Abstract
In healthy human subjects, descending motor pathways including the corticospinal tract were stimulated electrically at the level of the cervicomedullary junction to determine the effects on the discharge of motoneurones innervating the biceps brachii. Post-stimulus time histograms (PSTHs) were constructed for 15 single motor units following electrical stimulation of the corticospinal tract and for 11 units following electrical stimulation of large diameter afferents at the brachial plexus. Responses were assessed during weak voluntary contraction. Both types of stimulation produced a single peak at short latency in the PSTH (mean 8.5 and 8.7 ms, respectively) and of short duration (< 1.4 ms). In separate studies, we compared the latency of the responses to electrical stimulation of the corticospinal tract in the relaxed muscle with that in the contracting muscle. The latency was the same in the two conditions when the intensity of the stimulation was adjusted so that responses of the same size could be compared. Estimates of the descending conduction velocity and measurements of presumed peripheral conduction time suggest that there is less than 0.5 ms for spinal events (including synaptic delays). We propose that in response to electrical stimulation of the descending tract fibres, biceps motoneurones receive a large excitatory input with minimal dispersion and it presumably contains a dominant monosynaptic component.
Since its introduction, transcranial magnetic stimulation in awake subjects (Barker et al. 1985) has been used extensively in studies on the cortical control of human movement (reviewed by Rothwell, 1997). To distinguish cortical effects from spinal effects, motor evoked potentials (MEPs) induced by transcranial magnetic stimulation over the motor cortex are often compared with H reflexes. Both responses are influenced by the level of motoneuronal excitability. However, the H reflex does not test the same input axons or synapses as the corticospinal input to motoneurones.
In recent studies we have used the cervicomedullary motor evoked potentials (CMEPs) induced by electrical stimulation of descending fibres at the level of the mastoid processes to evaluate the ‘excitability’ of the spinal motoneurones innervating upper limb motoneurones (Gandevia et al. 1999; Taylor et al. 2000b). It tests many of the same input axons and synapses as the response to motor cortical stimulation (Ugawa et al. 1991; Gandevia et al. 1999). With appropriate timing, the antidromic volley from cervicomedullary stimulation can collide with and reduce the response to motor cortical stimulation and thus a subset of those axons activated by cervicomedullary stimulation belongs to the population activated by cortical stimulation (Ugawa et al. 1991; Taylor et al. 2002). Like electrical stimulation of a peripheral nerve, cervicomedullary stimulation at low intensities should preferentially activate fast-conducting, large-diameter axons. The available evidence suggests that the fastest conducting descending fibres are not subject to traditional presynaptic inhibition (Nielsen & Petersen, 1994). In contrast, presynaptic inhibition of the group Ia terminals needs to be considered for the H reflex (reviewed by Nielsen et al. 1999). As the behaviour of CMEPs is similar after brief and prolonged maximal voluntary contractions (MVCs), it is unlikely that activity results in significant changes in the excitability of the descending axons (Gandevia et al. 1999; cf. for the H reflex Burke & Gandevia, 1999).
Hence, the experiments described here were designed to gain information about the nature of the neural pathway activated by electrical stimulation of the corticospinal tract. Such information is important for future studies of motor control using CMEPs. Two approaches were used. First, the effects of stimulation of the descending motor tracts on the probability of firing of single motor units during weak voluntary contraction were examined and were compared to the effects of stimulation of peripheral sensory axons (Sears & Stagg, 1976; Kirkwood, 1979; Fetz & Gustafsson, 1983). Second, we compared the latency of compound muscle potentials to cervicomedullary stimulation in the biceps brachii during rest with that during weak elbow flexion. In addition, we measured changes in the latency of the CMEPs evoked at rest by electrical stimulation at different intensities up to those that activate motor roots directly. A preliminary account of some of the findings has been presented elsewhere (Petersen et al. 2001).
METHODS
Six subjects (including the authors) volunteered and gave written informed consent to participate in the experiments, which had received approval from the local ethics committee at the University of New South Wales. All procedures used conformed with the Declaration of Helsinki. Subjects were seated in a chair with the right arm strapped to a rigid myograph, the elbow flexed to 90 deg and the shoulder fixed at 90 deg (Allen et al. 1995; Gandevia et al. 1999).
Recording
EMG activity from the biceps brachii was recorded with surface Ag-AgCl electrodes (1 cm diameter), placed on the mid-belly of the muscle and over the distal tendon. The EMG signal was amplified, band-pass filtered (16-1000 Hz), digitized and sampled (rate 10 kHz) using a CED 1401 interface (Cambridge Electronic Design, Cambridge, UK).
Single motor unit activity was obtained using bipolar wire electrodes (75 μm Teflon-coated stainless steel) inserted into the muscle via a hypodermic needle (23 gauge). The needle was extracted once the wires were in place. The intramuscular EMG activity was amplified, band-pass filtered (60-3 000 Hz), digitized and sampled at 10 kHz. To help the subjects to maintain the activity of a single motor unit, visual and auditory feedback of the EMG activity was provided.
Stimulation
The corticospinal tract was stimulated by passing a high-voltage electrical current (duration 100 μs, Digitimer 180A, maximum output 750 V) between a set of cup electrodes attached to the skin (1-2 cm posterior and superior to the tip of the mastoid processes with the cathode on the left side). The electrodes were filled with conductive gel. The peripheral nerve was stimulated electrically (Digitimer DS7, 1 ms pulse width) using a surface cathode over the brachial plexus (2-3 cm above the clavicle and a third of its length from the medial end) and the anode on the acromion. For the studies of single units, the intensity of the cervicomedullary stimulation was initially set just below threshold for an evoked response in the surface EMG and for stimulation of the peripheral nerve the intensity was set just below motor threshold and thus presumably activated group I muscle afferents. For both types of stimulation, once the subject had the motor unit firing regularly, the intensity was adjusted to ensure that the motor unit discharged in response to the stimulus in ≈5 trials out of every 10.
Single motor unit responses to stimulation of the corticospinal tract or peripheral nerve
Subjects performed a weak elbow flexion (1-10 % MVC) such that one motor unit could be clearly distinguished in the recording from the wire electrode. Using auditory and visual feedback, subjects attempted to maintain the firing of the unit at a constant rate of around 10 Hz. A spike discriminator (dual amplitude, time-window; BAK DDIS-1) was used for on-line triggering from the motor unit and the trigger was used to control the timing of stimulation. Stimuli were controlled by computer and were delivered at selected intervals after a spontaneous firing of the unit. Intervals were set so that stimuli were delivered when the motoneurone was responsive to the test excitatory input and the number of stimuli that would not evoke a response (due for example to motoneuronal refractoriness) was minimized (Fournier et al. 1986). In practice, the interval from the trigger to the stimulus was adjusted so that the next spontaneous firing of the unit was expected at a slightly longer latency than that induced by the stimulus. Stimulus intensity was adjusted so that approximately half the stimuli resulted in firing of the motor unit at an appropriate latency. Stimuli were never delivered more frequently than once every 2 s. To allow the effect of stimulation to be determined, the spontaneous firing was measured using an identical number of triggers for which no stimuli were delivered. Triggers without stimulation were randomly mixed with those with stimulation and the timing for both was recorded. Stimulation of the corticospinal tract and stimulation of peripheral axons were carried out in separate runs, although the same units could not be examined.
Latency of CMEPs in the relaxed and contracting muscle
CMEPs were evoked in the relaxed and contracting biceps brachii in three subjects. In each subject, the maximal M-wave (Mmax) in the biceps brachii was elicited through supramaximal stimulation over the brachial plexus. The intensity of cervicomedullary stimulation was adjusted to produce a CMEP in the relaxed muscle of an amplitude of ≈25 % Mmax. At least five CMEPs were recorded at a high sampling rate (20 kHz). While subjects performed weak voluntary contractions (10 % MVC), the intensity of transmastoid stimulation was reduced to produce CMEPs that matched in size those evoked in the relaxed muscle, and five CMEPs were again recorded. The CMEPs were matched in size in an attempt to minimize the effects due to the variations in motor axon and muscle fibre conduction velocities. Subjects had visual feedback of elbow flexion force and made contractions to a target set at 10 % of the strength of the MVC based on prior assessments of maximal isometric efforts (see Allen et al. 1995). In additional studies, CMEPs were recorded from the relaxed muscle for a full range of stimulus intensities varying from threshold for a CMEP to maximal stimulator output. Again a sampling rate of 20 kHz was used to allow close examination of the onset latencies.
Analysis
Off-line analysis of the unit firing was performed using a spike template-matching algorithm (Spike2, CED) and PSTHs were constructed with 0.1 ms bin widths. Possible false triggers were revealed by visual inspection of individual sweeps in the 100 ms period after each stimulation. PSTHs were constructed for spontaneous firing (following triggers with no stimulation) and following the cervicomedullary or peripheral nerve stimuli.
The PSTHs from cervicomedullary stimulation included responses to an average of 70 stimuli (range 44-133) and the PSTHs from peripheral nerve stimulation included responses to 118 stimuli (range 50-275). To determine the stimulus-evoked increase in firing probability we subtracted the spontaneous firing probability from that associated with cervicomedullary or peripheral nerve stimulation. The onset and duration of the increase in the probability of firing were estimated visually from a ‘difference’ PSTH and its accumulated sum using cursors, to 0.1 ms resolution (see Fig. 1). The firing probability in the peak of the PSTH was taken as the number of counts in the peak divided by the number of stimuli.
Figure 1. The probability of firing in response to electrical stimulation of the corticospinal tract at the cervicomedullary level (A) or in response to electrical stimulation of the peripheral nerve at the brachial plexus (B) for single motor units in the biceps brachii for one subject.
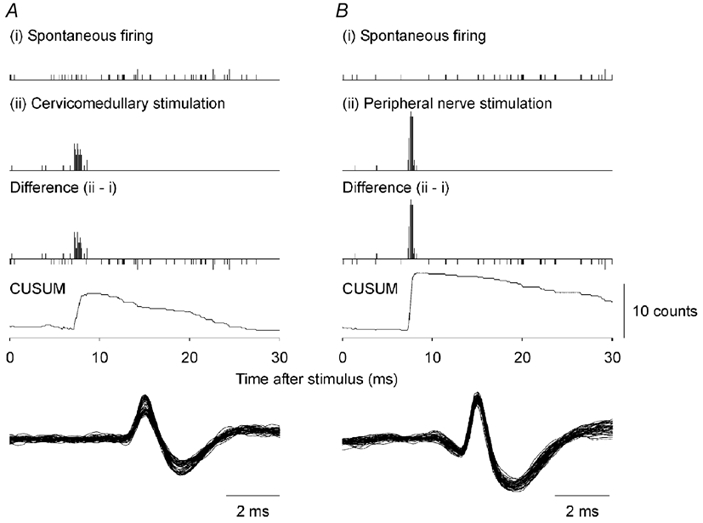
The resolution for each bin is 0.1 ms. The top plots (i) show the spontaneous firing. The second plots (ii) show the post-stimulus time histogram (PSTH) following stimulation. The third plots show the difference histograms (ii - i). The bottom plots show the cumulative sum (CUSUM) for the difference histogram. Here, and in Fig. 2, the onset latency has been corrected for the triggering delays (see Methods). The difference PSTH in A is based on 100 stimuli and the peak has a width of 1.2 ms. The difference PSTH in B is based on 83 stimuli and the peak has a width of 0.8 ms. In the lower panels the action potentials of all responses in the main PSTH peak have been superimposed.
The absolute latency of the onset of the response in a single motor unit is not revealed accurately by intramuscular recordings because conduction along the muscle fibre is slow compared with that along the motor axon. A better estimate was obtained from an average of the surface EMG triggered by the spike detected in the intramuscular recording. This spike-triggered averaging was performed for weak voluntary contractions not associated with the cervicomedullary stimulation. Hence, all PSTHs were corrected according to the latency difference between the triggering from the intramuscular recording and the onset of the motor unit potential seen in the surface recording.
In studies in which compound muscle potentials were recorded through surface electrodes, latencies of onset of individual potentials were measured by cursor.
RESULTS
Figure 1 shows the responses of single biceps brachii motor units following electrical stimulation of the corticospinal tract (Fig. 1A) and following stimulation of group I afferents at the brachial plexus (Fig. 1B). Stimuli were timed such that the next spontaneous firing of the motor unit just followed the expected latency of a peak induced by the stimulation of corticospinal tract (Fig. 1A, ii) or of group I afferents (Fig. 1B, ii). These PSTHs showed a single clear peak at short latency. This peak was still evident when the unit's spontaneous firing was subtracted to give the ‘difference’ histogram (ii, Fig. 1A and B).
The duration and onset latency of the peak of increased firing probability were derived for each unit, from their histograms and cumulated sums. To illustrate the variability between units, ‘difference’ PSTHs for six units are shown in Fig. 2.
Figure 2. Examples of firing probability for six single motor units in the biceps brachii in response to corticospinal tract (A) or to peripheral nerve stimulation (B).
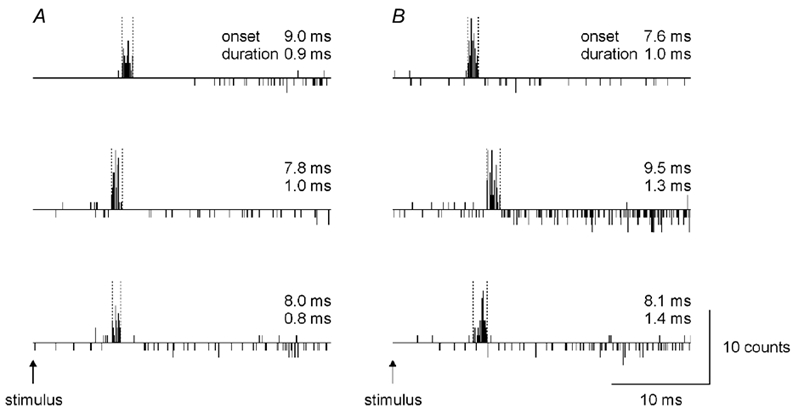
In the upper, middle and lower PSTHs in A, the responses are derived from 69, 84 and 73 stimuli, respectively. In B, the responses are from 64, 239 and 126 stimuli, respectively. The firing probabilities shown have been adjusted for the spontaneous firing, as explained in the legend to Fig. 1. Vertical dashed lines have been placed around the peaks. The onset latencies and the peak duration are given for each PSTH. Data taken from four subjects.
The mean ±s.d. duration of the peak following stimulation of the corticospinal tract was 1.3 ± 0.3 ms (n= 15) and following stimulation of the peripheral nerve 1.1 ± 0.4 ms (n= 11). There was no significant difference between the duration of the peaks induced by the different stimuli (P= 0.374; unpaired t test). The mean latencies for the peak onsets, when adjusted according to the potential measured in the surface EMG (see Methods), were 8.5 ± 1.1 and 8.7 ± 1.9 ms for the cervicomedullary and peripheral nerve stimulation, respectively.
In separate studies, the onset latencies of the compound CMEPs in the surface recordings obtained in the relaxed muscle were compared with those obtained during a contraction (10 % MVC). Several synapses inserted between the site of stimulation and the motoneurones may produce a longer latency of the CMEP in the relaxed muscle compared with the contracting one (see Discussion). Figure 3A shows recordings from the three subjects. The continuous traces show the CMEPs in biceps brachii obtained with the subject relaxed and the dashed traces show those as the subject performed a weak voluntary contraction. No consistent change in the onset latency was evident across the subjects, although a small reduction (< 0.2 ms) in latency during contraction may have occurred in one subject.
Figure 3. The effect of voluntary contraction on the responses to corticospinal stimulation and changes in the latency of the responses to increasing intensities of the stimulation.
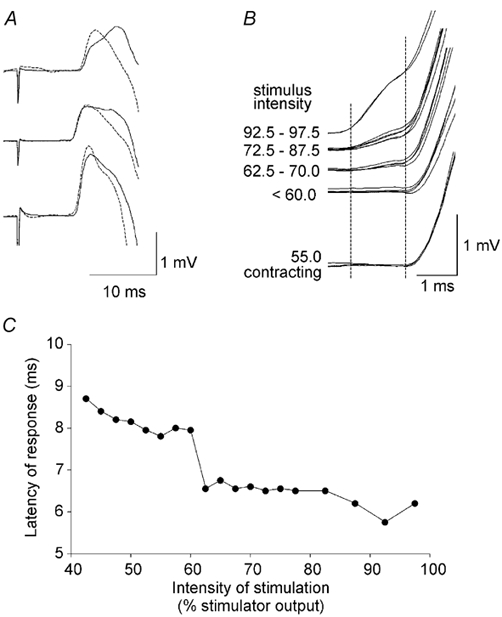
A, averaged responses (cervicomedullary motor evoked potentials, CMEPs) in the biceps brachii in three subjects. The continuous traces show responses when the subject was relaxed and the dashed traces show responses when the subject contracted isometrically at 10 % maximal isometric voluntary contraction. Stimulus intensity was adjusted to give responses of similar amplitude. The onset latency was similar in the two situations. Small changes in the later slope of the responses probably reflect the slightly different position of the muscle underneath the surface electrodes. B, individual CMEPs superimposed from a typical subject during contraction (lower traces), and above for progressively increasing stimulus intensities up to 97.5 % stimulator output with the muscle relaxed (i.e. 728 V). With intensities of 62.5 to 70 % there is a small ‘foot’ preceding the main response (left dashed vertical line), which probably reflects a shift in the preferred stimulus site from the cervicomedullary junction to the motor root. The right dashed vertical line depicts the onset of the trans-synaptic response. C, data derived from B to show the latency shift.
Figure 3B and C illustrates for one subject how a change in the intensity of the electrical stimulus at the cervicomedullary junction affects the latency of the CMEP. The intensity was raised in small increments from the lowest intensity at which the stimulus could produce a CMEP up to the maximal stimulator output. Below an intensity of 60 % of the maximum stimulator output, the latency of the initial response was between 8 and 9 ms, and it decreased as the intensity was increased. At an intensity just above 60 % of the maximum stimulator output a small ‘foot’ preceded the main response by ≈1.5 ms. As the intensity increased further, the ‘foot’ became clearer, but it shifted latency only minimally (with the stimulus intensities available). In Fig. 3C, the latencies of the responses in individual trials are plotted against the stimulus intensity. A clear shift in latency is seen at stimulus intensities around 60 % of the maximal stimulator output. Similar results with a latency jump of ≈1.5 ms were obtained in the other two subjects.
DISCUSSION
Electrical stimulation at the level of cervicomedullary junction can evoke an antidromic volley that collides with that activated by transcranial magnetic stimulation of the motor cortex when stimuli are timed appropriately (for biceps brachii see Gandevia et al. 1999; Taylor et al. 2002; see also Ugawa et al. 1991). It is therefore reasonable to suggest that axons in the corticospinal tract are activated by this stimulus (cf. Ugawa et al. 1997; Taylor et al. 2001). Here we present, from three lines of argument, evidence that such electrical stimulation of the human corticospinal tract activates a powerful monosynaptic pathway to the motoneurones of the biceps brachii.
Firstly, the latency of the CMEP during rest was changed only minimally when the muscle contracted. Secondly, a change in the intensity of the electrical stimulation of the corticospinal tract produced, at a critical intensity, a shift in latency of ≈1.5 ms, most probably representing a change in the site of stimulation from the descending corticospinal axons to the cervical ventral roots. Thirdly, we found essentially the same characteristics for the peaks of PSTHs in the firing of single motor units produced by electrical stimulation of either the corticospinal tract at the cervicomedullary level or the peripheral nerve at the brachial plexus. The peaks were of short duration, and when corrected according to the onset of the motor unit potential in the surface EMG, the latency was sufficiently short to suggest that a fast pathway is involved. There was no evidence of an earlier inhibition, a phenomenon that has been reported for transcranial magnetic stimulation (Davey et al. 1994). This may reflect our use of stimuli that produced an overt excitatory response.
Contraction versus rest
There was minimal change in the latency of the CMEP between rest and contraction. The onset of the CMEP depends on the fastest pathway from the site of stimulation to the site of recording. Hence, several factors determine how fast the volley is transmitted to the muscle. These include (i) the conduction velocity of descending axons, (ii) the number of synapses in the pathway and transmission time at those synapses and (iii) the conduction velocity of the motor axons. As we compared the latencies of responses of matched size, peripheral conduction time should have been the same in both conditions. Furthermore, the altered intensity of stimulation should have had little effect on the fastest conduction time in descending axons, as the fastest axons should be recruited by the low-intensity stimuli. However, the time across a series of synapses might decrease with increased excitability of the motoneurones in a voluntary contraction. With the muscle at rest, recruitment of the motoneurones by the stimulus-evoked volley is likely to be orderly, with the large motoneurones (which have the fastest axons) being recruited last, that is, close to the end of the rise time of the compound EPSP (Henneman et al. 1965; for review see Henneman & Mendell, 1981). With ongoing voluntary contraction, the excitability of active units will vary between refractoriness and threshold so that on average the membrane potential of motoneurones will be raised compared to rest. On average, the motoneurones will be activated slightly earlier. This decrease in average latency could be as much as half the rise time of the compound EPSP and should increase the variance of the onset latency. Each synapse in the active pathway increases the possible change in latency. Here, the absence of a consistent change in latency associated with voluntary contraction suggests that only one synapse is involved in the fastest pathway from the site of stimulation to the motoneurone. Due to the large number of electrical cervicomedullary stimuli that would be required, the variance of the onset latency was not examined here. However, when magnetic stimulation was used at the cervicomedullary level and up to 20 responses analysed, the variability of onset time of CMEPs was increased during contraction compared with rest, but again the mean onset was unchanged (Taylor et al. 2000a).
Changes to the intensity of cervicomedullary stimulation
Electrical stimulation between the mastoid processes is believed to activate the corticospinal tract at the level of the cervicomedullary junction (Ugawa et al. 1991). Small changes in the positions of the stimulating electrodes do not alter the latency of the response. This indicates that there is a particular site at which the descending axons are susceptible to stimulation. The difference in latency between responses evoked through transcranial electrical stimulation and transmastoid stimulation suggests that this site is at the pyramidal decussation where the axons bend (Ugawa et al. 1991). If stimulus intensity is increased sufficiently or if electrodes are placed over the cervical spine, there is a jump in the latency of the response as the site of stimulation moves to the cervical ventral roots (Mills & Murray, 1986; see also Plassman & Gandevia, 1989). That is, stimulation is no longer supra-motoneuronal, but activates the axons of the motoneurones. The conduction time from the cervicomedullary junction to the cervical roots is ≈1.5 ms (Fig. 2C), while the distance is ≈90 mm (Gandevia & Plassman, 1988). If the conduction velocity in the fastest axons of the corticospinal tract in human subjects is assumed to be around 80 m s−1 (e.g. Boyd et al. 1986; Gandevia & Plassman, 1988; Inghilleri et al. 1989; Fujiki et al. 1996), conduction over 90 mm should take at least 1.1 ms. Thus, ≈0.4 ms is left for synaptic transmission. This time is less than the 0.5 ms that is usually reported for synaptic delay at the motoneurone (Coombs et al. 1955).
Single-unit studies
The primary peak in the PSTH constructed from the increase in firing probability due to activation of an excitatory pathway to the motoneurone can reflect the rise time and amplitude of the underlying excitatory postsynaptic potential (EPSP; Fetz & Gustafsson, 1983; see also Poliakov et al. 1996). Group I muscle afferents are known to produce large composite monosynaptic EPSPs in motoneurones. These are reflected, as expected, in the PSTH as brief increases in firing probability occurring at short latency. Here we have compared the peaks induced by electrical stimulation of the peripheral nerve at an intensity below motor threshold with electrical stimulation of descending axons (presumably corticospinal tract fibres of the largest diameter). Our observations with single motor units apply to motoneurones with a low threshold to recruitment in a voluntary contraction (see also Binder et al. 1998). The peak produced by either stimulation had essentially the same latency, duration and shape, which suggests that the EPSPs produced on the motoneurone by the different pathways are similar. While the initial increase in firing probability following stimulation of group I afferents is due to activation of afferents with monosynaptic projections to the motoneurones, it is probable that the later part can be influenced by disynaptic inhibitory/excitatory connections (Burke et al. 1983, 1984; Marchand-Pauvert et al. 2002). It is notable that corticospinal stimulation produced only a single peak in the PSTHs - more than one peak would have been expected if the stimulus had activated different populations of axons synapsing on the motoneurones (see Taylor et al. 2001).
The mean duration of the peak to cervicomedullary stimulation (1.3 ms) agrees well with that reported for anodal stimulation over the motor cortex for biceps brachii motoneurones (1.1 ms; Maertens de Noordhout et al. 1999). Both studies used a bin width of 0.1 ms. However, transcranial magnetic stimulation over the motor cortex evokes peaks of longer duration in biceps motoneurones (3.2 ± 0.3 ms; Palmer & Ashby, 1992). This may reflect the use of a wide bin width (1 ms), but is more likely to result from the multiple descending volleys that are evoked by magnetic cortical stimulation in human subjects (e.g. Berardelli et al. 1990; Burke et al. 1993). Finally, the observed mean duration in our study may be slightly longer than the values observed for responses evoked by pyramidal tract stimulation in monkeys. Recordings from single motor units gave PSTH peaks with a duration of 0.74 ± 0.25 ms (Olivier et al. 2001) and recordings from the soma of single motoneurones revealed EPSPs with a mean rise time of 0.93 ± 0.18 ms (Maier et al. 1998).
While it is difficult to deduce the exact nature of the anatomical connection between corticospinal axons and motoneurones in studies on human subjects, the cortex-to-motoneuronal connection described here behaves in a directly comparable way to the known monosynaptic connection between Ia afferents and motoneurones. There is evidence that the corticospinal system has evolved such that in humans it includes a direct projection to many motor nuclei (e.g. Kuypers, 1981; Gandevia & Plassman, 1988; Porter & Lemon, 1993). If, as proposed, the response to cervicomedullary stimulation contains a strong monosynaptic component in human motoneurones, then this response should serve as a useful control in studies of motor cortical excitability.
Acknowledgments
The study received support from the National Health and Medical Research Council of Australia (no. 3206). N. T. Petersen was supported by the Weimann Foundation and the Danish Sports Research Council.
REFERENCES
- Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle and Nerve. 1995;18:593–600. doi: 10.1002/mus.880180605. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Cruccu G, Manfredi M. Descending volley after electrical and magnetic transcranial stimulation in man. Neuroscience Letters. 1990;112:54–58. doi: 10.1016/0304-3940(90)90321-y. [DOI] [PubMed] [Google Scholar]
- Binder MD, Robinson FR, Powers RK. Distribution of effective synaptic currents in cat triceps surae motoneurons. VI. Contralateral pyramidal tract. Journal of Neurophysiology. 1998;80:241–248. doi: 10.1152/jn.1998.80.1.241. [DOI] [PubMed] [Google Scholar]
- Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, Marsden CD. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. Journal of Neurology Neurosurgery and Psychiatry. 1986;49:251–257. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC. Properties of human peripheral nerves: implications for studies of human motor control. Progress in Brain Research. 1999;123:427–435. doi: 10.1016/s0079-6123(08)62878-2. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. Journal of Physiology. 1983;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. Journal of Physiology. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. erratum Journal of Physiology 476, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. Excitatory synaptic action in motoneurones. Journal of Physiology. 1955;130:374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey NJ, Romaiguère P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. Journal of Physiology. 1994;477:223–235. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FetZ EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. Journal of Physiology. 1983;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. Journal of Physiology. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki M, Isono M, Hori S, Ueno S. Corticospinal direct response to transcranial magnetic stimulation in humans. Electroencephalography and Clinical Neurophysiology. 1996;101:48–57. doi: 10.1016/0013-4694(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. Journal of Physiology. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Plassman BL. Responses in human intercostal and truncal muscles to motor cortical and spinal stimulation. Respiration Physiology. 1988;73:325–337. doi: 10.1016/0034-5687(88)90054-0. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its input. In: Brooks V, editor. Handbook of Physiology, section 1, The Nervous System. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 423–508. [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. Journal of Neurophysiology. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Priori A, Manfredi M. Corticospinal potentials after transcranial stimulation in humans. Journal of Neurology Neurosurgery and Psychiatry. 1989;52:970–974. doi: 10.1136/jnnp.52.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. Journal of Neuroscience Methods. 1979;1:107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kuypers H G J M. Anatomy of the descending pathways. In: Brooks V, editor. Handbook of Physiology, section 1, The Nervous System. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 597–666. [Google Scholar]
- Maertens De, Noordhout A, Rapisarda G, BogacZ D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain. 1999;122:1327–1340. doi: 10.1093/brain/122.7.1327. [DOI] [PubMed] [Google Scholar]
- Maier MA, Illert M, Kirkwood PA, Nielsen J, Lemon RN. Does a C3-C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey. Journal of Physiology. 1998;511:191–212. doi: 10.1111/j.1469-7793.1998.191bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E. Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. Journal of Physiology. 2002;542:963–976. doi: 10.1113/jphysiol.2002.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Murray NM. Electrical stimulation over the human vertebral column: which neural elements are excited? Electroencephalography and Clinical Neurophysiology. 1986;63:582–589. doi: 10.1016/0013-4694(86)90145-8. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? Journal of Physiology. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. Journal of Physiology. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Gandevia SC. Stimulation of the corticospinal tract activates descending neurones with direct connections to human motoneurones. XXXIV Congress of International Physiological Sciences. 2001 CD-Rom abstract 1853. [Google Scholar]
- Plassman BL, Gandevia SC. High-voltage stimulation over the human spinal cord: sources of latency variation. Journal of Neurology, Neurosurgery and Psychiatry. 1989;52:213–217. doi: 10.1136/jnnp.52.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov AV, Powers RK, Sawczuk A, Binder MD. Effects of background noise on the response of rat and cat motoneurones to excitatory current transients. Journal of Physiology. 1996;495:143–157. doi: 10.1113/jphysiol.1996.sp021580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon; 1993. [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. Journal of Neuroscience Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. Journal of Physiology. 1976;263:357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Magnetic stimulation of the descending tracts in human subjects. Proceedings of the Australian Neuroscience Society. 2000a;11:48. [Google Scholar]
- Taylor JL, Butler JE, Petersen NT, Gandevia SC. Unexpected reflex response to transmastoid stimulation in human subjects during near-maximal effort. Journal of Physiology. 2001;536:305–312. doi: 10.1111/j.1469-7793.2001.t01-1-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE, Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. Journal of Physiology. 2000b;525:793–801. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. Journal of Physiology. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Annals of Neurology. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of the descending and ascending tracts at the foramen magnum level. Electroencephalography and Clinical Neurophysiology. 1997;105:128–131. doi: 10.1016/s0924-980x(97)96141-5. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Morita H, Baumgartan J, Petersen N, Christensen LO. On the comparability of H-reflexes and MEPs. Electroencephalography and Clinical Neurophysiology. 1999;51(Supplement):93–101. [PubMed] [Google Scholar]
- Olivier E, Baker SN, Nakajima K, Brochier T, Lemon RN. Investigation into non-monosynaptic corticospinal excitation of macaque upper limb single motor units. Journal of Neurophysiology. 2001;86:1573–1586. doi: 10.1152/jn.2001.86.4.1573. [DOI] [PubMed] [Google Scholar]


