Abstract
Activation of the vestibular system can either increase or decrease ventilation. The objectives of the present study were to clarify whether these different responses are the result of activating different vestibular subnuclei, by addressing three questions. Do neurones within the medial, lateral and spinal vestibular nuclei (VNM, VNL and VNS, respectively) function differently in respiratory modulation? Is the ventral medullary nucleus gigantocellularis (NGC) required to fully express the VN-mediated respiratory responses? Is glutamate, by acting on N-methyl-d-aspartic acid (NMDA) receptors in the vestibular subnuclei, capable of modulating respiration? In anaesthetized, tracheotomized and spontaneously breathing rats, electrical stimuli (< 10 s) applied in the VNL and VNS significantly elevated ventilation by 35 % and 30 % (P < 0.05), respectively. However, VNM stimulation produced statistically significant (P < 0.05) changes that differed depending upon the stimulation site: either ventilatory inhibition (by 40 % in 57 % of the trials) or excitation (by 55 % in 43 % of trials), and which were often accompanied by a pressor response. These electrical-stimulation-evoked cardiorespiratory responses were almost eliminated following microinjection of ibotenic acid into the stimulation sites (P < 0.05) or bilaterally into the NGC (P < 0.05). As compared to vehicle, microinjection of NMDA into the unilateral VNM, VNL and VNS significantly increased ventilation to 74 %, 58 % and 60 % (P < 0.05), respectively, with no effect on arterial blood pressure. These data suggest that neurones within the vestibular subnuclei play different roles in cardiorespiratory modulation, and that the integrity of the NGC is essential for the full expression of these VN-mediated responses. The evoked respiratory excitatory responses are probably mediated by glutamate acting on NMDA receptors, whereas the neurotransmitters involved in VNM-mediated respiratory inhibition and hypertension remain unknown.
The vestibular nucleus (VN) is mainly responsible for sensing changes in head position with respect to gravity and producing relevant compensatory responses of muscles that control posture and eye movement. Recently, the VN has been reported to contribute to respiratory regulation. Electrical stimulation of the VN (Bassal & Bianchi, 1982; Mameli et al. 1988) or vestibular nerve (Yates et al. 1993; Mori et al. 2001) brought about either an increase or decrease in respiration by changing the respiratory amplitude and/or frequency in cats and rabbits. In agreement with this, lesions of the VN altered respiration in decerebrate cats and awake goats (Huang et al. 1991; Wenninger et al. 2001). Selective natural stimulation of vestibular receptors by rotation of the head elicited changes in the activity of nerves innervating inspiratory and expiratory muscles in cats (Rossiter et al. 1996) and ventilation in humans (Monahan et al. 2002). In the rat, the VN contains three major subnuclei: medial (VNM), lateral (VNL) and spinal (VNS). Previous studies have shown that different divisions of the VN receive different labyrinthine inputs and subserve different functions (Gilman & Newman, 1992). Although activation of the vestibular system could increase or decrease ventilation, no systematic investigation has been conducted to clarify whether these different effects are the result of activating different vestibular subnuclei.
Recently, Yates and his colleagues reported that in decerebrate cats removal of the medullary reticular formation (Mori et al. 2001) abolished the inhibitory components of the respiratory responses to stimulation of the vestibular nerve. These data strongly suggested that full expression of the respiratory responses evoked by activation of the vestibular nerve is dependent on the integrity of the medullary reticular formation. It is generally accepted that the VN receives not only labyrinthine inputs, but also inputs from other central regions (Gilman & Newman, 1992), such as the cerebellum known to be involved in respiratory control (Xu et al. 1998, 2001; Xu & Frazier, 2000). It remains unclear, however, whether the medullary reticular formation is also critical for the expression of VN-mediated respiratory responses. Our previous anterogarde tracing study in the rat documented that some VN neurones project monosynaptically to the nucleus gigantocellularis (NGC), which is located within the mid-ventral portion of the medullary reticular formation (Zhang et al. 1999). Since substantial evidence has now demonstrated NGC involvement in respiratory modulation in cats, rats and goats (Stremel et al. 1990; Mori et al. 2001; Wenninger et al. 2001; Xu et al. 2001), a logical question would be whether the respiratory responses elicited by stimulation of the vestibular subnuclei are dependent on the integrity of NGC neurones.
There is considerable evidence to show that N-methyl-d-aspartic acid (NMDA) is involved in afferent synaptic transmission in the VN. Vestibular subnuclear neurones contain NMDA receptors (Darlington & Smith, 1995; Vidal et al. 1996; Chen et al. 2000) and electrical stimulation of the vestibular nerve selectively elevates the release of glutamate in the VN (Yamanaka et al. 1997). Focal administration of excitatory amino acid into the VN significantly altered the tilt-induced firing behaviour of local neurones in rats (Takeshita et al. 1999) and enhanced ventilation in awake goats (Wenninger et al. 2001). However, the involvement of NMDA in the vestibular subnuclei in respiratory modulation has not been fully investigated.
The present study was conducted on anaesthetized and spontaneously breathing rats. We found that VNL or VNS stimulation elevated ventilation with little change in arterial blood pressure (ABP), whereas activation of the VNM produced either inhibitory or excitatory ventilatory responses that were often accompanied by a pressor response. These cardiorespiratory responses were eliminated or significantly attenuated following microinjection of ibotenic acid (IA) into the stimulating sites or bilaterally into the NGC. Furthermore, microinjection of NMDA into the vestibular subnuclei always significantly elevated ventilation. These data suggest that neurones within the vestibular subnuclei play different roles in the modulation of ventilation and that the integrity of the NGC is essential for the full expression of these VN-mediated responses. The evoked respiratory excitatory responses are probably mediated by glutamate acting on NMDA receptors in different vestibular subnuclei, whereas the neurotransmitters involved in VNM-mediated respiratory inhibition remain unknown.
METHODS
General protocol
The experimental protocols described in this study were approved by the Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and were in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals. The experiments were conducted on 44 anaesthetized (chloralose and urethane; 100 mg kg−1 and 500 mg kg−1; i.p.), tracheotomized and spontaneously breathing Sprague-Dawley rats (400-550 g). The left femoral vein and artery were cannulated, the former for anaesthetic administration and the latter for monitoring ABP. Supplemental anaesthetic was administered intravenously to suppress corneal and withdrawal reflexes. The trachea was cannulated below the larynx with a cannula connected to a one-way breathing valve. The tracheal pressure (Ptr) was recorded via a pressure transducer that was connected to a side-port of the tracheal cannula. The core temperature was monitored with a rectal probe and maintained at ≈37.5 °C by a heating pad and radiant heat. Respiratory variables including airflow and tidal volume (VT) were recorded via a pneumotachograph. The latter was made of stainless steel and had a linear flow-pressure relationship in the range 0–20 ml s−1 and a flow resistance of 0.046 cmH2O ml−1 s−1, with a dead space of ≈0.2 ml. A three-way switch was attached to the inspiratory inlet of the one-way breathing valve and used to manipulate the inhaled gas mixture to maintain end-tidal O2 and CO2 (PET,O2 and PET,CO2, respectively) at > 100 mmHg and ≈35 mmHg, respectively. PET,O2 and PET,CO2 were monitored via an infrared O2-CO2 analyser (Hewlett Packard 78356A).
Occipital craniotomy
Animals were placed in a rigid metal frame with the head fixed in a stereotaxic apparatus (David Kopf). A hole (< 8 mm diameter) was drilled at the midline (P-12.5 mm, to the Bregma) for stereotaxically inserting the electrode/needle into the vestibular subnuclei, according to the rat brain atlas (Paxinos & Watson, 1986). Bleeding was controlled with bone wax, absorbable haemostat (Surgicel and Gelfoam) and the use of a bipolar coagulator (Radionics, Model 440S). The underlying tissue was covered by cotton saturated with mineral oil to prevent drying.
Electrical stimulation of the vestibular subnuclei
After baseline cardiorespiratory variables became stable for at least 15 min, the following studies were conducted in 35 rats. Stereotaxic coordinates were used to position a stainless steel, concentric bipolar electrode into a given vestibular subnucleus. The electrode placements were: AP (rostral to the obex) = 3.5-4.0 mm, ML = 2-2.5 mm for VNL; AP = 2.5-3.0 mm, ML = 1.0-2.0 mm for VNS; AP = 2.5-3.5 mm, ML = 0.5-1.5 mm for VNM. The depth from the cerebellar dorsal surface was 6.5-7.0 mm. The stimulating electrode was placed and fixed in the site where reproducible respiratory responses were clearly detectable during electrical stimulation. The stimulating parameters were delivered by a digital stimulator (Grass S8800) at the beginning of either the inspiratory or expiratory phase. The stimulating profile was fixed (300 ms trains of 0.2 ms pulses at 100 μA) throughout the experiment, while stimulating frequency (20, 50, 100, 150 and 200 Hz delivered for < 10 s) varied randomly for a given trial. In some cases, the stimulating electrode was repositioned into other sites (contralateral or ipsilateral) of the vestibular subnuclei and the same stimuli repeated. A simulating threshold was defined as the lowest stimulating frequency at which a detectable change in respiration was elicited. The placement sites of the stimulating electrodes were mapped on a grid.
Microinjection of IA into the VN-stimulation sites
In six out of 35 rats, a dual electrode, which was composed of a tungsten electrode and a micropipette (i.d. ≈15 μm mounted < 20 μm away from it; Xu & Frazier, 2000), was placed into the different vestibular subnuclei. The former was used as a stimulating electrode and the latter for microinjection of IA. It has been reported that IA is neurotoxic, being capable of initially stimulating neurones and then subsequently destroying them (Kohler & Schwarcz, 1983). The electrode was fixed at a VN site where electrical stimulation elicited detectable respiratory responses. Subsequently, the same stimulating protocol was repeated 2 h after injection of IA (100 mm, in a solution of 2 % Chicago Sky Blue in 150 mm saline) to locally lesion cell bodies in the vicinity of the injection site (Kohler & Schwarcz, 1983). The volume of drug ejected (50-100 nl) was verified by using a microscope to view the meniscus in the micropipette against a calibrated reticule in the microscope eyepiece (Melles Griot, E3069).
Bilateral microinjection of IA into the NGC
A needle (0.5 μl, 25 ga., Hamilton) prefilled with IA (100 mm) or its vehicle was inserted into the NGC (AP 1.5; ML 0.5) in 29 of the 35 rats. After stabilization of baseline cardiorespiratory variables, electrical stimulation of the vestibular subnuclei via the concentric bipolar electrode was conducted. When reproducible respiratory responses were clearly detectable during electrical stimulation, the electrode was fixed. IA was used to selectively destroy local neurones in 26 rats. IA (50-100 nl), driven from the micropipette by a microinjection unit (KOPF, Model 5002), was administered bilaterally into the NGC over a 1 min period. The protocols for electrical stimulation were repeated 2 h after microinjections. To test the effects of vehicle and experimental time course, the same electrical stimuli were given before and 2 h after vehicle injection into the NGC in four rats, one of which subsequently received an injection of IA.
Microinjection of NMDA into the subvestibular nuclei
NMDA (100 mm) dissolved with mock cerebrospinal fluid (detailed in Xu et al. 1992) and mixed with 2 % Chicago Sky Blue was loaded in the needle (0.5 μl, 25 ga., Hamilton). The latter was sequentially positioned into the individual vestibular subnucleus in nine other rats. NMDA or its vehicle (30-50 nl), driven from the needle by a microinjection unit (KOPF, Model 5002), was delivered into a given subnucleus 15 min after stabilization of cardiorespiratory variables. A period of at least 10 min was allowed for the animals’ recovery from the NMDA-induced responses. The needle was randomly repositioned into another vestibular subnucleus (contralateral or ipsilateral) or the same nucleus on the contralateral side, and the injection repeated. Three to six trials (NMDA and/or vehicle) were performed in each animal with no more than one injection applied in the individual subnucleus (ipsilateral/contralateral).
General histological examination
After completion of protocols, the animals were killed by administration of additional anaesthetic and the brainstem and cerebellum were removed and placed in 10 % formalin. After at least 3 days of immersion fixation, the brainstem was frozen and 50 μm sections were cut and mounted. The tissue sections containing sites marked with Chicago Sky Blue were drawn with the aid of camera lucida.
Data acquisition and analysis
During an experiment, the raw data of ABP, mean arterial blood pressure (MABP), VT, respiratory frequency (fR) and minute ventilation (VI, product of VT and fR) were recorded on a polygraph (model 7D, Grass) as well as an on-line computer (PowerLab) for later analysis. The control (baseline) values, expressed as absolute values, were obtained by averaging the relevant variables within five breaths just before application of electrical stimuli and 1 min immediately before microinjection of NMDA or IA (vehicle) into the vestibular subnuclei or the NGC, respectively. The responses were collected and measured: (1) for three breaths immediately following electrical stimulation, and (2) during the 10 s period in which the greatest respiratory responses were recorded immediately after NMDA (vehicle) injection. The responses are presented as percentage change from control (Δ%). All data are presented as means ±s.e.m. A paired t test was used for comparing the differences between cardiorespiratory variables before and after vestibular subnuclei stimulation. One-way ANOVA and the Newman-Keuls test were used to identify the significance of the VN-mediated cardiorespiratory responses obtained before and after IA injection into the stimulating sites or the NGC. A P value less than 0.05 was considered significant.
RESULTS
Respiratory and ABP responses to electrical stimulation of the vestibular subnuclei
In the present study, 49 trials of electrical stimulation of different vestibular subnuclei were conducted before any drug injection in 35 rats (Table 1). Figure 1 displays typical respiratory and ABP responses to stimulation of the vestibular subnuclei. As shown, electrical stimulation of the VNM had either an inhibitory (Fig. 1A) or excitatory (Fig. 1B) effect on ventilation by altering VT and fR. In 28 trials, VNM stimulation led to a ventilatory decrease in 16 and an increase in 12 trials. In contrast, stimulation applied to the VNS (Fig. 1C) and VNL (Fig. 1D) increased ventilation by elevating both VT and fR. This consistent excitatory response was observed in 11 trials for VNL and 10 trials for VNS stimulation. In general, a greater stimulation always produced a stronger response, as depicted in Fig. 1A-D, in which the first and second stimulations were 100 and 150 Hz, respectively. The stimulating thresholds ranged from 50 to 150 Hz, with 100 Hz being the most predominant (VNM= 68 %; VNL= 73 %; VNS= 80 %). Three observations are noteworthy. First, a pressor response was evoked by VNM stimulation in 85 % of trials tested, regardless of whether the respiratory response was inhibitory or excitatory. Second, although there was no immediate alteration in ABP, activation of the VNL and VNS did produce a post-simulation depression in 29 % of the trials, as shown in Fig. 1C and D. Third, the threshold (95 % at 50–100 Hz) required for evoking the respiratory responses was smaller than that (≈85 % ≥ 150 Hz) necessary to produce a clearly detectable ABP change (Fig. 1A-D).
Table 1.
Distribution of the type of electrical stimulation applied in the different vestibular subnuclei
| Type of stimulation sequence | Stimulation trial | Animal number | ||
|---|---|---|---|---|
| VNM | VNL | VNS | ||
| A | 2 | 2 | 2 | 2 |
| B | 2 | 2 | — | 2 |
| C | 1 | — | 1 | 1 |
| D | 2 | — | — | 1 |
| E | — | 6 | 6 | 6 |
| F | — | 1 | — | 1 |
| G | — | — | 1 | 1 |
| H | 21 | — | — | 21 |
| Subtotal | 28 | 11 | 10 | — |
| Total | 49 | 35 | ||
In the present study, the type of electrical stimulation sequence was varied as shown in rows A-H. The trials applied in each subnucleus are summarized in the ‘Subtotal’ row, and the total number of trials and animals used are listed in the bottom row.
Figure 1. Experimental recordings of ventilatory and arterial blood pressure (ABP) responses to stimulation of the medial (VNM), lateral (VNL) and spinal (VNS) vestibular nuclei.
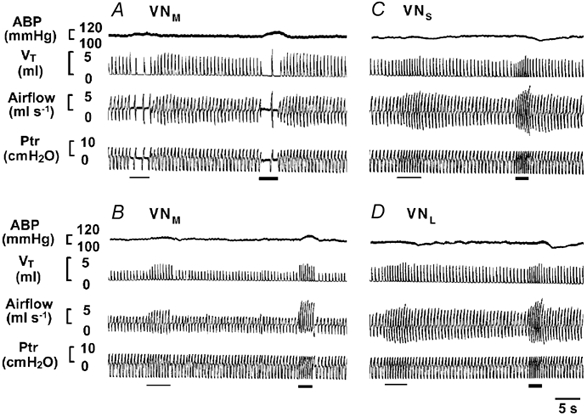
Experimental recordings of ventilatory and ABP responses to stimulation of the VNM (A and B), VNS (C) and VNL (D). In each panel, the traces from the top to bottom are ABP, tidal volume (VT), airflow and tracheal pressure (Ptr). Note that the thin and thick bars indicate stimulation at 100 and 150 Hz, respectively.
Figure 2 illustrates the group data with the respiratory (A) and ABP (B) responses to electrical stimulation of the vestibular subnuclei. As compared to control, 57 % of VNM stimulations significantly inhibited ventilation (by ≈40 %), whereas 43 % increased ventilation (by ≈55 %). However, stimulation of the VNS and VNL exclusively enhanced ventilation by ≈30 % and ≈35 %, respectively. With respect to breathing patterns, collectively, both VT and fR were altered in ≈50 % of the VN stimulations, with VT or fR singularly affected in ≈20 % and ≈30 % of VN stimulations, respectively. There was a significant increase (22 %) in MABP observed with 150 Hz stimulation of the VNM whether the respiration response was inhibitory or excitatory. In contrast, MABP was not significantly changed during VNL or VNS stimulation. The placements of the stimulating electrode are summarized in Fig. 2C. As shown, the sites responsible for inhibitory and excitatory responses seem to be localized primarily at the caudal and rostral regions of the VNM, respectively, although some of them are mingled.
Figure 2. Group data showing the effects of stimulation of the vestibular subnuclei on cardiorespiratory responses.
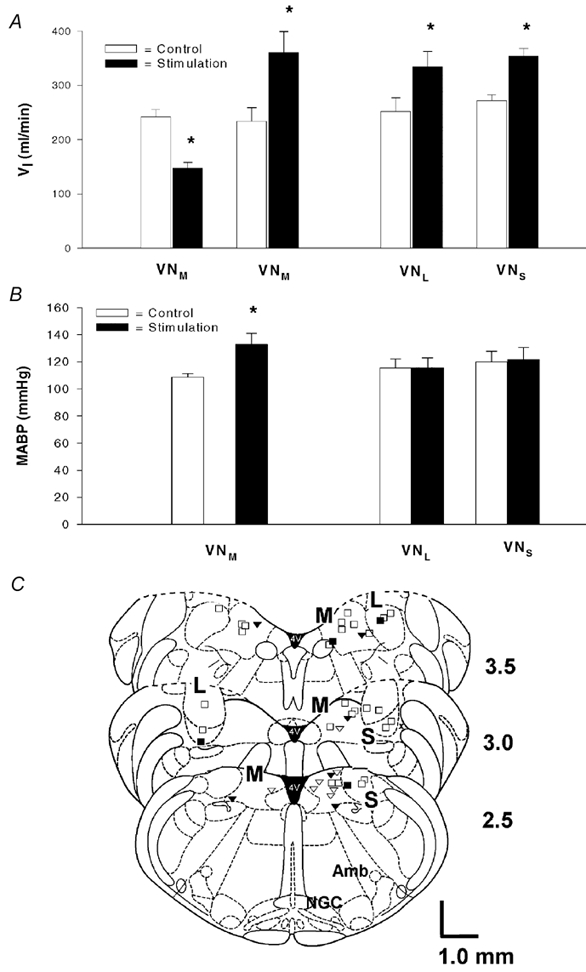
The responses of minute ventilation (VI) and mean arterial blood pressure (MABP) are illustrated in A and B, respectively. The number of animals used for VNM, VNL and VNS stimulation was 27, 11 and 10, respectively; data are presented as the mean ±s.e.m.; *P < 0.05 between the data obtained before and during electrical stimulation. C, placements of the stimulating electrode are mapped schematically in a group of cross-sections of the brainstem 2.5-3.5 mm rostral to the obex according to the rat brain atlas (Paxinos & Watson, 1986). M, L and S represent the VNM, VNL and VNS, respectively; Amb, nucleus ambiguus; NGC, gigantocellular nucleus. The locations of the electrodes referenced to stereotaxic coordinates and those derived from Chicago Sky Blue staining are represented by open and filled symbols, respectively. The triangles and squares denote the inhibitory and excitatory respiratory responses, respectively (complete overlap occurred at six sites: two inhibitory and four excitatory).
Comparison of VN-mediated respiratory and ABP responses before and after IA injection into the stimulating sites
In six rats, VN-mediated respiratory and ABP responses were compared before and after local injection of IA in 10 trials. In four of these rats, a second trial was performed in either the contralateral side or in a different ipsilateral subnucleus. Examples of experimental recordings are presented in Fig. 3A-C. As illustrated in the top panel of A, electrical stimulation of the VNM evoked significant respiratory and ABP responses. As compared to 100 Hz (the third stimulation), stimulation at 200 Hz (the first and second) elicited much greater cardiorespiratory responses. The amplitude of the cardiorespiratory responses was consistent when the same stimulation was applied at a given site. These responses were significantly attenuated 2 h after IA injection into the stimulating sites (the bottom panel of Fig. 3A). Similarly, the excitatory respiratory responses to stimulation of the VNL and VNS were almost eliminated 2 h following IA lesions (Fig. 3B and C). Figure 3D presents the group data in which the significant changes in ventilation (≈45 %) in response to VN stimulation were attenuated to less than 5 % following IA injections. In addition, the pressor response observed in six trials with VNM stimulation was almost abolished after IA injection. It should be noted that an injection of IA into the NGC usually resulted in an immediate excitatory respiratory response by increasing VT and fR (VI= 223.9 ± 29.0 ml min−1vs. 408.7 ± 46.6 ml min−1, P < 0.05). These excitatory effects disappeared 2 h after injection. Statistically, the control values (baseline) of VI and MABP were not significantly changed 2 h after NGC lesions (VI= 237.4 ± 37.4 ml min−1vs. 228.4 ± 26.2 ml min−1, P > 0.05; MABP = 110.3 ± 3.2 mmHg vs. 118.8 ± 7.4 mmHg, P > 0.05).
Figure 3. Effects of ibotenic acid (IA) microinjection into the vestibular subnuclei on the cardiorespiratory responses evoked by local electrical stimulation of VNM (A), VNL (B) and VNS (C).
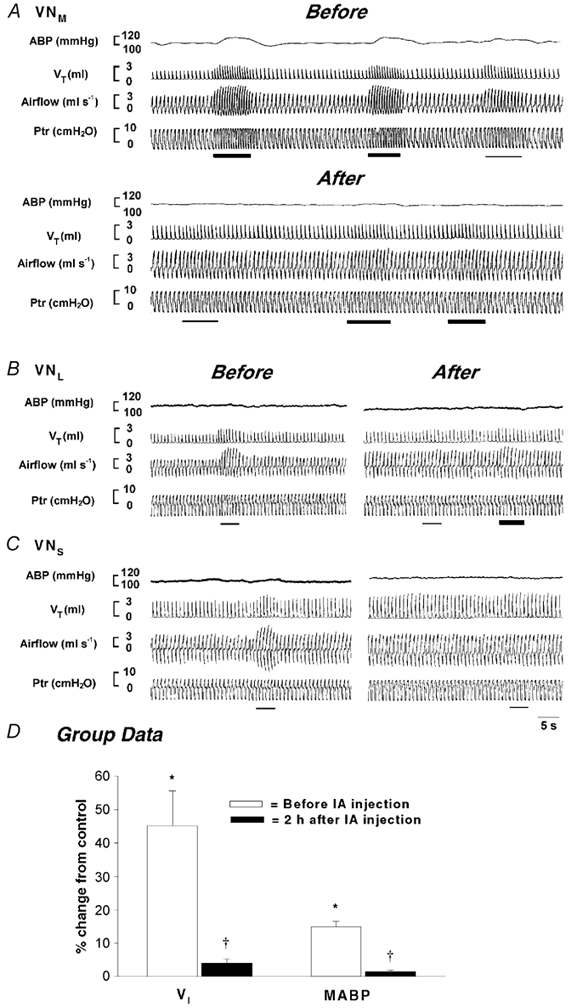
In each panel, the traces from the top to bottom are ABP, VT, airflow and Ptr. The thin and thick bars indicate stimulation at 100 and 200 Hz, respectively. Group data comparing cardiorespiratory responses before and after local IA injection are depicted in D. The number of rats used for stimulation of the VNM, VNL and VNS was six, two and two, respectively; the data are presented as means ±s.e.m.; *P < 0.05 between the responses to vestibular nucleus (VN) stimulation and control (without electrical stimulation); †P < 0.05 between the responses before and after IA injection. Please note that the MABP data were collected from six rats that exhibited a pressor response to VNM stimulation at 150 Hz.
Dependence of VN-mediated respiratory and ABP responses on the NGC
To establish whether NGC neurones are involved in the VN-mediated respiratory and ABP responses, microinjection of IA was used to selectively destroy neurones within the NGC in 26 rats. Figure 4 shows typical examples in which ventilation was inhibited by stimulation of the VNM (A), but excited by stimulation of the VNS (B) and VNL (C). Stimulation of the VNM elicited a pressor response, whereas stimulation of the VNS and VNL caused a delayed depressor response. Two hours after IA injections into the NGC, the previously evoked respiratory (inhibitory or excitatory) and ABP responses (pressor or depressor) were significantly diminished or eliminated. Representative lesioned areas on one side of the NGC are depicted in Fig. 4D (1-2 mm rostral and ≈0.5-1.0 mm lateral to the obex). Statistical results concerning the influence of NGC ablations on the VN-mediated ABP and respiratory responses are listed in Fig. 5. Electrical stimulation of the VNM significantly changed MABP (Fig. 5A), VT (Fig. 5B) and fR (Fig. 5C), as shown by open bars. However, these responses were nearly abolished following bilateral lesions of the NGC (filled bars). Stimulation of the VNL and VNS also altered VT and fR with no significant changes in MABP, and these responses were significantly diminished following NGC lesions. It should be emphasized that following NGC lesions, a maximal stimulating intensity (200 Hz) failed, in most of these cases, to evoke the responses observed previously (see Fig. 4).
Figure 4. Comparison of the evoked cardiorespiratory responses immediately before and 2 h after NGC lesions.
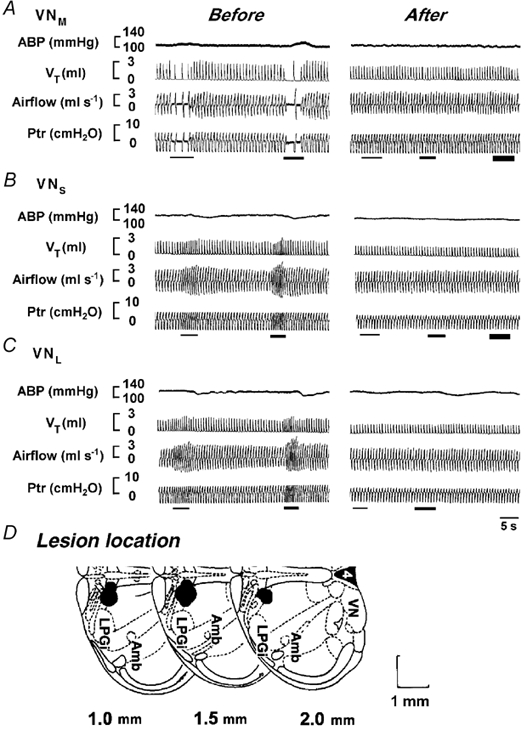
A-C, the cardiorespiratory responses to stimulation of the VNM, VNS and VNL. In each panel, the traces from the top to bottom are ABP, VT, airflow and Ptr. Note that the first, second and third (if presented) stimulation denoted by bars indicate a stimulus intensity of 100, 150 and 200 Hz, respectively. Representative areas stained by Chicago Sky Blue (IA) injections are illustrated schematically (one side) in D, where the measurements given at the bottom are the distances rostral to the obex. LPGi, lateral paragigantocellular nucleus (see Paxinos & Watson, 1986).
Figure 5. Group data showing the effects of NGC lesions on VN-stimulation-induced changes in MABP (A), VT (B) and respiratory frequency (fR; C).
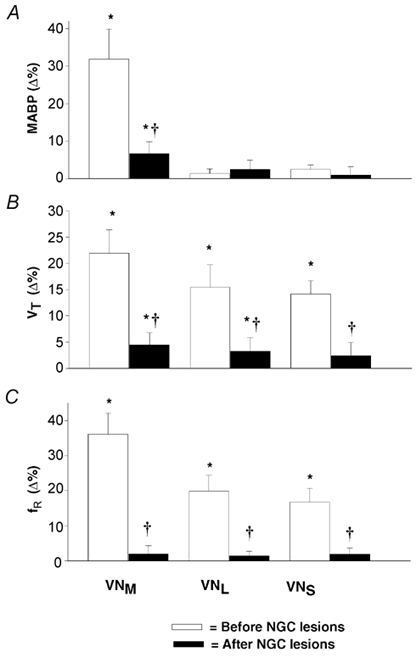
The numbers of rats for stimulation of the VNM, VNL and VNS were 20, 8 and 7, respectively; the data are presented as means ±s.e.m.; *P < 0.05 compared to control, †P < 0.05 between the responses before and after bilateral IA injection into the NGC.
To evaluate the effect of vehicle and experimental duration (time), stimulation of the VN was carried out over the same time course with vehicle injection in four rats. As depicted in Fig. 6, the cardiorespiratory responses to VN stimulation were not significantly different before (A) and 2 h after (B) vehicle injection into the NGC. The group data (numbers of trials for VNM, VNL and VNS were 2, 1 and 1, respectively) showed that the ventilatory responses to VN stimulation were 60.1 ± 10.2 % before and 56.2 ± 9.2 % 2 h after vehicle injection.
Figure 6. Cardiorespiratory responses to stimulation of the VNM before and 2 h after vehicle (100 nl) injection into the bilateral NGC in a rat.
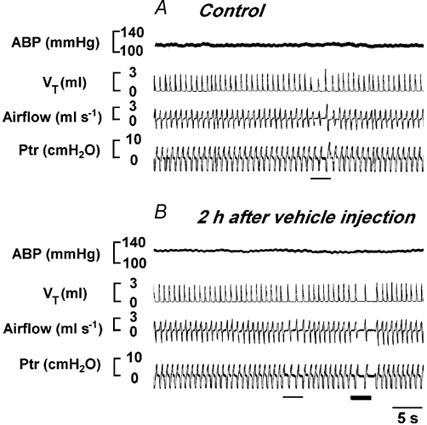
In each panel, the traces from the top to bottom are ABP, VT, airflow and Ptr. The thin and thick bars indicate stimulation at 100 and 200 Hz, respectively.
Respiratory and ABP responses to NMDA injection into the vestibular subnuclei
In the present study, injections of NMDA (32 trials) and its vehicle (6 trials) into different vestibular subnuclei were performed in nine rats. Figure 7 depicts experimental recordings of the respiratory and ABP responses to NMDA injection into the vestibular subnuclei in an anaesthetized rat. NMDA (50 nl) sequentially injected into the unilateral VNM (Fig. 7A), VNL (Fig. 7B) and VNS (Fig. 7C) significantly increased ventilation with little effect on ABP, and these responses returned to control levels within 2–3 min after injection. The locations of the NMDA injections are mapped in Fig. 7D. Similar results are also observed in the group data presented in Fig. 8. VNM, VNL and VNS stimulation significantly enhanced ventilation (Fig. 8A) by 74 %, 58 % and 60 %, respectively. The response amplitudes obtained from activation of individual subnuclei were not significantly different (P > 0.05). In no case did these injections markedly affect MABP (Fig. 8B). The cardiorespiratory responses to vehicle injections (two trials for each vestibular subnucleus over the same time period used for NMDA injection) were not significantly different from baseline (VI= 4.0 ± 5.8 %, P > 0.05; MABP = 1.0 ± 1.7 %, P > 0.05). In addition, three NMDA injections into the regions either 0.3 mm lateral to the VNL (two trials) or 0.2 mm ventral to the VNM failed to evoke significant cardiorespiratory responses (VI= 0.1 ± 4.3 %, P > 0.05; MABP = 1.4 ± 1.1 %, P > 0.05).
Figure 7. The cardiorespiratory responses to microinjection of N-methyl-d-aspartic acid (NMDA) into the VNM (A), VNL (B) and VNS (C).
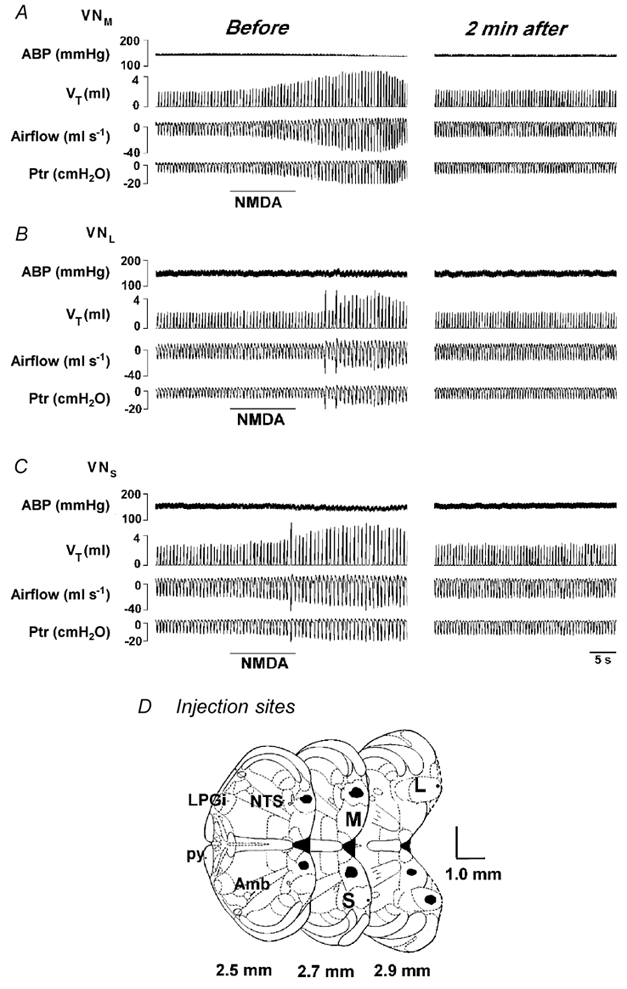
The immediate responses and their recovery (2 min after NMDA injection) are illustrated in the left and right columns, respectively. In each panel, the traces from the top to bottom are ABP, VT, airflow and Ptr. The horizontal bars represent the duration of microinjection of 50 nl NMDA into the given subnucleus. Injection sites stained by Chicago Sky Blue are marked in D, in which the numbers listed are the distances rostral to the obex. py, pyramidal tract (see Paxinos & Watson, 1986).
Figure 8. Group data presenting the effects of NMDA injection in the vestibular subnuclei on ventilation (A) and ABP (B).
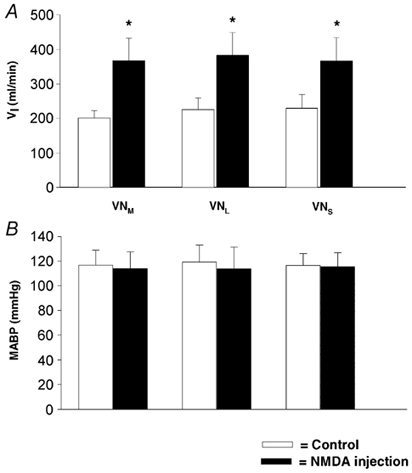
The number of animals in which NMDA was injected into the VNM, VNL and VNS was nine, nine and eight, respectively; data are presented as means ±s.e.m.; *P < 0.05 compared to control.
DISCUSSION
VN neurones are involved in respiratory modulations
We observed that electrical VN stimulation in the anaesthetized rat significantly altered ventilation. This VN-mediated respiratory response was the result of activation of local neurones rather than fibres of passage, because selective destruction of local VN neurones via microinjection of IA essentially abolished the respiratory responses previously observed in intact animals. In support of this, an elevated ventilatory response to microinjection of NMDA into the VN was observed in our subsequent studies and in awake goats (Wenninger et al. 2001). The cardiorespiratory responses to chemical stimulation of the VNM differ somewhat from those to electrical stimulation. NMDA injection into the subvestibular nuclei only increased ventilation without inhibitory respiratory and pressor responses. These differences may be due to the rather non-selective nature of electrical stimulation (neurones and fibres of passage) versus selective chemical stimulation of specific NMDA receptors. In addition, other types of receptors such as non-NMDA, neurokinin 1 and taurine, which are known to exist in the VN of the cat, guinea-pig and rat (Walberg et al. 1990; Vibert et al. 1996; Chen et al. 2000) may also play a role in cardiorespiratory modulation. The VN-mediated respiratory responses are not secondary to the changes in ABP since: (1) the responses can be either inhibitory or excitatory with ABP not changed or elevated; (2) the threshold required for evoking a detectable respiratory response is usually lower than that necessary to produce a detectable ABP alteration; (3) the respiratory responses always occur before the changes denoted in ABP. Our finding concerning VN-mediated respiratory responses is consistent with results obtained previously in other species. For example, electrical and chemical activation of the VN was able to alter respiratory motor output in cats (Bassal & Bianchi, 1982), rabbits (Mameli et al. 1988) and goats (Wenninger et al. 2001). The VN contains respiratory-modulated neurones, as demonstrated in studies on songbirds and budgerigar (Reinke & Wild, 1998). Activation of the vestibular system in the cat, via electrical stimulation of vestibular neurones or whole-body tilt, modulates the firing behaviour of respiratory-modulated neurones recorded in the pre-Bötzinger complex (Zheng et al. 1997) and medullary ventral respiratory group (Woodring & Yates, 1997). Anatomically, microinjection of the anterograde tracer Phaseolus vulgaris leukoagglutinin into the VN revealed projections to the vicinity of the nucleus ambiguus, nucleus tractus solitarius, and retrofacial nucleus in cats (Yates et al. 1995). Previous studies have also addressed the functional significance of the VN in respiratory modulation. Activation of the vestibular system by rotating the head in carotid-sinus-denervated, vagotomized and decerebrate cats significantly altered respiration (Rossiter et al. 1996; Woodring & Yates, 1997). More recently, studies carried out on humans indicated that stimulation of the semicircular canals, but not the otolith organs or neck muscle afferents, mediated the increased ventilatory responses predominantly by elevating fR (Monahan et al. 2002).
The vestibular subnuclei function differently in respiratory and ABP modulation
One of our major findings is that electrical stimulation of the VNL or VNS exclusively elevates ventilation with little effect on ABP, while activation of the VNM either inhibits or stimulates ventilation often with a pressor response. This finding provides the first experimental evidence to show the differential cardiorespiratory modulations of the vestibular subnuclei and explains some of the results noted in the literature. The question arises as to why stimulation of the VNM evokes different respiratory responses (either excitatory and inhibitory). A partial answer appears to be related to the different sites within the VNM activated by electrical stimulation. As shown in Fig. 2, the majority of sites responsible for excitatory and inhibitory respiratory response are localized at the rostral and caudal lateral portions of the VNM, respectively. Besides the differences in localization, our observation that NMDA injection into the VNM only elicits the excitatory respiratory responses reveals an involvement of different neurotransmitters in these varied responses. In addition, it is possible that some neurones receiving inputs from VNM neurones are excitatory, but others are inhibitory to inspiration. In fact, both bulbospinal expiratory (80 %) and inspiratory neurones (50 %) recorded in the medullary ventral respiratory group were responsive to electrical vestibular stimulation (Yates & Miller, 1996). Functionally, the role of the VN in the control of both inspiration and expiration has been investigated previously. Nose-up and nose-down rotation increased and decreased abdominal muscle activity, respectively, and these responses were abolished by ablation of the VN (Rossiter et al. 1996). In comparison, nose-up pitch with larger static tilt (50 deg) increased phrenic nerve activity, whereas horizontal rotation had little effect on abdominal muscle activity (Rossiter et al. 1996). The mechanisms underlying the different respiratory responses elicited by electrical stimulation of the VNM, and the reason why these responses are often accompanied by similar pressor responses, remain unknown. One of explanations would be that it simply reflects the different roles the postsynaptic neurones of the VNM play in the control of cardiorespiratory activity. The neurones receiving the inputs emanating from the given VNM site activated by electrical stimulation may be uniformly excitatory to the pressor response, but either excitatory or inhibitory to inspiration. With respect to ABP, it has been pointed out that the vestibular system influences the sympathetic nervous system and cardiovascular function (Uchino et al. 1970; Yates, 1992; Yates & Miller, 1994; Steinbacher & Yates, 1996b). Collectively, the fact that the vestibular subnuclei are capable of differentially influencing ventilation and ABP fits well with the compensatory cardiorespiratory responses noted with head movement and posture changes.
The VN-mediated respiratory and ABP responses are dependent on the integrity of the NGC
We observed that the respiratory (both excitatory or inhibitory) and/or pressor responses to electrical stimulation of different vestibular subnuclei were significantly attenuated or eliminated after selective destruction of NGC neurones via local IA injection. However, these VN-mediated responses were not significantly altered after vehicle injection over the same experimental time course. These data demonstrate that NGC neurones are essential for the full expression of the VN-mediated cardiorespiratory responses. These data differ somewhat from a previous report in which only the inhibitory components of the respiratory responses to electrical stimulation of vestibular nerve were eliminated following kainic acid injection into the medullary reticular formation (Mori et al. 2001). Several methodological differences may account for this discrepancy. First, the nature of the ventilatory responses to stimulation of the VN in the present study is different from the tonic activity of diaphragm and abdominal muscles in response to selective stimulation of the vestibular nerve. The fact that the VN receives not only labyrinthine inputs, but also inputs from other central regions raises the possibility that the mechanisms underlying the respiratory responses to stimulation of the VN and the vestibular nerve are not necessarily the same. Second, the regions lesioned were different (i.e. the NGC lesioned in this study is located in the mid-ventral portion of the medullary reticular formation). Finally, the species and animal preparations are different (anaesthetized rats vs. decerebrate cats). Nevertheless, our results confirm that the NGC and its adjacent area are important for the full expression of VN-mediated respiratory and ABP responses. Do the NGC neurones involved in the VN-mediated respiratory responses receive vestibular subnuclei inputs, either directly or indirectly? Our previous study, employing the anterograde tracing approach (HRP), has shown that some VN neurones project monosynaptically to the NGC (Zhang et al. 1999), suggesting a monosynaptic connection. However, further studies are required to clarify whether these monosynaptic connections are responsible for the VN-mediated cardiorespiratory responses we observed.
The NGC modulates respiration and ABP
IA is neurotoxic and is capable of initially stimulating neurones and then subsequently destroying them. Thus, the immediate respiratory excitatory responses to microinjection of IA into the NGC noted in the present study most probably reflect this non-specific excitatory cellular effect. There is a large body of evidence to support NGC involvement in respiratory control. Morphologically, neuronal pathways controlling the diaphragm and abdominal muscles have been investigated in the ferret and rat by transynaptic transport of the neuroinvasive pseudorabies virus (Dobbins & Feldman, 1994; Billig et al. 2001). The propriobulbar neurones that project to the bulbospinal neurones that innervate the phrenic and abdominal motor neurones were found in the NGC. Moreover, using retrograde tracer (HRP), it was found that the nasolabialis motoneurones of the facial nucleus, which are driven by the respiratory central network, receive monosynaptic afferents from NGC neurones (Chan, 1983). These results clearly suggest that NGC neurones innervate the premotor neurones responsible for the control of inspiration and expiration. Functionally, respiratory-modulated neurones have been recorded in the NGC in cats, and electrical stimulation of NGC neurones predominantly increased respiratory neuronal activity within the nucleus tractus solitarii and nucleus ambiguus (Vzaimnykh et al. 1980). Similar to our previous results (Xu et al. 2001), the NGC may not be critical for eupnoeic breathing control in the anaesthetized rat, since the eupnoeic breathing observed 2 h after IA injection into the NGC was not significantly different from the control. The role of the NGC in ABP modulation has also been evaluated. Stimulation of the NGC produces an immediate decrease (Chan, 1983; Stremel et al. 1990; Su et al. 1991; Xu et al. 2001) or increase in ABP (Wenninger et al. 2001; Xu et al. 2001), while no significant alteration was observed hours after microinjection of IA or kainic acid into the NGC (Wenninger et al. 2001; Xu et al. 2001). Taken together, these data support the assumption that the NGC may not be critical for maintaining baseline cardiovascular activity, but is involved in VN-mediated ABP responses.
Limitations of the experimental methods
A specific attempt was made to examine the consistency of the responses over the experimental time course. We believe that the diminished or abolished cardiorespiratory responses observed following IA injection into the VN sites or the NGC are not the result of a time-related variation in the responses because of the following reasons. First, the cardiorespiratory responses were consistent over time, as shown in Fig. 3 and Fig. 6. Second, the reduction of cardiorespiratory responses was dramatic (almost abolished). Third, in most cases, after IA lesions, maximal electrical stimulation failed to evoke the cardiorespiratory responses observed previously (Fig. 3 and Fig. 4). The gigantocellular reticular nucleus comprises a large region extending from the pons to the medulla (dorsal to ventral). In the present experiments, the lesions were made selectively in the mid-ventral portion of the medullary reticular formation because our previous anterograde tracing study in the rat documented that some VN neurones projected monosynaptically to this region. Since the lesioned areas were estimated by staining with Chicago Sky Blue, it is possible that the exact lesioned size is greater than those labelled in our figures. Owing to the variability in the location and extent of chemical lesions made in the NGC, we cannot discount the possibility of the involvement of the caudal ventrolateral medullary reticular formation in the VN-mediated ABP responses because this region is near the NGC. Indeed, previous studies have demonstrated that this region is critical to fully express vestibulosympathetic reflexes (Steinbacher & Yates, 1996a,b). The concentration of NMDA we used is unlikely to cause significant local neuronal lesions. We recently found that the excitatory ventilatory responses to the same concentration of NMDA injected into the fastigial nucleus were reproducible 10 min after the first injection in anaesthetized rats (F. Xu, T. Gibson & J. Zhuang, unpublished observation).
Summary
In the present study, we found that electrical stimulation of the VNL and VNS was excitatory to ventilation with limited effect on ABP, whereas stimulation of the VNM led to either an enhancement or reduction of ventilation that was often associated with a pressor response. These VN-mediated responses were significantly attenuated or eliminated following IA injection into the VN sites that had been stimulated electrically, or bilaterally into the NGC. Furthermore, microinjection of NMDA into the vestibular subnuclei always significantly elevated ventilation without ABP modulation. We conclude that neurones within the different vestibular subnuclei play different roles in the control of respiration and ABP. The integrity of the medullary NGC is essential for the full expression of these VN-mediated responses. Glutamate acting on NMDA receptors of the VN appears to contribute to the excitatory component of the VN-mediated respiratory responses. It is still questionable which neurotransmitters are involved in the VNM respiratory inhibitory and pressor responses.
Acknowledgments
The authors thank members of the University of Kentucky Respiratory Groups for helpful critiques. This study was supported by National Heart, Lung, and Blood Institute Grant HL-6342003 and a Career Investigator Award to F. Xu from the American Lung Association.
REFERENCES
- Bassal M, Bianchi AL. Inspiratory onset or termination induced by electrical stimulation of the brain. Respiration Physiology. 1982;50:23–40. doi: 10.1016/0034-5687(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Billig I, Hartge K, Card JP, Yates BJ. Transneuronal tracing of neural pathways controlling abdominal musculature in the ferret. Brain Research. 2001;912:24–32. doi: 10.1016/s0006-8993(01)02597-5. [DOI] [PubMed] [Google Scholar]
- Bystrzycka EK, Nail BS. The source of the respiratory drive to nasolabialis motoneurons in the rabbit: a HRP study. Brain Research. 1983;266:183–191. doi: 10.1016/0006-8993(83)90648-0. [DOI] [PubMed] [Google Scholar]
- Chan SH. Arterial pressure- and cardiac rhythm-related single-neuron activities in the nucleus reticularis gigantocellularis (NRGC) Journal of the Autonomic Nervous System. 1983;13:99–109. doi: 10.1016/0165-1838(85)90027-x. [DOI] [PubMed] [Google Scholar]
- Chen LW, Yung KK, Chan YS. Co-localization of NMDA receptors and AMPA receptors in neurons of the vestibular nuclei of rats. Brain Research. 2000;884:87–97. doi: 10.1016/s0006-8993(00)02913-9. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Metabotropic glutamate receptors in the guinea-pig medial vestibular nucleus in vitro. NeuroReport. 1995;6:1799–1802. doi: 10.1097/00001756-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rats. Journal of Comparative Neurology. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Gilman S, Newman SW. In: Clinical Neuroanatomy and Neurophysiology. 8. Gilman S, Newman SW, editors. Philadelphia: David Company; 1992. [Google Scholar]
- Huang Q, Zhou D, St John WM. Vestibular and cerebellar modulation of expiratory motor activities in the cat. Journal of Physiology. 1991;436:385–404. doi: 10.1113/jphysiol.1991.sp018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro M, Savaki HE, Kutyna FA, Davidsen L, Sokoloff L. Metabolic mapping in the sympathetic ganglia and brain of the spontaneously hypertensive rat. Journal of Cerebral Blood Flow and Metabolism. 1983;3:460–467. doi: 10.1038/jcbfm.1983.72. [DOI] [PubMed] [Google Scholar]
- Kohler C, SchwarcZ R. Comparison of ibotenic acid and kainite neurotoxicity in rat brain: a histological study. Neuroscience. 1983;8:819–835. doi: 10.1016/0306-4522(83)90013-1. [DOI] [PubMed] [Google Scholar]
- Mameli O, Tolu E, Melis F, Caria MA. Labyrinthine projection to the hypoglossal nucleus. Brain Research Bulletin. 1988;20:83–88. doi: 10.1016/0361-9230(88)90011-1. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Sharpe MK, Drury D, Ertl AC, Ray CA. Influence of vestibular activation on respiration in humans. American Journal of Physiology. 2002;282:R689–694. doi: 10.1152/ajpregu.00568.2001. [DOI] [PubMed] [Google Scholar]
- Mori RL, Bergsman AE, Holmes MJ, Yates BJ. Role of the medial medullary reticular formation in relaying vestibular signals to the diaphragm and abdominal muscles. Brain Research. 2001;902:82–91. doi: 10.1016/s0006-8993(01)02370-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Orlando: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Reinke H, Wild JM. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. Journal of Comparative Neurology. 1998;391:147–163. [PubMed] [Google Scholar]
- Rossiter CD, Hayden NL, Stocker HSD, Yates BJ. Changes in outflow to respiratory pump muscles produced by natural vestibular stimulation. Journal of Neurophysiology. 1996;76:3274–3284. doi: 10.1152/jn.1996.76.5.3274. [DOI] [PubMed] [Google Scholar]
- Steinbacher BC, Yates BJ. Brainstem interneurons necessary for vestibular influences on sympathetic outflow. Brain Research. 1996a;720:204–210. doi: 10.1016/0006-8993(96)00141-2. [DOI] [PubMed] [Google Scholar]
- Steinbacher BC, Yates BJ. Processing of vestibular and other inputs by the caudal ventrolateral medullary reticular formation. American Journal of Physiology. 1996b;271:R1070–1077. doi: 10.1152/ajpregu.1996.271.4.R1070. [DOI] [PubMed] [Google Scholar]
- Stremel RW, Waldrop TG, Richard CA, Iwamoto GA. Cardiorespiratory responses to stimulation of the nucleus reticularis gigantocellularis. Brain Research Bulletin. 1990;24:1–6. doi: 10.1016/0361-9230(90)90281-4. [DOI] [PubMed] [Google Scholar]
- Su CK, Kuo JS, Chai CY, Ten CT. Neurons in the medullary gigantocellular nucleus mediate cardioinhibition in cats. Chinese Journal of Physiology. 1991;34:399–412. [PubMed] [Google Scholar]
- Takeshita S, Sasa M, Ishihara K, Matsubayashi H, Yajin K, Okada M, Izumi R, Arita K, Kurisu K. Cholinergic and glutamatergic transmission in medial vestibular nucleus neurons responding to lateral roll tilt in rats. Brain Research. 1999;840:99–105. doi: 10.1016/s0006-8993(99)01775-8. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Kudo N, Tsuda K, Iwamura Y. Vestibular inhibition of sympathetic nerve activities. Brain Research. 1970;22:195–206. doi: 10.1016/0006-8993(70)90004-1. [DOI] [PubMed] [Google Scholar]
- Vibert N, Serafin M, Vidal PP, Muhlethaler M. Effects of substance P on medial vestibular nucleus neurons in guinea-pig brainstem slices. European Journal of Neuroscience. 1996;8:1030–1036. doi: 10.1111/j.1460-9568.1996.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Babalian A, De Waele C, Serafin M, Vibert N, Muhlethaler M. NMDA receptors of the vestibular nuclei neurones. Brain Research Bulletin. 1996;40:347–352. doi: 10.1016/0361-9230(96)00123-2. [DOI] [PubMed] [Google Scholar]
- Vzaimnykh O, Iader S, Tsentra D. Reciprocal connections of respiratory center nuclei. Biull Eksp Biological Medicine. 1980;90:652–654. [PubMed] [Google Scholar]
- Walberg F, Ottersen OP, Rinvik E. GABA, glycine, aspartate, glutamate and taurine in the vestibular nuclei: an immunocytochemical investigation in the cat. Experimental Brain Research. 1990;79:547–563. doi: 10.1007/BF00229324. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Martino P, Geiger L, Hodges M, Serra A, Feroah TR, Forster HV. Multiple rostral medullary nuclei can influence breathing in awake goats. Journal of Applied Physiology. 2001;91:777–788. doi: 10.1152/jappl.2001.91.2.777. [DOI] [PubMed] [Google Scholar]
- Woodring SF, Yates BJ. Responses of ventral respiratory group neurons of the cat to natural vestibular stimulation. American Journal of Physiology. 1997;273:R1946–1956. doi: 10.1152/ajpregu.1997.273.6.R1946. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Modulation of respiratory motor output by cerebellar deep nuclei in the rat. Journal of Applied Physiology. 2000;89:996–1004. doi: 10.1152/jappl.2000.89.3.996. [DOI] [PubMed] [Google Scholar]
- Xu F, Sato M, Spellman MJ, Jr, Mitchell RA, Severinghaus JW. Topography of cat medullary ventral surface hypoxic acidification. Journal of Applied Physiology. 1992;73:2000–2009. doi: 10.1152/jappl.1992.73.6.2631. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang Z, Frazier DT. Ventilation in shaker mutant rats with hereditary Purkinje cell degeneration. Society for Neuroscience Abstracts. 1998;24:379. [Google Scholar]
- Xu F, Zhou T, Gibson T, Frazier DT. Fastigial nucleus-mediated respiratory responses depend on the medullary gigantocellular nucleus. Journal of Applied Physiology. 2001;91:1713–1722. doi: 10.1152/jappl.2001.91.4.1713. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Sasa M, Matsunaga T. Glutamate as a primary afferent neurotransmitter in the medial vestibular nucleus as detected by in vivo microdialysis. Brain Research. 1997;762:243–246. doi: 10.1016/s0006-8993(97)00498-8. [DOI] [PubMed] [Google Scholar]
- Yates BJ. Vestibular influences on the sympathetic nervous system. Brain Research Reviews. 1992;17:51–59. doi: 10.1016/0165-0173(92)90006-8. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Balaban CD, Miller AD, Endo K, Yamaguchi Y. Vestibular inputs to the lateral tegmental field of the cat: potential role in autonomic control. Brain Research. 1995;689:179–206. doi: 10.1016/0006-8993(95)00569-c. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Research. 1993;629:209–217. doi: 10.1016/0006-8993(93)91322-j. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: Implications of cardiovascular control. Journal of Neurophysiology. 1994;71:2087–2092. doi: 10.1152/jn.1994.71.6.2087. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD. Vestibular respiratory regulation. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural Control of the Respiratory Muscles. Boca Raton, New York, London, Tokyo: CRC; 1996. pp. 271–282. [Google Scholar]
- Zhang Z, Xu F, Gibson T, Frazier DT. Hypercapnia-activated neurons in the fastigial nucleus (FN) project to medullary nucleus gigantocellularis (Gi) Society for Neuroscience Abstracts. 1999;25:938. [Google Scholar]
- Zheng Y, Umezaki T, Nakazawa K, Miller AD. Role of pre-inspiratory neurons in vestibular and laryngeal reflexes and in swallowing and vomiting. Neuroscience Letters. 1997;225:161–164. doi: 10.1016/s0304-3940(97)00208-5. [DOI] [PubMed] [Google Scholar]


