Abstract
In order to study the effects of the manipulation of various factors related to muscular activity on the concentration of kinins in muscular tissue, a microdialysis probe was implanted in the adductor muscle of the hindlimb in anaesthetized rats. After collection of baseline samples, the perfusion fluid was changed to a Ringer solution containing sodium lactate (10 or 20 mm), adenosine (50 or 100 μM) or a lower pH (7.0 or 6.6). Whereas perfusion with lactate did not have any significant effect on the concentration of kinins in the dialysate, the perfusion with a lower pH or with adenosine dose-dependently increased the kinin content in the samples. In a second microdialysis experiment, by using specific radioimmunoassays (RIA) for bradykinin and kallidin, we observed that about 70 % of the total kinins dialysed from rat muscle are a kallidin-like peptide. Also, the simultaneous perfusion with 100 μM caffeine totally abolished the increase in kinin levels induced by the perfusion at pH 6.6. In a third experiment, soleus muscles from rat were stimulated in vitro during 30 min in the presence or absence of 77 μM caffeine. Electrically stimulated contraction, but not the addition of 10 mU ml−1 insulin, induced an increase in the concentration of the kallidin-like peptide in the buffer. This effect was totally prevented by the addition of the adenosine antagonist caffeine. These results show that a kallidin-like peptide is released from rat muscle, and that its production is enhanced by muscle activity. Furthermore, the increase in kinin peptides during muscle contraction may be mediated by an increase in adenosine levels.
The kallikrein-kinin system generates the nonapeptide bradykinin and the decapeptide kallidin. Kallidin presents the same sequence as bradykinin, but with an additional lysine residue at the amino-terminal position. Bradykinin is liberated from plasma circulating high molecular weight kininogen (HMWK) by the action of the bound enzyme plasma kallikrein (EC 3.4.21.34). Kallidin is liberated preferentially from low molecular weight kininogen (LMWK) by tissue kallikrein (EC 3.4.21.35). Both kininogen varieties exhibit the sequences for bradykinin and kallidin, which are released by the differential cleavage of the sequence by the kallikrein enzymes. However, in the rat LMW and HMW kininogen, an Arg residue is located instead of the Lys residue at the N-terminal side of the kinin sequence. Hence, bradykinin has been considered to be the only kinin released from both kininogen forms in the rat (Kato et al. 1985; Hagiwara et al. 1995).
Bradykinin is liberated during inflammatory processes and its administration induces pain both in man and in animals. Bradykinin is one of the most potent algogenic substances known (Dray & Perkins, 1993). Its intramuscular administration can induce pain (Jensen et al. 1990; Babenko et al. 1999), and change the receptive fields to noxious stimuli (Hoheisel et al. 1993). Through activation of chemosensitive receptors in muscle, it can also excite primary and secondary muscle spindle afferents, affecting muscle stiffness and fusimotor reflexes (Djupsjöbacka et al. 1995).
Kinins can be released by muscle contractions in the cat (Stebbins et al. 1990), humans (Wicklmayr et al. 1988) and rats (Taguchi et al. 2000), most likely by an increase in tissue kallikrein activity (Koh et al. 1988). Bradykinin has important actions for muscle physiology and metabolism. Bradykinin, through B2 receptors, partially mediates the changes in the cardiovascular system (higher blood pressure and increased heart rate) caused by muscle contraction (Pan et al. 1993). It also increases muscle blood flow (Dietze et al. 1996). Furthermore bradykinin enhances the muscular glucose uptake stimulated by insulin or by muscular contraction (Dietze et al. 1996), even though this effect can be an indirect consequence of the increase in muscular blood flow induced by this kinin (Mayfield et al. 1996). Therefore, the contracting muscle liberates bradykinin apparently in order to improve energy delivery to the working muscle by increasing local blood flow (to enhance oxygen and glucose delivery), and facilitating glucose uptake by muscular tissue. Accordingly, inhibition of bradykinin synthesis compromises energy delivery to the muscle under a standard work load, which is restored when additional bradykinin is supplied (Dietze & Wicklmayr, 1977).
Muscular activity is accompanied by an increase in the concentration of lactate and adenosine (Bockman & McKenzie, 1983; Achike & Ballard, 1993; Rosdahl et al. 1993; Hellsten & Frandsen, 1997) and a decrease in the pH (Achike & Ballard, 1993; Mannion et al. 1995) in the muscle interstitial environment. Adenosine and lactate have been considered as waste by-products of muscle metabolism, but they are now thought to be important regulatory factors. Thus, adenosine has been implicated in the regulation of glucose uptake (Han et al. 1998; Derave & Hespel, 1999) and vasodilatation (Rådegran & Hellsten, 2000; Rådegran & Calbet, 2001) during muscle contraction. Lactate could be an important element in the adjustment of the muscular energy balance during muscle activity (Gladden, 2001; Shulman & Rothman, 2001).
The aim of the present study was to determine the role played by these physiological factors in the liberation of kinins in muscle. For this purpose, kinin liberation under alteration of the biochemical environment was assessed using in vivo microdialysis. In order to investigate the participation of adenosine further, the effect of caffeine on kinin release under muscle contraction was examined using electrically stimulated contraction of rat muscle in vitro.
METHODS
Male Wistar rats (B&K Universal AB, Sweden) were used throughout the study. The animals were housed under controlled laboratory conditions, with a standard 12 h day/night cycle (lights on 0700-1900). All experiments were conducted in accordance with the Norwegian laws and regulations on animal experimentation. After completion of the experiment, animals were killed by an overdose of anaesthetic.
Experiment 1. In vivo microdialysis and manipulation of muscle environment
Rats weighing 300–350 g were anaesthetized by an i.p. injection of 1 ml of a solution of 3 mg ml−1 acepromazin (Plegicil, Pherrovet, Sweden) and 50 mg ml−1 ketamine (Ketalar, Parke Davis, Sweden). Anaesthesia was maintained by subsequent i.p. administration when required (≈0.4 ml h−1). The rats were placed on their backs on a Plexiglas surface warmed by a blanket connected to a Harvard Homeothermic Blanket Control Unit (Harvard Instruments, MA, USA), keeping the body temperature of the rat at 36–37 °C. After exposing the medial side of the adductor muscle in the left or right hindlimb, a microdialysis probe (CMA/20, CMA/Microdialysis, Sweden) with a 10 mm polyethersylfone (PES) membrane was inserted longitudinally into the fascia using a Venflon 20G i.v. catheter. The inlet tubing was connected to a microinfusion pump (CMA/100 or CMA/102, CMA/Microdialysis, Sweden) and perfused with a modified Ringer solution containing (mm): 116 NaCl, 2.5 CaCl2, 4.6 KCl, 1.16 KH2PO4, 1.16 MgSO4, 11 Hepes (N-2-hydroxyethylpiperazine-N‘-2-ethanesulfonic acid)) at 4 μl min−1. After mixing the components, the pH of the perfusion buffers was adjusted with 1 m NaOH to 7.4, or to a lower pH when required. Treatments were administered through the microdialysis perfusion medium, by adding the pertinent substance (adenosine or lactate) at the desired concentration to the modified Ringer solution (pH 7.4), or adjusting its pH to the desired value. In the control group, the modified Ringer buffer, adjusted to pH 7.4, was used throughout the experiment. Samples were collected for 20 min (80 μl (sample)−1) in a plastic vial and immediately frozen for later analysis of kinin content. Kinin peptides were analysed by radioimmunoassay (RIA) using a commercial kit for bradykinin (JRR 1437, Advanced ChemTech Inc., KY, USA, no longer available).
Sample collection began ≈1.5 h after insertion of the microdialysis probe. Each animal received two doses of one treatment: pH, lactate or adenosine. First, three consecutive baseline samples were collected. Thereafter, the perfusion medium was switched to the one containing the first dose of one of the three treatments (pH 7.0, 10 mm lactate, or 50 μM adenosine). After three 20 min samples, the perfusion medium was changed to the one with the second dose of the same treatment (pH 6.6, 20 mm lactate, or 100 μM adenosine) and three more 20 min samples were collected. In addition, in some animals, samples were taken immediately after probe implantation to follow the time course of kinin concentration changes. In order to study the possible contribution of muscle blood flow on the results obtained with adenosine, a fifth group was added. This group had the same experimental design as the adenosine-treated animals, but 125 mg l−1 dihydralazine (Nepresol, Ciba, Basel, Switzerland) was added to all the perfusion solutions used, beginning straight after probe insertion and continuing throughout the whole experiment. In these animals, as well as in some animals of the adenosine group, tissue blood flow was measured by the laser-Doppler technique. In order to measure changes in blood flow in the tissue surrounding the dialysis site, a 120 mm optical cable-straight tip for implant (MTB500-0 L120), coupled to a 407 probe, was attached to the microdialysis probe and implanted together. A second 407 probe was inserted directly into the adductor muscle 0.5-1 cm from the dialysis site. Both probes were connected to a Periflux 4001 (all blood flow-measuring equipment was from Perimed, Stockholm, Sweden).
The blood flow data are the mean of two readings (in arbitrary units) carried out immediately before and after collection of the microdialysis samples. Kinin peptides were calculated as picograms (pg) of bradykinin per sample and recalculated as the percentage of the mean of the three baseline samples. Results were analysed after the recalculations applying a multivariate analysis of variance (MANOVA) for repeated measures. Three factors were used: treatment (vs. control or vs. dihydralazine), dose (two levels for each treatment), and sample (samples collected for each dose level.)
Experiment 2. In vivo microdialysis and effect of caffeine on pH-induced kinin release
Rats (340-390 g) were anaesthetized by an i.p. injection of 1 ml of a solution of 3 mg ml−1 acepromazin (Plegicil, Pherrovet, Sweden) and 50 mg ml−1 ketamine (Ketalar, Parke Davis, Sweden). Anaesthesia was maintained by constant i.p. administration of anaesthetics at 6.5 μl min−1. The microdialysis procedure was similar to that described above, with the exception that the microdialysis probes were implanted bilaterally in the hindlimbs. The perfusion medium was a Ringer-acetate solution (Braun, Melsungen, Germany; composition (mm): 1 MgCl2, 2 CaCl2, 4 KCl, 100 NaCl, 30 sodium acetate) containing 0.5 % bovine serum albumin (BSA) and adjusted to pH 7.4 or 6.6 with 1 m NaOH. The perfusion flow was 4 μl min−1 and samples were collected every 25 min (100 μl sample−1). Ninety minutes after probe implantation three baseline samples were collected. Afterwards, the perfusion medium was switched to one adjusted to pH 6.6, containing 100 μM caffeine in six of the eleven animals used. Subsequently three samples were collected. The samples were analysed for kinin content with RIAs using specific antibodies for bradykinin and kallidin (Hilgenfeldt et al. 1995). For each animal, bradykinin was measured in samples from one hindlimb and kallidin from the other.
Kinin peptide content was measured in picograms, recalculated and presented as the percentage of the mean of the three baseline samples. Three factors were considered for MANOVA analysis: pH (7.4 vs. 6.6), treatment (control vs. caffeine) and sample (three samples collected for each pH level).
Experiment 3. In vitro muscle stimulation and effects of caffeine
In order to investigate the effects of the adenosine antagonist caffeine on kinin release induced by muscle contraction, an in vitro protocol (Aslesen & Jensen, 1998) was used. Rats (130-150 g) were anaesthetized with 0.15 ml pentobarbitone (pentobarbital; 50 mg ml−1) i.p. and split soleus strips were dissected out. Smaller rats were used in this study in order to reduce muscle size and, therefore, the diffusion distance during incubation. The muscles were suspended on a contraction apparatus between two platinum electrodes at their approximate resting length. The muscles were preincubated for 30 min in 3 ml Krebs-Henseleit buffer (composition (mm): 116 NaCl, 2.5 CaCl2, 4.6 KCl, 1.16 KH2PO4, 1.16 MgSO4, 25.4 NaHCO3, 5.5 d-glucose, 2 sodium pyruvate, 5 Hepes with 0.1 % BSA), at pH 7.4. After the preincubation, the muscles were transferred to a fresh buffer and stimulated electrically to contract isometrically or kept resting for 30 min. In a factorial design, resting and contracting muscles were incubated with and without 10 mU ml−1 of insulin (Actrapid, Novo Nordisk, Denmark), and in the absence or presence of 77 μM caffeine (Sigma), added for the last 10 min of the preincubation. Contracting muscles were stimulated electrically with impulse trains of 200 ms (100 Hz, square-wave pulses of 0.2 ms duration and 10 V amplitude, resulting in an electrical field of 2500 V m−1) delivered at a rate of one train every 2 s for 30 min. All incubations were performed at 30 °C and an O2-CO2 mixture (95-5 %) was continuously bubbled through the buffer. At the end of the experiment, samples from the incubation medium were taken and frozen for later analysis of kinins.
Bradykinin and kallidin were assessed by RIA in 50 μl buffer samples. Since there were no differences in muscle weight between the different treatment groups, bradykinin and kallidin content in the buffer is presented as picomolar (pm). The presence of stimulation, insulin and caffeine, with two levels each, were used as factors for analysis of variance. Post hoc analyses were calculated for each treatment against control without caffeine using Dunnett's test (2 sided). The statistical package SPSS for Windows (SPSS, Chicago, IL, USA) was employed for all the statistical analyses in the present study. Results are quoted as means ±s.e.m.
The osmolarity of the solutions used in the experiments differed by no more than 12 %. Since the concentrations of the different ions were kept within the physiological range, these differences should not significantly affect the results.
RESULTS
Experiment 1. In vivo microdialysis and manipulation of muscle environment
Immediately after implantation of the microdialysis probe, the levels of bradykinin in the muscular tissue were about twice the ones observed 2 h later (Fig. 1A). These high levels declined steadily and stabilized 1 h afterwards. Thereafter, they remained fairly constant during the rest of the experiment, as can be observed from the control group (Fig. 2). During baseline samples, 90 min after probe implantation, the concentration of kinin peptides in dialysate, measured as bradykinin, was 7.33 ± 1.24 pg sample−1 (80 μl). These concentrations were well above the sensitivity level (0.5 pg sample−1) of the RIA kit used.
Figure 1. Kinin levels after insertion of a microdialysis probe in adductor muscle.
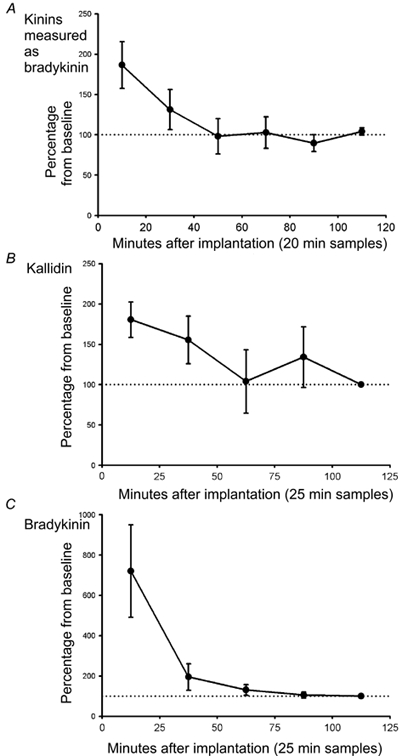
Changes (expressed as percentage of the mean concentration of the first baseline sample, mean ±s.e.m.) in the microdialysis concentration of kinin peptides (A; measured as bradykinin, n= 7), kallidin (B; n= 4) and bradykinin (C; n= 4) from the implantation of the microdialysis probe to the start of baseline sampling.
Figure 2. Effect of pH, adenosine and lactate on muscle kinin levels.
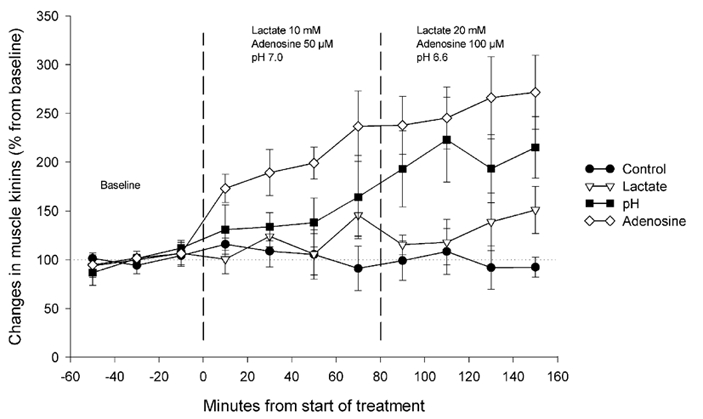
Changes (expressed as percentage of the mean concentration of the three baseline samples) in the microdialysis concentration of kinin peptides (measured as bradykinin), after perfusion with Ringer solution (control •, n= 5), Ringer solution containing 10 and 20 mm lactate (lactate ▿, n= 6), Ringer solution at pH 7.0 and 6.6 (pH ▪, n= 6), or Ringer solution containing 50 and 100 μM adenosine (adenosine ⋄, n= 7).
The perfusion with lactate had no significant effect on kinin levels (Fig. 2), despite a tendency towards a moderate increase during the two last samples. Perfusion with media of lower pH had a significant dose-dependent effect (dose: P= 0.036; treatment: P= 0.062; dose × treatment: P= 0.025). There was an increase in kinin levels up to 150 % after lowering the buffer's pH to 7.0. These levels went up to 200 % of baseline values when perfusing with buffer at pH 6.6.
A highly significant dose-dependent effect (dose: P= 0.006; treatment: P= 0.003; dose × treatment: P= 0.004) was observed when administering the two different concentrations of adenosine. Perfusion with 50 μM adenosine increased kinin levels in the dialysate to about 200 % of baseline values. Perfusion with 100 μM adenosine increased this level further to reach ≈270 % of the concentration observed during baseline sampling.
The addition of 125 mg l−1 of dihydralazine to the perfusion medium significantly increased blood flow in the area surrounding the microdialysis probe. Thus, during microdialysis baseline sampling (corresponding to the baseline period in Fig. 3) blood flow was 280 ± 98 % (P < 0.05, paired t test) higher than the values observed during the first reading immediately after probe implantation ≈1.5 h earlier. There were no significant changes in blood flow in the animals not treated with dihydralazine (143 ± 45 %; P > 0.4, paired t test).
Figure 3. Effect of dihydralazine on muscular kinin liberation induced by adenosine.
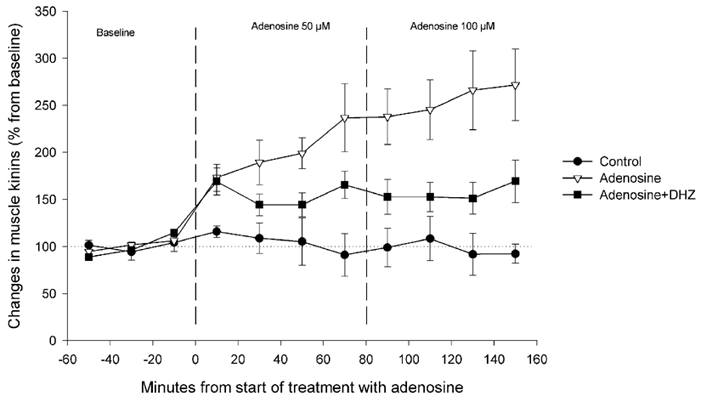
Changes (expressed as percentage of the mean concentration of the three baseline samples) in the microdialysis concentration of kinin peptides (measured as bradykinin) after the perfusion with Ringer solution (control •, n= 5), or 50 and 100 μM adenosine in Ringer solution (adenosine ▿, n= 7) or in Ringer solution containing 125 mg l−1 dihydralazine (adenosine + DHZ ▪, n= 5).
Dihydralazine significantly reduced the increase in kinin levels in dialysate induced by both doses of adenosine (treatment: P= 0.047; dose × treatment: P= 0.039; Fig. 3).
Experiment 2. In vivo microdialysis and effect of caffeine on pH-induced kinin release
The mean bradykinin level during baseline was 9.65 ± 1.86 pg sample−1 (100 μl), whereas the level measured by the kallidin antibody was 34.15 ± 7.39 pg sample−1. As seen in the first experiment, the levels of both kallidin and bradykinin were high immediately after probe implantation, falling continuously for 1 h, and then remaining fairly constant afterwards (Fig. 1B and C). This implantation effect was more obvious for bradykinin, which started out from levels nearly 800 % higher than the ones observed 60 min later.
As expected from the results of the previous experiment, the change to a perfusion medium at pH 6.6 increased the levels of bradykinin and kallidin to about 170 % (Fig. 4). Bradykinin showed an acute increase, returning to normal values during the second sample (pH: P= 0.960; pH × sample: P= 0.023). For kallidin, the increase was at a lower pace, reaching the observed maximum at the third sample (pH: P= 0.04; pH × sample: P= 0.553). The addition of 100 μM caffeine to the perfusion medium at pH 6.6 totally blocked the increase in both bradykinin (pH × treatment × sample: P= 0.027) and kallidin (pH × treatment: P= 0.009) induced by pH 6.6.
Figure 4. Effect of caffeine on the increase on muscle kinin levels induced by low pH.
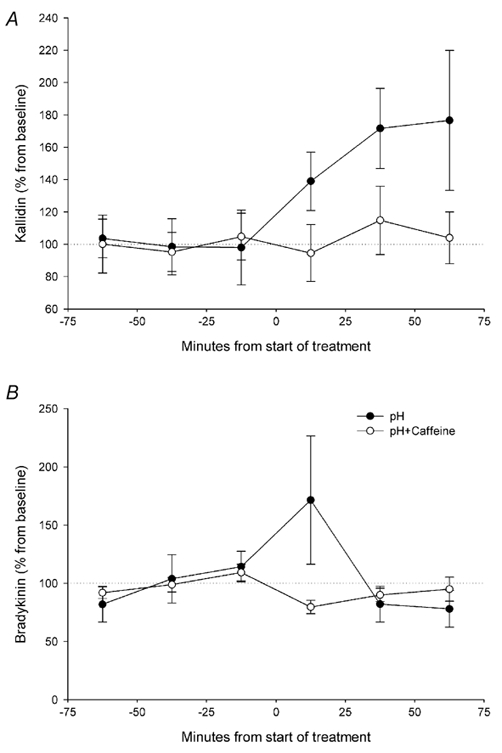
Changes (expressed as percentage of the mean concentration of the three baseline samples) in the microdialysis concentration of kallidin (A) and bradykinin (B) after the perfusion with Ringer-acetate solution at pH 6.6 (•; n= 5) or Ringer-acetate solution at pH 6.6 containing 100 μM caffeine (○; n= 6).
Experiment 3. In vitro muscle stimulation and effects of caffeine
Kallidin concentration in the buffer from non-stimulated control muscle was 42.4 ± 6.8 pm, while the levels of bradykinin were relative low (8.3 ± 0.9 pm), and around the sensitivity level for our assay. No differences between the diverse treatments were seen for this peptide (data not shown).
Electrically stimulated muscle contraction induced an increase in the kallidin concentration (stimulation: P < 0.001, Fig. 5), which was about 75 % higher than the concentration observed in controls. The addition of insulin did not significantly affect kallidin levels (insulin: P= 0.262), either with or without concurrent stimulation (stimulation × insulin: P= 0.367). The addition of caffeine to the buffer significantly blocked the increase in kallidin levels induced by electrical stimulation (caffeine: P= 0.003).
Figure 5. Effect of caffeine on kallidin levels after muscle stimulation in vitro.
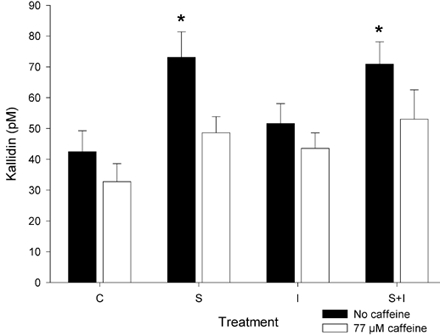
Levels of kallidin (expressed as pm) in the incubation medium of rat soleus muscle after 30 min rest (C), electrical stimulation during 30 min (S), addition of 10 mU ml−1 insulin (I), or electrical stimulation during 30 min with the addition of 10 mU ml−1 insulin (S + I), with (open columns) or without (filled columns) the addition of 77 μM caffeine (n= 12 for each treatment). *P < 0.05 vs. control without caffeine (2 sided Dunnett's test).
DISCUSSION
The present study shows that physiological changes in the muscle environment linked to muscle contraction, like higher adenosine levels or lower pH, are able to induce an increase in the intramuscular concentration of kinin peptides. It also shows the feasibility of measuring interstitial kinins in muscle tissue by the in vivo microdialysis technique. For this purpose, a commercial CMA/20 probe with PES membrane, with a high molecular cut-off (100 000 Da) was used. After insertion of the microdialysis probe in the muscle tissue, the levels of kinins in the dialysate declined steadily during 1 h, remaining rather stable after this time. The high kinin levels observed after the implantation are probably due to an acute response of the tissue to the insertion of the microdialysis probe. Thus, it is advisable to wait about 90 min after probe implantation before beginning sample collection, as done in the present study. Similarly, elevated neurotransmitter levels have been observed after insertion of a microdialysis probe into the brain of rats. This finding has been interpreted as being a consequence of an initial tissue lesion and rupture of cellular storage compartments (Ungerstedt, 1991). Since no storage of kinin peptides has been reported, and their half-life is very short (about 30 s), the high levels of peptides found after probe implantation probably originate from newly synthesized kinin due to tissue response to probe implantation. The contact of blood with dialysis membranes in patients subjected to haemodialysis activates the so called ‘contact system’, leading to an increased production of bradykinin (Coppo et al. 2000). The possibility that such a mechanism contributes to the higher kinin levels is sustained by the fact that bradykinin, and not kallidin, shows a greater response to insertion. It is established that, through activation of Factor XII, plasma kallikrein-HMWK, which liberates bradykinin (see below), quickly reacts to surfaces that initiate the ‘contact system’ (Kaplan et al. 1998). Nevertheless, the present data show that, if this is the case, it is an acute, time limited effect. It is also possible that the trauma from the microdialysis probe produces continuous leakage of kinins from the circulation to the interstitium. Hence the kinin levels measured by microdialysis would not represent physiological processes in the muscle. However, other authors have not observed any evidence of trauma or morphological changes in rat muscular tissue after implantation of microdialysis probes (Bangsbo, 1999). Furthermore, increasing the local blood flow by the vasodilator dihydralazine did not alter the baseline concentration of kinin peptides (data not shown). Also, it attenuated the adenosine-induced increase of bradykinin (Fig. 3) rather than promoting it, showing that simple filtration of kinins cannot explain the present findings. Nevertheless, other factors that can affect peptide concentration in the dialysate at the beginning of the experiment, like changes in the in vivo recovery of the microdialysis probes, cannot be ruled out.
The rats used for microdialysis were 3–4 weeks older than the ones used for the in vitro study, which were aged 5–6 weeks. Since changes in the regulation, coupled to postnatal development, of key components of the kinin- kallikrein system appear to take place primarily during the first 3 weeks of life (Yosipiv et al. 1994), this age difference should not affect the results obtained.
The kinin peptides are liberated by two different routes: (1) by a circulating plasma system, in which a plasma kallikrein, circulating as inactive kallikrein in a complex with HMWK, acts on kininogen and liberates bradykinin, and (2) by a tissue system, in which a locally secreted tissue kallikrein, encoded by a different gene than plasma kallikrein, liberates kallidin from LMWK (Kaplan et al. 1998). These two kinin peptides share the same sequence, with the exception of an additional lysine residue at the N-terminal side in kallidin (consequently also known as lys-bradykinin). Both have a similar potency at the bradykinin B2 receptor (Margolius, 1995). The antibody employed by the commercial kit used in Experiment 1 is directed against the C-terminus of the peptide, and, thus, has a very high cross reactivity between bradykinin and other analogues with differing N-terminus, like kallidin. On the other hand, the antibodies used for the kinin analysis in Experiments 2 and 3 were raised against both free ends of bradykinin or kallidin. These antibodies share very low cross reactivity, and therefore are highly specific for each peptide (Hilgenfeldt et al. 1995). The use of different antibodies in Experiments 1 and 2 can explain the disparity in the peptide concentrations in the dialysate observed during the baseline sampling. From the levels observed during baseline in the second in vivo microdialysis experiment, the larger part, at least 70 %, of the kinin reactivity recovered from muscle was due to a peptide that binds the kallidin antibody. This peptide also shows the same time course of response to low pH as the one observed when using the kinin-unspecific antibody, whereas bradykinin shows an acute response that returns to baseline values even when perfusion with the low pH solution continues. These results indicate that the kinin liberated from muscle under contraction (Wicklmayr et al. 1988; Stebbins et al. 1990; Taguchi et al. 2000) is preferentially kallidin, or a kallidin-like peptide. Since the in vitro muscle preparation is disconnected from the circulating system and, consequently, from the supply of new plasma kininogen and kallikrein, this kallidin-like peptide should be released from a locally synthesized and regulated kininogen-kallikrein system. However, the rat muscle tissue does not seem to express kininogen mRNA (Takano et al. 2000), but kininogen is known to be expressed in vascular tissue (Schölkens, 1996; Okamoto et al. 1998). Tissue kallikrein has been identified in rat muscle (Shimojo et al. 1987), but not localized in muscle cells (Figueroa et al. 1996), pointing to a localization in the adjacent vascular system. Other elements of the kinin system, like the B2 receptor and kinin metabolizing enzymes, have been identified in striated muscle cells (Vaghy et al. 1995; Figueroa et al. 1996). Thus, there may be a mechanism of cross talking between contracting muscle cells and the associated vascular system.
Unlike humans, rat kininogen does not show the kallidin sequence, since an Arg residue substitutes the Lys residue at the N-terminus of the kallidin sequence. On this basis, it has been argued that bradykinin would be the only kinin which can be released from low or high molecular weight kininogen in the rat (Kato et al. 1985; Hagiwara et al. 1995). It is highly probable that the kallidin-like peptide recovered in the present experiments is Arg-bradykinin, since the rat tissue kallikrein seems to be able to liberate this peptide from rat low molecular weight kininogen (U. Hilgenfeldt, personal communication). The kallidin antibody used presents an 80 % cross reactivity with this peptide (Hilgenfeldt et al. 1995). Another possibility is T-kinin, an undecapeptide with the same sequence as bradykinin but with additional Ile-Ser residues at the N-terminus and which is liberated from T-kininogen. However, T-kinin can be excluded, since the antibody does not show any significant cross reactivity with this peptide (Hilgenfeldt et al. 1995). Thus, the present study is one of the first to show a physiological release of a kallidin-like peptide in the rat, which can possess the same function as kallidin in other species, like humans.
Adenosine induced a dose-dependent increase in kinin peptides, and the adenosine antagonist caffeine prevented both the contraction- and the low pH-induced increase in these peptides. Accordingly, adenosine emerges as a central regulator of the kallikrein-kinin system in muscle. Under contraction, an increase in 5′ nucleotidase activity induces an extracellular accumulation of adenosine (Hellsten, 1999), which is enhanced by the presence of vascular endothelial cells (Hellsten & Frandsen, 1997). The perfusion at the highest adenosine dose (100 μM) used in the present experiment implies a maximal delivery (assuming a 100 % recovery for the microdialysis probe) of 0.4 nmol min−1 adenosine to the muscle. Taking into account that the length of the probe is 1 cm, and it is delivering to a large mass of muscle, this dose should be well inside the physiological formation rate of adenosine described for rat muscle cells (Hellsten & Frandsen, 1997; Hellsten, 1999). Adenosine has comparable effects on muscle metabolism to those of the kinin peptides. Adenosine, like bradykinin, could be a regulatory factor of glucose uptake (Han et al. 1998), and blood flow during muscle contraction (Rådegran & Calbet, 2001). Both adenosine and the kinin system have been associated with the mechanisms mediating cardiac ischaemic preconditioning (Nakano et al. 2000). Hence, the possibility arises that part of the aforementioned effects of adenosine may be mediated by its action on the kallikrein-kinin system. Moreover, adenosine and the kinin system may be two components of the mechanisms mediating the interaction between active muscle cells and the vascular system. Adenosine from contracting muscle would induce the liberation by the vascular bed of kinin peptides, which could talk back to muscular cells by its action over B2 receptors. Nevertheless, more studies should be done in order to validate such a mechanism.
Another metabolic element that should be considered as a possible regulatory factor in the liberation of kinin peptides during muscle contraction is low pH. The present study shows that low pH is also able to stimulate kinin production in a dose-dependent way. Low pH is known to stimulate the formation of bradykinin (Dray & Perkins, 1993; Dietze et al. 1996), and to inhibit its degradation (Edery & Lewis, 1962). However, caffeine prevented the increase of bradykinin and kallidin levels induced by low pH. This implies that the effect of pH on kinin liberation in muscle may be due to its stimulatory action on adenosine formation in muscular tissue (Ballard, 1995). Lactate did not affect kinin levels, indicating that it does not participate in the liberation of kinins under muscle activity. Also, lactate does not seem to affect the rate of formation of adenosine in contracting muscle cells (Hellsten, 1999). In normal conditions, the liberation of lactate is in the form of lactic acid, which decreases muscular pH. Thus, we cannot rule out the possibility of an indirect effect of the production of lactic acid on kinin peptides induced by lowering the intramuscular pH. In the present experiment, lactate was given as a sodium salt, in a buffered Ringer solution, which should prevent the decrease in pH in the perfused muscular tissue.
Despite the well known action of kinins as vasodilators, we observed a steady increase in kinin concentrations after addition of adenosine or lowering of pH. This suggests that the vasodilatory effects at these concentrations are insufficient to prevent the accumulation of kinins in the interstitial space. The adenosine-induced elevation was attenuated after increasing blood flow with the vasodilator dihydralazine, indicating that local blood flow is of significance for the regulation of kinin muscle concentration. This can be either due to a higher clearance rate from the extracellular environment, or to a feedback mechanism regulating kinin synthesis and/or degradation. However, a possible direct pharmacological interaction of dihydralazine with adenosine action and/or kinin synthesis cannot be ruled out from the present data.
Bradykinin and kallidin may be important factors controlling muscle metabolism during contractions (Dietze et al. 1996; Mayfield et al. 1996). In addition, bradykinin is a potent algogen, and can also produce allodynie by sensitization of nociceptors (Dray & Perkins, 1993). The intramuscular administration of bradykinin has an hyperalgesic effect (Jensen et al. 1990; Babenko et al. 1999), and is able to alter the perception of noxious stimuli (Hoheisel et al. 1993). Besides, during muscular ischaemia, which is known to induce muscle pain (Svensson & Arendt-Nielsen, 1995), similar changes in pH, adenosine levels and lactate as under anaerobic contraction can be also observed (Hagberg, 1985; Hara et al. 1988), accompanied by a larger liberation of kinin (Stebbins et al. 1990). Thus, the muscular kallikrein-kinin system may also be an important element in the appearance of muscular pain, and the knowledge of its regulation in active muscle can be central for understanding the appearance and development of musculoskeletal pain disorders.
In conclusion, the present data point out that the liberation of a kallidin-like peptide by contracting muscle can be mediated by the increase in adenosine levels concomitant with muscle activity. In the rat, this effect seems to be due to an increase in the activity of tissue kallikrein and the liberation of Arg-bradykinin from low molecular weight kininogen.
Acknowledgments
We thank Jørgen Jensen for his skillful assistance in the in vitro experiments and Ada Ingvaldsen for conducting the radioimmunoassay analysis. We thank also Elisabeth Paus from The Norwegian Radiumhospital for her help in setting up the RIA. The present study was partially supported by the Anders Jahres Fonds and the Norwegian Research Council. Laila Rosenborg was supported by a grant of the Norwegian Research Council.
REFERENCES
- Achike FI, Ballard HJ. Influence of stimulation parameters on the release of adenosine, lactate and CO2 from contracting dog gracilis muscle. Journal of Physiology. 1993;463:107–121. doi: 10.1113/jphysiol.1993.sp019586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslesen R, Jensen J. Effects of epinephrine on glucose metabolism in contracting rat skeletal muscles. American Journal of Physiology. 1998;275:E448–456. doi: 10.1152/ajpendo.1998.275.3.E448. [DOI] [PubMed] [Google Scholar]
- Babenko V, Graven-Nielsen T, Svensson P, Drewes AM, Jensen TS, Arendt-Nielsen L. Experimental human muscle pain and muscular hyperalgesia induced by combinations of serotonin and bradykinin. Pain. 1999;82:1–8. doi: 10.1016/S0304-3959(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Bangsbo J. Vasoactive substances in the interstitium of contracting skeletal muscle examined by microdialysis. Proceedings of the Nutrition Society. 1999;58:925–933. doi: 10.1017/s0029665199001238. [DOI] [PubMed] [Google Scholar]
- Bockman EL, McKenzie JE. Tissue adenosine content in active soleus and gracilis muscles of cats. American Journal of Physiology. 1983;244:H552–559. doi: 10.1152/ajpheart.1983.244.4.H552. [DOI] [PubMed] [Google Scholar]
- Coppo R, Amore A, Cirina P, Scelfo B, Giacchino F, Comune L, Atti M, Renaux JL. Bradykinin and nitric oxide generation by dialysis membranes can be blunted by alkaline rinsing solutions. Kidney International. 2000;58:881–888. doi: 10.1046/j.1523-1755.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- Derave W, Hespel P. Role of adenosine in regulating glucose uptake during contractions and hypoxia in rat skeletal muscle. Journal of Physiology. 1999;515:255–263. doi: 10.1111/j.1469-7793.1999.255ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze GJ, Wicklmayr M. Evidence for a participation of the kallikrein-kinin system in the regulation of muscle metabolism during muscular work. FEBS Letters. 1977;74:205–208. doi: 10.1016/0014-5793(77)80847-8. [DOI] [PubMed] [Google Scholar]
- Dietze GJ, Wicklmayr M, Rett K, Jacob S, Henriksen EJ. Potential role of bradykinin in forearm muscle metabolism in humans. Diabetes. 1996;45(suppl. 1):110S–114S. doi: 10.2337/diab.45.1.s110. [DOI] [PubMed] [Google Scholar]
- Djupsjöbacka M, Johansson H, Bergenheim M, Wenngren BI. Influences on the gamma-muscle spindle system from muscle afferents stimulated by increased intramuscular concentrations of bradykinin and 5-HT. Neuroscience Research. 1995;22:325–333. doi: 10.1016/0168-0102(95)00906-a. [DOI] [PubMed] [Google Scholar]
- Dray A, Perkins M. Bradykinin and inflammatory pain. Trends in Neurosciences. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Edery H, Lewis GP. Inhibition of plasma kininase activity at slightly acid pH. British Journal of Pharmacology. 1962;19:299–305. doi: 10.1111/j.1476-5381.1962.tb01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CD, Dietze G, Muller-Esterl W. Immunolocalization of bradykinin B2 receptors on skeletal muscle cells. Diabetes. 1996;45(suppl. 1):24S–28S. doi: 10.2337/diab.45.1.s24. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactic acid: New roles in a new millennium. Proceedings of the National Academy of Sciences of the USA. 2001;98:395–397. doi: 10.1073/pnas.98.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H. Intracellular pH during ischemia in skeletal muscle: relationship to membrane potential, extracellular pH, tissue lactic acid and ATP. Pflügers Archiv. 1985;404:342–347. doi: 10.1007/BF00585346. [DOI] [PubMed] [Google Scholar]
- Hagiwara Y, Kojima M, Kuraishi T, Hayashi I, Miyata T, Oh-Ishi S. Identification of rat urinary kinin as bradykinin. Life Sciences. 1995;57:997–1002. doi: 10.1016/0024-3205(95)02035-h. [DOI] [PubMed] [Google Scholar]
- Han DH, Hansen PA, Nolte LA, Holloszy JO. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes. 1998;47:1671–1675. doi: 10.2337/diabetes.47.11.1671. [DOI] [PubMed] [Google Scholar]
- Hara N, Mineo I, Kono N, Yamada Y, Kawachi M, Kiyokawa H, Yamasaki T, Wang YL, Nakajima H, Kuwajima M. Inosine and adenosine formation in ischemic and non-ischemic contracting muscles of rats: difference between fast and slow muscles. Research Communications in Chemical Pathology and Pharmacology. 1988;60:309–321. [PubMed] [Google Scholar]
- Hellsten Y. The effect of muscle contraction on the regulation of adenosine formation in rat skeletal muscle cells. Journal of Physiology. 1999;518:761–768. doi: 10.1111/j.1469-7793.1999.0761p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. Journal of Physiology. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeldt U, Linke R, Riester U, KÖNIG W, Breipohl G. Strategy of measuring bradykinin and kallidin and their concentration in plasma and urine. Analytical Biochemistry. 1995;228:35–41. doi: 10.1006/abio.1995.1311. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Mense S, Simons DG, Yu XM. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neuroscience Letters. 1993;153:9–12. doi: 10.1016/0304-3940(93)90064-r. [DOI] [PubMed] [Google Scholar]
- Kaplan AP, Joseph K, Shibayama Y, Nakazawa Y, Ghebrehiwet B, Reddigari S, Silverberg M. Bradykinin formation-Plasma and tissue pathways and cellular interactions. Clinical Reviews in Allergy and Immunology. 1998;16:403–429. doi: 10.1007/BF02737659. [DOI] [PubMed] [Google Scholar]
- Kato H, Enjyoji K, Miyata T, Hayashi I, Oh-Ishi S, Iwanaga S. Demonstration of arginyl-bradykinin moiety in rat HMW kininogen: direct evidence for liberation of bradykinin by rat glandular kallikreins. Biochemical and Biophysical Research Communications. 1985;127:289–295. doi: 10.1016/s0006-291x(85)80157-1. [DOI] [PubMed] [Google Scholar]
- Koh H, Uchida K, Waki M, Nambu S. Exercise-induced increase in glandular kallikrein activity in human plasma and its significance in peripheral glucose metabolism. Arzneimittelforschung. 1988;38:1181–1184. [PubMed] [Google Scholar]
- Mannion AF, Jakeman PM, Willan PL. Skeletal muscle buffer value, fibre type distribution and high intensity exercise performance in man. Experimental Physiology. 1995;80:89–101. doi: 10.1113/expphysiol.1995.sp003837. [DOI] [PubMed] [Google Scholar]
- Margolius HS. Theodore Cooper Memorial Lecture. Kallikreins and kinins. Some unanswered questions about system characteristics and roles in human disease. Hypertension. 1995;26:221–229. doi: 10.1161/01.hyp.26.2.221. [DOI] [PubMed] [Google Scholar]
- Mayfield RK, Shimojo N, Jaffa AA. Skeletal muscle kallikrein. Potential role in metabolic regulation. Diabetes. 1996;45(suppl. 1):20S–23S. doi: 10.2337/diab.45.1.s20. [DOI] [PubMed] [Google Scholar]
- Nakano A, Cohen MV, Downey JM. Ischemic preconditioning: from basic mechanisms to clinical applications. Pharmacology and Therapeutics. 2000;86:263–275. doi: 10.1016/s0163-7258(00)00058-9. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Yayama K, Shibata H, Nagaoka M, Takano M. Kininogen expression by rat vascular smooth muscle cells: stimulation by lipopolysaccharide and angiotensin II. Biochimica et Biophysica Acta. 1998;1404:329–337. doi: 10.1016/s0167-4889(98)00074-3. [DOI] [PubMed] [Google Scholar]
- Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. Journal of Applied Physiology. 1993;75:2061–2068. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- Rosdahl H, Ungerstedt U, Jorfeldt L, Henriksson J. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. Journal of Physiology. 1993;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RÅDEGRAN G, Calbet JAL. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiologica Scandinavica. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- RÅDEGRAN G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiologica Scandinavica. 2000;168:575–591. doi: 10.1046/j.1365-201x.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- Schölkens BA. Kinins in the cardiovascular system. Immunopharmacology. 1996;33:209–216. doi: 10.1016/0162-3109(96)00061-6. [DOI] [PubMed] [Google Scholar]
- Shimojo N, Chao J, Chao L, Margolius HS, Mayfield RK. Identification and characterization of a tissue kallikrein in rat skeletal muscles. Biochemical Journal. 1987;243:773–778. doi: 10.1042/bj2430773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. The ‘glycogen shunt’ in exercising muscle: A role for glycogen in muscle energetics and fatigue. Proceedings of the National Academy of Sciences of the USA. 2001;98:457–461. doi: 10.1073/pnas.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. Journal of Applied Physiology. 1990;69:1225–1230. doi: 10.1152/jappl.1990.69.4.1225. [DOI] [PubMed] [Google Scholar]
- Svensson P, Arendt-Nielsen L. Induction and assessment of experimental muscle pain. Journal of Electromyography and Kinesiology. 1995;5:131–140. doi: 10.1016/1050-6411(95)00019-v. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Kishikawa H, Motoshima H, Sakai K, Nishiyama T, Yoshizato K, Shirakami A, Toyonaga T, Shirotani T, Araki E, Shichiri M. Involvement of bradykinin in acute exercise-induced increase of glucose uptake and GLUT-4 translocation in skeletal muscle: Studies in normal and diabetic humans and rats. Metabolism: Clinical and Experimental. 2000;49:920–930. doi: 10.1053/meta.2000.6755. [DOI] [PubMed] [Google Scholar]
- Takano M, Sakanaka F, Yayama K, Okamoto H. Tissue-specific expression of rat kininogen mRNAs. Biological and Pharmaceutical Bulletin. 2000;23:1239–1242. doi: 10.1248/bpb.23.1239. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Introduction to intracerebral microdialysis. In: Robinson TE, Justice JB Jr, editors. Microdialysis in the Neurosciences. Amsterdam: Elsevier Science; 1991. pp. 3–22. [Google Scholar]
- Vaghy PL, Russell JS, Lantry LE, Stephens RE, Ward PE. Angiotensin and bradykinin metabolism by peptidases identified in cultured human skeletal muscle myocytes and fibroblasts. Peptides. 1995;16:1367–1373. doi: 10.1016/0196-9781(95)02034-9. [DOI] [PubMed] [Google Scholar]
- Wicklmayr M, Rett K, Fink E, Tschollar W, Dietze G, Mehnert H. Local liberation of kinins by working skeletal muscle tissue in man. Hormone and Metabolic Research. 1988;20:535–535. doi: 10.1055/s-2007-1010879. [DOI] [PubMed] [Google Scholar]
- Yosipiv IV, Dipp S, El Dahr SS. Ontogeny of somatic angiotensin-converting enzyme. Hypertension. 1994;23:369–374. doi: 10.1161/01.hyp.23.3.369. [DOI] [PubMed] [Google Scholar]
- Ballard HJ. The role of intracellular pH in the control of adenosine output from red skeletal muscle. Biological Signals. 1995;4:168–173. doi: 10.1159/000109437. [DOI] [PubMed] [Google Scholar]
- Jensen K, Tuxen C, Pedersen-Bjergaard U, Jansen I, Edvinsson L, Olesen J. Pain and tenderness in human temporal muscle induced by bradykinin and 5-hydroxytryptamine. Peptides. 1990;11:1127–1132. doi: 10.1016/0196-9781(90)90141-q. [DOI] [PubMed] [Google Scholar]


