Abstract
In cardiac muscle, β-adrenergic stimulation increases contractile force and accelerates relaxation. The relaxant effect is thought to be due primarily to stimulation of Ca2+ uptake into the sarcoplasmic reticulum (SR), although changes in myofilament properties may also contribute. The present study investigated the contribution of the myofilaments to the β-adrenergic response in isolated rat cardiac trabeculae undergoing either isometric or work-loop contractions (involving simultaneous force generation and shortening) at different stimulation frequencies (range 0.25-4.5 Hz). SR-dependent effects were eliminated by treatment with ryanodine (1 μM) and cyclopiazonic acid (30 μM). In isometric contractions during SR inhibition, isoprenaline increased the force but did not alter the time course of the twitch. In contrast, in work-loop contractions, the positive inotropic effect was accompanied by a reduced diastolic force between beats, most apparent at higher frequencies (e.g. diastolic stress fell from 58.6 ± 5.5 to 28.8 ± 5.8 mN mm−2 at 1.5 Hz). This relaxant effect contributed to a β-adrenoceptor-mediated increase in net work and power output at higher frequencies, by reducing the amount of work required to re-lengthen the muscle. Consequently, the frequency for maximum power output increased from 1.1 ± 0.1 to 1.6 ± 0.1 Hz. We conclude that the contribution of myofilament properties to the relaxant effect of β-stimulation may be of greater significance when force and length are changing simultaneously (as occurs in the heart) than during force development under isometric conditions.
Stimulation of myocardial β-adrenoceptors increases contractile force (positive inotropic effect) and accelerates relaxation. These effects are mediated by an increase in cAMP, which stimulates cAMP-dependent protein kinase A (PKA) to phosphorylate several intracellular proteins, including the sarcolemmal L-type Ca2+ channel, phospholamban and the Ca2+ release channel (ryanodine receptor, RyR) in the sarcoplasmic reticulum (SR) and troponin I and myosin-binding protein C on the myofilaments (reviewed by Bers, 2001). The positive inotropic effect has been attributed to the large rise in the intracellular Ca2+ transient, resulting mainly from the increased sarcolemmal Ca2+ influx following phosphorylation of the L-type Ca2+ channels (e.g. Tsien et al. 1986). The enhanced Ca2+ influx increases the Ca2+-induced release of Ca2+ from the SR, both by increasing the trigger Ca2+ and by enhancing SR Ca2+ loading, which increases the fractional release of Ca2+ and the amount of Ca2+ available for release. The phosphorylation of phospholamban removes its tonic inhibitory action on the SR Ca2+-ATPase, so promoting SR Ca2+ uptake. This may contribute to the increased Ca2+ transient during β-stimulation by increasing the SR Ca2+ load and hence increasing SR Ca2+ release. Phosphorylation of the RyR may also directly increase the release of Ca2+ (Valdivia et al. 1995; Marx et al. 2000), though the importance of this mechanism remains controversial (e.g. Eisner et al. 1998). The role of the SR in the positive inotropic effect may be substantial, since Shah et al. (1994) reported that the inotropic effect of β-stimulation was abolished by specific inhibition of the SR with ryanodine.
The phosphorylation of phospholamban also plays a major role in the relaxant effect of β-stimulation, by increasing the rate at which the Ca2+ transient declines. However, phosphorylation of the myofilament proteins may also contribute to the relaxant effect, either by increasing the rate of Ca2+ dissociation from troponin C (Robertson et al. 1982) or by increasing the rate of cross-bridge cycling (e.g. Hoh et al. 1988; Saeki et al. 1990; Strang et al. 1994; Fentzke et al. 1999; Herron et al. 2001; Kentish et al. 2001). In skinned cardiac muscles, in which the activity of the SR was eliminated completely, the intrinsic rate of myofibrillar relaxation after flash photolysis of the caged Ca2+ chelator, diazo-2, was found to be increased by PKA-induced phosphorylation in some studies (Zhang et al. 1995; Kentish et al. 2001), though not all (Johns et al. 1997). This acceleration of myofibrillar relaxation appears to be due to an increased rate of cross-bridge cycling resulting from phosphorylation of troponin I (Kentish et al. 2001). However, it has been difficult to determine the relative roles of the SR and myofilament proteins in the relaxant effect of β-adrenoceptor stimulation in intact cells. Some studies have suggested that myofilament mechanisms are unlikely to be involved, since the relaxant effects of β-stimulation may be uncoupled from the effects on myofilament Ca2+ responsiveness (McIvor et al. 1988; Okazaki et al. 1990) or from the phosphorylation of myofilament proteins (Talosi et al. 1993). In these studies the muscles or hearts were contracting isometrically. However, it is clear from the load dependence of cardiac relaxation (Brutsaert & Sys, 1989) that the properties of the myofibrils can determine the dynamics of relaxation when changes in cell length occur during contraction.
The relaxant effects of β-adrenergic stimulation have also been studied using intact (unskinned) cardiac preparations from phospholamban knockout (Pl-Ko) mice. In these preparations, the absence of phospholamban means that the decline of the Ca2+ transient is greatly accelerated, even in the basal state, which should enhance the ability to detect alterations in the rate of myofibrillar de-activation (see Li et al. 2000). However, results from Pl-Ko mice are contradictory. For example, β-stimulation increased the rate of relaxation of cardiac myocytes from Pl-Ko mice in the study by Wolska et al. (1996), but not in the experiments reported by Luo et al. (1994). Furthermore, Li et al. (2000) demonstrated that β-stimulation had no effect on relaxation of cardiac myocytes from Pl-Ko mice undergoing unloaded cell shortening but accelerated relaxation in papillary muscles contracting isometrically, which suggests that myofilament phosphorylation contributes to β-mediated acceleration of relaxation only when force is developed. On the other hand, convincing evidence for a role for myofilament phosphorylation (and more specifically troponin I phosphorylation) during β-stimulation has recently been provided by Pi et al. (2002) and Wolska et al. (2002). These studies used different approaches to generated transgenic mice in which cardiac troponin I could not be phosphorylated by PKA: Pi et al. by substituting non-phosphorylatable alanine at serine 23/24 (the PKA phosphorylation sites) and Wolska et al. by replacing cardiac troponin I with slow skeletal troponin I which lacks the PKA phosphorylation sites entirely. By crossing these non-phosphorylatable troponin I, transgenics with Pl-Ko mice, these groups were then able to examine the relaxant effects of isoprenaline in myocytes from mice in which either phospholamban phosphorylation or troponin I phosphorylation or both (or neither) were disrupted. Each study found that the effect of isoprenaline to accelerate relaxation was blunted when troponin I could not be phosphorylated and was abolished completely in the double knockout (Pl-Ko and non-phosphorylatable troponin I). In almost all the above studies, the contractions were either isometric or isotonic. However, cardiac muscles in the working heart undergo cyclical changes in length, such that force development and shortening occur simultaneously and net work is produced. The length changes are approximately sinusoidal (Semafuko & Bowie, 1975; Delhaas et al. 1993) and can be simulated in isolated muscles with the work-loop technique, in which the muscles undergo sinusoidal length changes and are stimulated at the same frequency. In a previous study (Layland & Kentish, 2000) we found that the net work production during work-loop contractions of rat trabeculae was increased by β-adrenoceptor stimulation and that this was partly due to a relaxant effect, which reduced the work required to re-lengthen the muscle after shortening. In the present study we investigated the relative contributions of SR and myofilament phosphorylation to this relaxant effect of β-adrenergic stimulation during work-loop contractions. We used trabeculae in which normal SR function was rendered ineffective by treatment with both ryanodine (to irreversibly open the SR Ca2+-release channel) and cyclopiazonic acid (to inhibit SR Ca2+ uptake). Under these conditions, any effects of β-stimulation on cardiac muscle relaxation are possibly attributable to myofilament phosphorylation. The effects of β-stimulation on work-loop contractions during SR inhibition were also compared with those observed during isometric contractions. Some of this work has previously been presented to the Physiological Society (Layland & Kentish, 2001).
METHODS
Muscle preparation
The muscle preparation and experimental apparatus were similar in most respects to those described previously (for details, see Layland & Kentish, 2000). In brief, Wistar or LBNF1 rats (either sex, ≈250 g) were stunned and then killed by cervical dislocation (Schedule 1 procedure in accordance with the UK Animal (Scientific Procedures) Act 1986). The hearts were removed and rinsed free of blood in modified Krebs-Henseleit solution (Krebs solution) which contained (mm): NaCl, 93; NaHCO3, 20; Na2HPO4, 1; MgSO4, 1; KCl, 5; CaCl2, 1; glucose, 10; sodium acetate, 20; with insulin, 5 U l−1; bubbled with 95 % O2-5 % CO2; pH 7.4 at 24 °C. EGTA (10 μM) was added to the Krebs solution to inhibit oxidation of catecholamines catalysed by heavy metal ions. Suitable trabeculae (strongly beating, free running, unbranched, diameter < 250 μm) were dissected from the right ventricle in Krebs solution containing 25 mm 2,3-butandenione-monoxime (BDM). Trabeculae attached horizontally between a force transducer element (SensoNor, Horten, Norway) and servomotor (300B, Cambridge Technology Inc., Watertown, MA, USA) in a muscle bath, were bathed in normal Krebs solution, and were stimulated at 0.33 Hz for > 90 min. This allowed time for any spontaneous oscillations, resulting from damage during the isolation procedure, to disappear and for force to stabilize. During this stabilization period, muscle length was increased to approximately 95 % of the length for maximum active force generation (Lmax). Muscle length and width were measured and cross-sectional area estimated, assuming that the muscle was cylindrical. Muscle wet weight was calculated from muscle volume, assuming that muscle density was 1.06 mg mm−3.
Inhibition of the SR Ca2+-release and Ca2+-uptake processes was achieved using simultaneous application of ryanodine (1 μM) and cyclopiazonic acid (CPA, 30 μM), respectively. Twenty minutes were allowed for these inhibitors to exert their effects and experiments were performed in the continued presence of the inhibitors. Stimulation of the β-adrenoceptors was achieved using isoprenaline (5 μM), a non-selective β-adrenoceptor agonist. Recordings were made 20–30 min after the start of isoprenaline application, when a new steady state had been achieved. All experiments were performed at 24 °C.
Work-loop protocol
A detailed description of the work-loop technique is given in a previous study (Layland & Kentish, 2000), in which we examined the effects of isoprenaline on work-loop contractions of rat cardiac trabeculae when the SR was functional. In the present study we used the same technique with the SR inhibited. In brief, muscles were subjected to sinusoidal length changes at various frequencies (0.5 to 1.75 Hz) and were stimulated to contract at a particular time (phase shift) in the sine wave. The range of frequencies investigated when the SR was inhibited (0.5 - 2.5 Hz) was lower than that used previously when the SR was functional (1-6.5 Hz), because the contraction was slowed by SR inhibition. The amplitude of the length change was ±5 % muscle length (10 % peak to peak), which is similar to the length changes of cardiac muscles in the working heart (e.g. Semafuko & Bowie, 1975; Delhaas et al. 1993). For a single cycle, a plot of developed force against length produced an anti-clockwise work loop, the area of which represented the net work done at that frequency (Josephson, 1985; Fig. 3). Timing of the stimulation was adjusted to maximise the area of the work loop. Cyclical contractions at each frequency were continued until a steady state was reached (≈2-3 min). The average force and length records of 10 or 20 consecutive work-loop cycles were then recorded using pCLAMP software (Axon Instruments). Any decline in net work with time was monitored by repeating the initial frequency (usually 1.5 Hz) after every third test frequency. A plot of work done at each repeated frequency indicated a linear deterioration with time and allowed us to derive correction factors for each test frequency (Layland et al. 1995; Layland & Kentish, 2000). Muscles were discarded if, during the course of an experiment, net work at the repeat frequency declined to less than 70 % of its initial value.
Figure 3. Effects of isoprenaline on work-loop contractions of two separate muscles.
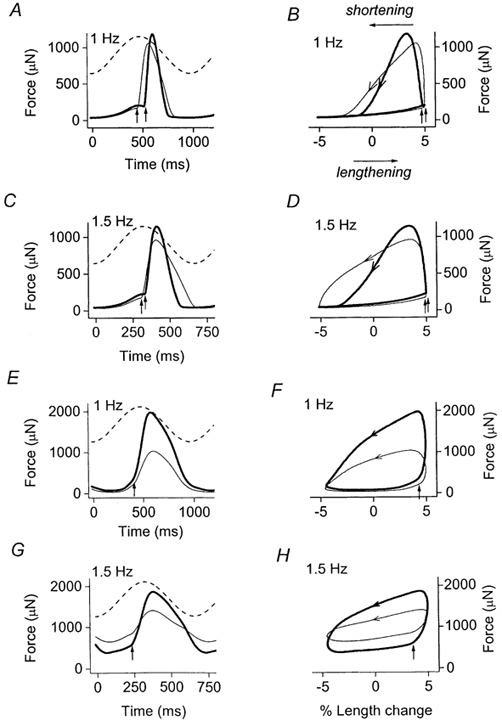
One muscle had the SR functional (A-D; data from Layland & Kentish, 2000) and the other had the SR blocked (E-H). Left panels, force records during control conditions (thin lines) and during β-adrenoceptor stimulation (thick lines) at the cycle frequencies indicated. Dashed lines, sinusoidal length trajectories (±5 % muscle length) at each frequency. Right panels illustrate the corresponding work loops at each frequency, derived from the force and length records illustrated on the left. Arrows drawn on loops, all work loops are anti-clockwise. Vertical arrows, timing of electrical stimulation in the work loop. Average of 10 traces. Note the varying time scales.
Loading muscles with fura-2
In order to investigate whether the SR was fully blocked by ryanodine and CPA, we used rapid caffeine application to assess the releasable pool of SR Ca2+ in muscles before and after application of the SR inhibitors. As previous described (Layland & Kentish, 1999), intracellular [Ca2+] was assessed using fura-2 fluorescence spectroscopy after iontophoresis of fura-2 (K+ salt) into one to three cells of each trabecula. The 340/380 fluorescence ratio was calculated on-line after subtraction of the initial autofluorescence at 340 and 380 nm (determined at 0.33 Hz with the muscle contracting isometrically). Muscles were initially stimulated isometrically at 0.33 Hz in order to record steady-state force and Ca2+ transients. Stimulation was then stopped and 40 mm caffeine, dissolved in Krebs solution, was applied to the muscle within 3–4 s to initiate the release of Ca2+ from the SR. Stimulation was then resumed at 0.33 Hz and normal Krebs solution applied to wash out the caffeine.
Chemicals and solutions
All chemicals used for Krebs solution were analytical grade and were obtained from BDH (Poole, Dorset, UK). BDM was obtained from Sigma (Poole, Dorset, UK), while the SR inhibitors CPA and ryanodine were from Calbiochem-Novabiochem Ltd (Beeston, Nottingham, UK). Isoprenaline was obtained in the form of a stabilised solution containing ascorbic acid and disodium EDTA (Saventrine I.V., Pharmax Ltd, Bexley, UK). Ryanodine was made up as a stock solution in de-ionised H2O whereas CPA was dissolved in dimethyl sulphoxide (DMSO). Stock solutions were stored at −20 °C. The final concentration of DMSO in the Krebs solution did not exceed 0.1 %. Fura-2 (K+ salt) was obtained from Molecular Probes Inc. (Eugene, OR, USA).
Data analysis
Results were analysed off-line using Clampfit (Axon Instruments) and Origin (Microcal) software. Active force was measured as the difference between peak contractile force and diastolic force (which was defined as the force immediately preceding stimulation). Twitch relaxation was assessed by measuring the time from peak-active force to 50 % relaxation (RT50). The time from the stimulus to 90 % relaxation (T90) was also measured as an index of twitch duration (where full relaxation was taken as the minimum diastolic force at that frequency). Averaged force traces (n= 10 or 20) recorded from work-loop contractions at different frequencies were plotted against their corresponding averaged length records to produce anti-clockwise work loops (Fig. 3). The area under the shortening portion of the loop (i.e. from +5 % to −5 % muscle length) represented the work done by the muscle during shortening (shortening work). The area under the lengthening portion of the loop (i.e. from −5 % to +5 % muscle length) represented the work done by the apparatus to stretch the muscle (lengthening work). Net work (μJ) was therefore the difference between shortening work and lengthening work, i.e. the area enclosed within the work loop, and was measured by integration. Net work values (μJ) at each frequency were divided by the calculated wet weight (mg) to give net work in μJ mg−1 (equivalent to J kg−1). Average power output (W kg−1) at each frequency was calculated by multiplying net work (J kg−1) by cycle frequency (Hz). Statistical analyses were performed using Student's paired or unpaired t test (Microcal Origin), or analysis of variance (ANOVA) with repeated measures (StatView version 5.0.1) as appropriate.
RESULTS
During isometric contractions at 0.33 Hz, inhibition of the SR with ryanodine and CPA reduced isometric twitch force significantly from 86.1 ± 7.4 to 23.9 ± 4.7 mN mm−2 (P < 0.01, n= 6) and prolonged the time from the stimulus to 90 % relaxation (T90) from 561 ± 36 to 829 ± 115 ms (P < 0.01, n= 6). These large changes reaffirm the pivotal role of the SR in the excitation-contraction process in rat cardiac muscle (Bassani et al. 1994). The smaller contraction remaining during SR inhibition can be attributed to sarcolemmal Ca2+ entry, largely via the L-type Ca2+ channels.
Figure 1 shows the effect of β-adrenergic stimulation with 5 μM isoprenaline on isometric twitch contractions (0.33 Hz) of two typical muscles, one with a functional SR (Fig. 1A) and one treated with ryanodine and CPA (Fig. 1B). When the SR was functional, isoprenaline induced the positive inotropic and relaxant effects characteristic of β-stimulation, as indicated by increased active isometric stress and smaller values for RT50 and T90 (Fig. 1A; Table 1). The large positive inotropic effect of isoprenaline was also observed in muscles treated with ryanodine and CPA (Fig. 1B; Table 1), though the initial force was of course lower. Indeed the percentage increase in force with isoprenaline tended to be greater when the SR was blocked (122.4 ± 46.6 %, n= 4) than when the SR was functional (71.3 ± 17.5 %, n= 6), although this difference was not statistically significant (unpaired t test, P > 0.05). In contrast to the inotropic effect of isoprenaline, the relaxant effect in isometric contractions was abolished if the SR was inhibited (Fig. 1B and Table 1).
Figure 1. Comparison of the effects of isoprenaline on isometric contractions of typical muscles with the SR functional (A) and the SR blocked (B).
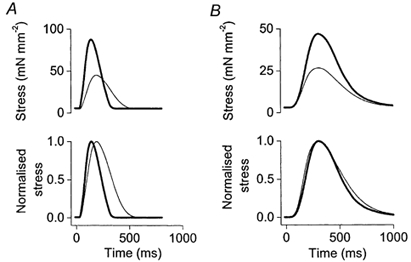
Bottom panels illustrate the same twitch data normalised to maximum active force to facilitate comparison of twitch duration. Thin lines indicate control conditions, thick lines indicate stimulation of the β-adrenoceptors with 5 μM isoprenaline.
Table 1.
Effects of isoprenaline on the amplitude and duration of the isometric twitch when the SR is functional and when the SR is blocked with ryanodine and CPA
| Control | Isoprenaline | |
|---|---|---|
| SR functional (n = 6) | ||
| Active stress (mN mm−2) | 37.1 ± 9.3 | 57.0 ± 10.6* |
| RT50 (ms) | 167 ± 33 | 109 ± 12* |
| T90 (ms) | 427 ± 53 | 294 ± 17* |
| SR blocked (n = 4) | ||
| Active stress (mN mm−2) | 19.6 ± 2.0 | 41.2 ± 4.3* |
| RT50 (ms) | 234 ± 28 | 206 ± 12 |
| T90 (ms) | 858 ± 165 | 859 ± 160 |
Stimulation rate 0.33 Hz.
P < 0.05 compared with the appropriate control value (paired t test).
The relationship between stimulation frequency and stress (force per unit cross sectional area) or T90 are shown in Fig. 2 for muscles with the SR active (Fig. 2A and C; data from Layland & Kentish, 2000) or muscles with the SR inhibited (Fig. 2B and D). The frequency range examined was necessarily much reduced when the SR was inhibited (compare Fig. 2A and B). As found previously (e.g. Ross et al. 1995; Layland & Kentish, 2000), when the SR is active β-stimulation shifts the isometric force-frequency relationship to higher frequencies. In Fig. 2A the frequency for maximum force production (determined after fitting the individual force-frequency data with 3rd or 4th order polynomial regression lines) increased from 2.1 ± 0.1 to 3.7 ± 0.3 Hz (n= 6, P < 0.01). Note that, as reported previously (Layland & Kentish, 1999, 2000), under our experimental conditions rat trabeculae usually show an increase in force as the stimulation frequency is increased (positive force-frequency relationship), at least over the lower frequency range. When the SR was inhibited (Fig. 2B), isoprenaline had a significant positive inotropic effect at all frequencies investigated but there was no longer an increase in the frequency for maximum force production (0.74 ± 0.04 Hz, n= 4, in control conditions; 0.64 ± 0.04 Hz, n= 4, during β-adrenergic stimulation). When the SR was functional, isoprenaline significantly reduced the twitch duration (as measured by T90) at all stimulation frequencies investigated (Fig. 2C). This effect was abolished by inhibiting the SR (Fig. 2D). With the SR blocked, T90 initially increased as the frequency rose from 0.25 to 0.75 Hz but then decreased at higher frequencies (Fig. 2D). The initial increase in T90 probably resulted from increased Ca2+ influx during the action potential and restricted Ca2+ removal from the cytosol, due to SR Ca2+-pump inhibition. The apparent shortening of twitch duration at higher frequencies was merely due to the fact that there was insufficient time for full relaxation.
Figure 2. Effects of isoprenaline on the isometric force-frequency (A and B) and duration-frequency (C and D) relationships in muscles with the SR functional (A and C) or inhibited (B and D).
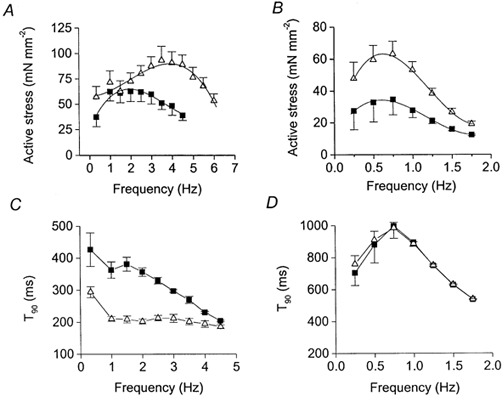
For both graphs, ▪, control data and ▵, data derived during stimulation with isoprenaline (5 μM). A and C are from Layland & Kentish (2000). Symbols indicate means ±s.e.m., n≥ 4. Force-frequency curves were fitted with 4th order polynomial regression lines.
Figure 3 shows examples of force and length records, with their corresponding work loops, at two different frequencies when the SR was functional (Fig. 3A-D) and when the SR was blocked (Fig. 3E and H). As a consequence of the slowing of contraction induced by SR inhibition, at the higher frequencies investigated (e.g. 1.5 Hz, Fig. 3H) work loops are elevated above the zero force level. This can be attributed to incomplete relaxation between beats, which is exacerbated when the SR is inhibited. This effect increases the work required to re-lengthen the muscle, which in turn tends to decrease net work production, since net work is the difference between the work done by the muscle during shortening and the work done on the muscle to re-lengthen it (lengthening work). As described previously (Layland & Kentish, 2000), during work-loop contractions with the SR functional, the effect of β-adrenergic stimulation to facilitate relaxation contributes to an increase in net work at higher frequencies by allowing more complete relaxation before re-lengthening occurs (Fig. 3B and D), thereby reducing the lengthening-work component. In the present study we were somewhat surprised to find that β-adrenergic stimulation also facilitates relaxation during work-loop contractions with the SR inhibited (Fig. 3F and H). However, unlike when the SR was functional, this relaxant effect was not manifest as a reduced twitch duration (e.g. compare Fig. 3A and E), but rather as a smaller diastolic force between beats (Fig. 3G and H), i.e. the extent of relaxation between beats was increased by β-stimulation even though twitch duration was little affected.
To quantify this observation, the effects of isoprenaline on the diastolic properties of the muscle were measured during both isometric and work-loop contractions. Figure 4A illustrates the diastolic stress of muscles contracting isometrically at different stimulation frequencies in control conditions and during β-stimulation. Diastolic force (measured as the force immediately before stimulation) increased dramatically with frequency above 0.75 Hz, due to incomplete relaxation between beats. Over this frequency range (0.25-1.5 Hz), isoprenaline had no significant effect on the diastolic force of isometrically contracting muscles (repeated measures ANOVA, P > 0.05). Severe impairment of relaxation meant that it was impossible to investigate frequencies above 1.5 Hz in isometric contractions. In contrast, during work-loop contractions isoprenaline significantly decreased the diastolic force at frequencies above 1 Hz (Fig. 4B; repeated measures ANOVA, P < 0.01). The effect of isoprenaline on the diastolic properties in work-loop contractions was also quantified by measuring the lengthening-work component at each frequency, calculated as the area enclosed between the lengthening portion of the work loop (from −5 to +5 % muscle length) and the zero force axis. Isoprenaline significantly reduced the lengthening-work component at frequencies above 1.25 Hz (Fig. 4C; repeated measures ANOVA, P < 0.01). This fall would contribute to an increased net work production at these frequencies (since net work = shortening work - lengthening work).
Figure 4. Relationships between diastolic stress and stimulation frequency.
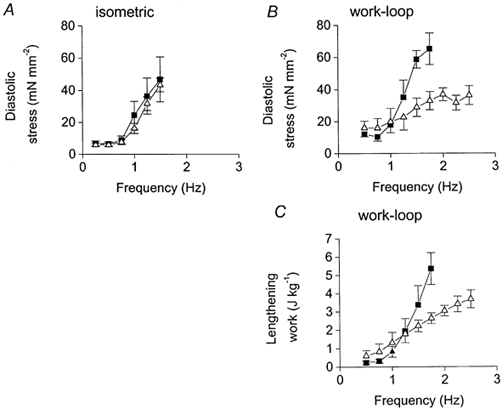
Relationships between diastolic stress (diastolic force per unit cross-sectional area) and stimulation frequency in isometric (A) and work-loop (B) contractions when the SR is inhibited. C, relationship between lengthening work (work required to stretch the muscle) and frequency during work-loop contractions with the SR inhibited. For all graphs, ▪, control data and ▵, data derived during stimulation with isoprenaline (5 μM). Symbols indicate means ±s.e.m., n= 4.
Figure 5 illustrates how the slowing of contraction induced by SR inhibition affects the work-frequency relationship (Fig. 5A) and power-frequency relationship (Fig. 5B) of the cardiac trabeculae under control conditions. The frequencies for maximum work and power output were determined for each muscle using 4th order polynomial regression lines. The net work production and power output were impaired above 1.25 Hz by SR inhibition, probably due to incomplete relaxation between beats. Indeed, the frequency for maximum power output (fopt) when the SR was functional (2.9 ± 0.3 Hz, n= 6) was reduced to 1.1 ± 0.1 Hz (n= 4) when the SR was inhibited (Fig. 5B). Furthermore, the maximum power generating capacity was reduced from 8.2 ± 1.1 W kg−1 (= 6) to 2.6 ± 0.4 W kg−1 (n= 4) by inhibition of the SR. This can be attributed both to the reduction in force at each frequency and also to the reduced range of frequencies over which the muscles can operate (since power = work × frequency).
Figure 5. Effects of SR inhibition on the work-frequency (A) and power- frequency (B) relationships.
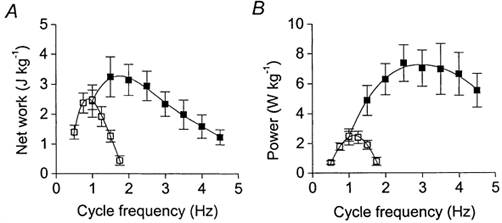
▪, averaged data from 6 muscles with the SR functional (data from Layland & Kentish, 2000). □, data from 4 muscles with the SR inhibited (this study). Net work and power output are expressed per unit wet weight. Symbols represent means ±s.e.m. Curves were fitted using 4th order polynomial regressions.
Figure 6 shows the effects of β-adrenoceptor stimulation on the averaged work-frequency (A) and power-frequency (B) relationships derived from four muscles in which the SR was inhibited. During SR inhibition, maximum net work in control conditions was 2.6 ± 0.4 J kg−1 and was achieved at a frequency of 0.9 ± 0.1 Hz (n= 4). Stimulation of the β-adrenoceptors increased maximum net work to 4.0 ± 0.4 J kg−1 (n= 4, P < 0.01, paired t test) and produced a small but not significant increase in the frequency for maximum work, to 1.0 ± 0.1 Hz. From the power- frequency relationships (Fig. 6B) it is clear that isoprenaline increased maximum power output, from 2.6 ± 0.4 W kg−1 in control conditions to 5.2 ± 1.0 W kg−1 (n= 4, P < 0.05, paired t test). In addition, β-adrenergic stimulation induced a rightward shift in the power- frequency curve, with fopt being raised by 45 % from 1.1 ± 0.1 to 1.6 ± 0.1 Hz (n= 4, P < 0.05, paired t test). It is probable that this shift is largely due to the enhanced degree of relaxation produced by β-adrenoceptor stimulation during SR inhibition (see Discussion).
Figure 6. Effects of β-stimulation on the work-frequency (A) and power-frequency (B) relationships of muscles with the SR inhibited (4 muscles).
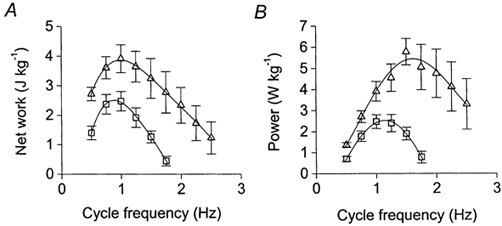
□, control data; ▵, data acquired during stimulation of the β-adrenoceptors with isoprenaline. Net work and power output are expressed per unit wet weight. Symbols, means ±s.e.m. Curves were fitted using 4th order polynomial regressions.
One potential source of error in these experiments is the possibility that treatment with CPA and ryanodine did not fully block the SR; in this case phosphorylation of phospholamban would still have been contributing to the effects of isoprenaline. In order to examine how effective the combination of CPA and ryanodine was at blocking the SR, we compared the caffeine-induced release of SR Ca2+ in fura-injected muscles before and after inhibition of the SR (Fig. 7). Before exposure to CPA and ryanodine, caffeine application released stored Ca2+ from the SR, as indicated by the increase in force and intracellular [Ca2+] (340/380 nm fluorescence ratio) in Fig. 7A. The increases in intracellular [Ca2+] elicited by caffeine were smaller than the electrically stimulated Ca2+ transients because in a multicellular preparation caffeine does not reach all the cells simultaneously (Smith et al. 1988). In the presence of CPA and ryanodine, caffeine application failed to release any detectable Ca2+ from the SR, regardless of whether isoprenaline was absent (Fig. 7B) or present (Fig. 7C). This indicates that the SR was unable to store Ca2+. Similar results were observed in three other muscles. In addition, the degree of SR inhibition produced by CPA and ryanodine was estimated in three muscles by comparing the electrically stimulated Ca2+ transients immediately before and after the injection of caffeine (i.e. before caffeine had washed out). Rate constants for the decline of the Ca2+ transient were derived by fitting a single exponential decay to the Ca2+ decline. For each of the three muscles, caffeine application was repeated at least three times for each condition (control, SR blocked, SR blocked + isoprenaline) and the corresponding rate constants averaged. When the SR was functional (Fig. 7A), the Ca2+ transients recorded during caffeine application were much prolonged compared with those recorded before, with the rate constant for Ca2+ decline falling from 3.43 ± 0.10 s−1 (n= 3 muscles) for the Ca2+ transient immediately before caffeine application to 1.14 ± 0.22 s−1 for the first electrically stimulated Ca2+ transient during caffeine (paired t test, P < 0.05). However, when the SR was blocked with CPA and ryanodine (Fig. 7B and C), the addition of caffeine did not produce any further slowing in the decline of the Ca2+ transient. This was true whether isoprenaline was absent (rate constants before and during caffeine were 1.96 ± 0.09 and 1.67 ± 0.19 s−1, respectively; n= 3; paired t test, P > 0.05) or present (rate constants before and during caffeine were 1.56 ± 0.24 and 1.39 ± 0.27 s−1, respectively; n= 3; paired t test, P > 0.05). These data also reveal that application of isoprenaline did not increase the rate constants of Ca2+ transient decline (either before or after caffeine) when the SR was inhibited (paired t tests, P > 0.05). Taken together, our data are consistent with the SR being completely blocked by the combination of ryanodine and CPA.
Figure 7. Examining the releasable pool of SR Ca2+.
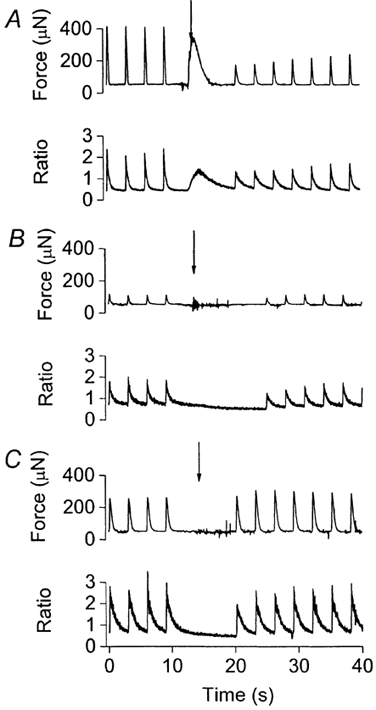
Examining the releasable pool of SR Ca2+ using caffeine application (40 mm) in a typical muscle with the SR functional (A), following inhibition of the SR with ryanodine and CPA (B), and during isoprenaline application with SR inhibition (C). For each figure, top panel, isometric force record and bottom panel, the corresponding fura-2 340/380 ratio (an index of intracellular [Ca2+]). Downward arrows, the onset of caffeine application. Muscles were stimulated electrically at 0.33 Hz except when caffeine was applied.
DISCUSSION
The main findings of the present study are: (1) in isometric contractions, the relaxant effect of β-adrenoceptor stimulation was abolished by inhibition of the SR, but the positive inotropic effect remained; and (2) in work-loop contractions during SR inhibition, the positive inotropic effect of β-adrenoceptor stimulation was accompanied by a relaxant effect, which was manifest as a greater extent of relaxation at higher frequencies and which resulted in an increase in the optimal frequency for power output. As we discuss below, it is likely that this relaxant effect results from PKA-induced phosphorylation of the myofibrils.
Do CPA and ryanodine block the SR completely?
Our conclusions depend critically upon the assumption that the Ca2+ sequestering activity of the SR was abolished by the combination of CPA and ryanodine. A number of previous studies have used ryanodine or CPA to block the SR, but used individually these may not have been completely effective (discussed below). However, our evidence suggests that the SR was fully inhibited by the combination of ryanodine and CPA, because under these conditions Ca2+ loading of the SR was prevented (Fig. 7), and the rate of decline of the electrically stimulated Ca2+ transient was not altered by the subsequent addition of caffeine (40 mm), which should eliminate any net uptake of Ca2+ by the SR. Furthermore, if there had been residual SR Ca2+ pumping activity in the presence of the SR inhibitors, phosphorylation of phospholamban during β-stimulation should have accelerated relaxation in the isometric twitches as well as in the work-loop contractions, but this was not observed (Fig. 1 and Table 1).
Effect of SR inhibition on the response to isoprenaline during isometric contractions
In cardiac muscle, the rate of relaxation is governed both by the rate of Ca2+ removal from the cytosol (either by SR uptake or sarcolemmal extrusion) and by the rate at which the myofilaments de-activate once Ca2+ is removed from troponin (see Bers, 2001). It is well established that with the SR functional, β-adrenergic stimulation increases force and accelerates relaxation (e.g. Fig. 1A). In muscles contracting isometrically, inhibition of the SR abolished the effect of isoprenaline to accelerate twitch duration over all frequencies investigated (Fig. 2D). While this indicates that stimulation of SR Ca2+ uptake (via phospholamban phosphorylation) contributes substantially to the acceleration of relaxation in isometric contractions during β-stimulation, it does not preclude an additional role for the myofilaments, since inhibition of the SR per se greatly prolongs relaxation (T90 is approximately doubled; see Table 1). As a consequence, the processes which limit the relaxation rate in the SR-inhibited muscles could be different from those operating when the SR is functional. Hence, the complete inhibition of the relaxant effect of isoprenaline by inhibition of the SR in the present study could merely reflect an inability to detect myofilament-based effects when twitch duration is much prolonged.
In contrast to the relaxant effect of isoprenaline, the positive inotropic effect was preserved when the SR was inhibited (Fig. 1B; Table 1). Most of this inotropic action is probably due to the increased sarcolemmal Ca2+ influx resulting from phosphorylation of the L-type Ca2+ channels by PKA (e.g. Tsien et al. 1986), which is sufficient to overcome the known decrease in myofibrillar Ca2+ sensitivity produced by PKA-induced phosphorylation (e.g. Robertson et al. 1982). In contrast to our results, Shah et al. (1994) reported that inhibition of the SR with ryanodine alone abolished the inotropic (and relaxant) effect of isoprenaline in isometrically contracting ferret papillary muscles. They concluded that the inotropic effect was largely due to an action on the SR. The use of ryanodine (1 μM) in their experiments would have eliminated the electrically stimulated release of Ca2+ from the SR by opening the Ca2+ release channels, but SR Ca2+ uptake would still have been operative and may have been buffering sarcolemmal Ca2+ entry (Janczewski & Lakatta, 1993; Lewartowski et al. 1994). Furthermore, during β-adrenoceptor stimulation this buffering could have been enhanced by the increased rate of SR Ca2+ re-uptake, so that a smaller fraction of the Ca2+ entering the cell would actually activate the contractile proteins. This interpretation is supported by data obtained previously in pilot experiments in our laboratory using rat papillary muscles (Fig. 8A). In accordance with Shah et al. (1994), these pilot experiments showed that isoprenaline had little positive inotropic effect when the SR was inhibited with ryanodine alone (triangles, Fig. 8A). If CPA was added to ryanodine in the absence of isoprenaline, there was a small increase in force (squares, Fig. 8A), resulting presumably from the elimination of the SR Ca2+-buffering activity (Lewartowski et al. 1994). If however the CPA was applied in the presence of both ryanodine and isoprenaline, there was a much greater positive inotropic effect, which suggests that the SR Ca2+ buffering had been substantial during β-adrenoceptor stimulation in the presence of ryanodine. Further evidence that isoprenaline can increase SR Ca2+ pump activity in the presence of ryanodine alone is given by the finding that isoprenaline significantly accelerated twitch relaxation in papillary muscles treated with ryanodine (Fig. 8B), though there was no change if isoprenaline was added in the presence of ryanodine and CPA (Fig. 1B; Table 1). Thus inhibition of both SR Ca2+ release and uptake may be required to eliminate SR function effectively and prevent the SR uptake from buffering Ca2+ entering the cell during the action potential. If ryanodine is the only SR ‘blocker’ present, any positive inotropic tendency of the increased Ca2+ entry during β-stimulation may be completely negated by the enhancement of SR Ca2+ buffering resulting from phospholamban phosphorylation (Fig. 8A). In the present study, maintaining the SR Ca2+-release channel in an open state (with ryanodine) while simultaneously inhibiting SR Ca2+ uptake (with CPA) allowed the increased sarcolemmal Ca2+ influx to be translated into an increased contractile force. The large inotropic effect (Fig. 1B; Table 1) suggests that phosphorylation of the SR proteins (either the Ca2+-uptake pump or the RyR - see Introduction) did not play a major role in the positive inotropic effect of β-stimulation in our experiments.
Figure 8. Effects of ryanodine and CPA in rat papillary muscles contracting isometrically.
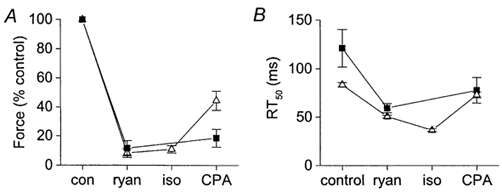
Effects of ryanodine and CPA on the inotropic (A) and relaxant (B) effects of isoprenaline in rat papillary muscles contracting isometrically. Muscles were bathed in Krebs buffer solution (composition as for trabeculae experiments) at 30 °C and stimulated at 0.33 Hz for at least 1 h to equilibrate. Ryanodine (ryan, 1 μM), isoprenaline (iso, 5 μM, if applied) and CPA (30 μM) were applied sequentially for 20 min each and the contraction recorded at the end of the 20 min period. ▪, control data (n= 3) and ▵, muscles stimulated with isoprenaline (n= 3). Symbols, means ±s.e.m.
Effects of SR inhibition on the isometric force-frequency relationship
Under our experimental conditions, the force-frequency relationship in rat ventricular trabeculae is bell-shaped, i.e. it exhibits both positive and negative components (Layland & Kentish, 1999, 2000). As in other species, the increase in force with increasing stimulation frequency can be attributed to the increased sarcolemmal Ca2+ influx per unit time (via ICa and perhaps reverse mode Na+-Ca2+ exchange) leading to increased SR Ca2+ loading and release. At higher frequencies this effect may become limited by the rate at which the SR can load with Ca2+, as the interval between beats becomes smaller. Beyond this point, mechanisms that tend to reduce SR Ca2+ release (e.g. reduced time for the SR Ca2+-release channel to recover from its adapted/inactivated state) would predominate, causing a secondary decline in force. β-Adrenergic stimulation shifts the force-frequency relationship, in that the positive inotropic effect is increased at higher stimulation frequencies and the frequency for maximum force production is raised (Ross et al. 1995; Layland & Kentish, 2000; see Fig. 2A). This may be partly attributed to an increased rate of SR Ca2+ uptake resulting from phospholamban phosphorylation (Layland & Kentish, 2000). This would increase the frequency at which the Ca2+ loading capacity of the SR becomes limited by the rate of SR Ca2+ uptake between beats, and would cause an increase in the frequency for maximum force production. The present experiments support this hypothesis since isoprenaline did not increase the frequency for maximum force production when the effects of phospholamban phosphorylation were eliminated by inhibition of the SR Ca2+-ATPase (Fig. 2B).
Effects of SR inhibition on the response to isoprenaline during work-loop contractions
In rat cardiac trabeculae with a functional SR, we demonstrated previously that during work-loop contractions, isoprenaline increased maximum power output and shifted the frequency for maximum power output (fopt) to a higher frequency (from 2.9 to 4.4 Hz at 24 °C, Fig. 6). Two major mechanisms will contribute to these phenomena. (1) The positive inotropic effect of β-stimulation is greater at higher stimulation frequencies (due to the shift of the force-frequency relationship described above). Since force is potentiated, net work (dependent on force and shortening) and power output (calculated as net work × cycle frequency) will also be greater at higher frequencies. This will increase both power output and fopt. (2) The effect of β-stimulation to facilitate relaxation between beats allows net work to be produced more effectively at higher frequencies, because it reduces the amount of work required to re-lengthen the muscle (lengthening work component). This in turn contributes to the increase in power output and fopt.
In the present study it was observed that, even when the SR was inhibited, β-adrenergic stimulation increased power output to a greater extent at the higher frequencies examined, such that the frequency for maximum power output (fopt) was increased (Fig. 6B). The positive inotropic effect of isoprenaline, which persists when the SR is blocked, contributes to the increase in power output per se. However, when the SR is blocked, mechanism 1 (above) cannot contribute to the increase in fopt, since the shift of the force-frequency relationship requires a functional SR (see Fig. 2B). However, the increase in fopt may be explained by a relaxant effect of β-stimulation (mechanism 2) that was found to be operative during work-loop, but not isometric, contractions. This relaxant effect was manifest as a reduction in the diastolic force between beats, rather than as an increased rate of relaxation, and was most pronounced at the higher frequencies examined (above 1.0 Hz, Fig. 3G, and Fig. 4B and C). Hence, during work-loop contractions with the SR inhibited, β-adrenoceptor stimulation leads to a more complete relaxation between beats and thus reduces the work required to re-stretch during the diastolic period. This, in turn, raises the frequency range over which the muscles can effectively produce work and power (mechanism 2).
The intracellular mechanisms responsible for this relaxant effect remain elusive. Phosphorylation of phospholamban should have played no part, because the SR was inhibited completely. Phosphorylation of the L-type Ca2+ channel also seems unlikely to contribute to this relaxant effect because isoprenaline increases the amplitude of the ICa (and hence contributes to the positive inotropic effect) without altering the rate of ICa inactivation (Sako et al. 1997). It therefore seems most probable that the relaxation effect arises from a change in myofilament properties. One possible explanation is that this results from isoprenaline increasing the rate of cross-bridge cycling (e.g. Hoh et al. 1988; Saeki et al. 1990; Strang et al. 1994; Herron et al. 2001), resulting from phosphorylation of troponin I (Fentzke et al. 1999; Kentish et al. 2001). This could potentially enhance the extent of relaxation between beats (reduced diastolic force), for example by increasing the rate at which the cross-bridges detach once intracellular Ca2+ is lowered. However, if this is a contributing factor, our findings suggest that an increased rate of cross-bridge detachment is of greater significance to relaxation when the muscles are undergoing shortening while developing force, rather than just contracting isometrically. Interestingly, it has recently been demonstrated that PKA-induced myofilament phosphorylation increases the power generating capacity of skinned cardiac myocytes undergoing loaded shortening (Herron et al. 2001). This effect was partly attributed to a PKA-mediated acceleration of steps in the cross-bridge cycle that limit the rates of loaded shortening (Herron et al. 2001). Another possible explanation for our results is that the effects of β-stimulation on the length dependence of myofilament Ca2+ sensitivity could contribute to the relaxant effect produced during shortening. Komukai & Kurihara (1997) found that the decrease in myofilament Ca2+ sensitivity induced by a reduction in muscle length is more apparent in the presence of β-adrenergic stimulation. If this mechanism was operative in our work-loop contractions, the 10 % decrease in muscle length during the cycle would be expected to produce a greater reduction in myofilament Ca2+ sensitivity, and therefore in force, in the presence of isoprenaline than in control conditions. It may also be possible that the effects of isoprenaline on relaxation may have more significance during work-loop than during isometric contractions because isoprenaline may increase the de-activating effect of shortening. A sudden decrease in muscle length during relaxation is known to increase cytosolic [Ca2+], probably due to Ca2+ release from the thin filaments as a result of a fall in the Ca2+ affinity of troponin C (e.g. Allen & Kurihara, 1982; Jiang et al. 1998). By increasing the rate of Ca2+ dissociation from troponin C and/or the rate of cross-bridge detachment, myofilament phosphorylation may increase the effects of shortening deactivation. Finally, it has recently been found that phosphorylation of the elastic subdomain (N2B) of titin by PKA can reduce passive force in rat cardiac myocytes (Yamasaki et al. 2002). It is possible therefore that titin phosphorylation may contribute to the reduced diastolic force between work-loop contractions during β-stimulation (Fig. 4B), although this is not consistent with a lack of change in passive force with isoprenaline in isometric contractions (Fig. 4A).
Since SR inhibition per se greatly slows cytosolic Ca2+ decline and relaxation, it is difficult to predict to what extent this myofilament-based effect contributes to relaxation in our work-loop experiments when the SR is functional. Further analysis (results not shown) of work-loop experiments conducted previously using muscles with a functional SR (Layland & Kentish, 2000) shows that isoprenaline significantly reduces the lengthening work component at higher frequencies compared with control values. This is similar to the effect of isoprenaline on the lengthening work component in the present study when the SR is inhibited (Fig. 4C), and suggests that myofilament phosphorylation enhances relaxation during work-loop contractions, similar to the effects recently demonstrated in isotonic contractions of single cells (Pi et al. 2002; Wolska et al. 2002).
Conclusion
Our work-loop experiments have unmasked an additional relaxant effect of β-adrenergic stimulation, absent in isometric experiments, that is possibly due to phosphorylation of myofilament proteins (probably troponin I). This myofilament-based effect may also play a part in the enhanced relaxation and power output in cardiac muscles with a functional SR. The relaxant effect becomes prominent during work-loop contractions at higher frequencies, which are the conditions that reflect those in the intact rat heart in vivo. Therefore, it is feasible that the myofilament-based effects revealed in our experiments could contribute to relaxation between beats during β-adrenergic stimulation and so aid the performance of cardiac muscle (in terms of enhanced power output) during sympathetic stimulation.
Acknowledgments
This work was supported by the British Heart Foundation and the Central Research Fund of London University. We would like to thank Sarah Davenport for obtaining the papillary muscle data and Dr Michael Shattock for helpful comments on the manuscript.
REFERENCES
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. Journal of Physiology. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. Journal of Physiology. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiological Reviews. 1989;69:1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- Delhaas T, Arts T, Prinzen FW, Reneman RS. Subepicardial fibre strain and stress as related to left ventricular pressure and volume. American Journal of Physiology. 1993;264:H1548–1559. doi: 10.1152/ajpheart.1993.264.5.H1548. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW, DiaZ ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovascular Research. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. Journal of Physiology. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron TJ, Korte FS, McDonald KS. Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes. Circulation Research. 2001;89:1184–1190. doi: 10.1161/hh2401.101908. [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Rossmanith GH, Kwan LJ, Hamilton AM. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circulation Research. 1988;62:452–461. doi: 10.1161/01.res.62.3.452. [DOI] [PubMed] [Google Scholar]
- Janczewski AM, Lakatta EG. Buffering of calcium influx by sarcoplasmic reticulum during the action potential in guinea-pig ventricular myocytes. Journal of Physiology. 1993;471:343–363. doi: 10.1113/jphysiol.1993.sp019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YD, Patterson MF, Morgan DL, Julian FJ. Basis for late rise in fura-2 R signal reporting [Ca2+]i during relaxation in intact rat ventricular trabeculae. American Journal of Physiology. 1998;274:C1273–1282. doi: 10.1152/ajpcell.1998.274.5.C1273. [DOI] [PubMed] [Google Scholar]
- Johns EC, Simnett SJ, Mulligan IP, Ashley CC. Troponin I phosphorylation does not increase the rate of relaxation following laser flash photolysis of diazo-2 in guinea-pig skinned trabeculae. Pflügers Archiv. 1997;433:842–844. doi: 10.1007/s004240050353. [DOI] [PubMed] [Google Scholar]
- Josephson RK. Mechanical power output from striated muscle during cyclic contraction. Journal of Experimental Biology. 1985;114:493–512. [Google Scholar]
- Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin-I by protein kinase A increases relaxation rate and cross-bridge cycling kinetics in mouse ventricular muscle. Circulation Research. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- Komukai K, Kurihara S. Length-dependence of Ca2+-tension relationship in aequorin-injected ferret papillary muscles. American Journal of Physiology. 1997;273:H1068–1074. doi: 10.1152/ajpheart.1997.273.3.H1068. [DOI] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. American Journal of Physiology. 1999;276:H9–18. doi: 10.1152/ajpheart.1999.276.1.H9. [DOI] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Effects of α1- or β-adrenoceptor stimulation on work-loop and isometric contractions of isolated rat cardiac trabeculae. Journal of Physiology. 2000;524:205–219. doi: 10.1111/j.1469-7793.2000.t01-1-00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Kentish JC. SR-independent acceleration of relaxation by isoprenaline during work-loop contractions in isolated rat cardiac trabeculae. Journal of Physiology. 2001;531 doi: 10.1113/jphysiol.2002.022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Young IS, Altringham JD. The effect of cycle frequency on the power output of rat papillary muscles in vitro. Journal of Experimental Biology. 1995;198:1035–1043. doi: 10.1242/jeb.198.4.1035. [DOI] [PubMed] [Google Scholar]
- Lewartowski B, Rozycka M, Janiak R. Effects of thapsigargin in normal and pretreated with ryanodine guinea pig cardiomyocytes. American Journal of Physiology. 1994;266:H1829–1839. doi: 10.1152/ajpheart.1994.266.5.H1829. [DOI] [PubMed] [Google Scholar]
- Li L, Desantiago J, Chu GX, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation. American Journal of Physiology - Heart and Circulatory Physiology. 2000;278:H769–779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of β-agonist stimulation. Circulation Research. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- McIvor ME, Orchard CH, Lakatta EG. Dissociation of changes in apparent myofibrillar Ca2+ sensitivity and twitch relaxation induced by adrenergic and cholinergic stimulation in isolated ferret cardiac muscle. Journal of General Physiology. 1988;92:509–529. doi: 10.1085/jgp.92.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N. PKA phosphorylation dissociates FKBP12. 6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Okazaki O, Suda N, Hongo K, Konishi M, Kurihara S. Modulation of Ca2+ transients and contractile properties by β-adrenoceptor stimulation in ferret ventricular muscles. Journal of Physiology. 1990;423:221–240. doi: 10.1113/jphysiol.1990.sp018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi YQ, KemnitZ KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics. Evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circulation Research. 2002;90:649–656. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin-I phosphorylation on the Ca2+-binding properties of the Ca-regulatory site of bovine cardiac troponin. Journal of Biological Chemistry. 1982;257:260–265. [PubMed] [Google Scholar]
- Ross J, Miura M, Kambayashi M, Eising GP, Ryu K-H. Adrenergic control of the force-frequency relation. Circulation. 1995;92:2327–2332. doi: 10.1161/01.cir.92.8.2327. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Shiozawa K, Yanagisawa K, Shibata T. Adrenaline increases the rate of cross-bridge cycling in rat cardiac muscle. Journal of Molecular and Cellular Cardiology. 1990;22:453–460. doi: 10.1016/0022-2828(90)91480-u. [DOI] [PubMed] [Google Scholar]
- Sako H, Green SA, Kranias EG, Yatani A. Modulation of cardiac Ca2+ channels by isoproterenol studied in transgenic mice with altered SR Ca2+ content. American Journal of Physiology. 1997;273:C1666–1672. doi: 10.1152/ajpcell.1997.273.5.C1666. [DOI] [PubMed] [Google Scholar]
- Semafuko WEB, Bowie WC. Papillary muscle dynamics: in situ function and responses of the papillary muscle. American Journal of Physiology. 1975;228:1800–1807. doi: 10.1152/ajplegacy.1975.228.6.1800. [DOI] [PubMed] [Google Scholar]
- Shah N, Than N, White E, Bennett KL, Orchard CH. The role of the sarcoplasmic reticulum in the response of isolated ferret cardiac muscle to β-adrenergic stimulation. Experimental Physiology. 1994;79:929–941. doi: 10.1113/expphysiol.1994.sp003818. [DOI] [PubMed] [Google Scholar]
- Smith GL, Valdeolmillos M, Eisner DA, Allen DG. Effects of rapid application of caffeine on intracellular calcium concentration in ferret papillary muscles. Journal of General Physiology. 1988;92:351–368. doi: 10.1085/jgp.92.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang KT, Sweitzer NK, Greaser ML, Moss RL. β-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circulation Research. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Talosi L, Edes I, Kranias EG. Intracellular mechanisms mediating reversal of β-adrenergic stimulation in intact beating hearts. American Journal of Physiology. 1993;264:H791–797. doi: 10.1152/ajpheart.1993.264.3.H791. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydropyridine calcium agonists. Journal of Molecular and Cellular Cardiology. 1986;18:691–670. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska BM, Arteaga GM, Peña JR, Nowak G, Phillips RM, Sahai S, De Tombe PP, Martin AF, Kranias EG, Solaro RJ. Expression of slow skeletal troponin I in hearts of phospholamban knockout mice alters the relaxant effect of β-adrenergic stimulation. Circulation Research. 2002;90:882–888. doi: 10.1161/01.res.0000016962.36404.04. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+ American Journal of Physiology. 1996;271:C391–397. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates cardiac-specific N2B domain of titin and reduces passive tension in rat cardiac myocytes. Circulation Research. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin-I phosphorylation increases the rate of cardiac muscle relaxation. Circulation Research. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]


