Abstract
The nucleus tractus solitarius (NTS) is a relay nucleus that integrates peripheral chemoreceptor input in response to hypoxia and hence influences the generation of respiratory rhythm. Several studies have shown that administration of progesterone stimulates ventilatory responses to hypoxia. There is some evidence that this steroid hormone can act at the level of the arterial peripheral chemoreceptors, whereas its action in the central nervous system remains unclear. To investigate a possible central involvement during hypoxia, we studied the effect of progesterone on neuronal activities recorded extra- and intracellularly in the NTS using brainstem slices. Central chemosensitivity was tested by comparing synaptic activity and intrinsic electro-responsiveness of 38 neurones during normoxia and hypoxia. In more than two-thirds of neurones recorded, hypoxia elicited a hyperpolarisation, a decrease in the input resistance and a decrease in spontaneous activity. In the remaining neurones (n = 12) hypoxia elicited a depolarisation and an increase in spontaneous activity. In all neurones tested, synaptic potentials evoked by stimulation of the tractus solitarius were decreased by hypoxia. While progesterone (1 μM) had no effect under normoxic conditions, it partially reversed all hypoxic neuronal responses. This effect developed over 2-3 min and reversed within 5 min suggesting a non-genomic mechanism of action. Taken together these results suggest that progesterone interacts with the hypoxia-induced cellular signalling. We conclude that in the NTS, transmission of afferent signals is reduced by hypoxia and restored by progesterone administration. Such a mechanism may contribute to the stimulation of breathing in response to hypoxia observed following progesterone administration in vivo.
Modulation of the ventilatory pattern by sex steroids, such as progesterone, has been widely investigated (Brodeur et al. 1986; Bayliss et al. 1987). Under normoxic conditions, progesterone is a ventilatory stimulant (Regensteiner et al. 1989). Ablative procedures show that the ventilation-stimulatory effect of progesterone may involve the hypothalamus where its nuclear receptors are expressed (Bayliss et al. 1990). Progesterone also acts on other brain regions related to respiration such as the nucleus tractus solitarius (NTS), which participates in the generation of respiratory rhythm (Bianchi et al. 1995), and where chemoafferent activities from the carotid bodies are integrated (Housley & Sinclair, 1988; Finley & Katz, 1992). In fact microinjection of progesterone in the cat NTS increases respiratory motor activity recorded from the phrenic nerve (Bayliss et al. 1987). However the cellular mechanisms underlying the action of progesterone under physiological conditions are poorly understood.
Several studies have shown that progesterone also stimulates the ventilatory response to hypoxia (Zwillich et al. 1978; Regensteiner et al. 1989; Tatsumi et al. 1997). Endogenous hormonal progesterone has been considered to be responsible for the sex difference that exists in the ventilatory response to hypoxia (Bayliss & Millhorn, 1992). However it has been shown that this sex difference also exists in ovariectomised and prepubertal animals (Mortola & Saiki, 1996). While progesterone-induced stimulation of the hypoxic ventilatory response is mainly mediated by the carotid bodies (Hannhart et al. 1990; Tatsumi et al. 1997), a direct effect of progesterone in the central nervous system has also been suggested (Hannhart et al. 1990). It has recently been shown that the brain expresses steroidogenic enzymes (Baulieu & Robel, 1995) and that neurones, astrocytes and oligodendrocytes can produce neurosteroids (Zwain & Yen, 1999).
There is compelling evidence that brainstem neurones are sensitive to oxygen deficits (Nolan & Waldrop, 1993; Sun & Reis, 1994). Neuronal responses to a decrease in PO2 seem to involve multiple molecular mechanisms such as changes in ionic conductances (Haddad & Jiang, 1993), cellular ATP levels (LaManna et al. 1996) and free radical levels (Chandel & Schumacker, 2000). In addition to stimulating the ventilatory response to hypoxia, progesterone exerts a neuroprotective effect on neurones during adaptive and pathological processes such as cortical hypoxia and ischaemia (Jiang et al. 1996). These stimulatory and neuroprotective properties may render progesterone useful as a treatment for patients suffering from chronic hypoxia (Sutton et al. 1975; Lombard et al. 1985; Stein, 2001). Brainstem chemosensitivity and neuroprotective properties of progesterone suggest that part of the stimulation by progesterone on the hypoxic ventilatory response may be due to a neuronal effect. In the present study we have investigated the possibility that progesterone exerts a direct effect on the NTS neuronal network under hypoxia.
Progesterone may interact with the cytoplasmic membrane of neurones (Ke & Ramirez, 1990) resulting in changes in membrane permeability (Joëls & Karst, 1995). In vivo, the effects of progesterone on ventilation are generally induced by prolonged administration (Zwillich et al. 1978; Regensteiner et al. 1989), and ventilatory changes are seen over long periods of time. Therefore, it is generally considered that excitation results from a genomic effect of progesterone (Bayliss et al. 1990). However, the mechanisms of this induction are not known, and early effects of the action of progesterone on ventilation and hypoxic ventilatory response have never been studied. Whether or not progesterone acts by a genomic or non-genomic mechanism, particularly in the central nervous system (Baulieu & Robel, 1995), remains to be determined.
To identify the central effect of progesterone, we used the isolated and superfused in vitro NTS slice preparation (Fortin & Chamapgnat, 1993). We studied the effect of progesterone on intrinsic membrane properties and synaptic activity under normoxic and hypoxic conditions.
METHODS
Slice preparation
The entire sequence of experimental treatment was performed on 38 Sprague-Dawley male rats (Janvier, LeGenest St Isle, France) weighing between 100 and 120 g. Thirty eight neurones (one per slice) were recorded. Males were used for these studies to exclude any influence of endogenous female hormones. All experiments were carried out according to ethical guidelines defined by the French agricultural ministry and the EU council directive for the care and use of laboratory animals (no. 2889). Rats were deeply anaesthetised with ether and decapitated at the second cervical vertebra. After transcollicular section the brain was rapidly chilled with cold (4 °C) artificial cerebrospinal fluid (ACSF), removed and separated from the cerebellum. During these surgical procedures cold ACSF was dripped onto exposed brain surfaces. Coronal slices 450 μm thick were cut with a vibratome (Leica, Wetzlar, Germany) in cold ACSF bubbled with 95 % O2-5 % CO2. Sections at the level of the obex, containing the NTS, were transferred to a warm (30 °C) recording chamber saturated with 95 % O2-5 % CO2. The upper surface was first exposed to a warm humidified atmosphere of 95 % O2-5 % CO2 for 5 min, whereupon sections were completely immersed and continuously superfused at a constant rate (2 ml min−1). The recording session began 1 h after the slice was prepared. Other slices containing the NTS were transferred to a chamber saturated with 95 %O2-5 % CO2 and maintained at 30 °C.
Electrophysiological recordings
Anatomical structures were identified through a binocular microscope (Leica). A stimulating tungsten concentric bipolar electrode was positioned on the dorsomedial part of the solitary tract. Every 6 s, a single stimulus of 50 μs duration was delivered from a programmable stimulator. When the stimulus intensity was higher than threshold, a single stimulus evoked an EPSP, the amplitude of which increased progressively with stimulus intensity until a maximal amplitude was reached. Recordings were performed in the NTS using conventional extracellular microelectrodes filled with 2 mNaCl (resistance 10 MΩ) or intracellular microelectrodes filled with 3 m KCl (resistance 50-90 MΩ) or 2 m potassium acetate (resistance 80-150 MΩ). Microelectrodes were pulled (o.d., 1.2 mm; Clark Electromedical Instruments, Pangbourne, UK) using a Sutter puller (model P-97, Sutter Instrument Co., Novato, CA, USA). Micropipettes were introduced into the slice in steps of 2 μm. The extracellular signal was amplified 50 000-fold with a Grass amplifier (P511 preamplifier, Grass Instrument Co., Quincy, MA, USA). Intracellular current clamp recordings and current injections were made through an active bridge circuit (bridge mode) while compensating for capacitance and resistance of the electrode. Single electrode voltage-clamp recordings were performed using a sample-and-hold amplifier (npi SEC 1L/H; npi-Advanced Electronic, Germany). The sampling frequency was 5 Hz. Steps of 50 pA were applied for measurement of input resistance.
Hypoxic exposure and drug application
Control perfusion solution (normoxic condition) was ACSF of the following composition (mm): NaCl 124, KCl 5, CaCl2 2, MgSO4 2.2, KH2PO4 1.26, NaHCO3 26 and glucose 10; bubbled with 95 % O2-5 % CO2. Studies using slices 300-400 μm thick, have shown that PO2 at the core of the slice is about 119 mmHg (Jiang et al. 1991; Nolan & Waldrop, 1993; Ballanyi et al. 1996). Two hypoxic levels were used: moderate hypoxia was achieved by superfusing ACSF saturated with 20 % O2-75 % N2-5 % CO2. This level of O2 produces a tissue PO2 of 15-20 mmHg in the core of a 400 μm slice (Jiang et al. 1991). Severe hypoxia was obtained with ACSF saturated with 95 % N2-5 % CO2, which produces a tissue PO2 level in the slice close to 0 mmHg (Jiang et al. 1991). Duration of hypoxic exposure was 35 min. Pilot studies revealed that the response to hypoxia was established after 5 min and remained quite stable for the next 30 min. Fifteen minutes after the onset of hypoxia, 1 μM progesterone (obtained from Sigma) was bath applied for a duration of 5 min. Progesterone was washed out under hypoxic conditions for 15 min, and recovery from hypoxia was observed under normoxia.
Neuronal activity was measured in the absence and presence of progesterone, under normoxic and hypoxic conditions. The protocol was repeated to verify reproducibility, but only the first application was used in the statistical analysis.
Data analysis
During the experiment, signals were displayed on an oscilloscope (Tektronics model 2221A, Les Ulis, France) and a chart recorder (Graphtec WR7600, Irvine, CA, USA) and were stored on tape for off-line analysis. Signal outputs were digitized on a PC using a LabMaster interface (Scientific Solution, Mentor, OH, USA) coupled to Acquis1 software. The activity of NTS neurones was evaluated by measuring (1) the frequency of spontaneous post-synaptic action potentials (Fig. 2 and Fig. 3A), and (2) the frequency of on-going synaptic potentials as an index of the activity of presynaptic neurones (Fig. 3B). Statistical analysis was performed using Wilcoxon's signed-rank test (P < 0.05 being considered significant).
Figure 2. Experimental protocol of progesterone application.
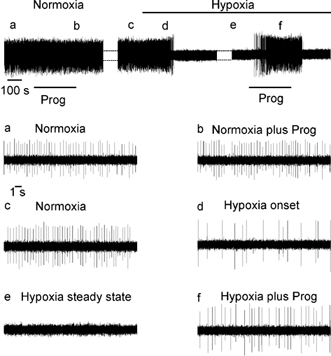
Extracellular recording of discharge of NTS neurone under normoxic and hypoxic conditions. Progesterone application is denoted by the bar. Top panel: a, normoxia; b, progesterone in normoxia (1 μM, 5 min); c, normoxia after progesterone wash out; d, onset of hypoxic response; e, steady state of the hypoxic response; f, progesterone during hypoxia. Lower panels show expanded time base of the top panel.
Figure 3. Progesterone partially reversed the hypoxia-induced decrease of spontaneous activity in the NTS.
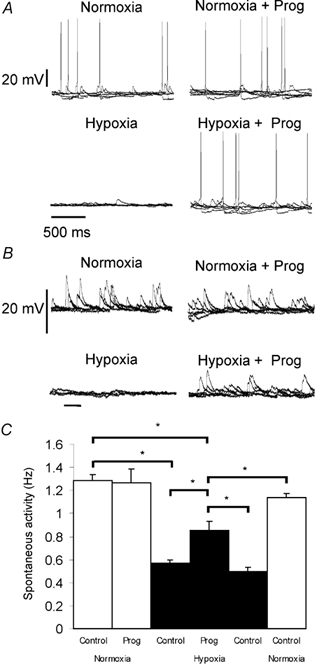
A, spontaneous firing of an NTS neurone and B, spontaneous synaptic activity of another NTS neurone. Intracellular records were made in normoxia, normoxia plus progesterone, hypoxia and hypoxia plus progesterone. C, histogram shows variations of spontaneous activity induced by progesterone, in normoxia (□) and hypoxia (▪). Mean frequency ± s.e.m. of n = 8 neurones; * P < 0.05.
RESULTS
Hypoxia induced different patterns of response in NTS neurones
Results were obtained from extracellular recordings from 16 neurones and intracellular recordings from 14 neurones, subjected to moderate hypoxia. In addition eight neurones recorded intracellularly were subjected to severe hypoxia.
In extracellular recordings, moderate hypoxia induced either an inhibition (n = 10-16) or an excitation (n = 6-16). Inhibition consisted of a decrease of spontaneous activity (44.3 ± 8.5 % of firing rate in normoxia), leading in five out of the 10 cells to a complete suppression of the spiking activity. In excited neurones, discharge frequency reached a maximal level, then decreased and stabilised at levels which were nevertheless always higher than in normoxia (157.7 ± 18.6 % of firing rate in normoxia).
Similar responses were obtained from cells recorded intracellularly; a majority (11/14) were hyperpolarised and stopped firing whereas a few (3/14) were depolarised (Fig. 1, and below). Both responses were observed throughout the NTS (Fig. 1D), that is there was no regionalisation of excitatory or inhibitory effects (Fig 1C).
Figure 1. Different effects of hypoxia on isolated NTS neurones in a brainstem slice.
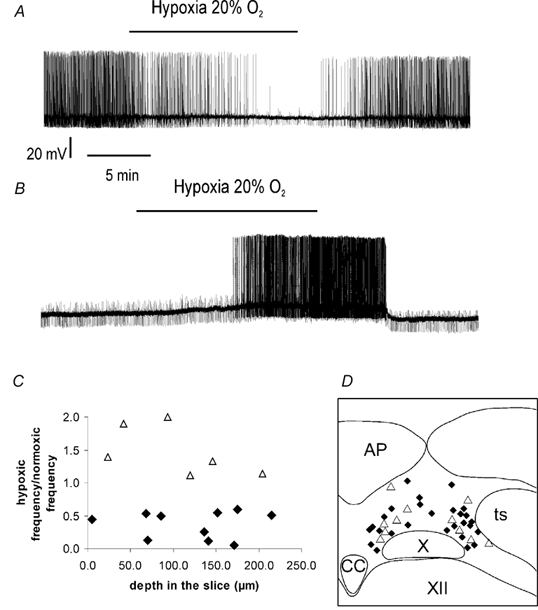
A, example of a neurone inhibited by hypoxia (intracellular recording). Moderate hypoxia (20 % O2) elicited a reversible hyperpolarisation accompanied by a reduction of spontaneous activity and of input resistance. B, example of a neurone excited by hypoxia (intracellular recording). Moderate hypoxia elicited a reversible depolarisation accompanied by an increase of spontaneous activity. C, type of neuronal responses to hypoxia, excitation or inhibition, according to the depth of the recording in the slice, expressed as the firing frequency ratio between normoxia and hypoxia (extracellular recording, n = 16). ♦, neurones inhibited by hypoxia; ▵, neurones excited by hypoxia. D, localisation of recorded neurones in the NTS (n = 38). ♦, neurones inhibited by hypoxia; ▵, neurones excited by hypoxia. AP, area postrema; CC, the medullary central canal; ts, the tractus solitarius; X, the dorsal motor vagal nucleus; XII, the hypoglossal nucleus.
In eight neurones, severe hypoxia induced either membrane hyperpolarisation (n = 5) or depolarisation (n = 3). Moderate and severe hypoxia induced the same type of effect (excitation, n = 1; inhibition, n = 2) when tested successively on the same neurone.
Progesterone restores spontaneous post-synaptic activity and synaptic potentials during hypoxia-induced inhibition
Figure 2 illustrates the protocol used to study the effect of progesterone under normoxic and hypoxic conditions. Progesterone was always applied once hypoxia-induced inhibition had reached a steady state. After hypoxia-induced suppression of firing (n = 16, Figs 2d and e, and 3), a short bath application of progesterone (1 μM, 5 min) reduced the inhibition by 38 % and therefore restored the neuronal activity to a level closer to that in normoxia (Fig. 2f and Fig. 3). During extracellular recording, this effect was restricted to neurones that were totally inhibited by hypoxia (n = 5) whereas it was always observed with intracellular recordings (n = 11). This effect of progesterone was always observed between 2 and 3 min after the beginning of drug application. Progesterone had no effect on NTS neurones under normoxic conditions (n = 30; Figs 2a and b, and 3) nor on neurones exhibiting a slight hypoxia-induced reduction of discharge activity (data not shown, n = 5 neurones recorded extracellularly).
Progesterone restores post-synaptic input resistance of cells inhibited by hypoxia
Under normoxia, NTS neurones exhibited a resting membrane potential of −60.0 ± 4.2 mV (n = 11). Moderate hypoxia induced membrane hyperpolarisation to −65.5 ± 4.0 mV, which was in all cases reduced by 45.5 % by progesterone (membrane potential during progesterone: −63.0 ± 4.1 mV, P < 0.01versus hypoxia).
In 8/11 neurones, input resistance decreased by approximately 40 % from 227.1 ± 19.6 MΩ in normoxia to 138.3 ± 5.2 MΩ in hypoxia (Fig. 4B). While progesterone had no effect on the input resistance of NTS neurones under normoxic conditions, progesterone reduced the hypoxia-induced decrease of input resistance by 20 % (input resistance, 156.3 ± 7.1 MΩ, P < 0.05, Fig. 4). The remaining neurones (3/11) showed no significant change in their input resistance under hypoxic conditions in the absence or presence of progesterone.
Figure 4. Progesterone partially reversed the hypoxia-induced increase of input resistance.
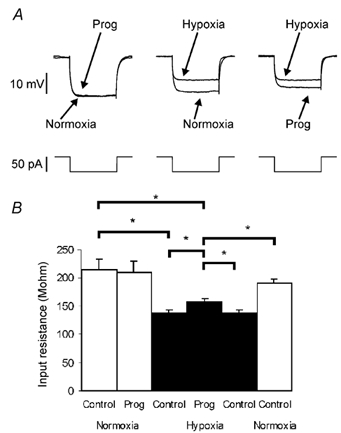
Negative current pulses (50 pA) were applied every 5 s. A, progesterone under normoxic conditions did not affect the membrane resistance. Hypoxia decreased membrane resistance, progesterone plus hypoxia partially reversed this effect. B, histogram shows variations of input resistance induced by progesterone, during normoxia (□) and during hypoxia (▪). Mean input resistance ± s.e.m. in n = 8 neurones; * P < 0.05.
Progesterone applied during severe hypoxia produced effects similar to those induced by moderate hypoxia (i.e. a partial restoration of input resistance and membrane potential; data not shown). Therefore, the influence of progesterone was not overcome by increasing the level of hypoxia.
Progesterone reduces the excitatory effects of hypoxia
In 6/22 neurones recorded intracellularly, moderate (n = 3-14) or severe hypoxia (n = 3-8) depolarised the cells and increased rate of action potential firing. These effects were partially reversed by progesterone (Fig. 5). Although membrane potential was depolarised by hypoxia from −62 ± 1.3 mV during normoxia to −56 ± 0.8 mV during hypoxic condition, this increase was reduced by 66 % (to −60 ± 1.2 mV) in the presence of progesterone.
Figure 5. Progesterone partially reversed the hypoxia-induced depolarisation.
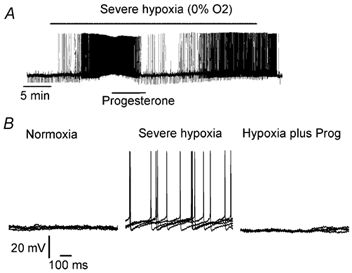
A, intracellular recording of a neurone excited by severe hypoxia (0 % O2). Hypoxia increased the membrane potential and spontaneous activity and progesterone reversed these modifications. B, progesterone (Prog) in hypoxic conditions restored the membrane potential and neuronal activity to a level close to that seen in normoxia (five superimposed traces).
The post-synaptic action of progesterone was investigated by injecting depolarising current steps and analysing the pattern of firing (Fig. 6). Normoxia was associated with a discharge accommodation that caused a time-dependent increase in the inter-spike intervals (Fig. 6A). This increase was reduced by hypoxia, resulting in an increase in the total number of action potentials per current step (Fig. 6B). Progesterone partially restored the firing accommodation in hypoxic neurones (Fig. 6D).
Figure 6. Progesterone partially reversed the hypoxia-induced loss of accommodation.
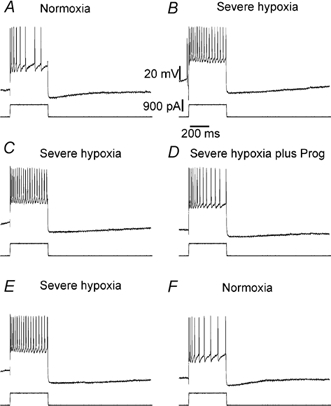
Positive current pulses (900 pA) were applied to neurones that were excited by hypoxia. In normoxia, spike accommodation occurred, which disappeared in hypoxic conditions (A and B), while an increased duration of the after-hyperpolarisation (AHP) was observed. Progesterone application restored accomodation but did not affect the kinetics of the AHP (C and D). In normoxic conditions both accommodation and the short duration of the AHP (D and E) were restored.
Taken together, these results show that progesterone reduces both hyperpolarising and depolarising responses to hypoxia, suggesting that the mechanism of action of progesterone is not necessarily linked to a particular electrophysiological response to altered levels of oxygen availability.
Progesterone reverses the synaptic depression induced by hypoxia
To investigate whether or not changes in synaptic transmission contribute to the effects of progesterone on neuronal responses during hypoxia, we recorded post-synaptic potentials elicited by a single electrical pulse of stimulation delivered to sensory afferent fibres in the tractus solitarius (Ts) (Fig. 7). For these experiments we selected those neurones that showed no change in input resistance during hypoxia or in response to progesterone treatment (n = 5). In these cells, changes in passive post-synaptic properties were thus expected to minimally affect the size of the synaptic potentials. The amplitude of synaptic potentials decreased from 3.89 ± 0.51 mV under control conditions to 2.40 ± 0.33 mV during hypoxia. This decrease was observed in neurones that were either inhibited or excited by hypoxia during both moderate and severe hypoxia. Progesterone, which was inactive under normoxic conditions, reduced the effect of hypoxia by 58.4 % and restored synaptic potentials to control levels (3.27 ± 0.14 mV; Fig. 7A). The same effect was obtained with different chloride ion gradients (KCl or potassium acetate) in the recording electrode, indicating that the response was not triggered by GABAergic transmission.
Figure 7. Progesterone reversed the hypoxia-induced decrease of evoked post-synaptic potentials (PSPs).
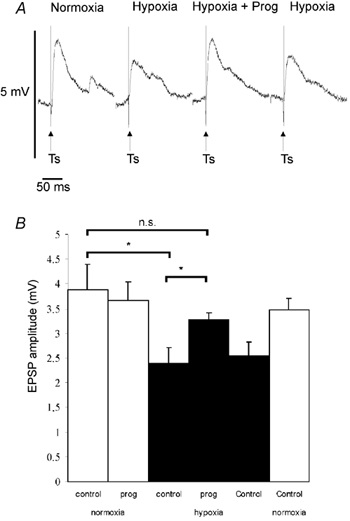
Stimulation of Ts-evoked PSPs in NTS neurones, which present no change in membrane resistance under hypoxia. A, hypoxia decreased the amplitude of evoked PSPs, while during hypoxia plus progesterone application, the amplitude of evoked PSPs was restored. B, histogram shows the variation of PSP amplitude evoked by Ts stimulation induced by progesterone in normoxic (□) and hypoxic (▪) conditions. Mean PSP amplitude ± s.e.m. of n = 5 neurones; * P < 0.05; n.s., non significant.
DISCUSSION
The major finding of this study is that progesterone, inactive under normoxic conditions, reduces both hyperpolarising and depolarising responses to hypoxia, suggesting that the acute effect of progesterone on the neuronal excitability is dependent on previous hypoxic challenge. We propose that progesterone interacts with the hypoxia-induced cellular signalling rather than through a selective membrane mechanism.
NTS neurones are sensitive to moderate hypoxia
There is compelling evidence that hypoxia can influence the activity of neurones in the central nervous system (Nolan & Waldrop, 1993, 1996; Sun & Reis, 1994). However, chemosensitivity of central neurones in vitro has generally been demonstrated using severe hypoxia (0 % O2, Nieber et al. 1995; Ballanyi et al. 1996). In the present study the effects of oxygen deficiency were examined using moderate hypoxia (20 % O2), which is closer to the levels of O2 availability observed during physiological hypoxic challenges, such as high altitude. Similar O2 levels (20 % O2) were typically used in early studies to demonstrate in vitro an increase in chemosensory output (Eyzaguirre & Koyano, 1965) and glucose consumption (Obeso et al. 1993) in glomus cells of the carotid body. We therefore conclude that the level of 20 % hypoxia used for studying chemosensitivity in carotid body is sufficient to investigate oxygen sensibility in NTS neurones. In the present study, neural responses to moderate hypoxia were compared to those induced by severe hypoxia. In fact it is difficult to decide whether hypoxic responses described in vitro are operational in vivo. However the present study shows that responses to progesterone during severe hypoxia are reversible and preserved when hypoxia is reduced to moderate levels. The data revealed that both stimuli produced either hyperpolarisation or depolarisation in individual cells. The main difference between the two hypoxic levels appeared to be quantitative whereas qualitatively the response to hypoxia was not affected by the level of the hypoxic exposure. In agreement with our results, previous studies have shown that in the brainstem, the level of hypoxia (10-20 % O2versus 0 % O2) does not change the type of response (excitation or inhibition) but rather increases the amplitude of the response (Nolan & Waldrop, 1993; O'Reilly et al. 1995).
Progesterone acts rapidly to alter neuronal responses to hypoxia
The effect on hypoxic responses starts 2-3 min after the onset of progesterone application. This delay is similar to that measured using the hydrophobic peptide substance P (Jacquin et al. 1989) and cholecystokinin (Branchereau et al. 1992) under the same experimental condition. We therefore conclude that lipophilic properties do not affect the accessibility of progesterone to its active site. This short response time also strongly suggests that progesterone is active at a membrane site (non-genomic). Most studies have examined the genomic effects of progesterone, since this hormone is a known regulator of gene transcription (Katzenellenbogen et al. 1996). The type of transcriptional activation observed following progesterone exposure is dependent on the type of nuclear receptor isoform present (Tora et al. 1988). In contrast to the rapid action of progesterone that we observed in the NTS, genomic effects are generally slow, exhibiting latencies longer than 30 min (Zakon, 1998). Non-genomic effects of progesterone have been attributed to binding to the plasma membrane (Wehling, 1997), which has also been observed with NTS neurones (Ke & Ramirez, 1990). Recently progesterone membrane receptors have been found and cloned in the CNS of rats (Gerdes et al. 1998; Krebs et al. 2000), opening new perspectives. Given the rapidity of the responses we observed, we suggest that the ability of progesterone to modify post-synaptic neuronal activity under hypoxic conditions is linked to a non-genomic effect of the steroid.
We found no effect of progesterone under normoxia whereas studies performed in vivo (Bayliss et al. 1987) have shown a prolonged stimulation of normoxic ventilation 45 min after the beginning of progesterone injection. This suggests that stimulation of ventilation during normoxia is elicited through a genomic mechanism (Bayliss et al. 1990) whereas the observed effect of progesterone on the hypoxic response in vitro is probably non-genomic.
Effects of progesterone are not channel specific
Different neuronal responses to hypoxia have been described in different brain structures; hyperpolarisation was reported in motoneurones in the dorsal motor vagal nucleus (Ballanyi et al. 1996), whereas depolarisation was described in hypoglossal neurones (Haddad & Donnelly, 1990; O'Reilly et al. 1995). In the present study, as in a previous study by Dean et al. (1991), both depolarising and hyperpolarising responses were observed in NTS neurones in response to hypoxia. These differential responses seem to be due to the heterogeneity of the NTS neurone properties. Many hypoxia-induced cellular responses have been explained by the involvement of channels sensitive to ATP. In particular ATP-dependent K+ (KATP) channels are activated by a reduction in ATP levels. These channels are responsible for the neuronal hyperpolarisation observed under hypoxic conditions (Mourre et al. 1989; Trapp & Ballanyi, 1995; Mironov et al. 1998). Alternatively, inactivation of Na+-K+-ATPase during energy depletion subsequent to anoxia or glucose deprivation might be a major factor involved in hypoxia-induced depolarisation (Donnelly et al. 1992). The type of neuronal response observed under hypoxic conditions may depend on the presence of the KATP subunit Kir6.2. In transgenic mice deficient in Kir6.2, neurones in the substantia nigra respond to hypoxia by depolarising, rather than hyperpolarising as in wild-type mice (Yamada et al. 2001). Consequently the type of hypoxic response observed in any given population of neurones may depend on the complement of KATP or other channels. Since application of progesterone tended to bring the membrane potential back to its pre-hypoxic value in all cases, this suggests that progesterone acts on a common hypoxia response pathway that can lead either to depolarisation or hyperpolarisation, depending on cell type.
This possibility is further strengthened by two other results. First, progesterone failed to alter neuronal activity under normoxic conditions. Second, the reversal by progesterone of hypoxia-induced effects was not overcome by increasing the level of hypoxia. These results suggest that progesterone selectively intervenes downstream of the O2-sensing or hypoxic-signalling pathway and upstream of the activated channels. During O2 deprivation, the major alteration affecting all cells is a decrease in the cellular content of ATP (LaManna et al. 1996). ATP production could therefore be one locus at which progesterone acts to restore neuronal metabolism during hypoxia. In fact, there is a progesterone binding site on proton ATP synthase (Ramirez et al. 1996) although its role in regulating ATP synthase activity is not understood.
Progesterone restores transmission of sensory afferent signals within the NTS during hypoxia
Sensory afferent signals were only modified on neurones presenting no change in input resistance during hypoxia suggesting that post-synaptic mechanisms were either weak or located at a long electrical distance from the cellular site of recording. Alternatively, it is possible that not all NTS neurones respond to progesterone in this preparation with a change in input resistance. In these cells, changes in passive post-synaptic properties were thus expected to minimally affect the size of the synaptic potentials.
The hypoxia-induced decrease of Ts-evoked potentials observed in this study may be explained by a decreased release of GABA and glutamate (Neubauer et al. 1990; Richter et al. 1999), which are the major neurotransmitters responsible for post-synaptic potentials (PSPs) in the NTS (Fortin & Champagnat, 1993). Thus, progesterone may restore synaptic responses during hypoxia by acting presynaptically on hypoxia-sensitive mechanisms controlling neurotransmitter release. However, an action of progesterone on GABAergic synapses can be excluded because the same type of response was achieved with KCl- and potassium acetate-filled electrodes. Therefore, we suspect that progesterone acts on glutamatergic transmission. In addition to altering responses evoked by afferent stimulation, on-going synaptic activity within the network, which was depressed by hypoxia, was also increased by progesterone. Thus, in this brainstem slice preparation, progesterone appears to act as a neuromodulator that counterbalances the gating influence of hypoxia on the integrative function of the NTS.
In vivo, the stimulatory effect of progesterone on hypoxic ventilatory responses can only partially be explained by stimulation of the carotid body. The acute effects described in this study would be expected to prevent or diminish hypoxic gating of chemosensory input to the NTS from the carotid body. Therefore progesterone may modulate chemosensitivity both at peripheral (carotid body) and central (NTS) sites.
Acknowledgments
We thank D. M. Katz for critically reading the manuscript. This work was supported by grants from CNRS, PICS (912), CEE (QLRT-2000-01467), ACI-BDPI No. 57, DRET No. 0034077, Fondation pour la Recherche Médicale. O. Pascual held a fellowship from the Ministry of National Education and Research (MENSR), France.
REFERENCES
- Ballanyi K, Doutheil J, Brockhaus J. Membrane potentials and microenvironnement of rat dorsal vagal cells in vitro during energy depletion. Journal of Physiology. 1996;495:769–784. doi: 10.1113/jphysiol.1996.sp021632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Non-genomic mechanisms of action of steroid hormones. Ciba Foundation Symposium. 1995;191:24–37. doi: 10.1002/9780470514757.ch3. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Cidlowski JA, Millhorn DE. The stimulation of respiration by progesterone in ovarectomized cats is mediated by an estrogen-dependant hypothalamic mechanism requiring gene expression. Endocrinology. 1990;126:519–527. doi: 10.1210/endo-126-1-519. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Millhorn DE. Central neural mechanisms of progesterone action application to the respiratory system. Journal of Applied Physiology. 1992;73:393–404. doi: 10.1152/jappl.1992.73.2.393. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Millhorn DE, Gallman EA, Cidlowski JA. Progesterone stimulates respiration through a central nervous system steroid receptor-mediated mechanism in cat. Proceedings of National Academy of Sciences of the USA. 1987;84:7788–7792. doi: 10.1073/pnas.84.21.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Böhme GA, Champagnat J, Morin-Surun M-P, Durieux C, Blanchard J-C, Roques BP, Denavit-Saubié M. Cholecystokinin A and cholecystokinin B receptors in neurones of the brainstem solitary complex of the rat: pharmacological identification. Journal of Pharmacology and Experimental Therapeutics. 1992;260:1433–1440. [PubMed] [Google Scholar]
- Brodeur P, Mockus M, McCullough R, Moore LG. Progesterone receptors and ventilatory stimulation by progestin. Journal of Applied Physiology. 1986;60:590–595. doi: 10.1152/jappl.1986.60.2.590. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria, old question, new insight. Journal of Applied Physiology. 2000;88:1880–1889. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- Dean JB, Gallman EA, Zhu WH, Millhorn DE. Multiple effects of hypoxia on neurons in dorsal motor nucleus (X) and nucleus tractus solitarii (NTS) Society for Neuroscience. 1991 Abstracts 17, 187.7. [Google Scholar]
- Donnelly DF, Jiang C, Haddad GG. Comparative responses of brainstem and hippocampal neurons to O2 deprivation: in vivo intracellular studies. The American Journal of Physiology. 1992;262:L549–554. doi: 10.1152/ajplung.1992.262.5.L549. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Koyano H. Effect of hypoxia, hypercapnia and pH on the chemoreceptor activity of the carotid body in vitro. Journal of Physiology. 1965;178:385–409. doi: 10.1113/jphysiol.1965.sp007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organisation of carotid body chemoafferent projections to the brainstem of the rat. Brain Research. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fortin G, Champagnat J. Spontaneous synaptic activities in rat nucleus tractus solitarius neurones in vitro: evidence for re-excitatory processing. Brain Research. 1993;630:125–135. doi: 10.1016/0006-8993(93)90650-c. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. Journal of Physiology. 1990;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two puative steroid membrane receptors. Biological Chemistry. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Progress in Neurobiology. 1993;40:277–318. doi: 10.1016/0301-0082(93)90014-j. [DOI] [PubMed] [Google Scholar]
- Hannhart CK, Picket CK, Moore LG. Effects of estrogens and progesterone on carotid body output responsiveness to hypoxia. Journal of Applied Physiology. 1990;68:1909–1916. doi: 10.1152/jappl.1990.68.5.1909. [DOI] [PubMed] [Google Scholar]
- Housley GD, Sinclair JD. Localization by kainic acid lesion of neurones transmitting the carotid chemoreceptor stimulus for respiration in rats. Journal of Physiology. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin T, Denavit-Saubié M, Champagnat J. Substance P and serotonin mutually reverse their excitatory effects in the nucleus tractus solitarius. Brain Research. 1989;20:214–222. doi: 10.1016/0006-8993(89)90616-1. [DOI] [PubMed] [Google Scholar]
- Jiang C, Agulian S, Haddad GG. O2 tension in adult and neonatal brain slices under several experimental conditions. Brain Research. 1991;568:159–164. doi: 10.1016/0006-8993(91)91392-e. [DOI] [PubMed] [Google Scholar]
- Jiang N, Choop M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Research. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H. Effect of estradiol and progesterone on voltage-gated calcium and potassium conductance in the CA1 hippocampal neurons. Journal of Neuroscience. 1995;15:4289–4297. doi: 10.1523/JNEUROSCI.15-06-04289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen JA, O'Malley BW, Katzenellenbogen BS. Tripartite steroid hormone receptor pharmacology: interaction with multiple effector site as a basis for the cell and promoter-specific action of these hormones. Molecular Endocrinology. 1996;10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- Ke FC, Ramirez VD. Binding of progesterone to nerve cell membrane of rat brain using progesterone conjugated to 125I-bovine serum albumin as ligand. Journal of Neurochemistry. 1990;54:467–472. doi: 10.1111/j.1471-4159.1990.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proceedings of the National Academy of Sciences of the USA. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna JC, Haxiu MA, Kutina-Nelson KL, Pundik S, Erokwu B, Yeh ER, Lust WD, Cherniack NS. Decreased energy metabolism in brainstem during central respiratory depression in response to hypoxia. Journal of Applied Physiology. 1996;81:1772–1777. doi: 10.1152/jappl.1996.81.4.1772. [DOI] [PubMed] [Google Scholar]
- Lombard RM, Zwillich CW, Creach CE, Pierson DJ, Weil JV. Medical therapy of obstructive sleep apnea. Medicial Clinics of North America. 1985;69:1317–1335. doi: 10.1016/s0025-7125(16)30989-0. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langhor K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. Journal of Physiology. 1998;509:755–766. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: gender differences. Respiration Physiology. 1996;106:21–34. doi: 10.1016/0034-5687(96)00064-3. [DOI] [PubMed] [Google Scholar]
- Mourre C, Ben Ari Y, Bernardi H, Fosset M, Lazdunski M. Antidiabetic sulfonyl urea: location of binding site in the brain and effect on the hyperpolarization induced by anoxia in hippocampal slices. Brain Research. 1989;486:159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. Journal of Applied Physiology. 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Nieber K, Sevcik J, Illes P. Hypoxic changes in rat locus coeruleus neurones in vitro. Journal of Physiology. 1995;486:33–46. doi: 10.1113/jphysiol.1995.sp020788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan PC, Waldrop TG. In vivo and in vitro responses of neurons in the ventrolateral medulla to hypoxia. Brain Research. 1993;630:101–114. doi: 10.1016/0006-8993(93)90648-7. [DOI] [PubMed] [Google Scholar]
- Nolan PC, Waldrop TG. In vitro responses of VLM neurones to hypoxia after normobaric hypoxic acclimatization. Respiration Physiology. 1996;105:23–33. doi: 10.1016/0034-5687(96)00033-3. [DOI] [PubMed] [Google Scholar]
- Obeso A, Gonzalez C, Rigual R, Dinger B, Fidone SJ. Effect of O2 level on glucose uptake in rabbit carotid body. Journal of Applied Physiology. 1993;74:2387–2393. doi: 10.1152/jappl.1993.74.5.2387. [DOI] [PubMed] [Google Scholar]
- O'Reilly JP, Jiang C, Haddad GG. Major differences in response to graded hypoxia between hypoglossal and neocortical neurones. Brain Research. 1995;683:179–186. doi: 10.1016/0006-8993(95)00373-x. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone and testosterone in the rat brain: fantasy or reality. Cellular and Molecular Neurobiology. 1996;16:175–178. doi: 10.1007/BF02088175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner RG, Woodward WD, Hagerman DD, Weil VD, Pickett CK, Bender PR, Moore LJ. Combined effect of female hormones and metabolic rate on ventilatory drives in women. Journal of Applied Physiology. 1989;66:808–813. doi: 10.1152/jappl.1989.66.2.808. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmit-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. Journal of Physiology. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen. Trends in Neurosciences. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Sutton FD, Zwillich CW, Creagh CE, Pierson DJ, Weil JV. Progesterone for outpatient treatment of Pickwickian syndrome. Annals of Internal Medicine. 1975;83:476–479. doi: 10.7326/0003-4819-83-4-476. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Pickett CK, Jacoby CR, Weil JW, Moore LG. Role of endogenous female hormones in hypoxic chemosensitivity. Journal of Applied Physiology. 1997;83:1706–1710. doi: 10.1152/jappl.1997.83.5.1706. [DOI] [PubMed] [Google Scholar]
- Tora L, Hinrich G, Turcotte B, Gaub M-P, Chambon P. The N-terminal region of chicken progesterone receptor specifies target gene activation. Nature. 1988;333:195–198. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurones in vitro. Journal of Physiology. 1995;487:37–50. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M. Specific, non genomic actions of steroid hormones. Annual Review of Physiology. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizi T, Seino S, Inagaki N. Protective role of ATP-sensitive channels in hypoxia-induced generalized failure. Science. 2001;292:1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- Zakon HH. The effect of steroidal hormones on electrical activity of excitable cells. Trends in Neurosciences. 1998;21:202–207. doi: 10.1016/s0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SSC. Neurosteroidogenesis in astrocytes, oligodendrocytes and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- Zwillich CW, Natalino MR, Sutton FD, Weil JV. Effects of progesterone on chemosensitiviy in normal men. Journal of Laboratory and Clinical Medicine. 1978;92:262–269. [PubMed] [Google Scholar]


