterial FA concentration and whole body FA rate of appearance (Ra) during rest, exercise and recovery.
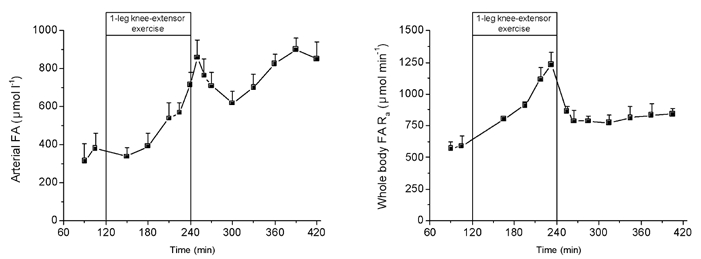
Values are means ± s.e.m. of five subjects.
Figure 2. Leg FA kinetics during rest, exercise and recovery.
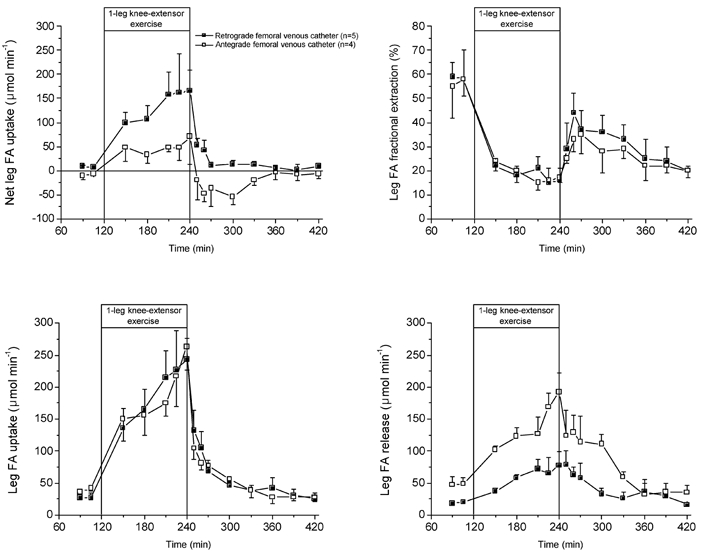
▪, FA kinetics when using femoral venous FA data from the femoral venous catheter in the retrograde position (n = 5), which excludes, to a large extent, contamination from subcutaneous adipose tissue (van Hall et al. 1998). □, FA kinetics when using femoral venous FA data from the femoral venous catheter in the commonly used antegrade direction (n = 3).
Leg FA kinetics depended substantially on the position of the femoral venous catheter. The leg net FA uptake was 2- to 3-fold higher when the calculation of leg FA kinetics was made with the retrograde femoral venous catheter. The higher net FA uptake was caused by a lower FA release, since fractional extraction and thus FA uptake, was not dependent on the catheter position.
The arterial FA concentrations did not increase during the first hour of exercise compared to rest, but nearly doubled during the second hour of exercise. After 10 min of recovery the FA concentration was higher than during exercise but decreased until 1 h after exercise, to increase again throughout the remaining recovery period. The FA Ra at rest was ≈600 μmol min−1 and increased continuously with exercise to reach 1200 μmol min−1 after 2 h of exercise. Immediately on cessation of exercise, FA Ra decreased to ≈850 μmol min−1 and remained at similar levels over the remaining 3 h of recovery.
A small but significant net FA uptake by the leg of 8 μmol min−1 was observed at rest. The leg net FA uptake increased during the first 1–1.5 h of exercise to plateau at ≈165 μmol min−1. During recovery, a rapid decrease in net FA uptake was observed, which reached pre-exercise values within 30 min of recovery. Fractional extraction of FA by the leg was ≈60 % at rest and dropped to ≈18 % with exercise. The fractional extraction of FA by the leg was markedly lower in recovery from exercise compared to pre-exercise levels and decreased continuously as recovery continued. The tracer-calculated FA uptake by the leg was ≈25 μmol min−1 at rest and increased 6- and 10-fold in the first and second hours of exercise, respectively. The decrease in FA uptake in recovery was rapid, but far lower than the net FA uptake. After 1.5 h of recovery the leg FA uptake reached pre-exercise levels. Leg FA release was slightly lower than FA uptake at rest and in recovery. However, the increase of leg FA release was only 1.5- to 3-fold compared to the 6- to 10-fold increase in leg FA uptake during exercise.
Whole body and leg glycerol kinetics (Figs 3 and 4)
Figure 3. Arterial glycerol concentration and whole body glycerol rate of appearance (Ra) during rest, exercise and recovery.
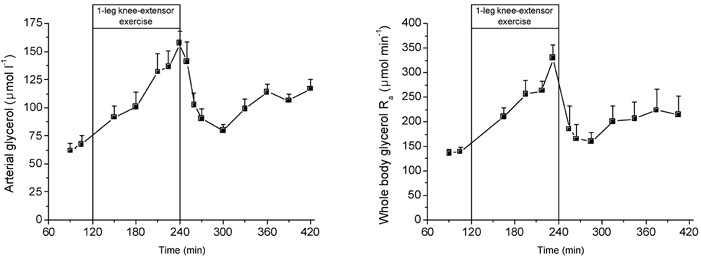
Values are means ± s.e.m. of five subjects.
Figure 4. Leg glycerol kinetics during rest, exercise and recovery.
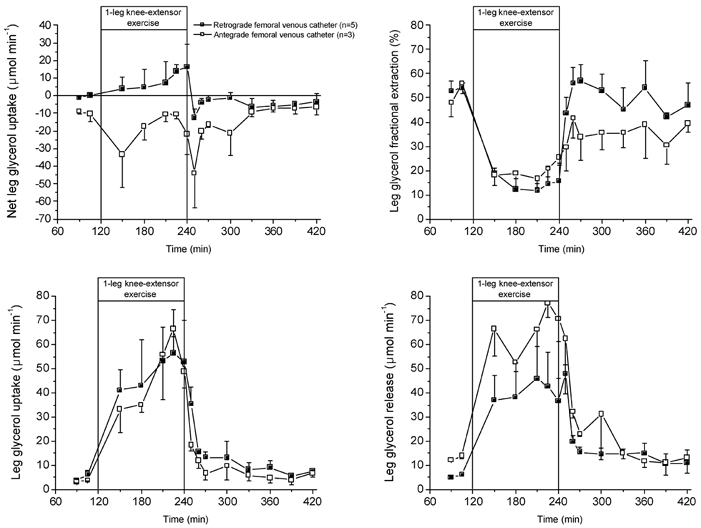
The closed squares represent FA kinetics when using for the femoral venous FA data from the femoral venous catheter in the retrograde position (n = 5), which excludes to a large extent contamination from subcutaneous adipose tissue (van Hall et al. 1998). The open squares represent the FA kinetics when using the femoral venous FA data from the femoral venous catheter in the commonly used antegrade direction (n = 3).
Leg glycerol kinetics, like leg FA kinetics, depended on the position of the femoral venous catheter. A non-significant leg net glycerol uptake was measured with the retrograde femoral venous catheter whereas a significant leg glycerol release was observed with the catheter in the antegrade direction. The difference between leg net glycerol exchange originated from a lower glycerol release from the leg with the catheter in the retrograde direction.
The arterial glycerol concentration was ≈65 μmol l−1 at rest and increased continuously with exercise to nearly 150 μmol l−1 after 2 h of exercise. The arterial glycerol concentration decreased substantially during the first hour of exercise to near pre-exercise values, but then started to increase throughout the remaining recovery period. The pattern of change in glycerol Ra during the entire study period was similar to that observed for the arterial glycerol concentration.
Net glycerol uptake or release was not different from zero during the entire study, implying no net glycerol exchange by the leg. However, there was a tendency for net glycerol uptake during exercise to become significantly different from pre-exercise levels after 105 min of exercise. Despite the absence of a net leg exchange there was a tracer-calculated uptake of glycerol. In fact, the femoral venous glycerol enrichment was about 31, 90 and 40 % of the femoral arterial glycerol enrichment during rest, exercise and recovery, respectively. Due to the absence of a net glycerol exchange, the glycerol uptake and release by the leg were similar, and approximately 10-fold higher during exercise compared to pre- and post-exercise values.
Whole body and leg oxygen utilization, and fat and FA oxidation (Table 1 and Fig. 5)
Table 1.
Whole body and leg oxygen uptake, total fat oxidation and FA oxidation
| Exercise | Recovery | |||||
|---|---|---|---|---|---|---|
| Rest | 30–60 min | 90–120 min | 30–60 min | 90–120 min | 150–180 min | |
| Whole body | ||||||
| Pulmonary oxygen uptake (l min−1) | 291 ± 22 | 846 ± 75 | 944 ± 52 | 291 ± 30 | 283 ± 30 | 272 ± 32 |
| Respiratory exchange ratio | 0.84 ± 0.02 | 0.90 ± 0.02 | 0.87 ± 0.01 | 0.79 ± 0.03* | 0.78 ± 0.01* | 0.78 ± 0.01* |
| Total fat oxidation (μmol FA min−1) | 279 ± 45 | 527 ± 130 | 598 ± 165 | 334 ± 61* | 353 ± 61* | 332 ± 35* |
| FA oxidation (μmol min−1) | 193 ± 48 | 470 ± 86 | 568 ± 164 | 350 ± 72* | 347 ± 38* | 345 ± 33* |
| FA oxidation/total fat oxidation (%) | 68 ± 10 | 89 ± 22 | 97 ± 13 | 105 ± 29* | 98 ± 19* | 105 ± 15* |
| Leg | ||||||
| Leg blood flow (l min−1) | 0.27 ± 0.01 | 3.86 ± 0.91 | 4.19 ± 0.76 | 0.52 ± 0.12* | 0.28 ± 0.06 | 0.26 ± 0.05 |
| Leg oxygen uptake (ml min−1) | 17 ± 3 | 503 ± 120 | 544 ± 112 | 36 ± 6* | 19 ± 3 | 17 ± 2 |
| Respiratory quotient | 0.84 ± 0.02 | 0.93 ± 0.01 | 0.92 ± 0.02 | 0.81 ± 0.02 | 0.80 ± 0.01* | 0.79 ± 0.01* |
| Total leg fat oxidation (μmol FA min−1) | 16 ± 3 | 195 ± 64 | 223 ± 73 | 40 ± 7* | 23 ± 4* | 22 ± 3* |
| Leg plasma FA oxidation (μmol min−1) | 10 ± 4 | 133 ± 28 | 219 ± 21 | 50 ± 9* | 25 ± 6* | 25 ± 5* |
| FA oxidation/total fat oxidation (%) | 65 ± 12 | 68 ± 32 | 98 ± 12 | 122 ± 14* | 109 ± 5* | 113 ± 12* |
Values are means ± s.e.m. of five subjects. The leg estimates are made with the retrograde femoral venous catheterization data. The leg data represent only the upper leg since the lower leg was occluded during measurements with a cuff inflated to ∼ 240 mmHg.
Significant differences between recovery and rest.
Figure 5. Percentage of whole body FA Rd and leg FA uptake oxidized to CO2.
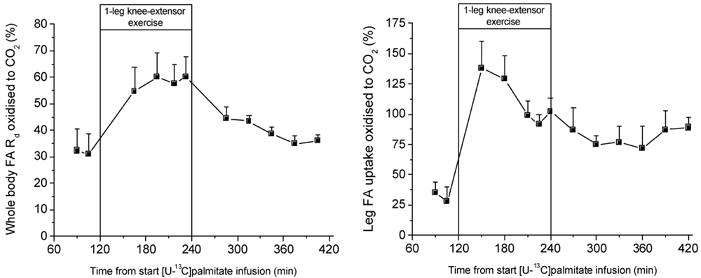
Values are means ± s.e.m. of five subjects. The leg FA uptake oxidized to CO2 is estimated only from the data from the retrograde femoral venous catheterization.
The pulmonary oxygen uptake increased from 290 ml min−1 at rest to ≈900 ml min−1 during exercise. The resting upper leg consumed ± 6 % of total oxygen uptake. However, the active upper leg used ≈80 % of the increase in total oxygen uptake with exercise; the remaining increase in oxygen utilization during exercise was most likely utilized by other muscles in the body that had increased activity. The non-exercising leg muscles are slightly activated to stabilize the body, and the respiratory muscles for the enhanced respiration.
Whole body and leg fat oxidation was lower pre- compared to post-exercise. During exercise the relative contribution of fat oxidation to total energy utilization decreased, but in absolute terms fat oxidation increased 2-fold. The increase in fat oxidation of the active upper leg could account for about 70 % of the systemic increase in fat oxidation. At rest, 32 % of the FAs leaving the circulation was oxidized to CO2, and similarly 35 % of the FAs taken up by the leg was oxidized to CO2. During exercise ≈60 % of the whole body FA Rd was oxidized compared to ≈95 % of the leg FA uptake. Whole body and leg FA oxidation could account for 65–68 % of total fat oxidation at rest and for virtually all of it during exercise and recovery.
Leg and skeletal muscle contribution to whole body FA and glycerol turnover and FA reesterification (Table 2)
Table 2.
Leg and total skeletal muscle contribution to whole body measurements
| Exercise | Recovery | |||||
|---|---|---|---|---|---|---|
| Rest | 30–60 min | 90–120 min | 30–60 min | 90–120 min | 150–180 min | |
| Leg FA uptake/WB FA Rd (%) | 4.5 ± 0.4 | 16.5 ± 1.8 | 21.6 ± 2.7 | 8.4 ± 2.9* | 4.9 ± 0.8 | 4.0 ± 1.1 |
| Leg FA release/WB FA Ra (%) | 3.2 ± 0.4 | 4.3 ± 1.2 | 9.9 ± 2.1 | 7.3 ± 2.7* | 4.0 ± 0.6* | 3.3 ± 0.9 |
| Leg glycerol uptake/WB glycerol Rd (%) | 3.7 ± 0.6 | 18.3 ± 1.3 | 19.4 ± 2.4 | 8.2 ± 3.1* | 4.3 ± 0.7 | 3.2 ± 1.3 |
| Leg glycerol release/WB glycerol Ra (%) | 4.1 ± 0.4 | 18.2 ± 2.1 | 15.1 ± 1.9 | 9.3 ± 2.4* | 7.4 ± 0.2* | 4.9 ± 0.9 |
| Muscle FA uptake/WB FA Rd† (%) | 25 | – | – | – | – | – |
| Muscle FA release/WB FA Ra† (%) | 18 | – | – | – | – | – |
| Muscle glycerol uptake/WB glycerol Rd† (%) | 21 | – | – | – | – | – |
| Muscle glycerol release/WB glycerol Ra† (%) | 23 | – | – | – | – | – |
| WB FA R a7sol;WB R a glycerol | 4.2 | 3.8 | 3.8 | 4.8 | 3.9 | 3.8 |
| Leg FA release7sol;leg glycerol release | 3.3 | 0.9 | 2.6 | 3.8 | 2.2 | 2.6 |
| WB FA reesterification ‡ (μmol min−1) | 393 ± 133 | 364 ± 114 | 416 ± 98 | 426 ± 38* | 467 ± 59* | 495 ± 58* |
| Leg FA reesterification §(μmol min−1) | 17 ± 6 | n.d. | 16 ± 4 | 11 ± 4* | 11 ± 3* | 9 ± 3* |
| Leg/WB FA reesterification (%) | 4.3 | n.d. | 3.7 | 2.7 | 2.3 | 1.6 |
| Musclesol;WB FA reesterification † (%) | 24 | – | – | – | – | – |
Values are the means of five subjects; n.d. is not detectable; WB, whole body. The leg estimates are only made with the retrograde femoral venous catheterization data. Leg values represent the upper leg, as the lower leg was occluded by a cuff inflated to ∼ 240 mmHg.
Muscle uptake, release and reesterification was estimated from leg data extrapolated to total body skeletal muscle mass, assuming that the upper leg contains 5 kg of muscle and 40% of body weight is skeletal muscle (28 kg).
Whole body FA reesterification is calculated as FA Rd— FA oxidized.
Leg FA reesterification is calculated as leg FA uptake — leg FA oxidized.
Significant differences between recovery and rest.
Skeletal muscle fat metabolism in the present study was measured using the leg model. Assuming that leg skeletal muscles are representative for all skeletal muscles in the body and that little or no contamination occurs from non-skeletal muscle tissues in the leg, it is possible to extrapolate the contribution of skeletal muscle to whole body FA and glycerol turnover. Since a cuff was placed under the knee, the leg data actually represents the upper leg, containing ≈5 kg of skeletal muscle. Whole body skeletal muscle of young healthy, non-obese individuals is assumed to be 40 % of body weight (for review see, van Hall et al. 1999). The contribution of leg FA and glycerol uptake and release to whole body FA and glycerol Rd and Ra was between 3.2 and 4.5 % at rest, with similar values in the third hour of recovery. During exercise the contribution of leg FA uptake to Rd increased 4- to 5-fold, whereas the contribution of leg FA release to Ra increased less, being increased 3-fold in the last 30 min of exercise. The contribution of leg glycerol uptake and release to whole body Rd and Ra increased similarly with exercise by 4- to 5-fold. At rest the leg data, representing skeletal muscle, can be used to extrapolate to whole body skeletal muscle. The contribution of total body skeletal muscle to FA and glycerol turnover and FA reesterification was between 18–25 %. Whole body FA reesterification was unchanged during exercise but increased in recovery. Leg FA reesterification was not determined in the first hour of exercise since FA oxidation rates are overestimated, mainly as a result of changes in the muscle bicarbonate pool size (van Hall, 1999). The last half-hour of exercise leg FA reesterification was similar compared to rest. Leg FA reesterification was lower during recovery.
Discussion
Lipid metabolism and the role of skeletal muscle is an important research area in health and disease. The utilization of extra-muscular lipids, plasma FA and very low density lipoprotein TAG by skeletal muscle can be determined by arterial-venous concentration differences across a limb and blood flow measurements. Using the Fick principle, the net uptake or release can be determined. The inclusion of 13C- or 14C-FA tracers in these experiments makes it possible to determine whether FA taken up by skeletal muscle is oxidized or esterified in TAG, and the contribution of mTAG to skeletal muscle fat utilization. However, for quantitative as well as qualitative measures of skeletal muscle fat utilization, it is critical that the venous blood sample represents blood from skeletal muscle with a minimum of contamination from other tissues. The forearm model can be used if the position of the deep venous catheter is controlled (Wahren, 1967) and shunting is prevented. However, a drawback of the model is that blood from the deep venous catheter drains a portion of the forearm muscles, which implies that a small muscle mass is studied from a rather specific muscle group. Therefore, possible contamination and shunting make a quantitatively large contribution, and the model cannot be used during exercise (van Hall et al. 1999). The leg mode does not have, or suffers less, from these limitations. However, the femoral venous catheter has commonly been used in the antegrade position, which does not avoid contamination from leg skin and adipose tissue, and, depending how far the catheter is advanced into the femoral vein, contamination from adipose tissue of lower abdomen. However, femoral venous catheterization in the retrograde direction, bypassing the great saphenous vein, prevents contamination from skin and subcutaneous adipose tissue. In addition, a cuff inflated to supra-diastolic pressure prevents contamination from the lower leg and shunting in the foot. The present study clearly shows that in order to study skeletal muscle fat metabolism it is highly important to avoid contamination from subcutaneous adipose tissue, and to a lesser extent skin. The net leg FA uptake was 2- to 3-fold higher with the catheter in the retrograde compared to antegrade position. Remarkably enough leg tracer-calculated FA fractional extraction, and thus the FA uptake, was not affected by the catheter position. As a consequence, the difference in the net leg FA uptake was solely caused by a difference in leg FA release. Coppack et al. (1999) have shown that in post-absorptive subjects tracer-estimated FA uptake by adipose tissue from the anterior abdominal wall was absent. In a study with a protocol similar to that in the present study, FA uptake by adipose tissue from the abdominal anterior wall could not be detected during rest, exercise and recovery (van Hall et al. 2002a). This implies that if subcutaneous adipose tissue of the leg behaves similarly to adipose tissue of the abdominal anterior wall no FA was taken up by subcutaneous adipose tissue of the leg, explaining the absence of a difference in FA uptake between the catheter positions. In addition, this also means that the leg FA uptake oxidized to CO2 was similar for the different catheter positions (not shown). Clearly, the catheter position may affect FA uptake and thus FA uptake oxidized to CO2 when the subjects are not studied in the post-absorptive condition. A tracer-estimated FA uptake by adipose tissue has been reported with glucose infusion (Coppack et al. 1999). Despite the precautions taken in the present study to avoid contamination from subcutaneous adipose tissue, it did not prevent a tracer-estimated leg FA release. Several reasons can be put forward to explain the mutual FA uptake and release by skeletal muscle. (1) Despite all precautions, some contamination may have existed from subcutaneous adipose tissue. (2) FA release from extramyocellular TAG (emcTAG) may have occurred. (3) There may have been hydrolysis of intramyocellular TAG (imcTAG). The upper leg of healthy young males contains ≈1 kg of subcutaneous adipose tissue (Gonzalez-Alonso et al. 2000). The imcTAG content is reported to be between 3–6 mmol (kg wet weight)−1 in healthy individuals, depending on fibre type and training status (Guo, 2001). The imcTAG content of cleaned muscle biopsies in healthy individuals is more or less comparable between measurements using 1H-MR spectroscopic imaging and biochemical TAG assay techniques (Rico-Sanz et al. 1999; Guo 2001; Hwang et al. 2001). The emcTAG content has been reported to be 2–3 times that of imcTAG (Guo, 2001) using biochemical assays, and 3- up to 14-fold using 1H-MR spectroscopic imaging (Rico-Sanz et al. 1999; Hwang et al. 2001). Obviously, most of the upper leg TAG is stored in subcutaneous TAG. In a study using a protocol similar to that in the present study, FA release from adipose tissue from the abdominal anterior wall was 1200 nmol (100 g tissue)−1 min−1 at rest (van Hall et al. 2002a). Assuming that leg subcutaneous adipose tissue behaves in a similar way to adipose tissue of the anterior abdominal wall, the total upper leg subcutaneous adipose tissue could have accounted for ≈12 μmol min−1 of the leg FA release at rest. Thus, even if leg subcutaneous adipose tissue is assumed to be much more active than subcutaneous adipose tissue from the abdominal wall, it is highly unlikely that the leg FA release (18 μmol min−1) can be explained by a little contamination from subcutaneous adipose tissue. Clearly, subcutaneous adipose tissue FA release can explain the difference in leg FA release between antegrade and retrograde femoral venous catheterization. The metabolic role of emcTAG is largely unknown. Limited information has been obtained with 1H-MR spectroscopic imaging, showing that during exercise emcTAG stores were unchanged while imcTAG decreased (Brechtel et al. 2000; Larson-Meyer et al. 2002). This may suggest that emcTAG is more like subcutaneous TAG than imcTAG. Recently, a fast imcTAG fractional synthesis rate of 3.8 % h−1 was reported (Sacchetti et al. 2002). Since the imcTAG pool at rest is relatively unchanged, this implies that imcTAG hydrolysis proceeded at a similar rate. Assuming an imcTAG pool of 6 mmol (kg wet weight)−1, this would have caused a leg FA release of 14 mmol min−1. Confirmative for the imcTAG synthesis and hydrolysis rate is also the estimated leg FA reesterficiation of 17 μmol min−1. Thus, the leg FA release mainly originates from imcTAG turnover in post-absorptive healthy subjects at rest. During exercise and early recovery, leg FA release was higher compared to rest, most probably caused by imcTAG degradation in excess of the active muscle need for FA oxidation. Alternatively, imcTAG turnover is enhanced during exercise and responsible for the increase in FA release during exercise.
On cessation of exercise an immediate drop in fat utilization occurs. Thus, if lipolysis is not inhibited immediately, a substantial increase in FA concentration occurs. Alternatively, the rate of FA reesterification increases substantially. It has been suggested that FA reesterification plays an important role in arterial FA homeostasis. A rapid decrease in the rate of FA reesterification during exercise makes FA available for oxidation, and a rapid increase in FA reesterification on cessation of exercise prevents a large increase in FA concentration (Wolfe et al. 1990). In the present study whole body FA reesterification was similar during rest and exercise and only increased during the latter part of recovery. Thus whole body FA reesterification does not seem to play a very important role in whole body FA homeostasis (Table 2), as reported previously during bicycle exercise and recovery (van Hall et al. 2002a). Instead, a rapid enhancement and inhibition in adipose tissue TAG lipolysis has to occur at the onset of exercise and recovery, respectively. This has indeed been shown to occur during cycle exercise and in recovery (Mulla et al. 2000; van Hall et al. 2002a). A remarkable observation from the present study was that the net leg FA uptake, but not the tracer-calculated FA uptake and release, decreased fast on cessation of exercise. Leg FA uptake and release decreased in an exponential fashion and reached pre-exercise values after approximately 1.5 h of recovery. It seems unlikely that non-steady-state conditions in early recovery can explain this observation, since the skeletal muscle free FA pool is small (Sacchetti et al. 2002). The enhanced FA uptake and release in recovery may indicate that imcTAG turnover was enhanced during exercise and persisted into recovery. Unfortunately in the present study, variability in FA oxidation rates in early recovery prevented accurate estimates of FA oxidation and thereby reesterification rates.
A leg net glycerol uptake or release could not be observed under any of the conditions. However, the isotopic enrichment of glycerol in the femoral vein was substantially lower than in the femoral artery, as shown previously (Elia et al. 1993; Landau et al. 1996; Jensen et al. 2001a). This implies a substantial and quantitatively similar uptake and release of glycerol by skeletal muscle. Several explanations can be put forward. First, skeletal muscle utilizes glycerol, and glycerol is synthesized at the same rate via independent reactions. Second, glycerol taken up can be utilized and a proportional amount of glycerol originates from the net breakdown of mTAG. Third, glycerol can be phosphorylated in skeletal muscle and then incorporated into mTAG. The enzymatic machinery for utilization of glycerol seems to be present in skeletal muscle. Glycerol dehydrogenase, the enzyme that could initiate glycerol oxidation by skeletal muscle, has been demonstrated in man (Hagenfeldt & Wahren, 1968), rat (Toews, 1966) and rabbit (Kormann et al. 1972). Indeed, oxidation of glycerol by skeletal muscle has been shown to occur in man (Hagenfeldt & Wahren, 1968) and rat (Toews, 1966; Pearce & Connett, 1980). De novo synthesis of glycerol may occur from the hydrolysis of glycerol-3-phosphate derived from glucose via glycerol-3-phosphate phosphatase, so far not demonstrated in skeletal muscle, or non-specific phosphatases present in skeletal muscle. However, a substantial increase in muscle glycerol has been shown in contracting muscle of patients with muscle-subunit lactate dehydrogenase deficiency (Kanno & Maekawa, 1995). These patients have a diminished lactate dehydrogenase activity (< 10 % of healthy individuals) and it was suggested that the NADH produced by glycolysis was in part oxidized by glycerol-3-phosphate dehydrogenase in order to maintain a high rate of glycolysis during muscle contraction. The high rate through glycerol-3-phospate dehydrogenase can only be maintained if glycerol-3-phosphate is hydrolysed to glycerol (Newsholme & Taylor, 1969). Therefore, human skeletal muscle has the ability for de novo glycerol synthesis, but whether this is quantitatively an important route in healthy individuals remains to be established. Glycerol kinase has been demonstrated in muscle of humans (Selzer et al. 1982) and several other species (Newsholme & Taylor, 1969). Thus, glycerol taken up from the circulation, or originating from complete mTAG breakdown, can be incorporated into mTAG as shown in rats (Guo & Jensen, 1999). It is of note, that in the present study the ratio of muscle FA to glycerol release was ≈3. This may suggest that the observed glycerol release from skeletal muscle originates mainly from mTAG breakdown and not as much from de novo synthesis in these healthy individuals.
Many studies investigating whole body fat metabolism and FA reesterification have used whole body glycerol Ra as a reflection of whole body lipolysis, with the assumption that all glycerol released in the process of lipolysis, whether in adipose tissue or skeletal muscle, appears in the plasma. Furthermore, glycerol cannot be produced in the body other then from net lipolysis. The present study clearly demonstrates that glycerol is utilized by skeletal muscle. Moreover, skeletal muscle releases glycerol without a net release or in far excess of net glycerol release, giving rise to whole body glycerol turnover. These findings are in line with those reported previously (Landau et al. 1996; Jensen et al. 2001a). Therefore, the assumption that whole body glycerol Ra, as a measure of total FA release into plasma, can be used for estimations of whole body FA reesterification and mTAG lipolysis is not entirely correct. Therefore, these estimates should be looked upon with caution. In addition, net leg glycerol release cannot be used as an estimate of mTAG utilization. In fact, previously reported net leg glycerol release during rest and exercise can most likely be attributed to contamination from subcutaneous adipose tissue, due to femoral venous antegrade catheterization.
In summary, skeletal muscle FA and glycerol metabolism, using the leg arterial-venous difference method, can only be studied if contamination from skin and subcutaneous adipose tissue is prevented. Despite avoiding contamination from skin and subcutaneous adipose tissue, a tracer-estimated leg FA release was observed, which seems to originate from imcTAG turnover. Whole body FA reesterification was unchanged when going from rest to exercise and vice versa, but increased in recovery. This suggests that the whole body TAG-FA cycle is not playing a very important role in amplifying the FA flux with the increased or decreased FA demand at the onset of exercise or recovery. Glycerol is utilized by skeletal muscle and the uptake increases with exercise. Therefore, the assumption that whole body glycerol Ra, as a measure of total FA release into plasma, can be used for estimations of whole body FA reesterification and mTAG lipolysis is not entirely correct. In post-absorptive humans, total body skeletal muscle contributes 17–24 % to whole body FA and glycerol turnover and FA reesterification at rest.
Acknowledgments
The Copenhagen Muscle Research Centre is funded by a grant from the Danish National Research Foundation (grant no. 504-14).
References
- Brechtel K, Niess AM, Machann J, Rett K, Schik F, Claussen CD, Dickhuth HH, Haering H-U, Jacob S. Utilisation of intramyocellular lipids (IMCLs). during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS) Hormone and Metabolic Research. 2000;33:63–66. doi: 10.1055/s-2001-12407. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. American Journal of Physiology. 1999;276:E233–240. doi: 10.1152/ajpendo.1999.276.2.E233. [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. Journal of Clinical Investigations. 1976;58:421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia M, Khan K, Calder G, Kurpad A. Glycerol exchange across the human forearm assessed by a combination of tracer and arteriovenous exchange techniques. Clinical Science. 1993;84:99–104. doi: 10.1042/cs0840099. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. Journal of Physiology. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z. Triglyceride content in skeletal muscle: variability and the source. Analytical Biochemistry. 2001;296:1–8. doi: 10.1006/abio.2001.5233. [DOI] [PubMed] [Google Scholar]
- Guo Z, Buergera B, Jensen MD. Simultaneous triglyceride synthesis and hydrolysis in human muscle during exercise. FASEB Journal. 1998;12:A364. [Google Scholar]
- Guo Z, Burguera B, Jensen MD. Kinetics of in tramuscular triglyceride fatty acids in exercising humans. Journal of Applied Physiology. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- Guo Z, Jensen MD. Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. Journal of Biological Chemistry. 1999;274:23702–23706. doi: 10.1074/jbc.274.34.23702. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Wahren J. Human forearm muscle metabolism during exercise II. Uptake, release and oxidation of individual FA and glycerol. Scandinavian Journal of Clinical and Laboratory Investigations. 1968;21:263–276. doi: 10.3109/00365516809076994. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Pernow B, Jones NL. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. Journal of Applied Physiology. 1967;23:90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Hwang J-H, Pan JW, Heydrari S, Hetherington HP, Stein DT. Regional differences in intramyocellular lipids in humans observed by in vivo1H-MR spectroscopic imaging. Journal of Applied Physiology. 2001;90:1267–1274. doi: 10.1152/jappl.2001.90.4.1267. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Chandramouli V, Schumann WC, Ekberg K, Previs SF, Gupta S, Landau BR. Sources of blood glycerol during fasting. American Journal of Physiology - Endocrinology and Metabolism. 2001a;281:E998–1004. doi: 10.1152/ajpendo.2001.281.5.E998. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ekberg K, Landau BR. Lipid metabolism during fasting. American Journal of Physiology - Endocrinology and Metabolism. 2001b;281:E789–793. doi: 10.1152/ajpendo.2001.281.4.E789. [DOI] [PubMed] [Google Scholar]
- Kanno T, Maekawa M. Lactate dehydrogenase M-subunit deficiencies: clinical features, metabolic background, and genetic heterogeneities. Muscle and Nerve. 1995;(suppl. 3):S54–S60. doi: 10.1002/mus.880181413. [DOI] [PubMed] [Google Scholar]
- Kormann AW, Hurst RO, Flynn TG. Purification and properties of an NADP+-dependent glycerol dehydrogenase from rabbit skeletal muscle. Biochimica et Biophysica Acta. 1972;258:40–55. doi: 10.1016/0005-2744(72)90965-5. [DOI] [PubMed] [Google Scholar]
- Landau BR, Wahren J, Previs SF, Ekberg K, Chandramouli V, Brunengraber H. Glycerol production and utilisation in humans: sites and quantitation. American Journal of Physiology. 1996;271:E1110–1117. doi: 10.1152/ajpendo.1996.271.6.E1110. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. American Journal of Physiology - Endocrinology and Metabolism. 2002;282:E95–106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. American Journal of Physiology. 1998;274:E978–983. doi: 10.1152/ajpendo.1998.274.6.E978. [DOI] [PubMed] [Google Scholar]
- Mulla NA, Simonsen L, Bülow J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: effect of exercise intensities. Journal of Physiology. 2000;524:919–928. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme EA, Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochemical Society Symposium. 1976;41:61–109. [PubMed] [Google Scholar]
- Newsholme EA, Taylor K. Glycerol kinase activities in muscle from vertebrates and invertebrates. Biochemical Journal. 1969;112:465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce FJ, Connett RJ. Effect of lactate and palmitate on substrate utilisation of isolated rat soleus. American Journal of Physiology. 1980;238:C149–159. doi: 10.1152/ajpcell.1980.238.5.C149. [DOI] [PubMed] [Google Scholar]
- Péronnet F, Massicotte D. Table of non protein respiratory quotient: an update. Canadian Journal of Sport Science. 1991;16:23–29. [PubMed] [Google Scholar]
- Rådegran G. Ultrasound doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. Journal of Applied Physiology. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. Journal of Applied Physiology. 1999;87:2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. American Journal of Physiology. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. Journal of Physiology. 2002;540:387–395. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer S, Stoch R, Marcus R, Jackson E. Alteration of human pain thresholds by nutritional manipulation and l-tryptophan supplementation. Pain. 1982;13:385–393. doi: 10.1016/0304-3959(82)90007-0. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Coggan AR, Gastadelli A, Wolfe RR. A new correction factor for use in a tracer estimations of plasma fatty acid oxidation. American Journal of Physiology. 1995;269:E649–656. doi: 10.1152/ajpendo.1995.269.4.E649. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR. Effect of hyperglycemia-hyperinsulimia on whole body and regional fatty acid metabolism. American Journal of Physiology. 1999;276:E427–434. doi: 10.1152/ajpendo.1999.276.3.E427. [DOI] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Medical Science. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Toews C. Evidence for the metabolism of glycerol by skeletal muscle and the presence of a muscle nicotinamide-adenine nucleotide phosphate-dependent glycerol dehydrogenase. Biochemical Journal. 1966;98:27–29. doi: 10.1042/bj0980027c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Bülow J, Sacchetti M, Al Mulla N, Lyngsø D, Simonsen L. Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. Journal of Physiology. 2002a;543:1033–1046. doi: 10.1113/jphysiol.2002.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, González-Alonso J, Sacchetti M, Saltin B. Skeletal muscle substrate metabolism during exercise: methodological considerations. Proceedings of the Nutritional Society. 1999;58:899–912. doi: 10.1017/s0029665199001202. [DOI] [PubMed] [Google Scholar]
- van Hall G, Sacchetti M, Rådergran G. Whole body and leg acetate kinetics at rest, during exercise and recovery in humans. Journal of Physiology. 2002b;542:263–272. doi: 10.1113/jphysiol.2001.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J. Quantitative aspects of blood flow and oxygen uptake in the human forearm during rhythmic exercise. Acta Physiologica Scandinavica. 1967;67(suppl. 269):5–88. [PubMed] [Google Scholar]
- Wolfe RR. Stable isotope approaches for study of energy substrate metabolism. Federation Proceedings. 1982;41:2692–2697. [PubMed] [Google Scholar]
- Wolfe RR, Klein S, Carraro F, Weber J-M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. American Journal of Physiology. 1990;258:E382–389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]


