Abstract
Surprising sensory stimuli have been found to attenuate one or two sympathetic discharges in human muscle nerves of some, but not all subjects, an effect suggested to be due to arousal. The aims of the present study were: (1) to provide evidence for or against an arousal mechanism by searching for evidence of habituation, and (2) to investigate if the presence or absence of inhibitory response is reproducible. To this end we recorded peroneal muscle sympathetic nerve activity (MSNA), electrocardiogram (ECG), finger blood pressure and changes of skin electrical resistance in 17 awake healthy subjects, while sensory stimuli consisting of five electrical pulses were delivered to a finger. The electrical pulses were triggered on five consecutive R waves of the ECG after a delay of 200 ms. Dummy stimuli, consisting of five trigger pulses without electrical pulses were used as controls, and the interval between two successive stimuli (real or dummy) was 30 s. On a group basis, the stimuli attenuated two initial and one late MSNA bursts. On an individual basis, significant attenuation of one or two initial bursts occurred in eight subjects, whereas in nine subjects there was no significant inhibition. In nine subjects the experiments were repeated once and in three subjects they were repeated twice. The effects on MSNA were reproducible in 11 of the12 subjects. In the group of subjects without significant MSNA inhibition the stimuli induced a small, transient increase in mean blood pressure, which was not present in the group with significant MSNA inhibition. Heart rate did not change in either group. In conclusion, the inhibitory effect on MSNA of five repeated electrical pulses to a finger is largely similar to that previously shown for one pulse, i.e. there is rapid habituation of the response, compatible with an arousal-induced effect. The inhibitory responsiveness shows marked interindividual differences, which are reproducible over several months and associated with different effects on blood pressure.
Human muscle sympathetic nerve activity (MSNA) is modulated by arterial baroreflex mechanisms: the sympathetic impulses are grouped in pulse synchronous bursts occurring preferentially during transient reductions of blood pressure (Delius et al. 1972a), and changes of arterial baroreceptor firing induce opposite changes in the strength of MSNA (Wallin & Eckberg, 1982). Recently, it was shown that a sensory stimulus delivered 200–400 ms after the R wave of the ECG led to an inhibition of one or two MSNA bursts, suggesting that the stimulus in some way potentiated the inhibitory effect of the afferent baroreceptor discharge evoked by the systolic pressure wave (Donadio et al. 2002). The effect was seen in some, but not in all subjects. Since the inhibition could occur either after visual or electrical skin stimuli, it was suggested that the response was most likely to be related to arousal.
An arousal reaction can be regarded as a response to external or internal stimulation, alerting the individual to a novel situation that may require some form of action. The patterns of arousal responses appear to be similar across mammalian species, including humans (Blanchard et al. 2001). Animal studies have shown that arousal responses to tactile stimuli are enhanced in situations perceived as threatening (Blanchard et al. 1986), and induce cardiovascular changes that may facilitate particular defensive behaviours (Carrive, 2000; Dielenberg et al. 2001). In humans arousal reactions can be evoked both in the awake state and during sleep in association with (spontaneous or induced) K-complexes in the electroencephalogram (Hornyak et al. 1991; Okada et al. 1991; Xie et al. 1999) and presumably, such reactions have considerable survival value. The manifestations of arousal may include autonomic and respiratory adjustments and skeleto-motor reactions (startle) but the variability is wide, depending on many factors including stimulus intensity (Turpin et al. 1999), cognitive load of the stimulus (Unrug et al. 1997) and aspects of personality (Lawler et al. 2001).
In order to extend knowledge of the inhibitory effects of sensory stimulation on MSNA the present experiments were undertaken with the following aims: (1) to provide further evidence for or against the hypothesis that the inhibition was due to arousal. To this end MSNA was recorded while a series of identical sensory stimuli were delivered with short intervals, the assumption being that arousal-induced effects would habituate with rapid repetition; and (2) to investigate if the interindividual differences in sympathetic responses to sensory stimulation, mentioned above, are reproducible over time. If such differences were present we also wanted to determine whether or not there were associated differences in cardiovascular effector responses.
METHODS
Subjects
We studied 17 awake, male, healthy individuals aged 28 ± 2 years (range 21–48 years) with arterial blood pressure below 140/90 (measured with a sphygmomanometer on the upper arm); no subject was on medication. The recordings were performed in the morning, approximately 3 h after a light meal. Room temperature was 22–25 °C. Tobacco, caffeine and alcohol were not allowed for 12 h before the examination. In 12 subjects the study protocol was repeated after 2–3 months, and in three subjects a third recording was made within 6 months. The experimental procedures were approved by the Human Ethics Committee at the University of Göteborg and all subjects gave their written, informed consent to the procedures, which conformed to the Declaration of Helsinki.
Measurements
Subjects were semi-reclining in a comfortable chair. ECG was recorded via Ag-AgCl electrodes on the chest and respiratory movements were monitored by a strain gauge belt around the lower part of the chest. Arterial finger blood pressure was measured non-invasively by the volume-clamp method (Portapress Model-2, Amsterdam, The Netherlands), with the cuffs around the middle phalanx of the third and fourth fingers on the same side as the microneurography recording. Changes in skin resistance (filter settings 0.3-100 Hz) were measured from the plantar and the dorsal sides of the foot ipsilateral to the microneurography recording side.
Multiunit efferent post-ganglionic muscle sympathetic nerve activity was recorded with an insulated tungsten microelectrode with a tip diameter of a few microns inserted into the left peroneal nerve, posterior to the fibular head. A low-impedance reference electrode was inserted subcutaneously a few centimetres away. In subjects in whom the study protocol was repeated, the second recording was obtained from the right and the third from the left peroneal nerve.
The nerve signal was amplified (× 50 000), filtered (band pass 700–2000 Hz) and fed through a discriminator for further noise reduction and audio-monitoring. A mean voltage (integrated) display was obtained by passing the original signal through a resistance-capacitance circuit (time constant 0.1 s). During the experiment, neural activity and arterial pressure were monitored on a storage oscilloscope. When a muscle-nerve fascicle had been identified, small electrode adjustments were made until a site was found in which sympathetic impulses with a good signal-to-noise ratio could be recorded. A recording of MSNA was considered acceptable when it revealed spontaneous, pulse-synchronous bursts of neural activity that fulfilled the criteria for MSNA previously described (Sundlöf & Wallin, 1977). The filtered and integrated nerve signals were sampled and stored together with other signals on a personal computer using a locally produced data acquisition system. In addition, all signals were stored on analogue tape.
Stimulation
Two types of stimuli were used. The real stimulus was a series of five electrical constant-voltage square wave pulses (0.2-10 ms duration, 100–150 V amplitude) triggered with a delay of 200 ms on five consecutive R waves of the ECG, and delivered via surface electrodes taped to the second finger of the hand opposite to the microneurography recording. The strength of the stimulus was adjusted to be as high as possible without causing pain. Approximately the same intensity of stimulation was kept in each subject for the first, second and third recordings. The dummy stimulus was a series of five trigger pulses without subsequent electrical shocks. All trigger pulses were recorded and stored in the computer and on the tape.
Electrical and dummy stimuli were randomly delivered every 30 s (without regard to the amount of activity) and the same order was used for all subjects. Intervals between real stimuli varied between 30 s and 3.5 min.
Procedure
After acquiring a stable recording site, resting MSNA was recorded for 15 min. After the rest period the subject was informed that the stimulation was about to start at the predetermined intensity. During all stimulation periods the loudspeaker was turned off but the experimenter monitored the integrated neurogram on an oscilloscope screen to detect artefacts caused by muscle tension, etc. In each experiment real and dummy stimuli were given 30 times. In six subjects the fifth electrical pulse of each stimulus was shifted and applied to the second finger of the opposite hand.
Analysis
Sympathetic activity
Sympathetic bursts occurring during the last 5 min of the rest period were identified by inspection of the mean voltage neurogram, and the amount of activity was expressed as burst incidence (bursts (100 heart beats)−1) and burst frequency (bursts min−1).
To describe the sequence of events associated with the stimuli, the following definitions were adopted (Fig. 1). The first electrical pulse (pulse 1) of a stimulus was delivered in a heart cycle (between R waves 0 and 1) denoted cardiac interval (CI) 1, and the subsequent four pulses were given in CI 2 to 5. A sympathetic burst generated in the central nervous system during CI 1 is defined as burst 1 (and those generated during CI 2 to 5 as bursts 2 to 5). Burst 1 will arrive at the recording electrode after a delay corresponding to the baroreflex latency (defined as the latency from the R wave of the ECG to the start of the inhibition (equal to the peak) of the appropriate burst in the mean voltage neurogram). When recording in the peroneal nerve at the fibular head, this delay is approximately 1.3 s (Fagius & Wallin, 1980). With this definition and since the duration of the average cardiac interval was shorter than the reflex delay in all subjects, the electrical artefacts from the five pulses of a stimulus usually contaminated bursts −1 to 3 (Fig. 1).
Figure 1. Relationship between sympathetic bursts, cardiovascular parameters and stimulus.
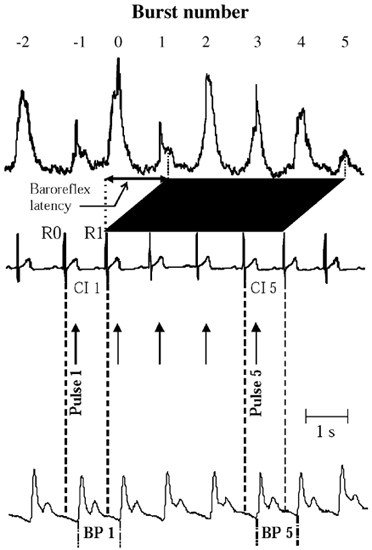
Definitions and terminology for the relationship between mean voltage neurogram, cardiovascular parameters and electrical stimulus consisting of five repeated square wave pulses triggered with a delay of 200 ms from the R wave of the ECG (note stimulus artefacts in mean voltage neurogram). Pulses 1–5 delivered in cardiac intervals 1–5 (CI 1-5). Baroreflex latency defined as time from R wave 1 of the ECG (R1) to peak of burst 1 in mean voltage neurogram, i.e. bursts 1–5 are terminated by the afferent baroreceptor discharges induced by the systolic pressure waves occurring in CI 2-6.
We quantified the effects of the stimulation on the amplitudes of bursts 0 to 5. In order to exclude the artefacts from the electrical pulses in the quantitative analysis, we averaged, for each experiment, all (usually around 25) artefacts occurring in segments of the mean voltage neurogram without bursts. This average artefact was then arithmetically subtracted from the neurogram by the computer (Fig. 2). After artefact removal all amplitudes of bursts 0 to 5 were normalised to the mean amplitude (equal to 100 units) of all bursts −4, −3 and −2 (which were uncontaminated by artefacts from the electrical pulses) for either real or dummy stimulations in each subject. Then, in each subject the mean normalised amplitude for each of bursts 0 to 5 was compared to the mean amplitude of all bursts −4, −3 and −2 both for real and dummy stimuli. Absent bursts were included and given the value of zero.
Figure 2. Artefact elimination.
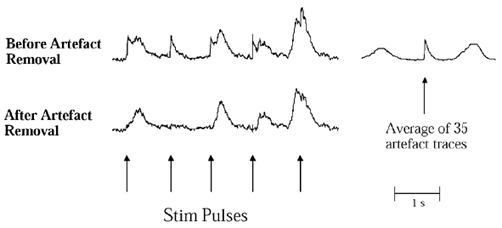
Technique for removal of artefacts induced by the electrical pulses. Upper left trace, mean voltage neurogram distorted by artefacts from five pulses. Average artefact (upper right trace) was obtained from sections of the neurogram without bursts (as in association with pulse 2). Lower trace, the same mean voltage neurogram after subtraction of averaged artefact.
Since a previous study has shown that the mechanisms determining the strength and occurrence of MSNA bursts are not identical (Kienbaum et al. 2001), the effects of the stimuli were also analysed separately for each variable. For burst strength (equal to amplitude) the calculation was identical to that for total amplitude, except that absent bursts, i.e. zero values, were not included in the mean. To determine if the occurrence of bursts was reduced by the stimuli we determined how often each of bursts 0 to 5 were present in the neurogram compared to the expected occurrence. The expected occurrence was derived from the baroreflex threshold diagram, calculated as described by Kienbaum et al. (2001). The threshold diagrams were based on bursts and diastolic blood pressures before (-4, −3 and −2) and after (11 to 25) all real and dummy stimuli.
Cardiovascular variables
For each electrical or dummy stimulation, the computer determined mean blood pressure and cardiac interval of all cardiac cycles −4 to 25. Individual values of blood pressure and cardiac interval were then normalised in the same way as burst amplitude, and for each cardiac cycle the difference between the respective mean value and corresponding mean value of heart cycle −4, −3 and −2 (equal to 100 units) were determined separately for real and dummy stimuli.
Skin resistance changes
Counts were made of the number of skin resistance responses occurring within 7 s from the first pulse of a stimulus. To be included as a response, the amplitude had to exceed 5 % of the biggest spontaneous skin resistance deflection occurring during the rest period. The occurrence of responses was expressed as a percentage of the total number of stimuli.
Statistics
All values are expressed as means ±s.e.m. Student's two-tailed t test for unpaired data was used to compare: (a) mean normalised burst amplitude of bursts −4, −3 and −2 with each of bursts 0 to 5 and (b) the difference between real and expected occurrence of bursts during the stimuli. Separate analyses were made for real and dummy stimuli.
Unpaired t tests were also used to compare the level of MSNA (in bursts (100 heart beats)−1 and bursts min−1) and the occurrence of skin resistance responses between subgroups of subjects. Bonferroni corrections were made in all t tests using a nominal level of significance at P= 0.05.
Analysis of variance followed by the Duncan test was used to compare time-dependent changes from the baseline in mean blood pressure between subgroups of subjects. The reproducibility of sympathetic inhibition between repeated recordings in the same subject were assessed with Pearson linear regression analysis. P < 0.05 was considered significant.
RESULTS
Resting levels of MSNA in the sitting position were 62 ± 3 (range 41-88) bursts (100 heart beats)−1, 39 ± 2 (range 21-50) bursts min−1. Heart rate was 61 ± 1 (range 49-74) beats min−1. Cuff blood pressure was 104(± 10)/ 62(± 8) mmHg.
Stimulation-induced effects on muscle sympathetic activity
On a group basis electrical skin stimulation caused a significant reduction of the averaged mean voltage amplitude of bursts 0, 1 and 4, when compared to the mean amplitude of the pre-stimulus control bursts (Table 1). In contrast, dummy stimuli induced no changes of the amplitudes of bursts 0-5. When burst occurrence and burst amplitude were analysed separately, there was a significant reduction of both, i.e. there was no evidence of differentiated effects on these variables (Table 1). Considering the whole data set, the amplitude of bursts 0 or 1 (choosing the one with the most marked attenuation) was reduced by 45 ± 4 % compared to the mean of control bursts −4, −3 and −2.
Table 1.
Group comparison of inhibitory effects on MSNA bursts
| Burst number | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Total amplitude | ** | *** | — | — | * | — |
| Burst amplitude | * | *** | — | — | * | — |
| Burst occurrence | * | *** | — | — | — | — |
Analysis made with six Bonferroni-corrected repeated t tests. Significance of burst inhibition compared to control (bursts −4 to −2) indicated by the following symbols: —, no significant effect
P < 0.05
P < 0.01
P < 0.001.
To compare on an individual basis the effects of the stimulation in the present study with those reported by Donadio et al. (2002) we analysed the effects on bursts 0 and 1 separately from those on bursts 2 to 5. In eight of the 17 subjects the mean voltage amplitude was reduced on burst 0 (one subject), burst 1 (four subjects) or both burst 0 and 1 (three subjects). Figure 3 shows superimposed averaged records (dummy and electrical stimulation) of sympathetic activity from subjects in whom one (A) and two (B) bursts were attenuated. Table 2 summarises individual data from all subjects. In subjects with significant amplitude reduction of bursts 0 and/or 1 the resting level of MSNA was higher than in subjects without amplitude reduction (68 ± 3 vs. 57 ± 4 bursts (100 heart beats)−1 and 52 ± 4 vs. 36 ± 3 bursts min−1, n= 8 vs. n= 9, P < 0.05 for both). In subjects with significant amplitude reduction the mean amplitude was reduced by between 40 and 87 % compared with the mean of the control bursts −4, −3 and −2. In subjects without significant amplitude reduction (n= 9), mean amplitudes were 19–44 % lower in eight and 25 % higher in one subject (the calculations were all based on the smallest of bursts 0 and 1). Furthermore, in subjects with statistically significant amplitude reduction of bursts 0 or 1 the decrease in amplitudes was greater during the first than during the last 15 stimuli (71 ± 3 vs. 52 ± 3 %, respectively, P < 0.01).
Figure 3. Stimulus-induced effects of MSNA in two subjects.
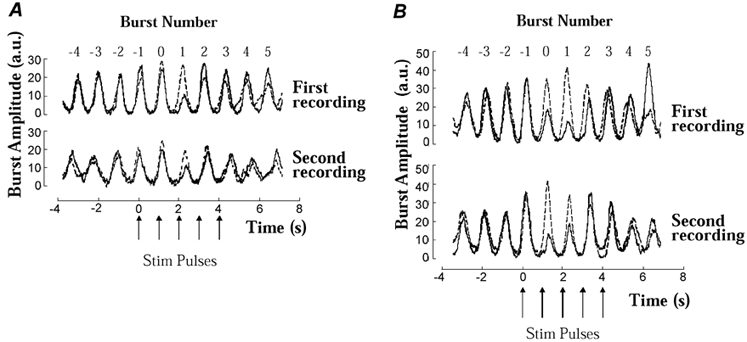
Examples of subjects (A and B corresponding to nos 2 and 12, respectively, in Table 2) showing amplitude reduction of burst 1 (A) or 0 and 1 (B) in the averaged mean voltage neurograms. Results of dummy and real stimuli shown by dashed and continuous lines, respectively. Note good agreement in all recordings between control bursts in records from dummy and real stimuli, and also similarity between inhibitory effects in first and second recordings.
Table 2.
Individual effects on MSNA burst amplitudes
| 1st recording Burst number | 2nd recording Burst number | 3rd recording Burst number | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | |
| Subject | ||||||||||||||||||
| 1 | — | *** | — | — | * | — | *** | — | — | — | — | †† | ||||||
| 2 | — | ** | — | — | — | — | — | *** | — | — | — | — | — | ** | — | — | — | — |
| 3 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| 4 | — | ** | — | — | — | — | ||||||||||||
| 5 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| 6 | — | — | — | — | — | — | *** | — | — | — | — | † | ||||||
| 7 | * | ** | * | — | — | — | — | *** | * | — | — | — | — | * | — | — | — | — |
| 8 | — | — | — | — | — | — | ||||||||||||
| 9 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| 10 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| 11 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 12+ | *** | *** | — | — | — | ††† | *** | ** | — | — | — | — | ||||||
| 13+ | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| 14+ | — | — | — | — | — | — | ||||||||||||
| 15+ | — | * | — | — | — | — | — | * | — | — | — | — | ||||||
| 16+ | — | * | — | — | ** | — | ||||||||||||
| 17+ | ** | — | — | — | †† | — | ||||||||||||
Significance of burst inhibition indicated with the following symbols: —, no significant effect
P < 0.05
P < 0.01
P < 0.001.
after the subject identification indicates that the last electrical pulse was shifted to the opposite hand. Augmentation of burst amplitude indicated by
P < 0.05
P < 0.01
P < 0.001.
In addition to effects on bursts 0 and/or 1 the stimuli also induced a significant reduction in the amplitude of burst 2 in one subject and of burst 4 in one subject. There were also significant increases in burst amplitudes (burst 4 in one subject and burst 5 in another) and these occurred in subjects in whom at least one previous burst had been attenuated (see Table 2).
In six subjects (indicated by ‘+’ after the subject number in Table 2) the fifth pulse of each stimulus was applied to the opposite hand. On a group basis, no significant reduction in amplitudes of burst 4 or 5 occurred, but on an individual basis burst 4 was attenuated in one subject.
To illustrate the time courses of the different types of responses, the material was divided into two groups, one comprising subjects with, and the other one comprising subjects without significant amplitude reduction in burst 0 or 1 in the individual analysis. As expected, the variations of burst amplitudes over time differed markedly between the two groups (Fig. 4A and B). In the group with amplitude reduction of bursts 0 and/or 1 there was also an approximately 20 % increase of the amplitudes of bursts 5 and 6 when compared with the three control bursts (P < 0.05). This effect was not present in subjects without amplitude reduction.
Figure 4. Stimulus-induced cardiovascular effects.
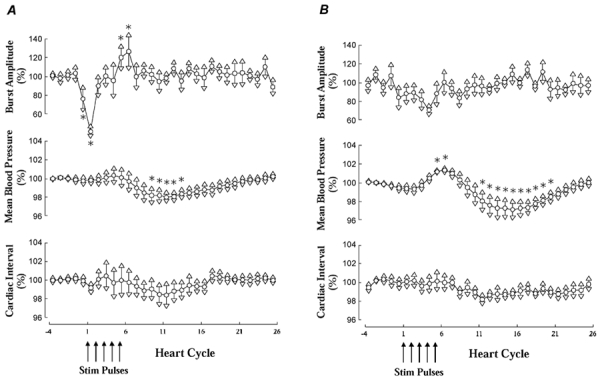
Average effects of five electrical pulses on burst amplitude, mean blood pressure and cardiac interval in all subjects with (A) or without (B) significant inhibition of burst 0 or 1 (or both) in first recording. All values expressed as percentages of mean values in bursts or cardiac intervals −4 to −2. *P < 0.05.
Reproducibility
In nine subjects the experiments were repeated once and in three subjects, twice. The stimulation-induced effects on MSNA were reproducible in 11 of the 12 subjects (for details see Table 2). All three subjects in whom three recordings were made had reproducible results in all recordings: in two subjects bursts 0 and/or 1 were attenuated and in one subject no attenuation occurred. Of the nine subjects in whom two recordings were made eight had reproducible results: three showed burst amplitude reduction (in one case different bursts in the two recordings) and five showed no amplitude reduction. In the remaining subject the amplitude of burst 0 was significantly attenuated in the second, but not in the first experiment.
Moreover, when plotting the relative amplitude reduction of bursts 0 or 1 in the first recording versus that in the second recording for all 12 subjects (using the burst with the most marked attenuation in each recording), the regression line (P < 0.01) fell close to the 45 deg line (Fig. 5). Thus, the degree of inhibition was also reproducible, even when subjects were included in whom the individual effect did not reach statistical significance.
Figure 5. Comparison of stimulus-induced responses in repeated recordings.
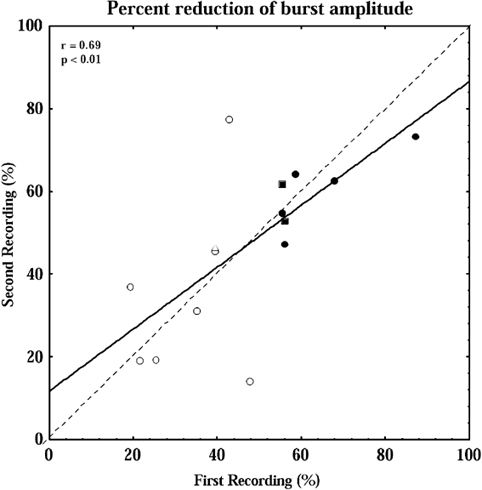
Relationship between degree of inhibition in subjects in whom repeated recordings were made. ○, comparison of first and second recordings; □, comparison of second (abscissa) and third (ordinate) recordings. Filled symbols indicate subjects in whom the amplitude reduction of burst 0 and/or 1 was statistically significant in both recordings. Amplitude reduction is expressed as percentage of mean amplitude of bursts −4 to −2 preceding the stimuli. The continuous line is the regression line and the dashed line is the line of identity.
Stimulation-induced effects on cardiovascular variables
As illustrated in Fig. 4, there were stimulus-induced changes in mean blood pressure that differed between subjects with and without significant attenuation of bursts 0 or 1 in the individual analysis. Subjects without burst amplitude reduction had a significant early increase in pressure (with a peak approximately 6–7 heart beats after the first electrical pulse) followed by a reduction in blood pressure. In subjects with burst amplitude reduction there was no early increase but only the late decrease. The reduction of pressure, which occurred at unchanged MSNA, was more marked in the group without burst amplitude reduction but the difference did not reach statistical significance.
In contrast with the stimulus-induced effects on blood pressure, there were no significant early or late group effects on heart rate in either group. On an individual basis there were significant changes of heart rate in 10 of 17 subjects, six in subjects with and four in subjects without significant MSNA response. These changes varied markedly; both accelerations and decelerations occurred, with decelerations being most common.
Stimulation-induced effects on skin resistance
Electrical stimuli generally evoked transient skin resistance responses, but all subjects showed a clear tendency to habituation. The incidence of responses was similar in subjects with and without MSNA response (66 ± 12 and 57 ± 8 %, respectively, P > 0.10). The tendency to habituation was quantified as the incidence of skin resistance responses evoked by the last 15 stimuli divided by the corresponding incidence during the first 15 stimuli. In subjects with significant MSNA inhibition in the individual analysis this ratio was 0.79 ± 0.17, and in subjects without inhibition the corresponding value was 0.65 ± 0.18 (P > 0.10).
DISCUSSION
Our main findings were: (1) the MSNA response to five electrical pulses time-locked to the ECG was remarkably similar to that evoked by one electrical pulse, suggesting that marked habituation occurred; (2) inhibitory MSNA responses were obtained in approximately 50 % of the subjects, and when the experiment was repeated after several months, the result was reproducible in 11 of 12 subjects; and (3) the group of subjects without significant MSNA inhibition had a transient increase in mean blood pressure after the stimulus, which was not seen in the group with MSNA inhibition.
Muscle sympathetic responses
The main sympathetic response to five ECG-triggered electrical skin pulses given with a delay of 200 ms was similar to that evoked by one pulse in the study of Donadio et al. (2002), i.e. burst 0, burst 1 or both bursts were attenuated (both on a group basis and in individual subjects). In fact, in the present study the initial MSNA inhibition to five ECG-triggered pulses occurred slightly less frequently (in 8/17 subjects, 47 %) than the inhibition by a single pulse in the study of Donadio et al. (2002) (in 11/19 subjects, 58 %). In addition to this initial effect induced by pulse 1, however, there were only minor indications that pulses 2 to 5 led to sympathetic inhibition: burst 2 was reduced in amplitude in one and burst 4 in two subjects and, on a group basis for all subjects, burst 4 was significantly attenuated. In addition, the increased amplitudes of bursts 5 and 6 in Fig. 4A may be a sign of post-inhibitory excitation, indicating that some inhibitory effect remained until the end of the stimulus. Thus, the findings indicate that when repeated electrical pulses are given with short intervals, the MSNA inhibition habituates markedly after the first pulse, supporting the view that the inhibitory effect is due to arousal.
When the last pulse in each stimulus was shifted to the opposite hand, no increase in the inhibitory effect occurred, suggesting that the habituation to the electrical pulses was not weakened. The explanation for this somewhat unexpected result may be that the surprise effect was too weak: after one stimulus the subject had already become aware of the experimental protocol, which then remained unchanged through all 30 stimuli.
Between experiments made at intervals of months, there may have been: (a) unrecognised differences in the number of sympathetic fibres recorded from; (b) unrecognised differences in the subject's estimate of the strength of the electrical stimulus; (c) unrecognised differences in the psychological state of the subject. In spite of this, the inhibition of burst 0 or 1 was remarkably reproducible (Table 2 and Fig. 5), suggesting that the underlying mechanism was stable and characteristic for the individual. Interindividual differences in MSNA responsiveness to stimuli or manoeuvres have not been demonstrated previously, but there are well-documented reproducible interindividual differences of resting levels of MSNA in supine subjects (Sundlöf & Wallin, 1977; Fagius & Wallin, 1993). We found higher resting levels of MSNA (in the sitting posture) in subjects with significant MSNA inhibition, raising the possibility that the two types of interindividual differences in some way are linked.
The mechanisms underlying the interindividual differences in arousal-induced MSNA inhibition are unclear. When the effects of electrical and visual stimuli were compared (Donadio et al. 2002) the MSNA inhibition was more common after electrical stimuli, which was also the mode of stimulation that evoked the most arousal reactions. On the other hand, in neither the study of Donadio et al. (2002) nor the present experiments were there differences in the occurrence of skin resistance responses between subjects with and without significant MSNA inhibition in the individual analysis, suggesting that the inhibition is not merely a question of high reactivity to stimuli. Arousal-induced changes of respiration may contribute to the MSNA effect (cf. Donadio et al. 2002) but this possibility cannot be evaluated from the present data. Personality-related factors could also be of importance but no supporting evidence is available. In the only published study of MSNA and personality type (Schroeder et al. 2000), there was no relationship between type A personality and the strength of MSNA at rest, or with increases of MNSA during mental arithmetic, isometric handgrip or the cold pressor test.
Cardiovascular responses
Electrical stimulation of certain areas in the hypothalamus and the brainstem evokes a defence reaction, the early cardiovascular component of which (the alerting stage) is seen as a preparation for fight or flight (Hilton, 1982). In conscious cats (Caraffa-Braga et al. 1973), monkeys (Forsyth, 1972) and rabbits (Yu & Blessing, 1997) similar vascular responses, graded in relation to stimulus intensity and usually comprising splachnic, renal and cutaneous vasoconstriction, muscle vasodilatation and a rise in blood pressure, are evoked both by short-lasting sensory stimuli (such as a sudden sound or a pain stimulus) and emotional stress of longer duration. In humans, emotional stress induced by mental arithmetic was found to evoke similar circulatory changes (Brod et al. 1959), and in microneurographic recordings, both arousal and mental arithmetic lead to increases in skin vasoconstrictor nerve traffic (Delius et al. 1972b; Haarth et al. 1972). Taken together the findings suggest that the vascular response induced by arousal is qualitatively similar to that evoked by more long lasting emotional stress.
Against this background it is likely that the MSNA inhibition seen in the present study is part of a more or less generalised stress response, which to some degree is similar to the defence reaction, and which probably contributes to the early phase of the muscle vasodilatation mentioned above. The arousal-induced MSNA effect probably contributes to the reduction of MSNA during the first 30 s of mental stress that was found previously (Callister et al. 1992). Later during a stress response (after one to several minutes) MSNA has been found to be unchanged in the arm and increase in the leg (Anderson et al. 1987; Callister et al. 1992) suggesting that during this phase, mechanisms other than a change of vasoconstrictor nerve traffic are important for evoking the vasodilatation. We found that the stimuli were followed by small (approximately 2 % of the resting level) but significant blood pressure changes. Interestingly, there was an early transient increase in mean blood pressure in the group of subjects without significant MSNA inhibition, which did not occur in the group with MSNA inhibition. A possible explanation may be that an arousal-induced excitation of other sympathetic subdivisions (e.g. renal, splanchnic) is counteracted by the inhibition of MSNA, thereby abolishing the early blood pressure increase seen in subjects without significant inhibition. The mechanism underlying the late reduction of blood pressure seen in both groups is also unclear, but may be related to an associated change of baroreflex threshold or sensitivity, since MSNA remained unchanged.
The electrical stimuli induced no significant changes of group-averaged heart rate, primarily because of marked interindividual differences in latency and type of response. Thus, changes in cardiac output are unlikely to contribute significantly to the blood pressure changes. The lack of consistent heart rate effects agrees with previous observations (Eves & Gruzelier, 1984; Turpin et al. 1999); sudden sound stimuli may evoke both early and late accelerator and decelerator responses, in part related to stimulus intensity. There is also evidence that the cardiovascular responses to stress (Brod et al. 1959; Mancia et al. 1972), as well as the habituation of such responses (Zborzyna, 1987), display interindividual differences both in animals and humans.
Methodological aspects
To demonstrate an inhibitory effect of a stimulus requires a certain level of background activity. In the present study the interindividual differences in resting activity ranged between 41 and 88 bursts (100 heart beats)−1, meaning that the electrical pulses of the stimulus often occurred in cardiac intervals without activity. Since all subjects received 30 stimuli, the consequence is that the statistical analyses of the significance of inhibition were based on different levels of background activity. Because of this interindividual variability one should expect the reliability of the significance analysis to be weaker in subjects with fewer bursts. Accordingly, our statistical methodology may underestimate the number of subjects with MSNA inhibition. This agrees with the good reproducibility of burst amplitude reduction in repeated recordings in some subjects without significant MSNA inhibition (Fig. 5). Thus, it is likely that the tendency to respond with MSNA inhibition is graded, ranging between weak or no responses in some subjects and marked responses in others.
In the present study, the method of initiating stimuli differed from that used by Macefield et al. (1998) and Donadio et al. (2002). In both these studies the experimenter delivered the stimulus at the start of a sequence of MSNA bursts, the effect being that the strength of activity in the averaged mean voltage neurogram varied over the period of averaging. An advantage of this procedure was that inhibitions occurred against a relatively high level of activity but, on the other hand, the intervals between stimuli (real or dummy) were subjective and quite variable. In the present study the subjectivity was avoided by using the computer to initiate the stimuli at 30 s intervals, regardless of the level of MSNA activity. As shown in Fig. 3 this resulted in a relatively constant level of activity in the averaged mean voltage neurogram (i.e. except for those bursts that were attenuated by the electrical pulses, burst amplitudes were fairly similar).
Since all subjects in the present study were males, the results cannot be generalised. There is, however, no indication that the inhibitory effect on MSNA is limited to males; in the study of Donadio et al. (2002) two of three females had significant MSNA inhibition to single sensory stimuli.
In summary, healthy human subjects differ in their sympathetic and haemodynamic responses to a sudden arousal stimulus. In some subjects MSNA is almost unaffected by the stimulus and the blood pressure shows a transient increase. Other subjects show a short-lasting inhibition of sympathetic activity, and they do not display a blood pressure increase following the stimulus. The mechanisms underlying these differences, as well as their putative long-term implications remain to be resolved.
Acknowledgments
We thank Göran Pegenius for excellent technical assistance. Supported by Swedish Medical Research Council Grant 12170. V.D. was supported by the Foundation Blanceflor Boncompagni-Ludovisi, née Bildt.
REFERENCES
- Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;6(suppl. III):114–119. doi: 10.1161/01.hyp.9.6_pt_2.iii114. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neuroscience and Behavioral Reviews. 2001;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behaviours of laboratory and wild Rattus norwegicus. Journal of Comparative Psychology. 1986;100:101–107. [PubMed] [Google Scholar]
- Brod J, Fencl V, Hejl Z, Jirka J. Circulatory changes underlying blood pressure elevation during acute emotional stress (mental arithmetic) in normotensive and hypertensive subjects. Clinical Science. 1959;18:269–279. [PubMed] [Google Scholar]
- Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. Journal of Physiology. 1992;454:373–387. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraffa-Braga E, Granata L, Pinotti O. Changes in blood flow distribution during acute emotional stress in dogs. Pflügers Archiv. 1973;339:203–216. doi: 10.1007/BF00587372. [DOI] [PubMed] [Google Scholar]
- Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Research. 2000;858:440–445. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth K-E, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiologica Scandinavica. 1972a;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth K-E, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiologica Scandinavica. 1972b;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Research. 2001;897:228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- Donadio V, Kallio M, Karlsson T, Nordin M, Wallin BG. Inhibition of human muscle sympathetic activity by sensory stimulation. Journal of Physiology. 2002;544:285–292. doi: 10.1113/jphysiol.2002.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eves FF, Gruzelier JH. Individual differences in the cardiac response to high intensity auditory stimulation. Psychophysiology. 1984;21:342–352. doi: 10.1111/j.1469-8986.1984.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. Journal of Neurological Sciences. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clinical Autonomic Research. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Forsyth RP. Sympathetic nervous system control of distribution of cardiac output in unanaesthetized monkeys. Federation Proceedings. 1972;31:1240–1244. [PubMed] [Google Scholar]
- Hagbarth K-E, Hallin RG, Hongell A, Torebjörk HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiologica Scandinavica. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hilton SM. The defence-arousal system and its relevance for circulatory and respiratory control. Journal of Experimental Biology. 1982;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in humans. Brain. 1991;114:1281–1295. doi: 10.1093/brain/114.3.1281. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? Journal of Physiology. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Taylor JL, Wallin BG. Inhibition of muscle sympathetic outflow following transcranial cortical stimulation. Journal of the Autonomic Nervous System. 1998;68:49–57. doi: 10.1016/s0165-1838(97)00117-3. [DOI] [PubMed] [Google Scholar]
- Mancia G, Bacelli G, Zanchetti A. Hemodynamic reponses to different emotional stimuli in the cat: patterns and mechanisms. American Journal of Physiology. 1972;223:925–933. doi: 10.1152/ajplegacy.1972.223.4.925. [DOI] [PubMed] [Google Scholar]
- Okada H, Iwase S, Mano T, Sugiyama Y, Watanabe T. Changes in muscle sympathetic nerve activity during sleep in humans. Neurology. 1991;41:1961–1966. doi: 10.1212/wnl.41.12.1961. [DOI] [PubMed] [Google Scholar]
- Schroeder KE, NarkiewicZ K, Kato M, Pesek C, Philips B, Davison D, Somers VK. Personality type and neural circulatory control. Hypertension. 2000;36:830–833. doi: 10.1161/01.hyp.36.5.830. [DOI] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. Journal of Physiology. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin G, Schaefer F, Boucsein W. Effects of stimulus intensity, risetime, and duration on autonomic and behavioural responding: Implications for the differentiation of orienting, startle, and defence responses. Psychophysiology. 1999;36:453–463. [PubMed] [Google Scholar]
- Unrug A, Bener J, Barry RJ, Van Luijtelaar EL, Coenen AM, Kaiser J. Influence of diazepam and buspirone on human heart rate and the evoked cardiac response under varying cognitive load. International Journal of Psychophysiology. 1997;25:177–184. doi: 10.1016/s0167-8760(96)00744-1. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in man. American Journal of Physiology. 1982;242:H185–190. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puelo DS, Morgan B. Arousal from sleep shortens sympathetic burst latency in humans. Journal of Physiology. 1999;515:621–628. doi: 10.1111/j.1469-7793.1999.621ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y-H, Blessing WW. Cutaneous vasoconstriction in conscious rabbits during alerting responses detected by hippocampal T-rhythm. American Journal of Physiology. 1997;272:R208–216. doi: 10.1152/ajpregu.1997.272.1.R208. [DOI] [PubMed] [Google Scholar]
- Zbrozyna AW. Habituation of cardiovascular responses. In: Taylor EW, editor. The Neurobiology of the Cardiorespiratory System. UK: Manchester University Press; 1987. pp. 241–260. [Google Scholar]
- Lawler KA, Klinne KA, Adlin RF, Wilcox ZC, Craig FW, Krishnamoorthy JS, Piferi RL. Psychophysiological correlates of individual differences in patterns of hemodynamic reactivity. International Journal of Psychophysiology. 2001;40:93–197. doi: 10.1016/s0167-8760(00)00155-0. [DOI] [PubMed] [Google Scholar]


