Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) has been shown to induce Ca2+ release in numerous cellular models, ranging from marine invertebrates to mammals. However, endogenous levels of this pyridine dinucleotide have yet to be demonstrated. In the sea urchin egg, NAADP receptors are abundant but have the peculiarity of being inactivated at low concentrations (picomolar) and activated at higher concentrations (nanomolar) which apparently rules out any possibility of the receptor being activated by concentration rises induced by a slow enzymatic formation in the cytosol. One of the most important events of fertilization is a Ca2+ transient in the egg, which leads to egg activation. The mechanisms which underlie the transient are still unclear and several theories persist including the existence of a sperm receptor and that soluble factors may pass from the sperm to the egg cytosol. We have investigated the possibility that NAADP might be present in sperm. Indeed, we found that sea water-activated spermatozoa are able to synthesize NAADP and that sperm extracts contain micromolar concentrations of the messenger. Although it is unlikely that NAADP alone mediates the fertilization wave, our data suggest that transfer of NAADP from spermatozoa to egg may play a role in this phenomenon.
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a pyridine nucleotide that has been shown to induce Ca2+ release in numerous cellular models, ranging from marine invertebrates to mammals (Lee, 2000; Patel et al. 2001). Although it is structurally similar to cyclic ADP ribose (cADPR), an agonist/modulator of ryanodine receptors, NAADP activates a distinct Ca2+ release mechanism on intracellular membranes (Genazzani & Galione, 1997; Lee, 2000; Patel et al. 2001). In the sea urchin, the best characterized model in which the pioneering experiments on this molecule were performed (Clapper et al. 1987; Lee & Aarhus, 1995; Chini et al. 1995), NAADP-induced Ca2+-release is insensitive to pharmacological agents that affect cADPR- or IP3-induced responses. Furthermore, it does not show heterologous desensitization with the IP3 and cADPR responses, while it does show homologous desensitization. In the sea urchin egg, NAADP exhibits two peculiarities. Firstly, NAADP, unlike IP3 and cADPR, releases Ca2+ from a store distinct from the endoplasmic reticulum (Genazzani & Galione, 1996; Lee & Aarhus, 2000). Secondly, low concentrations of NAADP that are unable to release Ca2+ can block any further responses even to maximal concentrations of the messenger (Genazzani et al. 1996; Aarhus et al. 1996). It would appear that the latter phenomenon is due to the irreversible binding of NAADP to the receptor (Aarhus et al. 1996; Billington & Genazzani, 2000; Patel et al. 2000). This inactivation process is observed both in homogenates and in intact eggs, suggesting that it might play a role in Ca2+ responses in the egg. Therefore, if NAADP receptors are to be activated in the egg, a rapid surge of messenger has to occur so as to bypass inactivation. One possibility is that NAADP is compartmentalized and released into the cytosol upon requirement.
In the present study, we have investigated the possibility that the separate compartment might be the spermatozoa. It is well known that, upon sperm-egg fusion, a Ca2+ wave initiates from the point of contact (Stricker, 1999). Furthermore, it is known that the spermatozoa release the contents of their cytosol (including the genetic material) into the egg. The association of these two phenomena is debatable, and while there is evidence that the Ca2+ wave might be dependent on soluble sperm factors, contradictory evidence associates the wave with the activation of a sperm receptor on the plasma membrane (Hogben et al. 1998). Here, we show that NAADP can be synthesized in sperm extracts, and more importantly, that micromolar NAADP levels are detectable in sea water-activated sperm cytosol. This evidence suggests that, at fertilization, NAADP receptors neighbouring the point of sperm-egg fusion could be activated by NAADP from the spermatozoa. This, coupled with the large volume of data showing the abundance of the NAADP-sensitive Ca2+ release mechanism in sea urchin eggs, suggests that the transfer of NAADP from spermatozoa to egg may play a role in the Ca2+ transient seen at egg fertilization.
METHODS
Collection of spermatozoa and eggs
Eggs and spermatozoa of Lytechinus pictus (Marinus Inc., Long Beach, CA, USA) were obtained by intracoelomic injection of 0.5 m KCl and collected in artificial sea water (ASW; 435 mm NaCl, 40 mm MgCl2, 15 mm MgSO4, 11 mm CaCl2, 10 mm KCl, 2.5 mm NaHCO3, 1 mm EDTA, pH 8.0). Egg homogenates were prepared as described previously (Dargie et al. 1990). Spermatozoa were treated in one of three ways prior to homogenization, so as to rule out possible artefacts of a single preparation. (1) Spermatozoa were collected in ASW and homogenized. (2) Spermatozoa were collected in ASW and centrifuged at 10 000 g. They were then resuspended and pelleted three times in 0 Ca2+ ASW before homogenization in intracellular medium (IM; 250 mm potassium gluconate, 250 mmN-methyl glucamine, 20 mm Hepes, 1 mm MgCl2, pH 7.2) + 25 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 50 μg ml−1 SBTI. (3) Spermatozoa were collected in ASW and pelleted at 10 000 g. They were then resuspended and pelleted 3 times in low osmolarity buffer (10 mm KCl, 20 mm Hepes, 100 μM EGTA, pH 7.5). Prior to preparation, spermatozoa were counted.
The spermatozoa were then freeze thawed 5 times in liquid N2 before homogenization in an Ultra Turrax homogenizer. Homogenates were aliquoted and frozen in liquid N2 for long term storage at -80 °C.
Sperm extract (cytosol) was prepared by a centrifugation protocol. Homogenates were spun at 20 000 g for 10 min at 4 °C and the supernatant was boiled for 10 min. After centrifugation at 100 000 g for 1 h, the supernatant was kept and the pellet discarded.
[32P]NAADP binding assays
[32P]NAADP and [32P]NADP synthesis was conducted as described previously (Aarhus et al. 1996; Billington & Genazzani, 2000). Binding reactions were carried out on ice in IM for 20 min. Reactions, final volume 200 μl, were initiated by the addition of egg homogenate (50 μg protein) to IM, 50 pm[32P]NAADP and sperm extract. After 20 min, reactions were terminated by centrifugation at 20 000 g, the pellet was washed in 1 ml ice-cold IM and resuspended in scintillation fluid before standard liquid scintillation counting. Preliminary experiments on spermatozoal membranes revealed that there was no detectable [32P]NAADP binding.
Desensitization experiments were performed as follows; 50 μg of homogenate was treated with sperm extract at concentrations below half-maximal for 20 min (final volume 40 μl). This was then diluted in 360 μl IM containing 50 pm[32P]NAADP to decrease the extract concentration to non-inhibitory concentrations. Reactions then proceeded as above.
Enzyme assays
Assays for the degradation of the active factor by alkaline phosphatase (AP; Sigma) and nucleotide pyrophosphatase (NPP; Sigma) were carried out in buffer consisting of 20 mm Tris and 2.5 mm Mg2+, pH 10.5 for AP and pH 7.5 for NPP. Sperm extract was incubated at 37 °C with either AP at a final concentration of 1 unit ml−1 or NPP at 0.01 units ml−1. Aliquots were removed at various time points and assayed for remaining inhibitory ability.
HPLC of sperm extract and production of NAADP
A 1 ml sample of sperm extract was separated by HPLC on AG-MP1 resin (Bio-Rad) as described previously (Aarhus et al. 1995). Fractions were collected at 1 min intervals and then completely dried in a vacuum drier. Each fraction was resuspended in 100 μl double-distilled H2O and frozen at −80 °C until needed for binding experiments.
To assess production of NAADP by sperm extracts, 800 μg of sperm protein (determined using the Bradford assay) were used and incubated under different conditions at room temperature. The subsequent reaction was analysed by HPLC as above and compared to standards of authentic nucleotides.
RESULTS AND DISCUSSION
[32P]NAADP binding to sea urchin egg homogenates has been characterized by several laboratories using a competition assay (Aarhus et al. 1996; Billington & Genazzani, 2000; Patel et al. 2000) and we have used this assay to test extracts of sea water-activated sperm for the presence of compounds able to inhibit [32P]NAADP binding. We found that sperm extracts were able to inhibit NAADP binding to egg membranes. Three different preparations (prepared as detailed in Methods) were tested and were found to have differing amounts of the active compound(s) (Fig. 1A). This can be explained, in part, by the different spermatozoa concentrations of the preparations (approximately 20 × 108, 0.13 × 108 and 5 × 108 spermatozoa ml−1 for Aug 00, Jul 01 and Aug 01 preparations, respectively) as the concentration of the inhibitory agent in each preparation closely mirrored the spermatazoa concentration.
Figure 1. A factor in sperm extracts is able to compete for [32P]NAADP binding.
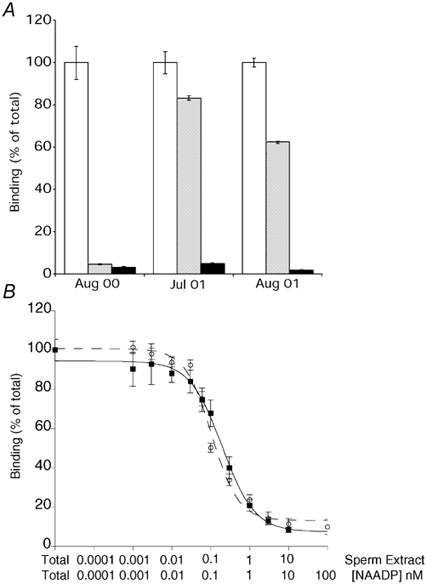
A, inhibition of [32P]NAADP binding to egg homogenates by the three different sperm homogenate preparations and authentic NAADP. B, representative concentration-response curves obtained from competition binding experiments with sperm extract (Aug 00, ▪-▪) and NAADP (○--○). Units for sperm homogenate are expressed as % volume/200 μl binding reaction. Data are presented as means ±s.e.m.; n = 3-9.
Using the most concentrated preparation (Aug 00), it was possible to perform a competition binding curve (Fig. 1B). The competition binding curve obtained with the sperm extract and with authentic NAADP were superimposable, since both showed a Hill slope of 1.
NAADP binding is virtually irreversible and therefore pre-incubation of egg homogenate with authentic NAADP results in the abolition of further binding (Aarhus et al. 1996; Billington & Genazzani, 2000; Patel et al. 2000). Nonetheless, high salt concentrations can both displace NAADP in competition binding (Patel et al. 2000) and can dissociate pre-bound NAADP (R. A. Billington & A. A. Genazzani, unpublished data). To test if the compound in sperm extract could be NAADP, experiments were performed based on the ability of the sea urchin receptor to bind NAADP practically irreversibly. When egg homogenates were pre-treated with sperm extract or with a concentration of NAADP with identical inhibitory potential, subsequent binding of [32P]NAADP was abolished to the same extent (Table 1). Furthermore, unlike high salt concentrations but like authentic NAADP, the sperm extract was unable to displace specifically pre-bound [32P]NAADP (Table 1). These results are consistent with the presence of a pyridine nucleotide in the sperm extract since the only compounds known to display these peculiarities are NAADP and NADP (although it has been shown that the latter effect could be due to contaminating NAADP (Dickey et al. 1998). The inhibitory agent was unlikely to be a protein, since boiling of the samples for 5–10 min did not alter the capacity of the extract to compete for binding (R. A. Billington & A. A. Genazzani, unpublished data). In order to verify that the active factor was a phosphate-containing nucleotide, the extract was incubated with alkaline phosphatase or nucleotide pyrophosphatase and then assayed for its ability to inhibit binding. After incubation with alkaline phosphatase for 1 h, 76.7 ± 1.29 % of the inhibitory activity had been lost. Similarly, when the extract was incubated with nucleotide pyrophosphatase, the inhibitory activity was completely abolished within 1 h, strengthening the idea that the active factor was indeed a nucleotide.
Table 1.
Effect of sperm extract and NAADP on [32P]NAADP bound to egg homogenate
| Displacement of bound [32P]NAADP(%of residual) | Inhibition of [32P]NAADP binding (% of total) | ||
|---|---|---|---|
| [32P]NAADP → agent | Agent → [32P]NAADP | ||
| Buffer | 100 ± 3.53 | Buffer | 100 ± 2.93 |
| Sperm extract | 70.51 ± 3.19 | Sperm extract | 15.37 ± 2.02 |
| NAADP 1 μM | 78.73 ± 1.98 | NAADP 1.5 nM | 11.23 ± 2.50 |
In the left columns, [32P]NAADP was pre-incubated with egg homogenate then challenged in an attempt to induce dissociation. In the right columns, the agent was incubated with homogenate and then [32P]NAADP was added in a large enough volume to decrease the agent to non-inhibitory concentrations. Values are given as means ± S.E.M. n= 6–8.
To investigate if the sperm extract contained NAADP, the extract was fractionated by HPLC using an anion-exchange protocol routinely used for separation of pyridine nucleotides (Aarhus et al. 1995). One minute fractions were collected and assayed for their ability to inhibit [32P]NAADP binding. In all three preparations, fractions which were able to inhibit [32P]NAADP binding to egg membranes co-eluted with authentic NAADP (Fig. 2) or with [32P]NAADP. These fractions were also able to completely desensitize the NAADP-induced Ca2+ release system in egg homogenates (R. A. Billington & A. A. Genazzani, unpublished data). The majority of the displacing factor eluted in one peak, but activity could also be observed in a smaller peak eluting earlier. The identity of this compound was not characterized, but it accounts for 18.7 % of total activity. The concentration of NAADP in spermatozoa can be calculated using the published value for the water volume of sea urchin spermatozoa of 0.71 μl per 108 spermatozoa (Christen et al. 1982). The concentration of NAADP in the extract can therefore be obtained by comparing the IC50 for authentic NAADP to the volume of extract necessary to inhibit 50 % of binding and by accounting for the fact that 18.7 % of the activity is not attributable to NAADP. Using 193 pm as the IC50 of NAADP (Billington & Genazzani, 2000), the calculated concentration of NAADP in the sperm cytosol from the three preparations was 4.0 ± 2.3 μM (values for the preparations ranged from 7.8 to 0.27 μM). The variation could be dependent on seasonal variations or different methods used for preparation of the extracts. Nonetheless, at present it is not possible to state whether NAADP levels were similar in non-activated sperm or whether sea water activation of spermatozoa induced a surge in levels of this compound.
Figure 2. The competing factor co-elutes with NAADP on HPLC.
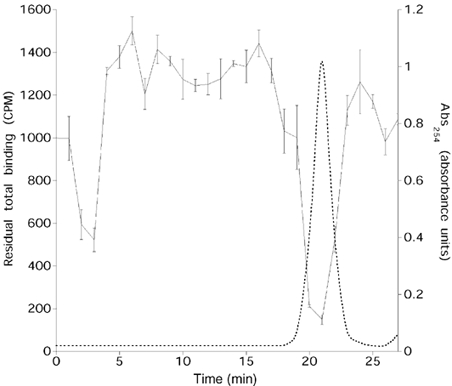
Inhibition of [32P]NAADP binding to egg homogenate by HPLC fractionated sperm extract (Aug 01; continuous line). The dashed line represents absorbance at 254 nm for authentic NAADP run on HPLC. Binding data are represented as means ±s.e.m.; n= 4. Similar data were obtained with the other two preparations.
It is indeed possible that the NAADP measured does not represent the true endogenous concentration since synthesis or degradation might occur during preparation. To exclude this possibility, we prepared extracts as described above in the presence of [32P]NADP. Since the only known route of NAADP synthesis is a base-exchange reaction starting from NADP and nicotinic acid (Aarhus et al. 1995; see below), we would expect that, if production did occur, part of the tracer would be converted to NAADP. After the separation of the extract by HPLC and subsequent determination of the radioactivity of the fractions, 92.9 ± 1.3 % of the radioactivity was recovered in a peak that co-eluted with NADP, while the remaining radioactivity was recovered in the membrane fraction (3.4 ± 0.2 %) and in the flow-through. No radioactivity co-eluted with NAADP, demonstrating that NAADP synthesis does not occur. To investigate whether NAADP degradation occurs, we performed identical experiments in the presence of [32P]NAADP. After HPLC separation, 83.8 ± 1.9 % of the radioactivity was recovered in a peak co-eluting with NAADP, with the remaining radioactivity in the membrane fraction (4.2 ± 0.4 %), and in small non-identifiable peaks. Therefore, it is likely that our determination of NAADP levels in sperm represents a conservative under-estimate of the content but is within our calculated standard error.
It has been previously shown that cADPR can be synthesized in sea urchin spermatozoa (Chini et al. 1997). Since it has been shown that similar or identical enzymes can also catalyse the synthesis of NAADP (Aarhus et al. 1995), and since we found that NAADP is present in spermatozoa, it follows that we should be able to detect NAADP production in our homogenates. Indeed, production of NAADP was detected by HPLC (a less sensitive method than the commonly used bio-assay) in the presence of exogenous NADP (2 mm) and nicotinic acid (6 mm) at pH 7 (Fig. 3). NAADP production in sperm extracts was over 350 times greater than in egg extracts. Eggs synthesized NAADP at a rate of 0.026 ± 0.02 pmol mg−1 min−1, while sperm synthesized NAADP at a rate of 9.7 ± 1.0 pmol mg−1 min−1. NAADP production was not detected in the absence of nicotinic acid (Fig. 3), indicating that the base-exchange reaction is the mechanism responsible. Furthermore, as expected, NAADP production was time dependent with maximal production observed at 1 h. Following on from earlier data (Aarhus et al. 1995), the pH dependence of NAADP production was investigated and it was found that NAADP production increased as the pH dropped (from 8 to 5), with maximal production at pH 5 (Fig. 3 inset). When the sperm homogenate was fractionated, NAADP synthesis was localized largely to the membrane fraction while little activity was detected in the cytosol (R. A. Billington & A. A. Genazzani, unpublished data). This distribution is similar to the one described in the sea urchin egg (Wilson & Galione, 1998).
Figure 3. Sperm homogenates can synthesise NAADP.
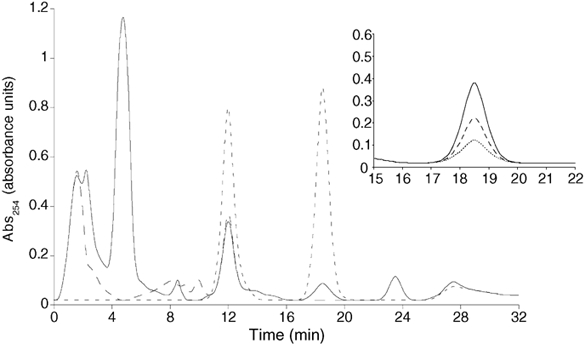
Representative HPLC traces (n= 6-8) showing production of NAADP by sperm homogenates after 1 h in the presence of 2 mm NADP and 6 mm nicotinic acid (NA) at pH 7.0 (continuous line), in the absence of NA (longer dashed line). Shorter dashed lines represent elution peaks of authentic NADP and NAADP standards. Other peaks on the trace have not been characterized. Inset, expanded view of the peak co-eluting with NAADP showing production of NAADP at pH 7 (dotted line), pH 6 (dashed line) and pH 5 (continuous line). Representative traces (n= 4) illustrated.
We then addressed the question of whether NAADP had a target receptor in the spermatozoa. When [32P]NAADP binding was performed, no sites were detected on sperm membranes, consistent with the idea that NAADP is released by sperm and binds to its target receptors in the egg. Nonetheless, because of the inactivation properties of the receptor, binding sites on sperm membranes might have been occupied by unlabelled NAADP in the intact sperm.
In conclusion, we have shown that NAADP can be produced in sea urchin spermatozoa and that sperm cytosol contains micromolar concentrations of this pyridine nucleotide. Furthermore, the rate of synthesis in sperm is over 350 times that in eggs. This represents the first report of NAADP levels in any tissue and strongly supports the notion that NAADP is an endogenous modulator of Ca2+ release. Considering that the sperm cytosol will be diluted more than 10 000-fold when its contents are released into the egg, it is unlikely that the NAADP concentrations reported here will produce a wave across the whole egg at fertilization. On the other hand, the NAADP from sperm will be at a high enough concentration around the site of sperm-egg fusion to activate NAADP receptors (EC50≈25 nm for Ca2+ release (Genazzani et al. 1996)), while more distal sites will, presumably, be inactivated (IC50≈250 pm for inactivation (Genazzani et al. 1996)). This model is substantiated by three previous observations: (i) the fertilization wave in sea urchin eggs can be abolished by co-injection of antagonists of the IP3 and ryanodine receptors, but residual Ca2+ release after fusion of the sperm still occurs (Galione et al. 1993); (ii) the NAADP response in fertilized eggs and homogenates is significantly reduced compared to unfertilized controls (Perez-Terzic et al. 1995; Genazzani, 1997) and (iii) the NAADP-sensitive store in starfish oocytes, a closely related echinoderm system, seems to be located just below the plasma membrane (Lim et al. 2001) and appears to release Ca2+ prior to the other two systems (Nusco et al. 2002). The experiments performed in starfish have revealed that while injection of NAADP leads to Ca2+ release from under the plasma membrane which spreads slowly to the centre of the egg, injection of IP3 leads to Ca2+ release and a subsequent fast moving wave emanating from the centre of the egg (Lim et al. 2001). Interestingly, it has been shown recently that release of Ca2+ by NAADP may prime the IP3 and cADPR sensitive stores for Ca2+ release (Churchill & Galione, 2001), providing a putative role for sperm NAADP. It has been shown previously that both IP3 and ryanodine receptors are activated at fertilization (Galione et al. 1993; Lee et. al., 1993) and, therefore, NAADP could act to fine-tune the Ca2+ wave.
For a fast-desensitizing messenger such as NAADP in the egg, the fact that it is produced in a separate compartment to the location of the receptor would validate its role.
Acknowledgments
R.A.B. is funded by a studentship from the Department of Pharmacology and A.A.G. is funded by a David Phillips Fellowship from the BBSRC. The authors are indebted to David Epel and Victor Vacquier for help in chasing references regarding sea urchin spermatozoa water volume and to Robin Hiley for critical reading of the manuscript.
REFERENCES
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+ Journal of Biological Chemistry. 1996;271:8513–8516. doi: 10.1074/jbc.271.15.8513. [DOI] [PubMed] [Google Scholar]
- Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. Journal of Biological Chemistry. 1995;270:30327–30333. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- Billington RA, Genazzani AA. Characterization of NAADP+ binding in sea urchin eggs. Biochemical and Biophysical Research Communications. 2000;276:112–116. doi: 10.1006/bbrc.2000.3444. [DOI] [PubMed] [Google Scholar]
- Chini E, Thompson M, Chini C, Dousa T. Cyclic ADP-ribose signaling in sea urchin gametes: metabolism in spermatozoa. American Journal of Physiology. 1997;272:C416–420. doi: 10.1152/ajpcell.1997.272.2.C416. [DOI] [PubMed] [Google Scholar]
- Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. Journal of Biological Chemistry. 1995;270:3216–3223. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- Christen R, Schackmann RW, Shapiro BM. Elevation of the intracellular pH activates respiration and motility of sperm of the sea urchin, Strongylocentrotus purpuratus. Journal of Biological Chemistry. 1982;257:14881–14890. [PubMed] [Google Scholar]
- Churchill G, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO Journal. 2001;20:2666–2671. doi: 10.1093/emboj/20.11.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. Journal of Biological Chemistry. 1987;262:9561–9568. [PubMed] [Google Scholar]
- Dargie PJ, Agre MC, Lee HC. Comparison of Ca2+ mobilizing activities of cyclic ADP-ribose and inositol trisphosphate. Cell Regulation. 1990;1:279–290. doi: 10.1091/mbc.1.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey DM, Aarhus R, Walseth TF, Lee HC. Thio-NADP is not an antagonist of NAADP. Cell Biochemistry and Biophysics. 1998;28:63–73. doi: 10.1007/BF02738310. [DOI] [PubMed] [Google Scholar]
- Galione A, McDougall A, Busa WB, Willmott N, Gillot I, Whitaker M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993;261:348–352. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- Genazzani A. 1997. Pyridine nucleotide metabolites as calcium mobilising messengers. D. Phil. Thesis. University of Oxford., GenaANIZZ. [Google Scholar]
- Genazzani AA, Galione A. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochemical Journal. 1996;315:721–725. doi: 10.1042/bj3150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AA, Galione A. A Ca2+ release mechanism gated by the novel pyridine nucleotide, NAADP. Trends in Pharmacological Sciences. 1997;18:108–110. doi: 10.1016/s0165-6147(96)01036-x. [DOI] [PubMed] [Google Scholar]
- Hogben M, Parrington J, Schevchenko V, Swann K, Lai F. Calcium oscillations, sperm factors and egg activation at fertilisation. Journal of Molecular Medicine. 1998;76:548–554. doi: 10.1007/s001090050249. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. Journal of Biological Chemistry. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. Functional visualization of the separate but interacting calcium stores sensitive to NAADP and cyclic ADP-ribose. Journal of Cell Science. 2000;113:4413–4420. doi: 10.1242/jcs.113.24.4413. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R, Walseth TF. Calcium mobilization by dual receptors during fertilization of sea urchin eggs. Science. 1993;261:352–355. doi: 10.1126/science.8392749. [DOI] [PubMed] [Google Scholar]
- Lim D, Kyozuka K, Gragnaniello G, Carafoli E, Santella L. NAADP+ initiates the Ca2+ response during fertilisation of starfish oocytes. FASEB Journal. 2001;15:2257–2267. doi: 10.1096/fj.01-0157com. [DOI] [PubMed] [Google Scholar]
- Nusco GA, Lim D, Sabala P, Santella L. Ca2+-response to cADPR during maturation and fertilization of starfish oocytes. Biochemical and Biophysical Research Communications. 2002;290:1015–1021. doi: 10.1006/bbrc.2001.6286. [DOI] [PubMed] [Google Scholar]
- Patel S, Churchill G, Galione A. Coordination of Ca2+ signalling by NAADP. Trends in Biochemical Sciences. 2001;26:482–489. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Churchill GC, Galione A. Unique kinetics of nicotinic acid-adenine dinucleotide phosphate (NAADP) binding enhance the sensitivity of NAADP receptors for their ligand. Biochemical Journal. 2000;352:725–729. [PMC free article] [PubMed] [Google Scholar]
- Perez-Terzic CM, Chini EN, Shen SS, Dousa TP, Clapham DE. Ca2+ release triggered by nicotinate adenine dinucleotide phosphate in intact sea urchin eggs. Biochemical Journal. 1995;312:955–959. doi: 10.1042/bj3120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Developmental Biology. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Wilson HL, Galione A. Differential regulation of nicotinic acid-adenine dinucleotide phosphate and cADP-ribose production by cAMP and cGMP. Biochemical Journal. 1998;331:837–843. doi: 10.1042/bj3310837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AA, Empson RM, Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. Journal of Biological Chemistry. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]


