Without a continuous supply of nutrients, the brain dies within a few minutes. Blood flow and glucose uptake are so tightly coupled to neuronal function that techniques which image these processes (e.g. PET) are valuable monitors of neuronal activity. However, in order to understand brain metabolism completely, and to find specific molecular targets for therapeutic intervention, it is also essential to study the dynamics of metabolism in single living brain cells.
In this issue of The Journal of Physiology, Ainscow and colleagues (Ainscow et al. 2002) show that it is possible to measure changes in cytosolic ATP concentration in both neurons and glia by adenovirus transfection of luciferase, a firefly enzyme that catalyses the generation of light from luciferin and ATP. This technique is an extremely valuable addition to our repertoire of tools for studying brain function, and will enable investigators to examine the energetic consequences of physiological and pathological interventions at the cellular and subcellular level. Glucose elevation stimulated a rise in intracellular ATP in glial cells isolated from the hypothalamus and cerebellum and in cerebellar neurons, but surprisingly not in hypothalamic neurons. When luciferase was targeted to the plasma membrane using SNAP25, a small decrease in ATP was even observed in hypothalamic neurons. Interestingly, a similar anomaly is seen in the pancreatic islet, where glucose-dependent increases in cytosolic and submembrane ATP occur in β-cells but not in α-cells. Why this is the case remains unclear, but it is possible that it simply reflects the greater consumption of ATP by hypothalamic neurons, so that although metabolic flux increases the absolute concentrations of ATP do not change. In favour of this view is the finding that the Na+-K+-ATPase inhibitor oubain unmasked a glucose-induced elevation in ATP in hypothalamic neurons.
Glial cells, once considered the poor relations of neurons, are now known to play a vital role in the brain by removing the by-products of neuronal electrical activity from the extracellular space and secreting substances crucial for neuronal function. Tight glial envelopes at synaptic junctions mediate uptake of glutamate released from nerve terminals, thereby ensuring spatial and temporal precision of synaptic transmission, and preventing glutamate excitotoxicity. Likewise, K+ ions lost during the action potential are siphoned away from the extra-neuronal space to prevent their build-up producing membrane depolarisation and over-excitability. Indeed, it may be detrimental for neurons to become excited when glia lack the energy to buffer such changes. The data of Ainscow et al. (2002) provide a rationale for how neurons know when it is safe to be active.
Their results indicate that glia respond to elevation of extracellular glucose with a large increase in anaerobic, but not aerobic, glycolysis. This is consistent with previous studies showing that lactate is released in large amounts from glia (Pellerin et al. 1998) and suggests that lactate might act as the messenger that communicates the metabolic status of glia to adjacent neurons. Ainscow et al. (2002) provide strong evidence in support of this idea. First, they show that neurons (but not glia) respond to lactate with elevation of cytosolic ATP. Second, they demonstrate that both glia and neurons in the hypothalamus express high levels of the lactate transporter MCT1. Finally, they find that lactate inhibits ATP-sensitive potassium (KATP) channels in cell-attached patches on hypothalamic neurones.
KATP channels are expressed widely in the brain, where they couple excitability to energy status of the neuron by hyperpolarising the plasma membrane and so diminishing neuronal excitability when intracellular energy supplies are low (Ashcroft, 1988). The findings of Ainscow et al. (2002) suggest an exciting new role for neuronal KATP channels: that of synchronising neuronal activity with the energy status of the surrounding glia. When glial metabolism is compromised by low fuel supplies, lactate release decreases, leading to opening of KATP channels in adjacent neurons and a reduction in their firing rate (Fig. 1A). Consequently, a toxic build-up of glutamate and potassium is prevented. Because of the high input resistance of the neuron, even a tiny outward current is sufficient to silence the cell, and thus only a very small number of K+ ions exit with little effect on extracellular potassium levels. Conversely, when glia have sufficient fuel to safeguard neuronal function, they release lactate as a metabolic by-product which shuts neuronal KATP channels, thereby allowing adjacent neurons to fire freely, as dictated by synaptic inputs and intrinsic pacemaker mechanisms (Fig. 1B).
Figure 1.
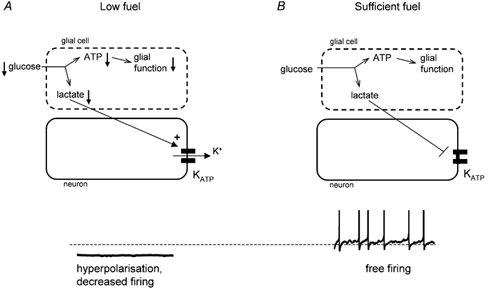
Several questions remain to be clarified with regard to this model. For example, it is important to determine how lactate affects spontaneous firing in different types of neurons, and how this is correlated with changes in intracellular ATP and KATP channel activity. Combining the cytosolic and submembrane measurements of ATP pioneered by Ainscow et al. (2002) with perforated patch recordings in the same cell within a brain slice will be the next step towards tackling such questions.
References
- Ainscow EK, Mirshamsi S, Tang T, Ashford MLJ, Rutter GA. Journal of Physiology. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. Annual Review of Neuroscience. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Developmental Neuroscience. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]


